Abstract
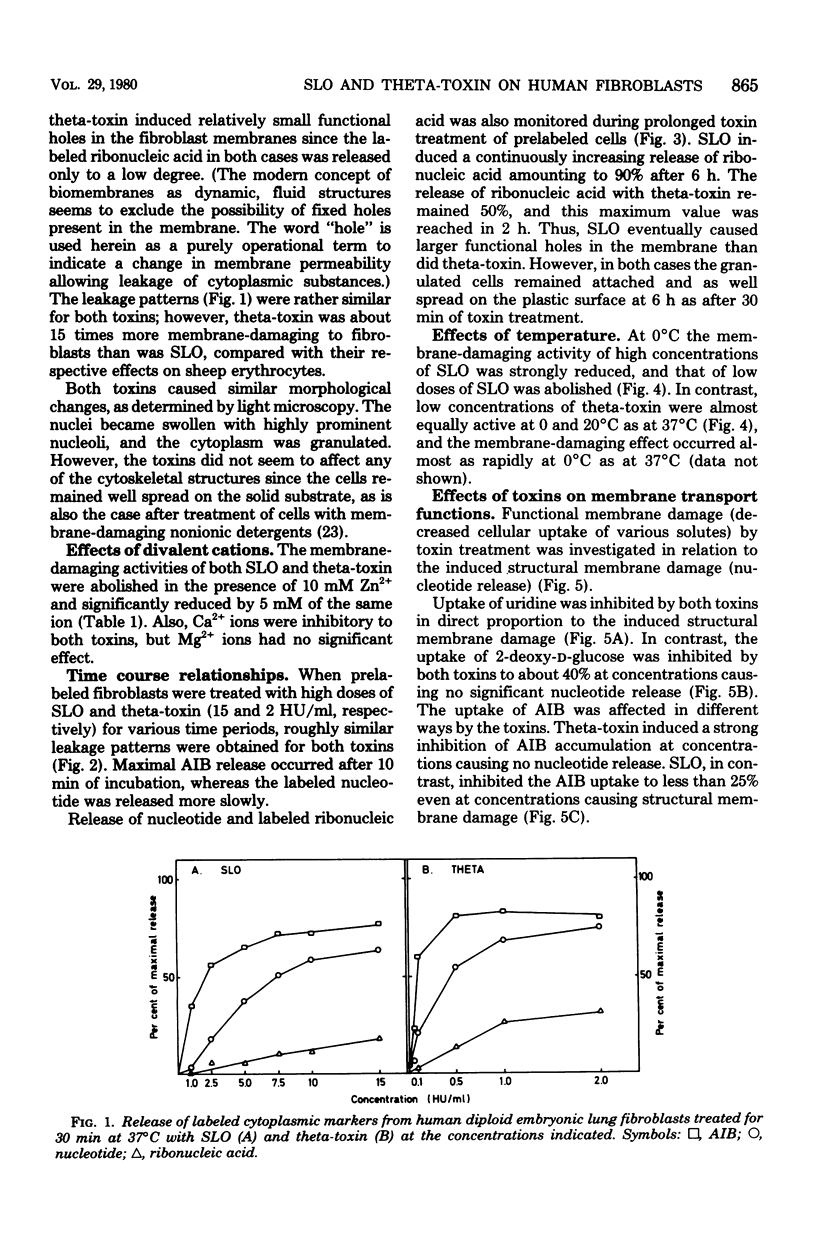
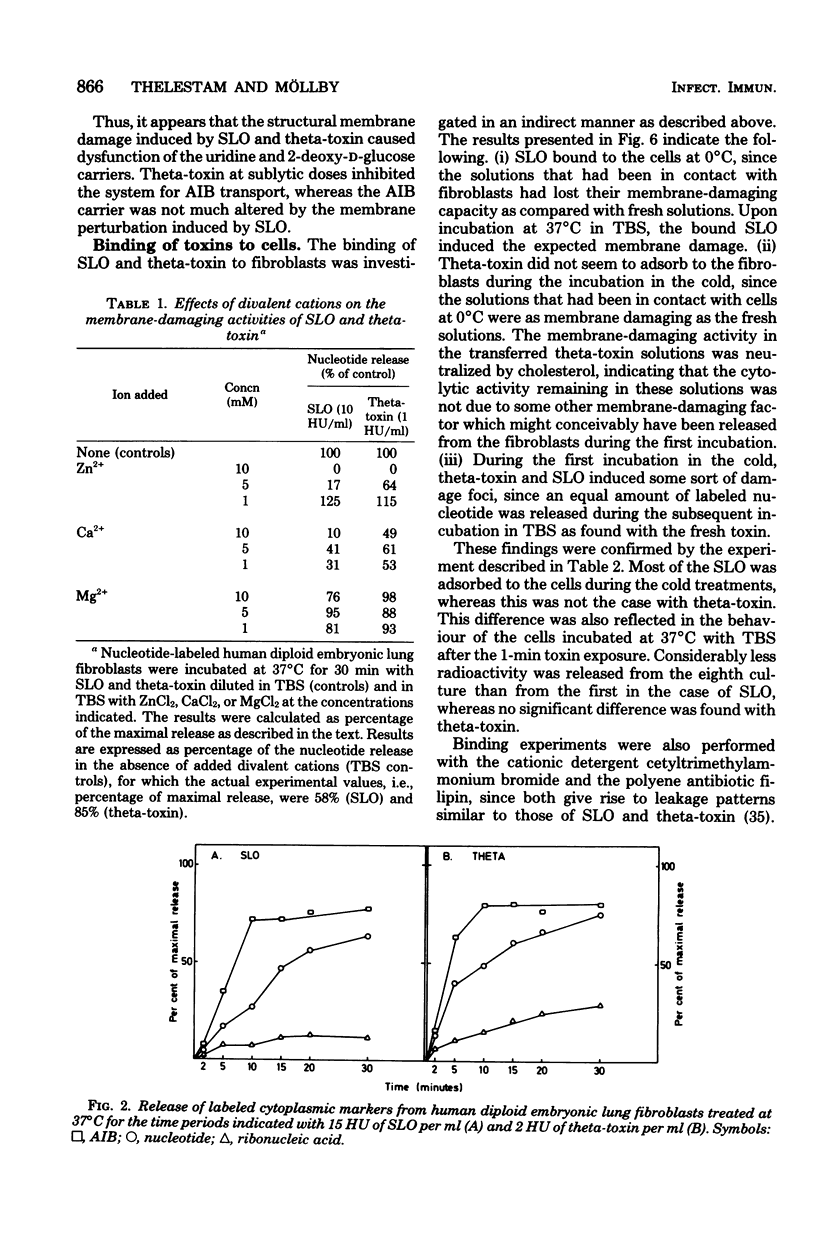
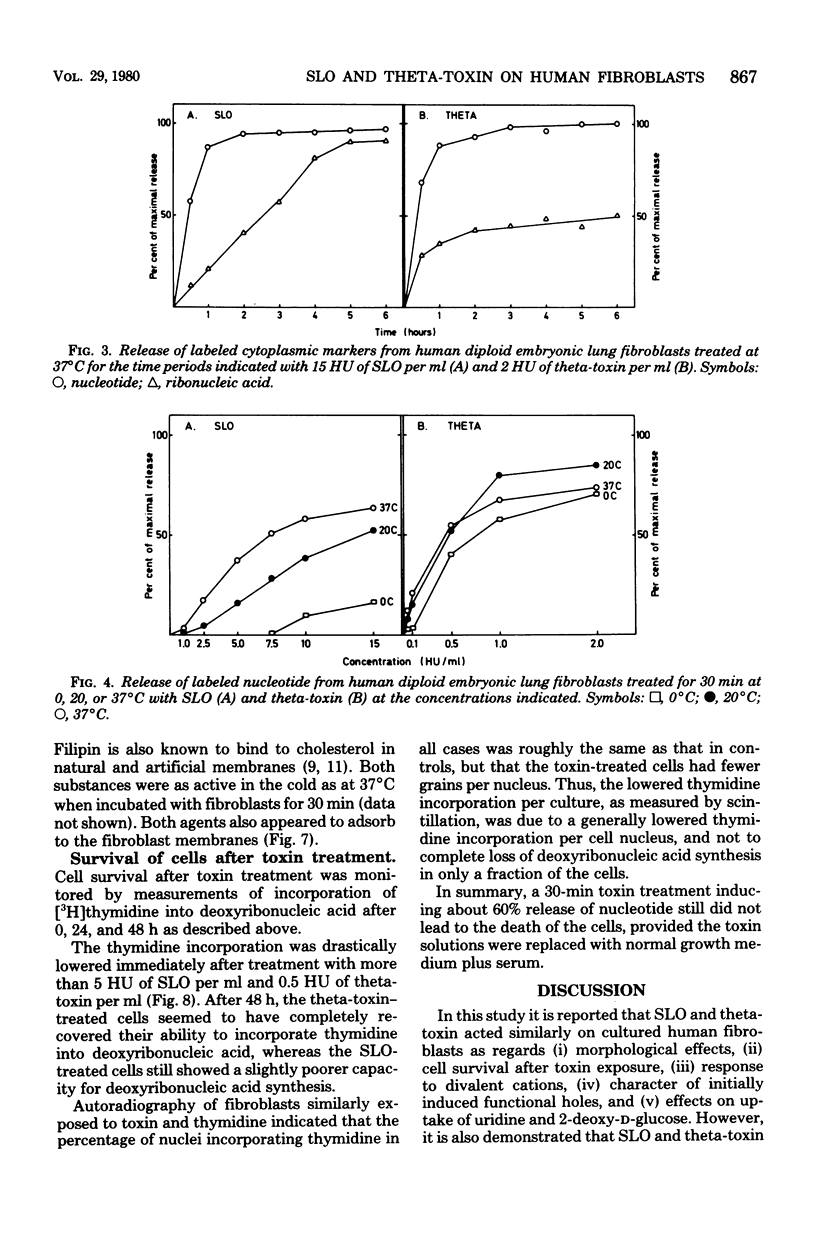
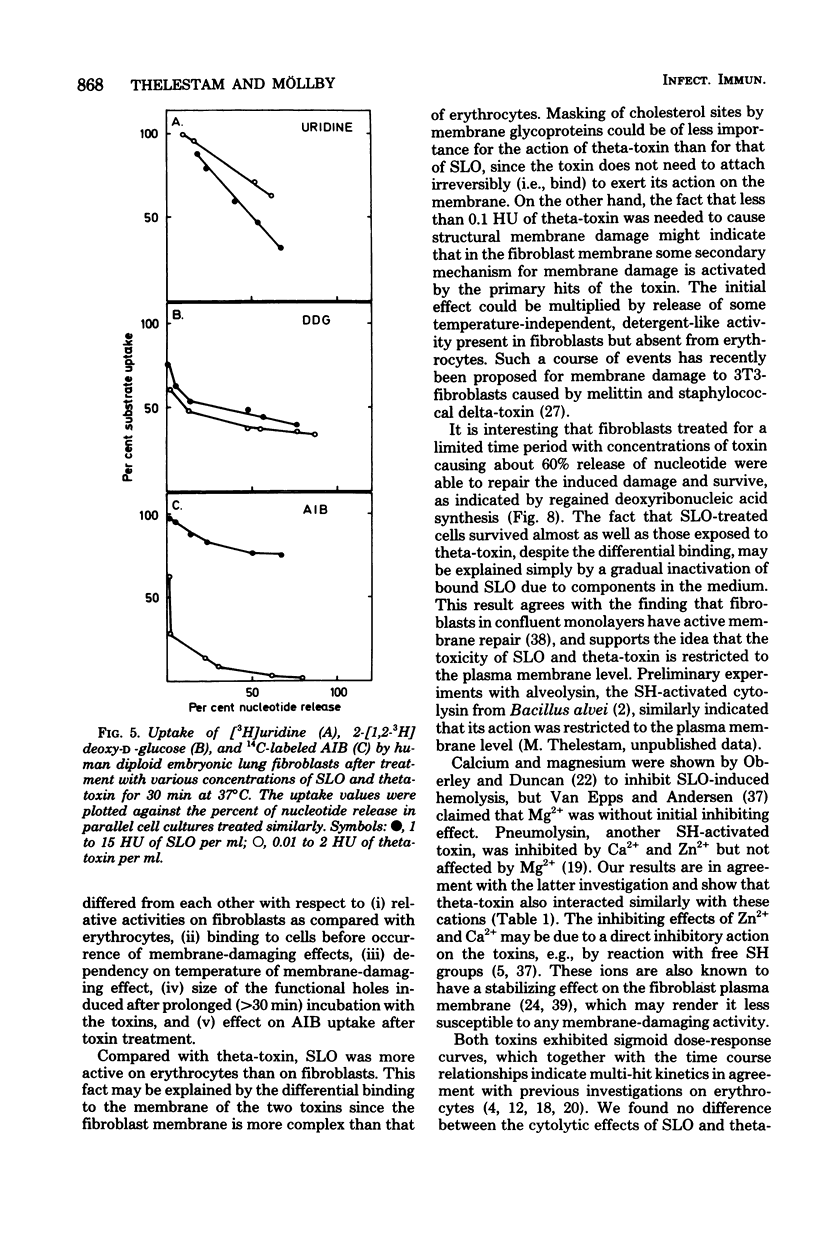
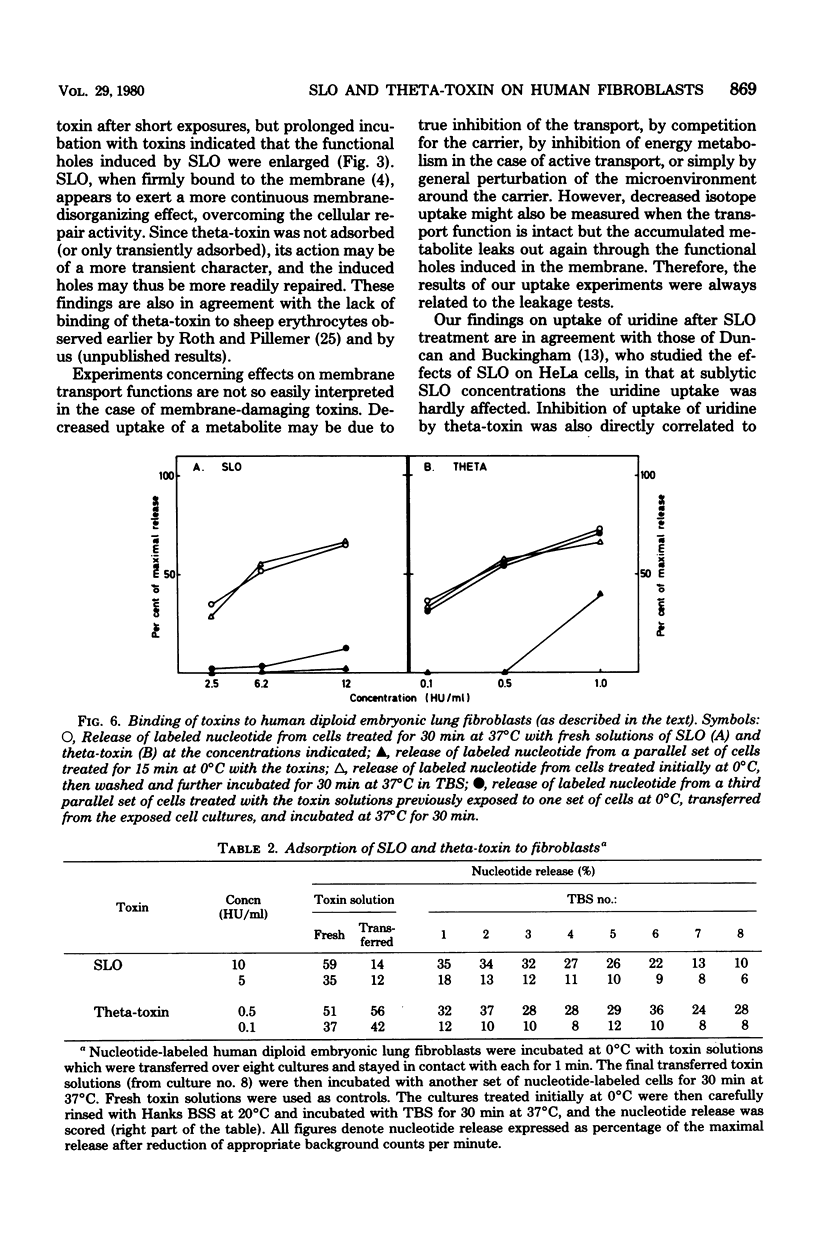
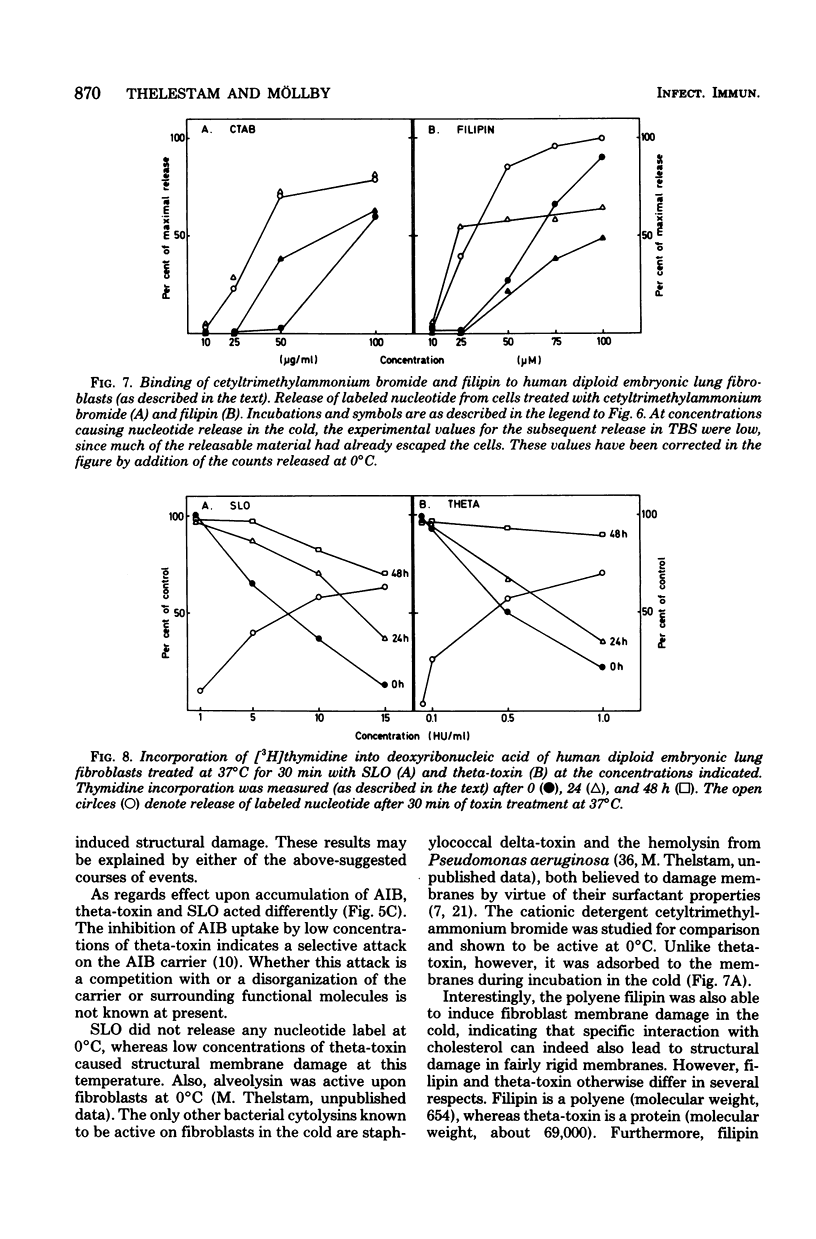
The membrane-damaging properties on human diploid embryonic lung fibroblasts of streptolysin O (from Streptococcus pyogenes) and theta-toxin (from Clostridium perfringens) were compared. The results are consistent with the suggested mechanism for hemolysis by streptolysin O involving one fixation site and one lytic site of this cytolysin. However, the membrane-damaging activity of the two toxins differed with respect to (i) relative cytolytic activity on human diploid lung fibroblasts compared with that on sheep erythrocytes, (ii) binding to the fibroblast membrane, (iii) activity at 0 degrees C, (iv) membrane repair after more than 30 min, and (v) effect on influx of amino acids. It is concluded that the mechanism of membrane damage caused by theta-toxin differs from that of cytoplasmic membrane. These results question the current concept that all thiol-activated, cholesterol-inactivated bacterial toxins are similar both structurally and functionally.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Kiredjian M., Geoffroy C. Purification de l'hémolysine thiol-dépendante extracellulaire de Bacillus alvei. Biochimie. 1977;59(3):329–336. doi: 10.1016/s0300-9084(77)80150-8. [DOI] [PubMed] [Google Scholar]
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires . II.--Cinétique de la lyse érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jul;115(1):97–121. [PubMed] [Google Scholar]
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires. I. Fixation sur la membrane érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jun;114(6):812–827. [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition of hemolysis by zinc and its reversal by L-histidine. Infect Immun. 1978 Mar;19(3):1101–1103. doi: 10.1128/iai.19.3.1101-1103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau L., Bittman R. Interaction of filipin with cholesterol in vesicles of saturated phospholipids. Biochemistry. 1977 Sep 20;16(19):4139–4144. doi: 10.1021/bi00638a001. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Amino acid transport systems in animal cells: interrelations and energization. J Supramol Struct. 1977;6(2):205–213. doi: 10.1002/jss.400060206. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Buckingham L. Effects of streptolysin O on transport of amino acids, nucleosides, and glucose analogs in mammalian cells. Infect Immun. 1977 Dec;18(3):688–693. doi: 10.1128/iai.18.3.688-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Hammond S. M. Biological activity of polyene antibiotics. Prog Med Chem. 1977;14:105–179. doi: 10.1016/s0079-6468(08)70148-6. [DOI] [PubMed] [Google Scholar]
- Hase J., Mitsui K., Shonaka E. Clostridium perfringens exotoxins. III. Binding of theta-toxin to erythrocyte membrane. Jpn J Exp Med. 1975 Dec;45(6):433–438. [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbayashi Y., Hotta M., Koyama J. Kinetic study on streptolysin O. J Biochem. 1972 Feb;71(2):227–237. doi: 10.1093/oxfordjournals.jbchem.a129759. [DOI] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol. 1967 Feb;93(2):670–674. doi: 10.1128/jb.93.2.670-674.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- ROTH F. B., PILLEMER L. Purification and some properties of Clostridium welchii type A theta toxin. J Immunol. 1955 Jul;75(1):50–56. [PubMed] [Google Scholar]
- Reynolds J. A. Are inorganic cations esential for the stability of biological membranes? Ann N Y Acad Sci. 1972 Jun 20;195:75–85. [PubMed] [Google Scholar]
- Shany S., Bernheimer A. W., Grushoff P. S., Kim K. S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol Cell Biochem. 1974 May 30;3(3):179–186. doi: 10.1007/BF01686643. [DOI] [PubMed] [Google Scholar]
- Shier W. T. Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):195–199. doi: 10.1073/pnas.76.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Fehrenbach F. J. Isoelectric analysis of haemolysins and enzymes from streptococci of groups A, C and G. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):860–870. doi: 10.1111/j.1699-0463.1974.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Freer J. H., Arbuthnott J. P. Interaction of Clostridium perfringens theta-haemolysin, a contaminant of commercial phospholipase C, with erythrocyte ghost membranes and lipid dispersions. A morphological study. Biochim Biophys Acta. 1975 Apr 8;382(4):479–493. doi: 10.1016/0005-2736(75)90216-3. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Classification of microbial, plant and animal cytolysins based on their membrane-damaging effects of human fibroblasts. Biochim Biophys Acta. 1979 Oct 19;557(1):156–169. doi: 10.1016/0005-2736(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Cytotoxic effects on the plasma membrane of human diploid fibroblasts--a comparative study of leakage tests. Med Biol. 1976 Feb;54(1):39–49. [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Determination of toxin-induced leakage of different-size nucleotides through the plasma membrane of human diploid fibroblasts. Infect Immun. 1975 Apr;11(4):640–648. doi: 10.1128/iai.11.4.640-648.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Sensitive assay for detection of toxin-induced damage to the cytoplasmic membrane of human diploid fibroblasts. Infect Immun. 1975 Aug;12(2):225–232. doi: 10.1128/iai.12.2.225-232.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R., Wadström T. Effects of staphylococcal alpha-, beta-, delta-, and gamma-hemolysins on human diploid fibroblasts and HeLa cells: evaluation of a new quantitative as say for measuring cell damage. Infect Immun. 1973 Dec;8(6):938–946. doi: 10.1128/iai.8.6.938-946.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O II. Relationship of Sulfyhdryl Groups to Activity. Infect Immun. 1971 May;3(5):648–652. doi: 10.1128/iai.3.5.648-652.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]


