Summary
Objective
Evidence indicates that thyroid hormones have effects on the inhibitory GABAergic system. The aim of this study was to investigate whether brain GABA levels are altered in patients with hypothyroidism compared with healthy controls.
Design/Methods
Fifteen patients with primary hypothyroidism and 15 matched healthy controls underwent single-voxel MEGA-PRESS magnetic resonance spectroscopy at 3T, to quantify GABA levels in the median prefrontal cortex (mPFC) and posterior cingulate cortex (PCC). All participants underwent thyroid function test. Neuropsychological performances were evaluated by administration of the Montreal Cognitive Assessment (MoCA) and the 21-item Beck Depression Inventory-II (BDI-II).
Results
The patients with hypothyroidism had significantly lower GABA+ levels in the mPFC compared with healthy controls (P = 0·016), whereas no significant difference (P = 0·214) was observed in the PCC. Exploratory analyses revealed that mPFC GABA+ levels were negatively correlated with the BDI-II scores in patient group (r = −0·60, P = 0·018). No correlations were found between GABA+ levels and TSH or fT3 or fT4 levels in either region (all P > 0·05).
Conclusion
This study suggests that alteration of GABAergic neurotransmission may play an important role in the pathophysiology of primary hypothyroidism, providing intriguing neurochemical clues to understand thyroid–brain interactions.
Introduction
It is generally accepted that thyroid hormones (TH) have significant effects on normal functioning of adult brain. Hypothyroidism is a common endocrine disorder defined by underproduction of TH and is often associated with neuropsychiatric and cognitive changes. Multimodal imaging studies have shown altered brain structure and function in patients with hypothyroidism,1 with decreased hippocampal volume,2 microstructural changes in white matter,3 brain metabolic changes,4 decreased regional cerebral blood flow (rCBF),5 lower cerebral glucose metabolism,6 decreased functional connectivity7 and decreased cortical excitability.8 The underlying mechanisms responsible for these alterations are largely unknown.
γ-Aminobutyric acid (GABA), the main inhibitory neurotransmitter in human brain, plays a crucial role in various physiological functions. In particular, changes in brain GABA have been demonstrated in a variety of psychiatric and neurological disorders including depression disorder, Alzheimer’s dementia and schizophrenia. As reviewed by Wiens and Trudeau,9 multiple lines of evidence indicate that TH affect the GABAergic neurotransmission, for example through the rate-limiting synthesizing enzyme glutamic acid decarboxylase (GAD). Pre-clinical studies suggested thyroxine (T4) and triiodothyronine (T3) could modulate activity of GABA-A receptor and selectively affect GABAergic phasic and tonic currents in rat hippocampal cultures via GABA-A receptor.10 A recent animal study found decreased GABA levels in the hippocampus of the hypothyroid group.11 However, an inconsistent result was observed by another group.12 Electrophysiological recordings also revealed reduced GABAergic synaptic transmission in the anterior cingulate cortex (ACC) of hypothyroid mice.13 Dysregulation of TH-mediated modulation in GABAergic neurotransmission may contribute to the pathophysiology of brain dysfunction in hypothyroidism. Thus, investigating in vivo brain GABA levels is key to understanding the brain of hypothyroidism.
Proton magnetic resonance spectroscopy (1H-MRS) provides an opportunity to noninvasively measure brain metabolites, including N-acetylaspartate (NAA), myo-inositol (mI), choline (Cho), glutamate–glutamine (Glx) and total creatine (tCr). GABA is present at low millimolar concentrations and is difficult to resolve from overlapping signals of other more concentrated metabolites at 3T.14 A spectral editing technique (MEGA) has been established to detect GABA selectively15 and has been successfully applied in a wide variety of studies.
In this study, we used MEGA-edited detection of GABA to investigate brain GABA levels in patients with hypothyroidism compared to matched healthy controls. We chose to explore two brain regions, one medial prefrontal region, which included portion of anterior cingulate cortex, and one posterior cingulate region. These two regions are important for emotion regulation and cognition functioning in normal brain,16–18 and have also been implicated in one previous fMRI study of patients with hypothyroidism.19 We hypothesized that the patients with hypothyroidism would have lower GABA levels than matched healthy controls. Exploratory analyses examined whether GABA levels were associated with TH levels or psychological measures.
Subjects and methods
Subjects
Fifteen patients with primary hypothyroidism (3 male/12 female, age 42·4 ± 7·4, range 22–54) and 15 matched healthy controls (4 male/11 female, age 43·7 ± 6·3, range 24–49) participated in this study.
Patients with hypothyroidism were diagnosed on the basis of elevated serum thyroid-stimulating hormone (TSH) and lowered free thyroxine (fT4) and free triiodothyronine (fT3). All patients were newly diagnosed and untreated and were recruited from Department of Endocrinology, Provincial Hospital Affiliated to Shandong University. The controls were matched with the patients for sex, age, body mass index (BMI) and years of education and were recruited from local community via fliers. No female participant was postmenopausal. Exclusion criteria for patients and control group included the following: current or past personal neurological or psychiatric disorders, head injury, hypertension, diabetes, pregnancy, intake of any psychotropic medication or hormonal preparation (including oral contraceptives) or cigarettes in the 6 months before enrolment.
All participants completed neuropsychological tests and MR scanning sessions on the same day when thyroid function tests were administrated. This study was approved by the local institutional review board. Written informed consents were obtained from all participants after a detailed description of the study.
Thyroid function test
Thyroid function tests were administrated to all the patients with hypothyroidism and healthy controls. Serum TSH, fT3 and fT4 were measured with chemiluminescent enzyme immunoassay methods (ADVIA Centaur, Siemens Healthcare Diagnostics Inc., NY, USA). The normal reference ranges were 0·55–4·78 mIU/l for TSH, 3·5–6·5 pmol/l for fT3 and 11·5–22·7 pmol/l for fT4.
Neuropsychological evaluation
The Montreal Cognitive Assessment (MoCA) was performed to assess global cognitive functions. The 21-item self-rated Beck Depression Inventory-II (BDI-II) was used to assess possible depressive symptoms in all participants.
Imaging protocol
Participants were imaged on a 3T scanner (Philips Achieva TX, Best, The Netherlands), equipped with an eight-channel phased-array head coil. T2-FLAIR images were acquired to rule out neurological abnormalities in participants. A T1-weighted three-dimensional TFE scan was acquired for MRS voxel placement and brain tissue segmentation. The scanning parameters were as follows: repetition time (TR) = 8·2 ms; echo time (TE) = 3·7 ms; slice thickness = 1 mm; matrix = 256 × 256; field of view = 24 × 24 cm2; and flip angle = 8°. Images were reconstructed with 1 × 1×1 mm3 isotropic voxels.
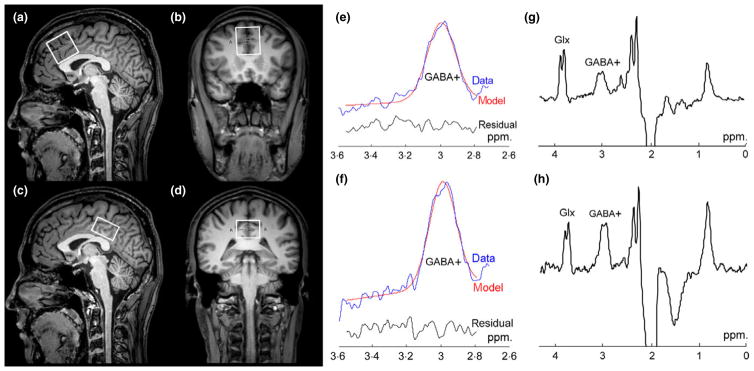
Edited 1H MR spectra were acquired from two brain regions: (1) the medial prefrontal cortex (mPFC) encompassing portions of the anterior cingulate cortex (ACC) (3 × 3×3 cm3); and (2) the posterior cingulated cortex (PCC) (3 × 3×2 cm3), as shown in Fig. 1a–d. The mPFC and PCC voxels were positioned with the inferior edge of the voxels parallel to the anterior and posterior descending surfaces of the truncus corporis callosi, respectively, and were located on the medial aspect of the axial plane with maximum inclusion of grey matter.
Fig. 1.
MRS voxel placement and representative spectra. T1-weighted TFE images show single-voxel placements centred on the median prefrontal cortex (mPFC) in the sagittal (a) and coronal (b) projections and on the posterior cingulate cortex (PCC) in the sagittal (c) and coronal (d) projections. (e, f) Curve-fitting of the GABA+ peaks of the mPFC and PCC; the red lines in the panels are the results of the GannetFit curve-fitting, the blue lines show the processed GABA+ spectra and the black line is the residual difference between the experimental data and the curve-fit. (g, h) Representative GABA+ spectra.
GABA-edited Mescher–Garwood point-resolved spectroscopy (MEGA-PRESS) was performed to detect GABA signal with the following experimental parameters: TR = 2000 ms, TE = 68 ms, acquisition bandwidth = 2000 Hz, 256 averages and scan duration 8 min 48 s. During odd-numbered acquisitions, a frequency-selective, Gaussian inversion pulse was applied to GABA-3CH2 resonance at 1·9 ppm, affecting the weakly J-coupled triplet peak of GABA-4CH2 at 3·01 ppm (ON). During even-numbered acquisitions, the same pulse was applied symmetrically to the other side of the water peak, at 7·5 ppm (OFF), to reduce baseline artefacts. The GABA-edited spectrum was obtained from the difference between the ON and OFF spectra (Fig. 1g, h). Unsuppressed water signals were also recorded using same parameters as an internal concentration reference (4 averages). This sequence also allows reliable detection of a co-edited fraction of Glx-2CH at 3·75 ppm because of J-coupling to the Glx-3CH2 signal at 2·1 ppm. The cleanly co-edited Glx-2CH signal was used for measurement of Glx. FASTMAP shimming of the voxels was performed automatically before each acquisition, yielding water signal line widths of 6 to 9 Hz.
1H MRS data processing and quantification
As the detected GABA signal at 3·01 ppm is also expected to contain a contribution from co-edited macromolecular (MM) resonances,14 we will refer to the GABA signals as GABA+. The GABA+ quantification was processed using the Gannet 2·0 toolkit in Matlab 2013b (Mathworks).20 Gannet comprised two main modules: GannetLoad, which is used to analyse the raw data, applying line broadening of 3 Hz, and frequency and phase correcting the individual spectra using Spectral Registration; and GannetFit, which uses a single-Gaussian model to fit the edited GABA+ peak (Fig. 1e, f), and a Gaussian–Lorentzian model for water spectrum. The final measurement of interest, GABA+ concentration ([GABA+]), was estimated relative to water amplitude in institutional unit (IU), accounting for metabolite relaxation times and tissue fractions, as discussed by Gao et al.21 We quantified the Glx and tCr amplitudes from the GABA+ spectra and nonedited spectra, respectively, using a nonlinear least-squares fitting algorithm: AMARES within jMRUI v4.0 software. A 3-Hz exponential line broadening was applied, and the Glx peak was modelled as a doublet. Glx levels were evaluated as ratios with respect to tCr (Glx/tCr). For MRS fit quality, the spectra were rejected if the Gannet FitError was > 10% (GABA+ measurements) or the relative Cramér–Rao lower bounds (CRLBs) was >10% (Glx, tCr).
Tissue segmentation
To obtain the tissue fractions of grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF), the 3D high-resolution T1-weighted images were segmented using an automatic brain segmentation program, FAST (FMRIB’s automated segmentation tool) in the FSL package (Oxford University, Oxford, UK).22 VOIs were superimposed on the structural images using the ‘Recreation of VOI’ Matlab tool.23 The segmentation data were used to scale for differences in brain tissue compositions between cohorts.
Statistical analysis
Statistical analyses were conducted using SPSS 16.0 (Chicago, IL, USA). We used two-tailed independent-samples t-tests to compare GABA+ and Glx levels between patients with hypothyroidism and healthy controls. In secondary analyses, we performed Pearson correlations to assess associations between GABA + levels and neuropsychological scores, and between GABA+ levels and thyroid hormone levels (TSH, fT4, fT3) in patients and controls. All the variables were presented as mean ± SD, and the significance level was set at a P-value of 0·05.
Results
Participant characteristics
The demographic and clinical characteristics of the participants are summarized in Table 1. There was no significant group difference with respect to age (P = 0·62), gender distribution (P = 0·5), BMI (P = 0·51) or education background (P = 0·81). Patients with hypothyroidism showed significantly lower self-rating scales of depressive symptoms (BDI-II) (P < 0·001) and cognition functioning (MoCA) (P < 0·001) than healthy controls.
Table 1.
Demographic and clinical characteristics of the subjects
| Characteristic | Hypothyroid (n = 15) | Control (n = 15) |
|---|---|---|
| Age (years) | 42·4 ± 7·4 | 43·7 ± 6·3 |
| Gender (male/female) | 3/12 | 4/11 |
| BMI | 23·5 ± 0·8 | 23·3 ± 1·2 |
| Education (years) | 13·3 ± 1·5 | 13·2 ± 1·5 |
| BDI-II | 11·0 ± 2·2 | 3·5 ± 1·8 |
| MoCA | 23·3 ± 1·8 | 26·3 ± 1·2 |
| TSH (mIU/l) | 97·8 ± 32·5 | 2·32 ± 0·8 |
| fT3 (pmol/l) | 1·96 ± 0·60 | 4·79 ± 0·68 |
| fT4 (pmol/l) | 6·17 ± 1·14 | 16·3 ± 2·11 |
Data are presented as mean ± SD. BMI, body mass index; TSH, thyroid-stimulating hormone; fT3, free triiodothyronine; fT4, free thyroxine; BDI-II, Beck Depression Inventory-II; MoCA, Montreal Cognitive Assessment.
Segmentation results
No significant differences were found between the patients with hypothyroidism and the healthy controls in terms of GM%, WM%, CSF% and GM:WM ratios in either region (see Table 2).
Table 2.
Tissue composition
| mPFC | PCC | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hypothyroid | Control | P | Hypothyroid | Control | P | |
| %GM | 45·8 ± 1·9 | 45·4 ± 1·7 | 0·59 | 48·5 ± 2·1 | 49·4 ± 2·7 | 0·31 |
| %WM | 39·0 ± 2·4 | 40·1 ± 2·5 | 0·22 | 41·2 ± 2·2 | 40·8 ± 2·9 | 0·66 |
| %CSF | 15·2 ± 2·1 | 14·5 ± 2·3 | 0·37 | 10·3 ± 2·7 | 9·8 ± 2·4 | 0·67 |
| GM:WM | 1·18 ± 0·11 | 1·14 ± 0·10 | 0·27 | 1·18 ± 0·09 | 1·21 ± 0·15 | 0·38 |
Data are presented as mean ± SD. mPFC, medial prefrontal cortex; PCC, posterior cingulated cortex; GM, grey matter; WM, white matter; CSF, cerebral spinal fluid.
Comparisons of GABA+ and Glx levels between groups
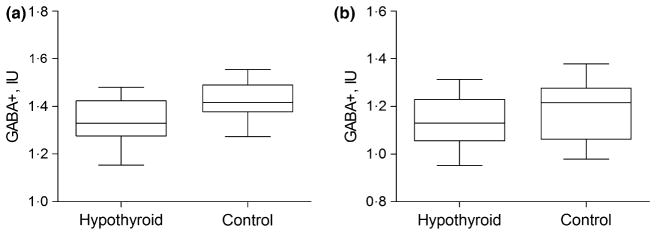
GABA+ difference spectra were successfully quantified from all 30 participants, resulting in a maximum FitError of 9·1%. The GABA+ FitError did not differ between patients with hypothyroidism and controls in the mPFC (5·61% ± 0·72% vs 5·24% ± 0·90%; P = 0·23) and PCC (6·24% ± 1·12% vs 6·56%± 1·14%; P = 0·46). In the mPFC, the patients with hypothyroidism (1·34 ± 0·09 IU) demonstrated significantly decreased GABA+ levels compared to healthy controls (1·42 ± 0·08 IU) (P = 0·016) (Fig. 2), whereas the PCC GABA+ levels were not significantly different (P = 0·214) in patients with hypothyroidism (1·14 ± 0·11 IU) compared to the control group (1·19 ± 0·13 IU).
Fig. 2.
Box and whisker plots show absolute GABA+ levels in institutional unit (IU) of (a) medial prefrontal cortex (mPFC) and (b) posterior cingulated cortex (PCC) in patients with hypothyroidism and control group. The within-box horizontal lines represent median values. The box extremities correspond to the 25th and 75th percentiles.
All Glx and tCr amplitudes were successfully quantified with a maximum relative CRLBs 9·2%. Glx/tCr levels did not differ between patients and controls in the mPFC (0·24 ± 0·03 vs 0·23 ± 0·03, P = 0·16) or the PCC (0·22 ± 0·03 vs 0·22 ± 0·02, P = 0·69).
Correlation analyses
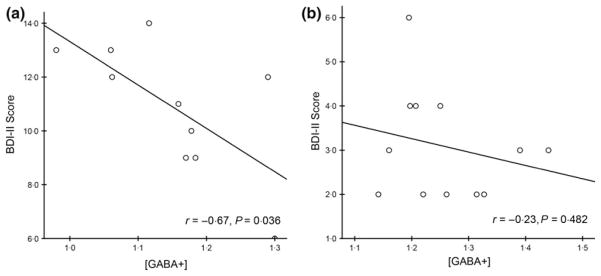
Exploratory analyses revealed that mPFC GABA+ levels were negatively correlated with BDI-II scores (r = −0·60, P = 0·018) for patients with hypothyroidism (Fig. 3). In the control group, GABA+ levels were unrelated to BDI-II scores in mPFC (r = −0·10, P = 0·72) or PCC region (r = −0·25, P = 0·36). And we found no correlations between GABA+ levels and TSH or fT3 or fT4 levels in either region (all P > 0·05).
Fig. 3.
Relationships between GABA+ levels and BDI-II scores in the hypothyroid group in (a) medial prefrontal cortex (mPFC) and (b) posterior cingulated cortex (PCC).
Discussion
This study indicates that GABA+ levels were decreased in the mPFC region of patients with hypothyroidism compared to age-and gender-matched healthy controls. A significant negative correlation was also found between mPFC GABA+ levels and depressive symptoms as rated by BDI-II scores in patients with hypothyroidism. To the best of our knowledge, this is the first in vivo demonstration of GABA+ changes in hypothyroidism.
Our finding of decreased GABA+ is in line with a recent rat study in which decreased hippocampal GABA levels were found in the hypothyroid group relative to the control group.11 However, our result is in contrast to the findings of Chapa et al. and Upadhyaya L et al., who reported increased GABA in the whole brain,24 cortex and hypothalamus of adult rats,12 respectively. The discrepancies may be due to methodological differences in establishment of animal models, duration of hypothyroidism or the brain regions between studies. Moreover, decreased GABAergic neurotransmission, as demonstrated by the decrease in the recorded mIPSCs, was detected in the ACC of hypothyroid mice.13 The present study showed decreased GABA+ in the ACC region, which is consistent with decreased synaptic currents, although MRS does not report directly on GABA receptor activity. Converging with prior preclinical studies on the interactions of TH and GABAergic system,9 our study may further indicate that TH participate in the modulation of the inhibitory GABAergic neurotransmission.
Accumulated evidence from other neuroimaging studies has shown hypothyroidism-related brain dysfunctions. Our study demonstrated reduced GABA+ levels that overlapped with diminished task-induced deactivation (TID), as reported in a previous fMRI study,19 in the mPFC area, and colocalized with rCBF aberrations in frontal areas.5 High regional GABA is associated with enhanced negative TID25,26 and CBF-weighted ASL signals27 (negative correlation), so we predict that altered GABAergic neurotransmission may be a neural correlate underlying these brain dysfunctions in hypothyroidism. Combination of MRS measurements and other functional MR imaging modalities may be an informative approach to uncover how GABA transmitter underlies these abnormalities.
Importantly, we find a negative correlation between mPFC GABA+ levels and BDI-II scores in patients with hypothyroidism. A similar decrease in GABA levels was also found in patients with major depression,28 suggesting a shared neurochemical mechanism. Although the relationship between hypothyroid state and mood disorders was inconclusive, T3 has been used to accelerate, augment and enhance the therapeutic effect of antidepressants in depression.29 Combining our findings, we assume that a facilitation of TH-mediated GABAergic neurotransmission by TH could be a possible mode of action in reducing affective symptoms in depression. However, given that the relationship between GABA+ and depression is already established, and hypothyroidism co-occurs with depression, we concede that the observed decrease in GABA+ may also be a consequence of depression rather than hypothyroidism per se.
GABA is localized mostly in GABAergic interneurons and synthesized from glutamate by its rate-limiting synthesizing enzyme GAD, namely GAD65 and GAD67. Reduced GABA levels may result from lower GAD activity. GAD65 levels were found to reduce to less than 50% of control in the hippocampus of hypothyroid rats.30 Moreover, anti-GAD antibodies in hypothyroidism may also be a potential factor influencing the efficiency of GABA production.31
The GABA metabolic pathway is part of glutamate/GABA–glutamine cycle.32 Glutamate and GABA released from neurons are predominantly taken up into astrocytes, where the glutamate/GABA is converted to glutamine. In turn, glutamine is released to GABAergic neurons for formation of GABA. TH have an influence on uptake and recycling of neurotransmitters in astrocytes by regulating glutamate uptake,33 and by increasing glutamine-synthetase activity.34 Thus, TH deficiency may cause impairments in the exchange of glutamate, glutamine and GABA between the neuronal and glial compartments.24 We found no glutamate–glutamine (Glx) differences between two groups. While it might be possible for altered glutamate or glutamine levels to exist in this patient group, new1H-MRS methods for reliable glutamate or glutamine discrimination would be required. Furthermore, we did not find associations between brain GABA+ levels and fT3, fT4 or TSH levels. It must be acknowledged that deiodinase II activity in the brain is increased in condition of hypothyroidism so that conversion of T4 to T3 is higher. In this case, TH levels measured in the blood may not reflect their levels in the brain. The relationship between brain GABA+ and brain TH levels merits further study.
This study is the first to report decreased brain GABA+ levels in patients with hypothyroidism. Dysregulation of GABAergic neurotransmission may culminate to disturb regional cortical microcircuitry and underlie neuropsychiatric and cognitive changes in hypothyroidism. Further elucidation of interactions between TH and GABAergic system is needed to interpret our results. Furthermore, our results should be cautiously interpreted. GABA is mainly found in two major pools, cytoplasm and presynaptic vesicular.35 Only vesicular GABA plays a role in inhibitory synaptic neurotransmission. MRS is only capable of detecting a total concentration of a certain metabolite within a defined brain region. It cannot distinguish between these separate functional pools of GABA. Recently, a [11C] Ro15-4513 PET study was proposed to detect synaptic GABA, which may serve as a promising approach for future research.
The present study has several limitations regarding study design and data acquisition, as follows: first, no follow-up GABA data were collected after treatment with levothyroxine (L-T4). L-T4 reversible effects have been reported in other imaging modalities, so a longitudinal study may be more informative. Second, our results may be confounded by menstrual phase among female participants, although the proportion of women in each menstrual phase was matched between groups. Third, the detected GABA+ signal contains a significant contribution (~50%) from co-edited macromolecules (MMs). New methods for MMs suppression are being developed, which can explain to what extent our results can be driven by changes in macromolecules instead of GABA itself. Fourth, our conclusions were further limited by the small sample size and, therefore, these results should be viewed as preliminary and will require confirmation in larger samples. In addition, the MRS voxels used were relatively large, which improves SNR at the cost of regional specificity.
In conclusion, we found reduced GABA+ levels in the mPFC region and also a significant negative correlation between mPFC GABA+ levels and depressive symptoms in patients with hypothyroidism. The observations suggest that altered GABAergic neurotransmission may be implicated in the pathophysiology of hypothyroidism, and the relationship between central TH and GABAergic system needs to be clarified. Our study provides clues to better understand the thyroid–brain interaction on the neurotransmitter level, and future multimodal imaging techniques could be informative.
Acknowledgments
The authors thank the patients and healthy participants who volunteered for this study.
Funding statement
The project was financially supported by the National Natural Science Foundation of China (grant nos. 81171380/H1807 and 81371534/H1802), Shandong Provincial Natural Science Foundation of China (grant no. BS2015YY003), Shandong Provincial Science and Technology Development Plans (grant no. 2013GSF11851) and Shandong Provincial Medical and Healthy Technology Development Program of China (grant no. 2015WS0176). This study applies tools developed under NIH R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants.
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interests, financial or personal, with other people or organizations that can inappropriately influence our work.
References
- 1.Pilhatsch M, Marxen M, Winter C, et al. Hypothyroidism and mood disorders: integrating novel insights from brain imaging techniques. Thyroid Research. 2011;4(Suppl 1):S3. doi: 10.1186/1756-6614-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooke GE, Mullally S, Correia N, et al. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid. 2014;24:433–440. doi: 10.1089/thy.2013.0058. [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Trivedi R, Singh K, et al. Diffusion tensor tractography in hypothyroidism and its correlation with memory function. Journal of Neuroendocrinology. 2014;26:825–833. doi: 10.1111/jne.12193. [DOI] [PubMed] [Google Scholar]
- 4.Modi S, Bhattacharya M, Sekhri T, et al. Assessment of the metabolic profile in Type 2 diabetes mellitus and hypothyroidism through proton MR spectroscopy. Magnetic Resonance Imaging. 2008;26:420–425. doi: 10.1016/j.mri.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Schraml FV, Beason-Held LL, Fletcher DW, et al. Cerebral accumulation of Tc-99 m ethyl cysteinate dimer (ECD) in severe, transient hypothyroidism. Journal of Cerebral Blood Flow and Metabolism. 2006;26:321–329. doi: 10.1038/sj.jcbfm.9600191. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Tang YY, Feng HB, et al. A behavioral and micro positron emission tomography imaging study in a rat model of hypothyroidism. Behavioural Brain Research. 2014;271:228–233. doi: 10.1016/j.bbr.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Kumar M, Modi S, et al. Alterations of Functional Connectivity Among Resting-State Networks in Hypothyroidism. Journal of Neuroendocrinology. 2015;27:609–615. doi: 10.1111/jne.12282. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo V, Crupi D, Bagnato S, et al. Neural response to transcranial magnetic stimulation in adult hypothyroidism and effect of replacement treatment. Journal of the Neurological Sciences. 2008;266:38–43. doi: 10.1016/j.jns.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Wiens SC, Trudeau VL. Thyroid hormone and gamma-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comparative Biochemistry and Physiology Part A Molecular Integrative Physiology. 2006;144:332–344. doi: 10.1016/j.cbpa.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 10.Puia G, Losi G. Thyroid hormones modulate GABA (A) receptor-mediated currents in hippocampal neurons. Neuropharmacology. 2011;60:1254–1261. doi: 10.1016/j.neuropharm.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Abd Allah ES, Gomaa AM, Sayed MM. The effect of omega-3 on cognition in hypothyroid adult male rats. Acta Physiologica Hungarica. 2014;101:362–376. doi: 10.1556/APhysiol.101.2014.3.11. [DOI] [PubMed] [Google Scholar]
- 12.Upadhyaya L, Agrawal JK. Effect of L-thyroxine and carbimazole on brain biogenic amines and amino acids in rats. Endocrine Research. 1993;19:87–99. doi: 10.3109/07435809309033016. [DOI] [PubMed] [Google Scholar]
- 13.Yi J, Zheng JY, Zhang W, et al. Decreased pain threshold and enhanced synaptic transmission in the anterior cingulate cortex of experimental hypothyroidism mice. Molecular Pain. 2014;10:38. doi: 10.1186/1744-8069-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Progress in Nuclear Magnetic Resonance Spectroscopy. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Stan AD, Schirda CV, Bertocci MA, et al. Glutamate and GABA contributions to medial prefrontal cortical activity to emotion: implications for mood disorders. Psychiatry Research. 2014;223:253–260. doi: 10.1016/j.pscychresns.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Hayden BY, Smith DV, Platt ML. Cognitive control signals in posterior cingulate cortex. Frontiers in Human Neuroscience. 2010;4:223. doi: 10.3389/fnhum.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz KP, Bedard AC, Czarnecki R, et al. Preparatory activity and connectivity in dorsal anterior cingulate cortex for cognitive control. NeuroImage. 2011;57:242–250. doi: 10.1016/j.neuroimage.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He XS, Ma N, Pan ZL, et al. Functional magnetic resource imaging assessment of altered brain function in hypothyroidism during working memory processing. European Journal of Endocrinology. 2011;164:951–959. doi: 10.1530/EJE-11-0046. [DOI] [PubMed] [Google Scholar]
- 20.Edden RA, Puts NA, Harris AD, et al. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of Magnetic Resonance Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao F, Wang G, Ma W, et al. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. NeuroImage. 2015;106:311–316. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 23.Montelius M, Ljungbreg A, Carlsson G, et al. Matlab tool for segmentation and re-creation of MRS volumes of interest in MRI image stacks. In: Montelius M, Ljungberg M, Carlsson Å, Starck G, Forssellaronsson E, editors. ESMRMB. Antalya/TR; 2008. Oct 1–3, [Google Scholar]
- 24.Chapa F, Kunnecke B, Calvo R, et al. Adult-onset hypothyroidism and the cerebral metabolism of (1,2-13C2) acetate as detected by 13C nuclear magnetic resonance. Endocrinology. 1995;136:296–305. doi: 10.1210/endo.136.1.7828544. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Chen X, Gu H, et al. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. Journal of Neuroscience. 2013;33:18566–18573. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 27.Donahue MJ, Near J, Blicher JU, et al. Baseline GABA concentration and fMRI response. NeuroImage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Schur RR, Draisma LW, Wijnen JP, et al. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of 1 H-MRS studies. Human Brain Mapping. 2016;37(9):3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joffe RT. Hormone treatment of depression. Dialogues in Clinical Neuroscience. 2011;13:127–138. doi: 10.31887/DCNS.2011.13.1/rjoffe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawano E, Takahashi M, Negishi T, et al. Thyroid hormone-dependent development of the GABAergic pre- and post-synaptic components in the rat hippocampus. International Journal of Developmental Neuroscience. 2013;31:751–761. doi: 10.1016/j.ijdevneu.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Marwaha RK, Garg MK, Tandon N, et al. Glutamic acid decarboxylase (anti-GAD) & tissue transglutaminase (anti-TTG) antibodies in patients with thyroid autoimmunity. Indian Journal of Medical Research. 2013;137:82–86. [PMC free article] [PubMed] [Google Scholar]
- 32.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. Journal of Neurochemistry. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 33.Dezonne RS, Lima FR, Trentin AG, et al. Thyroid hormone and astroglia: endocrine control of the neural environment. Journal of Neuroendocrinology. 2015;27:435–445. doi: 10.1111/jne.12283. [DOI] [PubMed] [Google Scholar]
- 34.Ruel J, Dussault JH. Triiodothyronine increases glutamine synthetase activity in primary cultures of rat cerebellum. Brain Research. 1985;353:83–88. doi: 10.1016/0165-3806(85)90025-2. [DOI] [PubMed] [Google Scholar]
- 35.Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Communicative & Integrative Biology. 2011;4:573–575. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]