Abstract
Autism spectrum disorder (ASD) is recognized as a global public health concern, yet almost everything we know about ASD comes from high-income countries. Here we performed a scoping review of all research on ASD ever published in sub-Saharan Africa (SSA) in order to identify ASD knowledge gaps in this part of the world. Fifty-three publications met inclusion criteria. Themes included the phenotype, genetics and risk factors for ASD in SSA, screening and diagnosis, professional knowledge, interventions for ASD, parental perceptions, and social-cognitive neuroscience. No epidemiological, early intervention, school-based or adult studies were identified. For each identified theme, we aimed to summarize results and make recommendations to fill the knowledge gaps. The quality of study methodologies was generally not high. Few studies used standardized diagnostic instruments, and intervention studies were typically small-scale. Overall, findings suggest a substantial need for large-scale clinical, training, and research programmes to improve the lives of people who live with ASD in SSA. However, SSA also has the potential to make unique and globally-significant contributions to the etiology and treatments of ASD through implementation, interventional, and comparative genomic science.
Keywords: autism, autism spectrum disorder, low- and middle-income countries, LMIC, low resource environments, Africa
Introduction
Autism spectrum disorder (ASD) is recognized by the World Health Organization as a growing global public health concern [World Health Organization, 2013] and may represent some of the greatest burden of disease in children and adolescents. In the Singapore Global Burden of Disease study [Epidemiology & Disease Control Division Ministry of Health Singapore, 2014], for instance, ASD contributed the highest disability adjusted life years (DALY) in those under the age of 15 years. The developmental and mental health needs of children and adolescents in low- and middle-income countries remain neglected [Kieling et al., 2011]. In a special Lancet Series on global mental health, Kieling and colleagues illustrated the so-called “90-10” divide— although 90% of the world’s children and adolescents live in low- and middle-income countries, only 10% of research is performed there [Kieling et al., 2011].
Sub-Saharan Africa (SSA), the focus of this review, includes African countries south of the Sahara desert. We focused on SSA, as opposed to the African continent, given that SSA is seen as a distinct geographic region by the World Bank, the United Nations (UN), and the World Health Organization (WHO), and is specified as such in the UN Millennium Development Goals. North Africa is classified as part of the Eastern Mediterranean Region (EMRO) [The World Bank, 2015; United Nations, 2014]. SSA is a heterogeneous region of ~24 million km2 comprising 46 countries, the majority of which are low-income countries. In 2014, 50% of the more than 970 million people living in SSA were under the age of 18 [UNICEF, Division of Data, Research, & and Policy, 2014]. The UN predicts that, by 2050, 40% of the world’s children will live in Africa [UNICEF et al., 2014].
To date, almost all clinical interventions, service developments, research and policy work for children and adolescents in SSA have focused on communicable diseases, such as Human Immunodeficiency Virus (HIV), Tuberculosis (TB), and Malaria, and on reducing infant mortality [United Nations, 2015], with almost no focus on neurodevelopmental disorders such as ASD. Over the past few decades, communicable disease interventions in SSA have resulted in a marked decline in mortality among children under 5 years of age [Garenne & Gakusi, 2006; Mathers & Loncar, 2006; Murray, Laakso, Shibuya, Hill, & Lopez, 2007; Rajaratnam et al., 2010]. With more children surviving in SSA, there are increasing calls to attend to the morbidity and needs of those with developmental delays or disabilities, including ASD [Engle et al., 2007; Scherzer, Chagan, Kauchali, & Susser, 2012]. The global prevalence of ASD is now around 1–2% [Centers for Disease Control and Prevention, 2014; Elsabbagh et al., 2012; Kim et al., 2011]. Although no population-based prevalence studies of ASD have been performed in Africa to date, at least in part due to barriers associated with access to “gold standard” diagnostic tools [Abubakar, Ssewanyana, de Vries, & Newton, 2016; Durkin et al., 2015], there is no reason to believe that rates of ASD will be lower than elsewhere.
Given the increasing recognition of ASD and related neurodevelopmental disabilities around the world, and the growing SSA population, it seemed crucial to synthesize what is known about ASD in SSA. Therefore, we set out (1) to compare publication output about ASD in SSA relative to other continents, and (2) to perform a comprehensive scoping review of ASD in SSA. The aim of the scoping review was to identify and summarize all peer-reviewed publications ever written about ASD in SSA, in order to generate an overall impression of the ASD landscape in SSA. The scoping review data were then used to identify knowledge gaps to guide further clinical, training, research and policy needs about ASD in this region of the world.
Scoping Review Methodology
The primary aim of this report was to generate a comprehensive scoping review of knowledge about ASD in SSA, as outlined above. The method for this is outlined here.
In order to place SSA data in a global context, we started with a broad comparison of the number of publications about ASD in SSA relative to other continents. We therefore searched for all articles on ASD across the seven continents. We identified citations by running searches in both PubMed and Embase. The search strategy included subject headings for autism (Autistic Disorder [MeSH] in PubMed and autism/exp in Embase) and either subject headings for countries and continents or country names (and state names, in the case of the United States). PubMed contains address information for only the first author up to 2014 and for all authors from 2014 onward. All articles up to 16 October 2015 were included and publications in all languages were included. Article details were downloaded into an EndNote library for each continental region and duplicates were removed within continental regions. Some articles contained authors from multiple continental regions or discussed multiple continental regions within one paper. Therefore, it was possible that a paper could be “counted” in multiple continental regions. For that reason, article counts were presented as absolute numbers for each continent or continental region rather than as a proportion of the whole. We did not perform a detailed review with additional inclusion/exclusion criteria for the purpose of this search.
The primary objective of the comprehensive scoping review was to establish the current broad landscape of peer-reviewed evidence on ASD in SSA, in order to formulate evidence-based recommendations for future research and clinical priority-setting in this region of the world. The methodological framework for scoping reviews [Arksey & O’Malley, 2005; Levac, Colquhoun, & O’brien, 2010] was followed. This included identifying the research question, searching for relevant studies, selecting studies, charting and summarizing the data, and reporting the results. The review objective, inclusion criteria, and study methods for this scoping review were specified in advance. Inclusion criteria were (1) ASD was the primary focus of the paper, (2) participants lived in SSA, (3) the article was data driven, (4) the article had been peer reviewed, and (5) full text of the article was available. Given that this was a comprehensive scoping review and not a systematic review, no articles were excluded on the basis of any quality criteria.
With the assistance of a research librarian (MvI) we therefore next searched PubMed, Embase, the Cochrane Library, Web of Science, PsycInfo, Global Health, African NiPAD, and African Index Medicus for published reports on ASD in SSA up to October 16, 2015. Given the variable use of terminology relating to ASD since the 1970s, we used a broad range of search terms to capture ASD-related articles. Search terms included database-specific subject headings and keyword variants for both autism (e.g., “pervasive developmental disorders,” “autism,” “autistic,” and “Asperger”) and the SSA region and individual countries. See Supporting Information Appendix S1 for the full search terms. We applied no language or date restrictions. Citations were imported into EndNote and duplicates were removed. We identified 801 publications potentially relevant to ASD in SSA after removing 509 duplicates. The high number of duplicates was attributable to the number of databases searched. Two authors (LF and NC) independently reviewed each title and abstract for inclusion. Where decision on inclusion could not be reached based on review of title and abstract alone, the full text was reviewed to determine if the study met the inclusion criteria specified above. Any disagreement resulted in joint review of the full text article with reconciliation. Where agreement could not be reached, full text review and decision on inclusion or exclusion of the article were performed by the senior author (PJdV). The senior author was required to review only one manuscript. Of all manuscripts, only one was not in English. The article was written in Afrikaans, one of the official languages of South Africa. Translation was unnecessary as three of the authors were fluent in English and Afrikaans. All full texts from the articles included were reviewed by three authors (LF, NC, PJdV). Fifty-three articles met criteria for inclusion in the final review. Examples of reasons for article exclusion were that they were not peer reviewed (e.g. abstracts, poster presentations, book chapters, dissertations), the study population was not from SSA (e.g., African-Americans, African immigrants in European countries, North African studies), and the papers did not focus on ASD (e.g. they focused on other disorders such as Schizophrenia or Tourette’s). Agreement between raters for inclusion was excellent (κ = 0.936). The flow diagram that outlines the study selection process is shown in Figure 1.
Figure 1.
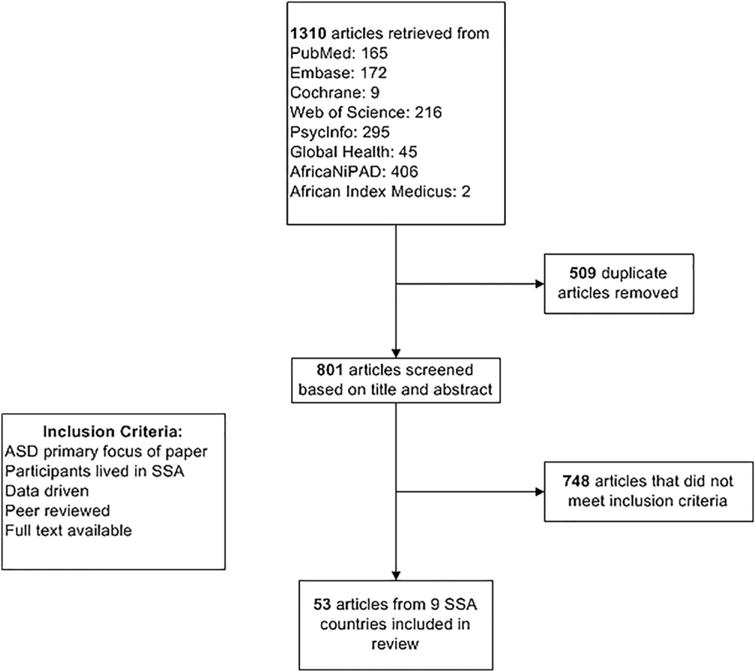
Flow diagram of studies included in this comprehensive scoping review.
A table for data extraction was developed at the protocol stage of the review to chart key information (first author, year of publication, country of publication, study design, description of study participants, and summary of study findings) (see Table I). Results are presented both in Table I and as a narrative summary. Articles were grouped thematically and classified under a number of main conceptual categories, some of which were specified prior to data extraction (e.g., phenotype, genetic studies, intervention), while others (e.g., family perspectives and professional knowledge) emerged through the data extraction process. Final decisions about the primary theme of articles were made after consensus agreement by three of the authors (LF, NC, PJdV) and with helpful suggestions by anonymous reviewers. We acknowledge that thematic overlap may have occurred, particularly where an article may have been relevant to more than one theme.
Table I.
Summary of All Studies Included in This Comprehensive Scoping Review
| First author (Year), Region, Country | Study Design | Participants | Key Methodological Aspects
|
Summary of Findings | ||
|---|---|---|---|---|---|---|
| Systematic diagnosis | Translation/Validation | Replication (Intervention) | ||||
| Phenotype of ASD | ||||||
|
| ||||||
| Lotter (1978), Ghana, Kenya, Nigeria, South Africa, Zambia, Zimbabwe | Descriptive/case report | 9 ASD according to “Western criteria” of the time 6 male 3 female 30 “autistic behavior” (gender breakdown not reported), age not reported 1312 children screened |
✓ (one ASD screening tool) | X | Lotter concluded that ASD exists in Africa and that in sex ratio, occurrence of epilepsy and socioeconomic status it was similar to ASD in the British population. He suggested that some repetitive movements and ritualistic behaviors were less common in the African sample. | |
| Bakare (2008), Enugu State, Nigeria | Descriptive/case report | 1 male 13 years |
X | N/A | Reports a case of comorbid childhood autism, oculocutaneous albinism, and severe intellectual disability. | |
| Bakare (2012), Enugu State, Nigeria | Group descriptive | 4 male 1 female mean age of all children assessed 13 years 11% of 44 children with ID |
X | N/A | Assessed the number of children with ASD and intellectual disability in a privately owned special education center for children with intellectual disabilities in Nigeria. 5 of 44 (11.4%) of children met DSM–IV criteria for ASD. | |
| Bello-Mojeed (2011), Lagos, Nigeria | Descriptive/case report | 1 male 1 female 14 and 17 years |
X | N/A | Report of 2 cases of ASD seen at a child and adolescent psychiatry clinic. Both children were identified late. The report aimed to raise awareness on the pattern of clinical presentation and factors associated with identification of ASD. | |
| Dhadphale (1982), Nairobi, Kenya | Descriptive/case report | 2 males 1 female 3–5 years |
X | N/A | Clinical features of ASD in 3 children are described. Authors concluded that onset, ASD features, and socioeconomic status of parents, participants are comparable to “western” children. | |
| Ezegwui (2014), Enugu State, Nigeria | Descriptive/case report | 13 male 5 female 5–15 years |
X | N/A | Reported on refractive errors in children diagnosed with ASD. Significant refractive error, mainly astigmatism was noted. | |
| Holford (1994), South Africa | Descriptive/case report | 9 male 4 female 3–13 years |
X | N/A | The author describes clinical presentation of 13 children with mild/high functioning AS Dusing 3 different sets of diagnostic criteria: (1) Gillberg and Gillberg’s (1989) diagnostic criteria elaborated; (2) Szatmari, Brenner and Nagy’s (1989) Diagnostic Criteria; and (3) ICD-10 (1992) Diagnostic Criteria. The utility of these criteria within context of limited service provision in South Africa is discussed. | |
| Izuwah (2015), Port Harcourt, Nigeria | Descriptive/case report | 60 male 15 female 2–25 years |
X | N/A | The pattern of presentation of ASD and presence of risk factors in cases referred to a private ASD centre in Nigeria are described. Risk factors, clinical features, and comorbidities of ASD in the group were described as “similar” to individuals from other parts of the world. | |
| Khan (1996), Matabeleland, Midlands and Harare, Zimbabwe | Descriptive/case report | 15 male 3 female 8–17 years |
✓ (1 ASD screening tool and an observational assessment of level of functioning) | X | Described clinical characteristics of “autistic behaviors” in relation to DSM-III-R criteria using one screening tool and an observational assessment. Concludes that the cases support the criteria but that behaviors such as abnormal responses to sensory stimuli should also be considered. | |
| Lagunju (2014), Ibadan, Nigeria | Descriptive/case report | 45 male 5 female mean age at diagnosis 45 months 2.3% of 2320 new cases |
X | N/A | Described clinical characteristics of all new cases of ASD among children in pediatric neurology and child psychiatry University clinics in Nigeria over a 6-year period (January 2007–December 2012). Mean age of diagnosis was 45 months (range 16–121 months). 22% had a family history of ASD and 75% had neurological comorbidities. | |
| Springer (2013), Cape Town, South Africa | Descriptive/retrospective chart review | 45 male 13 female median age at diagnosis 42 months 5.7% of 1010 files reviewed |
✓ (CARS was “utilized in cases of diagnostic uncertainty”) | X | Described demographic characteristics, clinical features, comorbidity and diagnostic yield of investigations in children with a “pervasive developmental disorders” in a developmental pediatric University clinic in South Africa from a retrospective chart review (April 2008–March 2010). Mean age of diagnosis was 42 months (range 15–106 months); 40% had “complex” autism with dysmorphism. 72% were non-verbal and 89% had behavioral problems. | |
| Szabo (1992), Johannesburg, South Africa | Descriptive/case report | 1 male 13 years |
X | N/A | The author presented a clinical description of one child’s symptoms as a basis for discussion of the utility of Asperger syndrome as a separate clinical category prior to publication of DSM-IV. A brief description of intervention strategies to target poor school performance and poor social integration was described. | |
| Wessels (1979), South Africa | Descriptive/case report | 2 male birth to 7 years |
X | N/A | Described clinical features of monozygotic twins with “early infantile autism”. Both twins showed similar features until 4 years “when one of them spontaneously improved”. Subsequently the twin with epilepsy demonstrated a poorer outcome. | |
| Genetic Studies | ||||||
| Arieff (2010), Western Cape and Gauteng Provinces, South Africa | Group descriptive | 96 male 13 female no ages reported |
X | N/A | The *S/*S genotype of the promoter region of the 5-HTTLPR gene was significantly associated with all South African ASD ethnic groups. Allele frequency of the South African ASD group differed from Japanese, Korean and Indian but not French, German, Israeli, Portuguese and USA ASD groups. | |
| Sharma (2013), Western Cape and Gauteng provinces, South Africa | Group descriptive | 129 male 13 female no ages reported |
✓ (CARS was “administered for the assessment of cases”) | X | A significant association for the rs736707 SNPs of the RELN gene in South African participants with a diagnosis of ASD was reported. | |
| Risk Factors | ||||||
| Claassen (2008), Pretoria, South Africa | Retrospective case study | 1 male 4 years |
X | N/A | Case study of dizygotic twin siblings one of whom had ASD. Authors assert that maternal stress contributed to the pathogenesis of ASD in one of a pair of dizygotic twins as the blood plasma of the child with ASD demonstrated elevated glucocorticoids and serotonin relative to the unaffected sibling. | |
| Mankoski (2006), Dar es Salaam, Tanzania | Case series, descriptive | 10 male 4 female 8 to 22 years (mean age 12 years) 70% of 20 children screened |
✓ | ✓ (ADI-R was translated into Kiswahili – not an official translation) | In a case series in Tanzania 20 participants were recruited from an Autism Unit at a primary school, from the Autism Unit wait list and from the community. Authors were able to confirm an ASD diagnosis on 14 of the children. 3 of the 14 children developed ASD following severe infections, including malaria. | |
| Van Wijngaarden (2013), Republic of Seychelles | Longitudinal descriptive | 1784 mother-child pairs screened for ASD 7–24 years (mean age 19 years)gender not reported |
N/A (no ASD diagnosis was made) | ✓ (Social Communication Questionnaire translated into Creole – not an official translation) | Participants included 1784 mother-child pairs in the Seychelles for whom: (1) a prenatal maternal hair sample was available, and (2) data on ASD phenotype were provided by a biological parent. No association between prenatal methylmercury exposure and ASD phenotype behaviors as measured by scores on two ASD screening instruments were identified. | |
| Screening and Diagnosis | ||||||
| Kakooza-Mwesige (2014), Kampala and Wakiso districts, Uganda | Cross-sectional | 8 children diagnosed with ASD Gender not reported 2–9 years 0.7% of 1169 children screened |
✓ (23-question screener used to identify cases. ASD diagnosis was guided by a “standard data collection form”) | ✓ (for the 23-quesiton screening tool) | Authors compiled a 23-question screener that included the Ten Questions Questionnaire (TQQ) and 13 questions on ASD. They conducted household screening of 1169 children 2–9 years, followed by clinical assessment of children who screened positive and a sample that screened negative. 27% (n5320) screened positive, 68 received a diagnosis of moderate to severe neuro-developmental disorder, 8 were diagnosed with ASD. A combination of a positive screen on ASD items and TQQ was modestly successful in identifying children at high risk of ASD. Although an estimated ASD prevalence of 1.2/100 – 1.3/100 was reported authors note that as the study was not designed to investigate complete population-based prevalence. | |
| Harrison (2014), Moshi and Dar es Salaam, Tanzania | Cross-sectional | 30 participants diagnosed with ASD “83%” male 2–21 years (mean age 7 years) |
✓ | X (although cultural adaptations to the test battery were described) | Described the implementation of a systematic ASD diagnostic procedure with adaptations for the local context. Evaluation included a brief intake interview, CARS-2 rating following a semi-structured 30-minute play observation, and the CARS-2 Questionnaire for Parents or Caregivers administered as a semi-structured interview with an interpreter, measure of adaptive functioning and DSM-5 checklist. Of the 41 consecutive, ethnically diverse, children in a walk-in pediatric specialty clinic in Tanzania, 30 met criteria for ASD, 11 met criteria for global developmental delay. | |
| Professional Knowledge | ||||||
| Bakare (2008), Enugu State, Nigeria | Group design with repeated measures | Psychiatric nurses 38 female 12 male mean age 33 years |
N/A | ✓ (test-retest reliability and internal consistency of questionnaire reported) | The knowledge of ASD among health workers (KCAHW) questionnaire was developed and administered to 50 psychiatric nurses at two time points, two weeks apart. Internal consistency of the four domain scores was high (Cronbach’s alpha = .97). No aspects of validity were evaluated. | |
| Bakare (2009), South-east and south-south regions of Nigeria | Group descriptive | Psychiatric and pediatric nurses 63 female 71 male mean age 36 years |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed the opinions of nurses in Nigeria on etiology, treatability and preventability of ASD. etiology was believed to be natural (27%), preternatural (14%), and supernatural (27%). Those with higher scores on the KCAHW were more likely to subscribe to natural causes. 55% believed ASD was treatable. Previous ASD experience was associated with opinions on treatability. | |
| Bakare (2009), South-east and south-south regions of Nigeria | Group descriptive | Psychiatric and pediatric nurses 63 female 71 male mean age 36 years |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed the knowledge of nurses about ASD symptoms, availability of services and legal aspects of care of children with ASD. Knowledge gaps across ASD symptom domains were reported. Scores on KCAHW were higher for older nurses and those with previous experience with ASD. Majority believed facilities and laws regarding care of children with ASD were lacking. | |
| Igwe (2011), Ebonyi State, Nigeria | Group descriptive | Psychiatric and pediatric nurses 56 female 24 male mean age 37 years (psychiatric) mean age 34 years (pediatric) |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed knowledge of ASD among pediatric and psychiatric nurses and factors related to level of knowledge. Psychiatric nurses had greater ASD knowledge than pediatric nurses. Increased experience working with children with ASD was associated with greater ASD knowledge. | |
| Igwe (2011), Enugu State, Nigeria | Group descriptive | Final year undergraduate health and life sciences students 163 female 137 male mean age 25 years |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed knowledge of ASD among final year undergraduate health and life sciences students. Medical students had the highest ASD knowledge followed by nursing students, and then psychology students. Total scores correlated with number of weeks posting in psychiatry and pediatrics, and number of credit hours in psychiatry/abnormal psychology and pediatrics. | |
| Bakare (2015), 5 out of 6 geopolitical regions of Nigeria | Group descriptive | Final year medical students 300 female 457 male mean age 25 years |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed ASD knowledge of 757 final year Nigerian medical students. 29% had participated in the evaluation and management of a child with ASD. Those with previous experience had higher knowledge scores. Gender and regional variations in scores were noted. | |
| Eseigbe (2015), Northwest Nigeria | Group descriptive | Medical doctors 48 female 119 male mean age 38 years |
N/A | ✓ (test-retest reliability of KCAHW questionnaire referenced) | Assessed knowledge of ASD in medical doctors attending an annual scientific meeting in Nigeria. Higher scores were associated with being male, a pediatrician or psychiatrist, previous experience managing ASD, and practicing in a tertiary health facility. Knowledge of age of onset and comorbidities was low. Challenges in managing children with ASD were identified. | |
| Audu (2010), Benin City, Edo State, Nigeria | Group descriptive | 131 primary school teachers Age and gender not reported |
N/A | ✓ (split-half reliability for Autism among primary school pupils questionnaire assessed) | Described school teacher reports of the presence and characteristics of ASD in Nigeria. 23% of teachers reported ASD did exist in school children, 77% reported there are more boys with ASD than girls, 32% reported ASD symptoms differed in occurrence by age. Parental ASD knowledge was thought to be low. | |
| Denkyirah (2010), Eastern and central regions of Ghana and 8 counties in a Midwestern State in the USA | Group descriptive | 65 teachers from Ghana 210 teachers in USA All female Age not reported |
N/A | X | Compared opinions of preschool teachers from Ghana and the USA on characteristics of effective transition programs for children with ASD. Teachers agreed on the effectiveness of most strategies, but differed with regard to the usefulness of assistive technology and parent training. | |
| Interventions for ASD | ||||||
| Alant (2013), Pretoria, South Africa | Within-subjects group descriptive design | 18 male 4 female 9– 20 years |
X | N/A | ✓ | Described the translucency (degree to which the symbol represents its referent) ratings of children with ASD over 3 consecutive exposures. Statistically significant differences in total translucency ratings of the Blissymbols by the children with ASD between day 1 and day 3 were reported. |
| Akande (1998), Nigeria | Pre-post, quasi-experimental group design | 4 male 3 female 4–5 years |
X | N/A | ✓ | Examined the influence of self-monitoring on tutor’s provision of reinforcement and in turn the effect of this on frequency of play behaviors in children with ASD in Nigeria. More reinforcement occurred during the self-monitoring condition on the part of the tutors which resulted in more play behaviors in the children. |
| Akande (1999), South Africa | Pre-post, quasi-experimental group design | 4 male 3 female 4–5 years |
X | N/A | ✓ | Replication of Akande (1998) above with the same results. |
| Akande (2000), South Africa | Multiple baseline design across training Sets | 3 male 7–11 years |
X | N/A | ✓ | Assessed the differential effects of presentation mode on the acquisition of color labels. Colors presented with cues were easier to learn than without the assistance ofcues. |
| Silver (1970), South Africa | Case study | 1 male |
X | N/A | X | Described the use of operant conditioning to teach sounds and words in a severely impaired child with substantial self-injurious behavior. Treatment consisted of 139 days of operant conditioning of vocalizations, sounds and words. |
| Bunning (2014), Kilifi, Kenya | Pre-post-quasi-experimental design | 1 male 12 years 1/9 children who took part in the study had ASD |
✓ | ✓ (the Communication Profile-Adapted was translated to Kiswahili, Giriama, and Chonyi - not an official translation) | ✓ | Examined caregiver perceptions of changes in communication following a caregiver-implemented intervention using low tech AAC strategies. One participant had ASD. Statistically significant positive changes in caregiver perceptions of communication and expansion of participant’s social activities were reported. |
| Panzegrouw (1996), Pretoria, South Africa | Quasi-experimental single subject A-B-A design with withdrawal phase | 1 male 15 years |
X | N/A | ✓ | Reports a parent-implemented communication intervention with adolescent with profound cognitive impairment and ASD features. Positive changes were seen in mother’s skills in communication and training and son’s skills in communication and 3 activities of daily living. |
| Geils (2008), South Africa | Qualitative study | 1 male 6 years |
X | N/A | ✓ | Examined conversational exchanges between a child with ASD and familiar co-participants to determine features leading to communicative success or breakdown. A playful, activity-based interactive style characterized by non-verbal turns, affection, and short, simple utterances enhanced coordinated interaction. Discordant interaction resulted from a tendency of the co-participants to dominate the interaction which directed and constrained interaction and resulted in the child’s withdrawal. |
| Travis (2010), Western Cape, South Africa | Single subject multiple baseline design | 2 male 9 years |
X | N/A | ✓ | Describes effectiveness of PECS on the frequency of requesting and commenting and length of verbal utterances in two children with ASD. Positive effects were noted for requesting and mixed results for commenting and length of verbal utterances. |
| Louw (2013), Western Cape, South Africa | Group descriptive | 60 male 5 female 5–18 years median age 9 years |
X | N/A | ✓ | Described patterns of medication use in children and adolescents with ASD in South Africa. Children were recruited from schools catering for children with ASD and a NGO database. 25% took psychotropic medication. Antipsychotics were most common, followed by stimulants, antidepressants and mood stabilizers. 40% took over the counter medication. 15% were on special diet. The Caucasian and Asian group were more likely to take over the counter medication than Black/Colored group. |
| Wong (2014), 30 countries, including South Africa (the only country in sub-Saharan Africa) | Descriptive, quantitative record review | 384 medical doctors Age and gender of participants not reported |
X | N/A | ✓ | Investigated psychopharmacological treatment patterns for ASD psychiatric comorbidities in 30 countries. In South Africa, antipsychotic medication was the most frequently prescribed followed by antidepressant and stimulant medication. |
| Family Perspectives | ||||||
| Alli (2015), Gauteng, South Africa | Descriptive, qualitative, in-depth individual interviews | Parents of children with ASD 9 mothers 1 father mean age 41 years |
X | N/A | Having a child with ASD influenced the daily functioning of immediate family members. Speech language pathologists were providing communicative strategies for parents; however, these strategies were not generalized to all settings, suggesting the importance of providing home-based strategies that could be utilized by parents in all settings. | |
| Fewster (2015), KwaZulu-Natal, South Africa | Descriptive, semi-structured interviews | 11 parents of children with ASD Gender and age not reported |
X | N/A | The aim of the study was to gain deeper understanding of the experiences and coping strategies of parents of children with ASD. The theme “a road map for coping with ASD” was used in this article to map out guidelines for practitioners when dealing with parents or children with ASD. | |
| Mitchell (2014), KwaZulu-Natal, South Africa | Descriptive, individual interviews | Parents of children with ASD 7 mothers 5 fathers Age not reported |
X | N/A | Parents felt that providers demonstrated a lack of knowledge of ASD, and an unwillingness to diagnose their child. They reported a lack of available support and services, and significant difficulties in obtaining an ASD diagnosis. | |
| Kapp (2011), Eastern Cape, Gauteng, Western Cape, South Africa | Descriptive, questionnaire including open-ended questions | 19 mothers of children with ASD 25–48 years |
X | N/A | The study examined resilience-promoting factors in families with children with ASD. These included social support, supportive spousal relationships, family time, togetherness and routines. Resilience resources included hardiness, family problem-solving communication, family time and routines. | |
| Greef (2010), Western Cape, South Africa | Descriptive, questionnaire including open-ended questions | 24 mothers 4 fathers 6 participants did not include gender 34–43 years |
X | X | The study examined resilience factors in families with children with ASD. These included higher socioeconomic status, social support, open and predictable patterns of communication, a supportive family environment, family hardiness, internal and external coping strategies, a positive outlook on life, and family belief systems. | |
| Oliver (2009), Eastern Cape, South Africa | Descriptive, individual semi-structured interviews | Parents of children with ASD 2 mothers 6 fathers Age not reported |
X | N/A | Parents reported that an understanding of ASD was essential to their adjustment to the diagnosis. Parents needed professional and social support. Parents of children with ASD faced specific behavioral, personal, familial, financial, social, and societal challenges. | |
| Du Toit (1999), South Africa | Descriptive, qualitative, in-depth individual interviews | Parents of children with ASD 3 mothers 3 fathers Age not reported |
X | N/A | Parents reported limited ASD knowledge, experienced a lack of social support, and a shortage of information and practical assistance. The study recommended that parental guidance be individualized. | |
| Gona (2015), Kilifi and Mombasa, Kenya | Descriptive, in-depth interviews and focus groups | 51 parents of children with ASD 25–75 years 52 service providers 30–52 years Gender not reported |
X | N/A | Explored parents’ and professionals’ (special needs teachers, clinicians, social workers) views of the perceived causes and treatment of ASD on the Kenyan Coast. Regardless of cultural background, all participants had similar views on causes (preternatural and biological) and treatment (traditional and spiritual healing to treatment in modern health facilities). | |
| Social-cognitive and Developmental Neuroscience | ||||||
| Noach (1971), South Africa | Descriptive/cross-sectional | 4 female 8–14 years |
X | N/A | Explored the development of concept formation in verbal children with ASD. The investigators concluded that ASD participants showed impaired concept formation. | |
| Hoogenhout (2014), Western Cape, KwaZulu-Natal, and Gauteng, South Africa | Descriptive/cross-sectional | 76 male 10 female 4–16 years |
X | N/A | Authors report that a single ASD category (as opposed to DSM-IV subgroups) with verbal IQ as a specifier captured the bulk of variation in individual theory of mind development. | |
| Pileggi (2013), South Africa | Descriptive/cross-sectional | 14 male 6 female 6–14 years |
X | N/A | Children with ASD showed no preference for cradling side whereas typically developing children showed strong left-sided preference. | |
| Pileggi (2015), Western Cape, South Africa | Descriptive/cross-sectional | 44 male 9 female 6–16 years |
X | N/A | ASD diagnosis was the only significant predictor of atypical cradling preference. Authors suggest the results support the hypothesis that leftward cradling bias is associated with basic social-affective capacities. | |
| Van Zyl (1985), Pretoria, South Africa | Descriptive/cross-sectional | 4 children with ASD Gender not reported 5–11 years |
X | N/A | A categorical system was designed to rate children’s interactive behavior. Immediate echolalia was found to be relevant, and displayed meaningful information and the maintenance of the social interaction. | |
ASD, autism spectrum disorder; DD, developmental disorder; ID, intellectual disability; TD, typically developing; AAC, augmentative and alternative communication; PECS, Picture Exchange Communication System; NGO: non-governmental organization.
The purpose of a scoping review is to provide an overview of the existing evidence, regardless of quality [Arksey & O’Malley, 2005; Levac et al., 2010]. Therefore, as mentioned earlier, no studies were excluded based on any “quality” evaluation. However, given that the purpose of this review was to describe the state-of-the-art in SSA with the aim of identifying future needs, we decided to comment on three key methodological aspects of the 53 included peer-reviewed articles. First, we noted whether clinical samples were identified or diagnosed systematically using any standardized instruments. This is important as it helps to determine whether the phenotype of clinical samples under study is valid and comparable to phenotypes described in global ASD research. Second, where pre-existing instruments developed in western contexts were used (such as rating scales or diagnostic instruments), we noted whether they had been translated and validated in the setting where they were applied. This helps to determine the cultural sensitivity of ASD research in SSA to date. Finally, for intervention studies, we noted whether the interventions used were described in sufficient detail to allow for replication. This is admittedly only one of many quality indicators of an intervention study, but one that is well-documented for intervention studies using group experimental, quasi-experimental [Gersten et al., 2005] and single subject experimental designs [Horner et al., 2005]. These three methodological aspects are included in Table I and commented on in the narrative summary.
Findings
In order to get a general sense of the research output about SSA in a global context, we started with a topline exploration of the number of articles ever written about ASD from different continents. Figure 2 shows the publication output about ASD in SSA (first column) relative to other continents. Of a total of 24,467 publications identified, 120 were from SSA. When the number of peer-reviewed publications from SSA was compared to those from Europe (n = 7,577) or North America (n = 11,560) the geographic disparity in publication output about ASD in SSA relative to other continents is clear.
Figure 2.
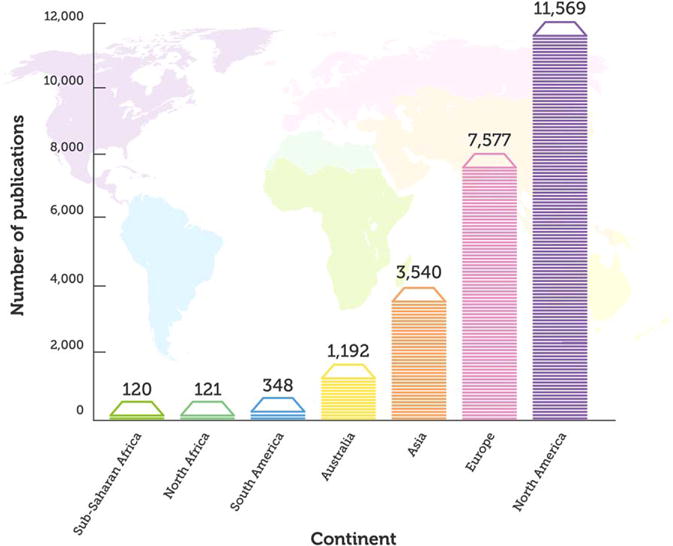
Publications about ASD around the globe. The figure shows the number of publications ever published on ASD by continent.
For the comprehensive scoping review, using the detailed search strategy outlined above, fifty-three articles (published between 1970 and 2015) were identified. These reported data from nine SSA countries (see Figure 3). More than three quarters of the studies (n = 42, 79%) were from South Africa or Nigeria. A summary of all articles is presented in Table I and a narrative summary of findings is presented below.
Figure 3.
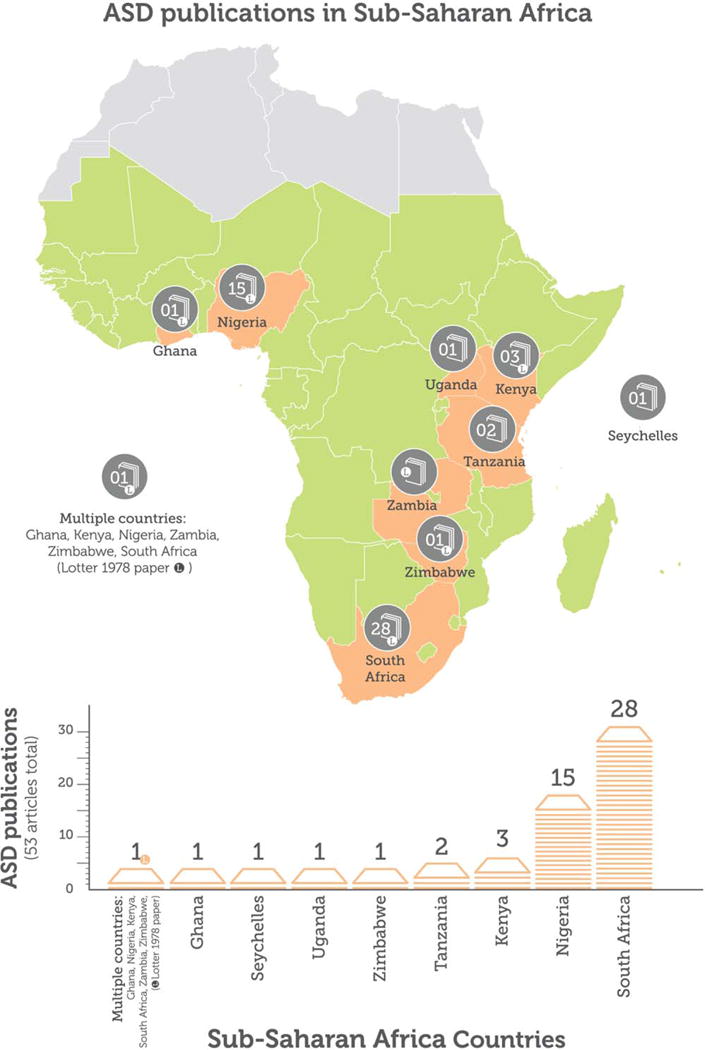
Publications about ASD in sub-Saharan Africa. The figure shows the number of peer-reviewed, data-containing publications ever published on ASD by country.
Thematic Grouping of Articles about ASD in SSA
Articles were grouped into eight thematic categories: (a) phenotype (n = 13), (b) genetics (n = 2), (c) risk factors (n = 3), (d) screening and diagnosis (n = 2), (e) professional knowledge (n = 9), (f) interventions (n = 11), (g) family perspectives (n = 8), and (h) social-cognitive neuroscience (n = 5). All articles included are shown in Table I, grouped under the primary theme of the article. The table summarizes each study included by first author, year of publication, country of publication, study design, description of study participants, methodological aspects, and study findings.
Phenotype of ASD in SSA
Viktor Lotter was the first to describe autism in SSA [Lotter, 1978]. In his study published in 1978 Lotter screened 1,312 children with intellectual disability in Ghana, Nigeria, Kenya, Zambia, South Africa, and Zimbabwe. He aimed to identify individuals with autism, subclassify them based on how well they met diagnostic criteria of the time, explore differences amongst subgroups, and compare phenotypic characteristics (motor, auditory, and repetitive-ritualistic behavior) in the African sample with those of a UK sample. Thirty of the children screened had “autistic-like” behaviors, and nine met criteria for the disorder. Lotter concluded that ASD behavior was found in “speaking and non-speaking” African children, and that it was associated with varying degrees of intellectual ability. He reported that the African group was broadly comparable to the British group in sex ratio, occurrence of epilepsy and socioeconomic status. He observed that certain repetitive movements and ritualistic behaviors involving objects were uncommon in the African sample [Lotter, 1978].
Since Lotter’s first phenotypic description, 12 articles (from Nigeria, South Africa, Kenya, and Zimbabwe) examined the phenotype of ASD in SSA [Bakare, Ebigbo, & Ubochi, 2012; Bakare & Ikegwuonu, 2008; Bello-Mojeed, Ogun, Omigbodun, Adewuya, & Ladapo, 2011; Dhadphale, Lukwago, & Gajjar, 1982; Ezegwui et al., 2014; Holford, 1994; Izuwah, Okoh, & Alikor, 2015; Khan & Hombarume, 1996; Lagunju, Bella-Awusah, & Omigbodun, 2014; Springer, van Toorn, Laughton, & Kidd, 2013; Szabo & Aber, 1992; Wessels & Pompe van Meerdervoort, 1979]. Most participants were drawn from tertiary level developmental, neurology, or child psychiatry clinics, and the majority were from higher socioeconomic families with access to clinical and educational services. Samples were relatively small (max. 75), and all participants were under 25 years of age. Speech delay was the most common initial symptom described by parents and eight of the 12 phenotype studies reported that participants were diagnosed between the ages of 7 and 17 years [Bakare et al., 2012; Bakare & Ikegwuonu, 2008; Bello-Mojeed et al., 2011; Holford, 1994; Izuwah et al., 2015; Khan & Hombarume, 1996; Lagunju et al., 2014; Lotter, 1978; Springer et al., 2013; Szabo & Aber, 1992]. The majority of children had intellectual disability, were non-verbal, and required substantial functional support for activities of daily living. The diagnosis of ASD was clinical, and mostly made using DSM and ICD criteria without the use of any standardized ASD diagnostic instruments [Bakare & Ikegwuonu, 2008; Bello-Mojeed et al., 2011; Izuwah et al., 2015; Khan & Hombarume, 1996; Lagunju et al., 2014; Springer et al., 2013; Szabo & Aber, 1992]. Four studies utilized autism rating scales (e.g., the Childhood Autism Rating Scale) as part of their diagnostic process [Izuwah et al., 2015; Khan & Hombarume, 1996; Lotter, 1978; Springer et al., 2013]. However, none of these rating scales had been translated or assessed for cultural appropriateness in the novel setting, and studies applied the published US, UK, or European cut-off values of tools.
Despite Lotter’s early assertion that repetitive and ritualistic behaviors were uncommon in the SSA population, multiple studies refuted this observation [Bakare & Ikegwuonu, 2008; Bello-Mojeed et al., 2011; Dhadphale et al., 1982; Holford, 1994; Izuwah et al., 2015; Lagunju et al., 2014; Springer et al., 2013; Szabo & Aber, 1992; Wessels & Pompe van Meerdervoort, 1979]. Lotter made an effort to include only black African children in his study, and deliberately excluded anyone of “mixed” or “Caucasian” ancestry. There have been suggestions that ASD may be more severe in black Africans [Becerra et al., 2014]. In a retrospective case review in South Africa, Springer and colleagues reported racial variation in verbal ability in a cohort of 58 children with ASD who attended a public tertiary developmental pediatric clinic over a 2-year period. Seventy-two percent of these children were non-verbal. There was a significantly higher proportion of black African children who were non-verbal at presentation (94%) compared with children of mixed ancestry (77%) and Caucasian children (42%). The authors, however, suggested that racial variation in verbal ability in these children was likely to be due to methodological and socioeconomic variables acting as barriers to care, rather than to true ethnic differences in verbal ability [Springer et al., 2013].
Phenotype – Knowledge Gaps in SSA
In high-income countries, cohorts of well-phenotyped individuals with ASD across the age and ability range, and across different languages have been established [Lowe, Werling, Constantino, Cantor, & Geschwind, 2015; Maestrini & IMGSAC, 2001; Simons VIP Consortium, 2012]. These cohorts, phenotyped with “gold standard” diagnostic instruments such as the Autism Diagnostic Observational Schedule, Second Edition (ADOS-2) [Lord et al., 2012] and Autism Diagnostic Interview, Revised (ADI-R) [Lord, Rutter, & Le Couteur, 1994] have allowed for large-scale examination of the ASD phenotype, genotype, associations between these two, and of longitudinal trajectories in phenotype and outcome [Chang, Gilman, Chiang, Sanders, & Vitkup, 2015; Lord, Bishop, & Anderson, 2015]. Therefore, in the majority of high-income countries there is a well-established “shared language” to discuss and compare the phenotype of individuals with ASD for clinical and research purposes.
The findings in SSA highlighted that there have been no large-scale or population-based studies, and that almost no studies have phenotyped participants using standardized diagnostic instruments. Findings have been biased to tertiary settings and high socioeconomic families. Importantly, almost no investigations of the phenotype of ASD have included adult individuals and no longitudinal studies of phenotype and phenotypic change have been performed in SSA. As a result, it is not possible to draw any firm conclusions about the phenotype, severity, comorbidities and functional outcomes of individuals who live with ASD in SSA.
We recommend that, in order to improve our understanding of the phenotype of ASD in SSA, researchers should use validated and accepted phenotyping instruments wherever possible. It will also be of the utmost importance that researchers receive appropriate training and supervision in the use of phenotyping instruments. At the same time, we recognize the barriers associated with the use of these instruments in the SSA setting [Abubakar et al., 2016; Durkin et al., 2015]. However, using DSM/ICD criteria is not sufficient to quantify the range and impact of ASD characteristics. We recommend a move away from categorical descriptions (ASD yes/no) to more dimensional descriptions including ASD specifiers, levels of severity, levels of expressive language and comorbidities as outlined in DSM-5 and related diagnostic systems. It is clear that large-scale, high-quality, population-based studies across the age range would add significantly to the knowledge gaps about the phenotype of ASD in SSA.
Genetics of ASD in SSA
Two papers on the genetics of ASD in SSA were identified [Arieff, Kaur, Gameeldien, van der Merwe, & Bajic, 2010; Sharma et al., 2013], both from South Africa. Arieff and colleagues compared allelic frequencies and genotypes of the 5-HTT Linked Polymorphic Region (5-HTTLPR) in African, mixed race, and Caucasian groups with ASD, matched controls without ASD, and an international group with ASD [Arieff et al., 2010]. The *S/*S genotype was significantly associated with all South African ethnic ASD groups. Allelic frequency of the South African groups differed from the Japanese, Korean, and Indian groups but not from the French, German, Israeli, Portuguese and USA groups with ASD. Sharma et al. (2013) reported significant genetic association for SNPs of the RELN gene in South African participants with a diagnosis of ASD.
Genetics – Knowledge Gaps in SSA
Many high-income countries have participated in a range of investigations into the genetic basis of ASD. Consortia such as the International Molecular Genetics Study of Autism Consortium (IMGSAC) [Maestrini & IMGSAC, 2001]), Autism Genetics Research Exchange (AGRE) [Lowe et al., 2015], and others [Simons VIP, 2012] have allowed for large-scale and cutting-edge genetic and molecular studies of candidate genes, genome-wide association studies, gene network, and next-generation sequencing studies. An increasing number of genetic causes of ASD have been identified, particularly as copy number variant [Sykes et al., 2009], and microdeletion/duplication syndromes [Miller et al., 2009]. One of the keys to this success has been the combination of sample size and well-phenotyped cohorts.
The two candidate gene studies performed in SSA were small-scale and neither had a well-phenotyped sample. There is, however, a growing interest in population genomics in Africa given that anatomically modern humans originated in SSA before migrating across the globe [Campbell, Hirbo, Townsend, & Tishkoff, 2014; Lachance & Tishkoff, 2013]. The ability to integrate genomic and phenotype data from SSA populations may identify novel variation underlying the complexity of ASD. We agree with a recent commentary [Abubakar et al., 2016] that SSA may therefore be able to make unique contributions towards understanding of the etiology of ASD. There is scope for researchers to explore the genetic heterogeneity of ASD in SSA populations as well as to examine the interplay between genetic and environmental factors in SSA. However, we recommend that well-phenotyped, large-scale cohorts will be required to make meaningful contributions to the global evidence-base on the genetics and genomics of ASD.
Risk Factors for ASD in SSA
Three articles described potential risk factors for ASD in SSA [Claassen, Naud e, Pretorius, & Bosman, 2008; Mankoski et al., 2006; van Wijngaarden et al., 2013]. Risk factors studied included prenatal stress, infectious disease, and mercury exposure, exemplifying some of the specific health concerns prevalent in SSA. Sample sizes varied widely from 2 to 1,784 participants and a range of methodologies were used. Claassen et al. [2008] proposed that maternal stress may contribute to ASD pathogenesis based on findings from a pair of dizygotic twins, one of whom had ASD, and whose blood plasma showed elevated glucocorticoids and serotonin relative to their non-affected sibling. In a case series in Tanzania, Mankoski et al. [2006] suggested that severe infection, for instance severe malaria, may contribute to the development of ASD, as three of the 14 children in their case series developed ASD following severe infection. This group used the ADI-R, a standardized diagnostic tool for ASD [Lord et al., 1994], translated into Kiswahili, to assist the diagnostic process. Unfortunately, no information was provided about the training of Kiswahili researchers, their reliability in use of the instrument, or on the validity of the instrument in the East African setting [Mankoski et al., 2006]. In a study conducted in the Republic of Seychelles, van Wijngaarden et al. [2013] failed to identify an association between prenatal methylmercury exposure and phenotypic scores on the Social Responsiveness Scale (SRS) [Constantino et al., 2003] and Social Communication Questionnaire (SCQ) [Berument, Rutter, Lord, Pickles, & Bailey, 1999] in four large cohorts of children. It was not clear from the article whether the SRS or SCQ had been translated or validated in the Seychelles, where the majority of inhabitants speak Seychellois Creole, one of the three official languages on the island.
Risk Factors – Knowledge Gaps in SSA
In high-income settings, there is an acceptance that ASD is caused by the interplay between genetic and environmental factors [Sandin et al., 2014; Volk et al., 2014]. There has been an increased interest in recent years in environmental risk factors such as toxins in herbicides and pesticides [Roberts et al., 2007]. Given the relatively low rates of many infectious diseases in high-income countries, few of these have been explored as possible risk factors for ASD.
In SSA, there is still a very high rate of infectious diseases including malaria, tuberculosis and human immunodeficiency virus (HIV), all of which are associated with central nervous system complications [McDonald, Elphinstone, & Kain, 2013; Rohlwink et al., 2016; Van Rie, Harrington, Dow, & Robertson, 2007]. Communicable diseases are public health priorities in SSA, given their high prevalence. Whilst the articles identified in this scoping review did not provide robust data about the role of infection as risk factors for ASD, studies in SSA may provide opportunities to examine the interplay between communicable and non-communicable diseases, such as between malaria and neurodevelopmental disability, and between genetic and environmental factors specifically associated with ASD. Similar to our recommendation for genetic studies, high-quality investigations will require high-quality phenotyping and standardized and well-validated outcome measures.
Screening and Diagnosis of ASD
To date, only two publications have focused specifically on ASD screening and diagnosis in SSA—one each from Tanzania and Uganda [Harrison, Zimak, Sheinkopf, Manji, & Morrow, 2014; Kakooza-Mwesige et al., 2014]. Both studies were cross-sectional. In Tanzania, Harrison et al. [2014] used the Childhood Autism Rating Scale, Second Edition (CARS-2), an observational diagnostic aid for ASD [Schopler, Van Bourgondein, Wellman, & Love, 2010] as part of a larger test battery to diagnose ASD. A process of cultural adaptation of the test battery for the target population was described.
In the only large-scale tool development study identified in this scoping review, Kakooza and colleagues piloted a 23-question screener (the 23Q), which included the Ten Questions Questionnaire (TQQ) [Durkin et al., 1995] and 13 additional questions specifically aimed at ASD detection [Kakooza-Mwesige et al., 2014]. The authors conducted household screening of 1,169 children between 2 and 9 years in Uganda, followed by clinical assessment of children who screened positive and a sample that screened negative. The authors provided an estimated ASD prevalence of 1.2–1.3/100 in this population, but noted that the study was not designed to determine population-based prevalence, and commented that these numbers may not reflect the true prevalence in this population. The 23Q was modestly successful in identifying children at high risk of ASD, but showed a relatively low positive predictive value of only 8%. That is, only 8% of the children who screened positive on the 23Q met criteria for ASD, suggesting that the majority of children who screened positive and were referred for in-depth clinical assessment (in a setting already challenged by limited resources) did not have ASD [Kakooza-Mwesige et al., 2014].
Screening and Diagnosis – Knowledge Gaps in SSA
In high-income countries, the use of standardized screening and diagnostic instruments is now well-established. For example, the United Kingdom has a well-established system for early detection of those presenting with “red flags” for ASD [National Institute for Health and Care Excellence, 2011]. A range of research investigations have been performed in high-income countries on screening instruments such as the Modified Checklist for Autism in Toddlers (M-CHAT) [Robins et al., 2014], SCQ [Berument et al., 1999], Childhood Autism Spectrum Test (CAST) [Scott, Baron-Cohen, Bolton, & Brayne, 2002], and on diagnostic instruments such as the ADOS-2 [Lord et al., 2012] and ADI-R [Lord et al., 1994]. Many of these instruments have been translated into a range of predominantly European languages [Western Psychological Services, 2016].
Only two SSA studies in this scoping review have examined screening or diagnostic instruments and both illustrated some of the challenges around tool evaluation in a novel setting. In the Tanzanian study by Harrison et al. [2014], for instance, the authors noted that the lead clinician, who did not speak Kiswahili, minimized verbal communication with the child during the play observation and primarily relied on social gestures and introduction of novel toys. Needless to say, a comprehensive ASD assessment requires assessment of verbal and non-verbal aspects of social communication. The Ugandan study by Kakooza et al. [2014] used a very clear two-stage design to evaluate their new 23Q screening tool, yet the investigating team did not have any gold standard diagnostic tools to confirm diagnoses. No published studies from SSA to date have used a combination of developmental history and observational “gold-standard” diagnostic instruments such as the ADI-R [Lord et al., 1994] and the ADOS-2 [Lord et al., 2012], as typically used in high-income country ASD research cohorts [Ashwood, Buitelaar, Murphy, Spooren, & Charman, 2015; Hallmayer et al., 2011; Lamb et al., 2005].
In order to develop a valid body of knowledge of ASD in SSA, standardized, validated and accessible tools for screening and diagnosis are critical. We acknowledge the challenges of translation, validation, and cost of training on existing tools such as the ADI-R and ADOS (see comments by Durkin and colleagues and Abubakar and colleagues) [Abubakar et al., 2016; Durkin et al., 2015]). We therefore agree that the SSA research agenda needs to include development and validation of open access, free, culturally fair, and globally relevant screening and diagnostic tools for ASD. We are delighted that such a process is underway supported by the International Society for Autism Research (INSAR) and Autism Speaks [Durkin et al., 2015]. However, we also recommend validation studies of existing screening and diagnostic tools in SSA settings to act as external benchmarks during the development of novel instruments. Researchers need to consider the cultural appropriateness, acceptability, and affordability of all screening and diagnostic tools and should evaluate the potential of introducing method bias when evaluating tools developed in high-income settings in low-income settings (e.g., language use, materials used, literacy levels, activities, expectations of the child). In some SSA settings, we may find that instruments can be used with high fidelity and validly with only minor modifications [Chambers et al., 2016; Smith, Malcolm-Smith, & de Vries, 2016].
Professional Knowledge
Nine publications focused on assessing the knowledge of health workers, teachers, and undergraduate health sciences students about ASD. All nine were performed in West Africa (Nigeria and Ghana) and almost all utilized the Knowledge of Childhood Autism Among Health Workers (KCAHW) questionnaire [Bakare, Ebigbo, Agomoh, & Menkiti, 2008]. This questionnaire comprises 19 Yes/No/Don’t know items across four “domains.” The first domain asks 8 questions about social reciprocity diagnostic criteria, the second asks 1 question assessing whether the individuals with ASD have “delay or total lack of spontaneous language,” domain 3 asks 4 questions about repetitive and stereotyped behaviors, and domain 4 asks 6 questions about terminology (“autism is childhood schizophrenia”), etiology (“autism is an auto-immune condition”), comorbidity (“autism could be associated with mental retardation”) and age of onset (“onset of autism is usually in (A) neonatal age, (B) infancy, (C) childhood”). The authors reported high internal consistency (Cronbach’s alpha = .97), and showed that total mean and domain mean scores (using t-tests) remained stable on re-administration 2 weeks later. No evaluation of the validity of the instrument has been performed.
Three studies [Bakare, Agomoh, et al., 2009; Bakare, Ebigbo, et al., 2009; Igwe, Ahanotu, Bakare, Achor, & Igwe, 2011] assessed ASD knowledge in pediatric and psychiatric nurses. In one of these studies, Bakare and colleagues [Bakare, Agomoh, et al., 2009] found that 27% of nurses believed the etiology of ASD to be supernatural, for example, lineage curses, actions of the devil, and cursed ancestral spirits. However, those with higher scores on the KCAHW were more likely to subscribe to natural causes for ASD. The majority believed ASD was treatable. Two studies found those with previous experience working with children with ASD had greater ASD knowledge relative to the group [Bakare, Ebigbo, et al., 2009]. Similar observations were made with medical professionals and health and life sciences students [Bakare et al., 2015; Eseigbe et al., 2015; Igwe, Bakare, Agomoh, Onyeama, & Okonkwo, 2010].
Audu and Egbochuko [2011] surveyed 131 primary school teachers on their knowledge about ASD. Twenty-three percent of teachers reported that they taught children with ASD, 77% reported more boys were affected than girls, and 32% reported that ASD symptoms differed by age. Teachers thought that parental awareness about ASD was low. Denkyirah and Agbeke [2010] explored opinions of 65 preschool teachers from Ghana and 210 preschool teachers from the United States to assess characteristics of successful transition from preschool to kindergarten for children with ASD, identifying many similarities, and few dissimilarities between the two countries.
Professional Knowledge – Knowledge Gaps in SSA
There has been a growing interest in quantification of knowledge about ASD in recent years [Harrison, Slane, Hoang, & Campbell, 2016] and a number of different instruments have been used to measure knowledge and change in knowledge about ASD. Given the absence of a widely accepted knowledge measure with good psychometric properties, relatively little is known about ASD knowledge around the globe, including in high-income countries.
It was therefore interesting to see a number of studies on knowledge about ASD, primarily from one group in West Africa. The Knowledge of Childhood Autism Among Health Workers (KCAHW) was evaluated in the review by Harrison et al. [2016], but was unfortunately found not to have very strong support for global use. Apart from knowledge, it is also important to consider skills and competencies [Liu et al., 2016]. No such evaluations have yet been performed in a SSA setting. There has also been no evaluation of the knowledge and skills of other key stakeholders, such as policy-makers, faith healers or other traditional practitioners. While most studies evaluating knowledge in professionals generally describe low knowledge of ASD and the need for improved education and training, no attempts have been made to document changes in knowledge following suitable training programmes. It is also unclear how much/what knowledge is necessary to improve skills and competencies, an aspect that would improve validity of knowledge measures [Harrison et al., 2016]. It is therefore important for researchers to determine what relevant professional groups currently know about ASD and develop strategies to improve their knowledge, skills, and attitudes. The development of standardized curricula for healthcare, social care and educational professionals, and evaluation of the impact of training on their knowledge, skills, and attitudes should be included in this endeavor. We recommend that, in a SSA setting, researchers should also include evaluation of cultural beliefs and stigma associated with ASD. Importantly, the knowledge, skills and attitudes of policy-makers and government officials need to be targeted.
Interventions for ASD
Eleven intervention studies were identified from SSA, nine from South Africa, one from Kenya, and one from Nigeria. Studies included single subject experimental designs [Akande, 2000; Travis & Geiger, 2010], pre-post quasi-experimental designs [Akande, 1998, 1999; Bunning, Gona, Newton, & Hartley, 2014; Pansegrouw & Alant, 1996; Silver, 1970], within-subjects design with repeated measures [Alant et al., 2013], non-experimental descriptive group designs of medication use [Louw, Bentley, Sorsdahl, & Adnams, 2013; Wong et al., 2014], and one qualitative design [Geils & Knoetze, 2008]. The sample sizes for experimental studies were very small with eight of the nine non-pharmacological intervention studies having fewer than 10 participants. None of the intervention studies used standardized assessment tools to diagnose ASD or to monitor ASD-related outcomes. Participant diagnoses were generally made independently of the study, often by clinical teams working at schools for children with ASD. Interventions were described with sufficient detail to allow for replication in 10 of the 11 studies.
Two studies focused on using augmentative and alternative communication (AAC). Travis and Geiger [2010] described the effects of teacher-implemented Picture Exchange Communication System (PECS) for two 9-year-old children with ASD. Improvements were noted in both participants for requesting with mixed results for commenting and length of verbal utterances. Bunning and colleagues [2014] also found positive changes following caregiver training in the use of the PECS in rural Kenya. A further study incorporating parent coaching found positive changes in both mother and child skills with an adolescent with profound cognitive impairment and “autistic features” [Pansegrouw & Alant, 1996]. Alant et al. [2013] described the translucency (degree to which the symbol represents its referent) ratings of Blissymbols by children with ASD over 3 consecutive exposures. Akande reported the differential effects of presentation mode on the acquisition of color labels in three children with ASD [Akande, 2000]. In two additional studies, Akande reported the impact of tutor’s self-monitoring of reinforcing statements on the frequency of play behaviors in children with ASD [Akande, 1998, 1999]. These latter two studies comprised an original study in Nigeria and a replication in South Africa. An early case study by Silver [Silver, 1970] described the use of operant conditioning to teach sounds and words to a severely impaired child with ASD and self-injurious behavior. Geils and Knoetze [2008] presented a naturalistic, qualitative case study examining conversational exchanges between a child with ASD and his familiar caregivers to determine features leading to communicative success or breakdown. Finally, two studies described the use of medication for individuals with ASD [Louw et al., 2013; Wong et al., 2014]. Both studies reported high rates of medication use with antipsychotics being the most commonly prescribed agent, followed by stimulants and antidepressants. Louw and colleagues [Louw et al., 2013] also found that 40% of individuals with ASD were taking over-the-counter supplements and that 15% were on a special diet.
Interventions – Knowledge Gaps in SSA
In high-income countries access to intervention for ASD varies, but a range of interventional options are available, from early intervention for infants and young children, to social skills training, special education and a growing support-base for services for adults with ASD [Dawson et al., 2010; Hedley et al., 2016; Laugeson, Frankel, Mogil, & Dillon, 2009; Spaulding, Lerner, & Gadow, 2016]. The contemporary conceptualization of naturalistic developmental behavioral interventions (NDBI) [Schreibman et al., 2015] is supported by a range of intervention studies, for example the Early Start Denver Model (ESDM) [Dawson et al., 2010], Social Communication, Emotional Regulation and Transactional Support (SCERTS) [Prizant, Wetherby, Rubin, & Laurent, 2003], and Pivotal Response Training (PRT) [Koegel & Koegel, 2006], with a more recent interest in parent-coaching [Kasari, Gulsrud, Paparella, Hellemann, & Berry, 2015; Rahman et al., 2016], and classroom-based implementation of NDBIs [Vivanti et al., 2014].
Considering the needs of individuals with ASD in SSA, the intervention studies reviewed reveal a number of important gaps. Very little has been published on the impact of family or provider beliefs in “cursed ancestral” on treatment-seeking behaviors. As discussed in the next theme on Family Perspectives [Gona et al., 2015] many people in Africa hold pluralistic beliefs about the cause and treatment of illness. We can postulate that these beliefs may prevent referral to medical or healthcare systems in which a child with ASD may receive intervention services, or that families may simultaneously pursue traditional and biomedical treatment approaches. The small number of intervention studies coupled with the wide range of intervention strategies investigated means that studies to date do not meet proposed evidence-based standards for any one intervention practice [Odom, Collet-Klingenberg, Rogers, & Hatton, 2010]. In addition, there were no studies investigating more comprehensive programmes such as inclusive education programmes, or low intensity interventions that can be implemented in community-based programmes. No intervention study in SSA to date has examined any of the NDBIs. There is therefore a pressing need to identify, develop, implement, and evaluate evidence-based interventions across the lifespan for individuals with ASD living in SSA. In order to address the wide range of needs that exist, it will be important to investigate interventions from low to high intensity, with an emphasis on making low-intensity interventions available to all families [de Vries, 2016]. A small start has been made on parent/caregiver-led interventions but this needs to be expanded, and the most appropriate parent/caregiver “coaches” in individual countries/regions need to be identified [Franz, Guler, Seris, Shabalala, & de Vries, 2016]. There is also a need to integrate interventions into existing systems of care to ensure that interventions can be scaled-up in communities [World Health Organization, 2013].
Family Perspectives About ASD
To date, eight papers on family perspectives have been published in SSA, seven from South Africa [Alli, Abdoola, & Mupawose, 2015; Du Toit & Kok, 1999; Fewster & Gurayah, 2015; Greeff & van der Walt, 2010; Kapp & Brown, 2011; Mitchell & Holdt, 2014; Olivier & Hing, 2009], and one from Kenya [Gona et al., 2015]. Qualitative and quantitative data were included in these articles, typically as in-depth interviews, focus groups, written open-ended questions, and questionnaires. In South Africa parents reported lack of social support, limited knowledge about ASD, lack of practical assistance, and uncertainty about their child’s prognosis [Du Toit & Kok, 1999; Fewster & Gurayah, 2015; Olivier & Hing, 2009]. Many experienced financial and marital strain in the context of raising a child with ASD [Du Toit & Kok, 1999; Mitchell & Holdt, 2014] and reported neglecting their own physical, emotional, and psychological needs [Fewster & Gurayah, 2015]. Parents felt unheard and inadequately informed by service providers, who often lacked knowledge about ASD and provided the diagnosis in a brash manner painting a bleak picture of the child’s prognosis [Du Toit & Kok, 1999; Fewster & Gurayah, 2015]. Parents reported significant delays in obtaining an ASD diagnosis, and highlighted the shortage of facilities and support services for children with ASD [Mitchell & Holdt, 2014; Olivier & Hing, 2009]. Parents noted their children had difficulty fitting into educational systems, and required specialized, individualized educational programs. They felt that the absence of a physical marker of disability in their child caused people to blame them for the child’s “poor behavior.” These negative experiences from the broader community resulted in increased family isolation [Du Toit & Kok, 1999]. Parents spoke of the need to “develop a rhino skin” providing them with the strength to fight stigma by asserting their rights to socialization and freedom of movement for their children [Fewster & Gurayah, 2015]. Resilience factors included higher socioeconomic status, social support, open and predictable patterns of communication between parents, supportive family environment, family hardiness, a positive outlook on life, and a family belief system [Greeff & van der Walt, 2010; Kapp & Brown, 2011; Olivier & Hing, 2009]. Parents also noted that their child’s communicative skills were not generalized to all settings [Alli et al., 2015].
Gona and colleagues conducted in-depth interviews and focus groups with 103 parents and professionals to explore their thoughts on causes and treatment of ASD in Kilifi and Mombasa, two ethnically, religiously, and socioeconomically diverse counties on the Kenyan coast [Gona et al., 2015]. Evil spirits, witchcraft, and curses as well as infections (for e.g. malaria), drug abuse, birth complications, malnutrition, and genetic factors were mentioned as possible causes of ASD. The treatment expectation from parents was the hope for a cure. Cultural and biomedical beliefs were not mutually exclusive on the Kenyan Coast, and indicated that parents from various cultural backgrounds, across educational levels, faiths and literacy levels, held pluralistic views about the causes and treatment options (traditional and biomedical) for their child with ASD [Gona et al., 2015].
Family Perspectives – Knowledge Gaps in SSA
There have been investigations of family perspectives in various high-income countries, including some recent surveys of UK families [Fletcher-Watson et al., 2016].
It was encouraging to see qualitative studies of family perspectives in SSA, although all but one of the studies was performed in South Africa. Given the socioeconomic, linguistic and cultural heterogeneity in SSA, it will be important to examine and understand family needs and perspectives across SSA communities, without assuming that it will be the same for all. The studies identified in the scoping review provide invaluable information regarding the needs and desires of families that should inform service development, intervention-planning, policy-making and research. We recommend that high-quality qualitative and mixed-method approaches should be used to expand our understanding of parental and family perspectives across SSA to inform and drive local, regional, national, and global ASD strategies. In addition, inclusion of the perspectives of individuals with ASD is paramount.
Social-Cognitive and Developmental Neuroscience of ASD
Five articles, all from South Africa, described research in various aspects of social-cognitive and developmental neuroscience in individuals with ASD [Hoogenhout & Malcolm-Smith, 2014; Noach, 1971; Pileggi, Malcolm-Smith, Hoogenhout, Thomas, & Solms, 2013; Pileggi, Malcolm-Smith, & Solms, 2015; van Zyl, Alant, & Uys, 1985]. All studies were cross-sectional, relatively small in sample size and examined only young people. In a 1971 paper, Noach compared the structure of concept formation in four verbal female children with ASD to a group of matched (but not for IQ) typically developing controls. Results suggested that the ASD group showed impaired ability to respond to common features of categories of objects relative to the typically developing control group [Noach, 1971]. Hoogenhout and colleague compared theory of mind in children with and without ASD. Participants were diagnosed using DSM-IV/5 criteria. Greater verbal ability was associated with increased theory of mind in the ASD but not in the typically developing group. The authors suggested that the single ASD category in DSM-5, with verbal IQ as a specifier, captured the bulk of the variation in individual theory of mind development [Hoogenhout & Malcolm-Smith, 2014]. In a pilot study, Pileggi and colleagues compared cradling bias in children with ASD with a matched typically developing control group. They reported that children with ASD showed no preference for cradling side whereas typically developing children showed a strong left-sided preference [Pileggi et al., 2013]. In a follow-up study, Pileggi and colleagues extended this investigation by including a control group with intellectual disability as well as measures of higher cognitive functioning. They again found that ASD diagnosis was the only significant predictor of atypical cradling preference and suggested that their results support the hypothesis that leftward cradling bias is associated with basic social-affective capacities [Pileggi et al., 2015]. Van Zyl and colleagues investigated whether echolalia could have a communicative purpose, by coding children’s interactive behavior (for e.g., initiation, turn-taking, and eye contact) with a familiar adult. They reported that immediate echolalia was relevant, and displayed meaningful information that maintained social interaction [van Zyl et al., 1985].
Social-Cognitive and Developmental Neuroscience – Knowledge Gaps in SSA
A very significant body of research has emerged from high-income countries on the social-cognitive and developmental neuroscience of ASD [Loth et al., 2016; Milosavljevic et al., 2016; Thomas, Davis, Karmiloff-Smith, Knowland, & Charman, 2016]. Many of these fundamental studies have contributed to the development of diagnostic and screening tools, and interventions for ASD [Mundy et al., 2007; Mundy, Sullivan, & Mastergeorge, 2009].
The handful of studies identified in this scoping review, all from South Africa, examined a few specific themes (theory of mind, cradling bias, and the development of concept formation) in small samples. We recommend that neuroscience research programmes in SSA aim to examine questions of significance to local communities, use methods that are applicable in low-resource environments, and ensure that findings can be translated into these low resource environments. Needless to say, neuroscience studies should clearly be embedded in high-quality phenotyping of participants.
Future Directions
In this comprehensive scoping review, we aimed to find all peer-reviewed articles ever published on ASD in SSA. The goal was to generate a map of the ASD research landscape in SSA so that we could identify knowledge gaps relevant to clinical service development, education and training, research, and policy.
In comparison to other continents, SSA had the lowest number of articles on ASD (n = 120), with North Africa (n = 121) and South America (n = 348) in second and third place. This observation supports earlier findings indicating the dearth of research on ASD and other disabilities in these regions of the world [Elsabbagh et al., 2012]. In spite of the fact that Africa is the second largest continent after Asia, we were able to identify only 53 peer-reviewed articles about ASD that reported data on participants who lived in SSA. The majority of outputs were from South Africa or Nigeria, yet represented only 42 articles. We summarized findings based on a number of themes, and presented key aspects of the knowledge gaps for each theme.
Given the low number of peer-reviewed ASD publications from SSA, we are cautious not to draw any firm conclusions about the state of ASD in this region. We hope that the knowledge gaps (Table II) identified can form the basis for “next steps” to improve knowledge about ASD and quality of life for those living with ASD in SSA.
Table II.
Recommendations for Future Research on Autism Spectrum Disorders in Sub-Saharan Africa Based on Knowledge Gaps Identified in This Review
| Theme | Recommendation for Next Steps |
|---|---|
| Phenotyping studies | Using DSM/ICD criteria is not sufficient to quantify the range of ASD characteristics • Use validated and accepted phenotyping instruments wherever possible • Move from simple categorical descriptions (ASD yes/no) to more dimensional descriptions including for example ASD specifiers, levels of severity, level of expressive language and comorbidities • Ensure that researchers receive appropriate training and supervision in the use of phenotyping tools |
| Genetics of ASD | SSA may be able to make unique contributions to understanding of the genetics of ASD • Explore the genetic heterogeneity of ASD in SSA populations • Examine the interplay between genetic and environmental factors in SSA • Ensure that this is done in well-phenotyped populations |
| ASD risk factors | SSA may be able to make unique contributions to understanding of ASD risk factors • Examine the interplay between genetic and environmental factors in SSA • Examine the interplay between communicable and non-communicable diseases in SSA |
| Screening and diagnosis | Standardized, validated and globally-accessible tools for screening and diagnosis are required • Perform validation studies of existing screening and diagnostic tools in settings where they have not been used • Consider the cultural appropriateness, acceptability, and affordability of screening and diagnostic tools • Evaluate the potential of introducing method bias when evaluating tools developed in high-income settings in low-income settings (e.g. language use, materials used, literacy levels, activities, expectations of the child) • Support the development of globally-acceptable, culturally-fair and open-access tools for screening, diagnosis and phenotyping of ASD and related neurodevelopmental disorders |
| Professional knowledge | Determine what relevant professional groups currently know about ASD and develop strategies to increase their knowledge, skills and attitudes • Develop standardized curricula for the education of healthcare, social care and educational professionals • Evaluate the impact of training on knowledge, skills and attitudes • Perform studies to evaluate cultural beliefs of professionals about ASD (including stigma) • Target knowledge, skills and attitudes of policy-makers and government officials |
| Intervention for ASD | Identify, develop and implement evidence-based interventions that are accessible to all • Interventions should range from ultra-low to ultra-high intensity, with an emphasis on making low-intensity interventions available to all families • Focus on parent/caregiver-led interventions • Identify the most appropriate parent/caregiver “coaches” in individual countries/regions • Integrate interventions into existing systems of care to ensure that interventions can be scaled-up in communities |
| Family perspectives | Listen to families who live with ASD and incorporate their needs and desires into service development, intervention-planning, research, and policy-making • Perform studies to understand parental perspectives across SSA • Do not assume that all perceptions and journeys will be the same • Use high-quality qualitative and mixed-method approaches • Use contextual knowledge gained from families to inform local/regional programmes |
| Social-cognitive and developmental neuroscience of ASD | Develop high-quality, meaningful social-cognitive and developmental neuroscience research programmes • Ensure that this research can be translated in low-resource environments • Consider carefully the benefit to individuals with ASD, their families and their communities • Ensure social-cognitive and developmental neuroscience studies are embedded in high-quality phenotyping of participants |
| Unexplored themes in sub-Saharan Africa | The scoping review identified a number of important themes that have never been explored in sub-Saharan Africa to date • Epidemiology of ASD • Early interventions, including naturalistic, developmental behavioral interventions (NDBIs) • Education and education systems • Transitional care from childhood to adult services • Adults with ASD • Use of technology for screening, diagnosis, training and monitoring |
ASD, Autism spectrum disorder; SSA, sub-Saharan Africa.
Apart from the themes summarized in the review, there were a number of notable thematic absences, which we believe are equally important to include in future ASD research agendas in SSA. Firstly, it remains the case that there are no true studies of the prevalence of ASD in SSA. It is clear from the review that standardized phenotyping tools will be required before high-quality epidemiological research can be performed. In the interim, whole-population counts of children known with disabilities or in specialist schools for ASD across SSA may start to give a sense of the scale and distribution of the needs of those with ASD and other neurodevelopmental disorders in SSA whilst avoiding many of the ascertainment biases from clinic samples.
We were struck by the absence of peer-reviewed data on early intervention and education-related aspects of ASD. We were not able to identify any articles on early intervention programmes, types of schools, access, training, curricula, in-school ASD interventions, or on health/education systems interfaces. We also could not find any publications on transitional care arrangements from school-aged to adult services for individuals with ASD in SSA. We found no publications that examined any aspects of ASD exclusively in adults. Clearly, these are all themes of great importance to all communities who live with ASD.
A further theme that was absent from the ASD literature in SSA was on the potential use of technology for screening, diagnosis, training, or treatment of ASD. Given the rapid developments in technologies for ASD around the globe [Grynszpan, Weiss, Perez-Diaz, & Gal, 2014], and given the increasing access in SSA to internet and mobile technologies [Betjeman, Soghoian, & Foran, 2013], this too may be an important avenue for future exploration in SSA.
Whilst the lack of data about ASD in SSA presented in this scoping review and noted elsewhere in the literature [Abubakar, 2016; Elsabbagh et al., 2012; van Schalkwyk, Beyer, & de Vries, 2016] risk presenting a bleak and insurmountable scenario, we propose that, from a global health perspective, SSA may present many unique and exciting opportunities to contribute to the ASD knowledge-base and to make a meaningful clinical impact. The very low baselines in clinical services, knowledge, and pathways to care may present unique opportunities to develop clinical care, training programmes and integrated health/education systems “from the ground up” using community-based participation and high-quality implementation science approaches [Bird et al., 2011; Bowen et al., 2009; Lund, Tomlinson, & Patel, 2016; Petersen et al., 2016; Proctor et al., 2011]. Another compelling opportunity in SSA may be integration of genetic/genomic and environmental data (Abubakar et al., 2016). This may allow us to study a range of putative environmental contributors to ASD. For instance, parenting, child-rearing practices, diets and environmental toxins may differ vastly in a very rural SSA community from a highly industrialized high-income country. Such information could contribute to our global understanding of these factors in relation to ASD and related neurodevelopmental disability. SSA has some of the highest rates of infectious diseases, and this may therefore allow powerful opportunities to investigate interactions between communicable (such as HIV, TB, and Malaria) and non-communicable diseases (such as ASD and related neurodevelopmental disorders).
Apart from expanding the thematic breadth and depth of ASD research in SSA, it is important to emphasize the broader needs in order to achieve these goals in SSA and in other low resource environments. As outlined in an earlier commentary (Abubakar et al., 2016) we need to enhance awareness about ASD in all communities and to all stakeholders from parents to policymakers. We need to increase our advocacy in a positive way to engage clinical, educational, research, and policy-making stakeholders around ASD and related neurodevelopmental disabilities. Education and training about ASD should be implemented across all levels of health, education, and the social care sector. This way, we will build knowledgeable leadership to improve health, education and social care systems to meet the needs of people with ASD in SSA. The very limited research on ASD in SSA to date emphasizes the need to develop research infrastructure, research funding, and research capacity in ASD across the lifespan. We believe that training a highly-skilled next generation of neurodevelopmental clinicians, educators, and researchers working in partnership with families, communities, and governments in SSA can truly transform the ASD landscape in this region of the world.
Acknowledgments
This work was supported by grants from Grant Sponsor: National Research Foundation (PJdV); Grant Sponsor: UCT PERC Programme (PJdV); Grant Sponsor: Struengmann Fund (PJdV); Grant Sponsor: National Institute of Mental Health (NIMH) Grant Number: K01-MH-104370 (LF); Grant Sponsor: Duke Global Health Institute (LF); Grant Sponsor: Florida State University Autism Institute (PJdV and NC).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Contributor Information
Lauren Franz, Division of Child and Family Mental Health and Developmental Neuroscience, Duke Global Health Institute, Duke University, Durham, North Carolina.
Nola Chambers, Division of Child and Adolescent Psychiatry, University of Cape Town, Cape Town, South Africa.
Megan von Isenburg, Medical Center Library, Duke University, Durham, North Carolina.
Petrus J. de Vries, Division of Child and Adolescent Psychiatry, University of Cape Town, Cape Town, South Africa
References
- Abubakar A, Ssewanyana D, de Vries PJ, Newton CR. Autism spectrum disorders in sub-Saharan Africa. Lancet Psychiatry. 2016;3:800–802. doi: 10.1016/S2215-0366(16)30138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akande A. Self-monitoring of autistic behavior. Psychology. 1998;35:23–29. [Google Scholar]
- Akande A. Autism - A case in early childhood: A South African investigation. Early Child Development and Care. 1999;155:71–78. [Google Scholar]
- Akande A. Assessing color identification in children with autism. Early Child Development and Care. 2000;164:95–104. [Google Scholar]
- Alant E, Zheng W, Harty M, Lloyd L. Translucency ratings of Blissymbols over repeated exposures by children with autism. AAC: Augmentative and Alternative Communication. 2013;29:272–283. doi: 10.3109/07434618.2013.813967. [DOI] [PubMed] [Google Scholar]
- Alli A, Abdoola S, Mupawose A. Parents’ journey into the world of autism. South African Journal of Child Health. 2015;9:81–84. [Google Scholar]
- Arieff Z, Kaur M, Gameeldien H, van der Merwe L, Bajic VB. 5-HTTLPR polymorphism: Analysis in South African autistic individuals. Human Biolology. 2010;82:291–300. doi: 10.3378/027.082.0303. [DOI] [PubMed] [Google Scholar]
- Arksey H, O’malley L. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology. 2005;8:19–32. [Google Scholar]
- Ashwood KL, Buitelaar J, Murphy D, Spooren W, Charman T. European clinical network: Autism spectrum disorder assessments and patient characterisation. European Child & Adolescent Psychiatry. 2015;24:985–995. doi: 10.1007/s00787-014-0648-2. [DOI] [PubMed] [Google Scholar]
- Audu EIV, Egbochuko EO. Autism among primary school pupils in Benin metropolis: Implications for counselling. Edo Journal of Counselling. 2011;3:261–272. [Google Scholar]
- Bakare MO, Agomoh AO, Ebigbo PO, Eaton J, Okonkwo KO, Onwukwe JU, Onyeama GM. Etiological explanation, treatability and preventability of childhood autism: A survey of Nigerian healthcare workers’ opinion. Annals of General Psychiatry. 2009;8:6. doi: 10.1186/1744-859X-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Ebigbo PO, Agomoh AO, Eaton J, Onyeama GM, Okonkwo KO, Aguocha CM. Knowledge about childhood autism and opinion among healthcare workers on availability of facilities and law caring for the needs and rights of children with childhood autism and other developmental disorders in Nigeria. BMC Pediatrics. 2009;9:12. doi: 10.1186/1471-2431-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Ebigbo PO, Agomoh AO, Menkiti NC. Knowledge about childhood autism among health workers (KCAHW) questionnaire: Description, reliability and internal consistency. Clinical Practice and Epidemiology in Mental Health. 2008;4:17. doi: 10.1186/1745-0179-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Ebigbo PO, Ubochi VN. Prevalence of autism spectrum disorder among Nigerian children with intellectual disability: A stopgap assessment. Journal of Health Care for the Poor and Underserved. 2012;23:513–518. doi: 10.1353/hpu.2012.0056. [DOI] [PubMed] [Google Scholar]
- Bakare MO, Ikegwuonu NN. Childhood autism in a 13 year old boy with oculocutaneous albinism: A case report. Journal of Medical Case Reports. 2008;2:56. doi: 10.1186/1752-1947-2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakare MO, Tunde-Ayinmode MF, Adewuya AO, Bello-Mojeed MA, Sale S, James BO, Orovwigho AO. Recognition of Autism Spectrum Disorder (ASD) symptoms and knowledge about some other aspects of ASD among final year medical students in Nigeria, sub-Saharan Africa. BMC Research Notes. 2015;8:454. doi: 10.1186/s13104-015-1433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, von Ehrenstein OS, Heck JE, Olsen J, Arah OA, Jeste SS, Ritz B. Autism spectrum disorders and race, ethnicity, and nativity: A population-based study. Pediatrics. 2014;134:e63–e71. doi: 10.1542/peds.2013-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Mojeed MA, Ogun OC, Omigbodun OO, Adewuya AO, Ladapo HTO. Late Identification of Autistic Disorder in Nigeria: An Illustration with 2 Case Reports. Nigerian Journal of Psychiatry. 2011;9:2. [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Betjeman TJ, Soghoian SE, Foran MP. mHealth in Sub-Saharan Africa. International Journal of Telemedicine and Applications. 2013;2013:482324. doi: 10.1155/2013/482324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird P, Omar M, Doku V, Lund C, Nsereko JR, Mwanza J, MHaPP Research Programme Consortium Increasing the priority of mental health in Africa: Findings from qualitative research in Ghana, South Africa, Uganda and Zambia. Health Policy and Planning. 2011;26:357–365. doi: 10.1093/heapol/czq078. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Fernandez M. How we design feasibility studies. American Journal of Preventive Medicine. 2009;36:452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning K, Gona JK, Newton CR, Hartley S. Caregiver Perceptions of children who have complex communication needs following a home-based intervention using augmentative and alternative communication in rural Kenya: An intervention note. Augmentative and Alternative Communication. 2014;30:344–356. doi: 10.3109/07434618.2014.970294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MC, Hirbo JB, Townsend JP, Tishkoff SA. The peopling of the African continent and the diaspora into the new world. Current Opinion in Genetics & Development. 2014;29:120–132. doi: 10.1016/j.gde.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 Sites, United States, 2010. 2014 Retrieved 24 November 2015 from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6302a1.htm?s_cid=ss6302a1_w. [PubMed]
- Chambers NJ, Wetherby AM, Stronach ST, Njongwe N, Kauchali S, Grinker RR. Early detection of autism spectrum disorder in young isiZulu-speaking children in South Africa. Autism. 2016 doi: 10.1177/1362361316651196. [DOI] [PubMed] [Google Scholar]
- Chang J, Gilman SR, Chiang AH, Sanders SJ, Vitkup D. Genotype to phenotype relationships in autism spectrum disorders. Nature Neuroscience. 2015;18:191–198. doi: 10.1038/nn.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen M, Naud e H, Pretorius E, Bosman MC. The contribution of prenatal stress to the pathogenesis of autism as a neurobiological developmental disorder: A dizygotic twin study. Early Child Development and Care. 2008;178:487–511. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ. Thinking globally to meet local needs: Autism spectrum disorders in Africa and other low-resource environments. Current Opinion in Neurolology. 2016;29:130–136. doi: 10.1097/WCO.0000000000000297. [DOI] [PubMed] [Google Scholar]
- Denkyirah AM, Agbeke WK. Strategies for transitioning preschoolers with autism spectrum disorders to kindergarten. Early Childhood Education Journal. 2010;38:265–270. [Google Scholar]
- Dhadphale M, Lukwago MG, Gajjar M. Infantile autism in Kenya. Indian Journal of Pediatrics. 1982;49:145–148. [PubMed] [Google Scholar]
- Du Toit Z, Kok JC. Belewenis en begeleidingsbehoeftes van ouers van kinders met Aspergersindroom: ’n opvoedkundige sielkundige perspektief. South African Journal of Education. 1999;19:185–191. [Google Scholar]
- Durkin MS, Elsabbagh M, Barbaro J, Gladstone M, Happe F, Hoekstra RA, Shih A. Autism screening and diagnosis in low resource settings: Challenges and opportunities to enhance research and services worldwide. Autism Research. 2015;8:473–476. doi: 10.1002/aur.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin MS, Wang W, Shrout PE, Zaman SS, Hasan ZM, Desai P, Davidson LL. Evaluating a ten questions screen for childhood disability: Reliability and internal structure in different cultures. Journal of Clinical Epidemiolology. 1995;48:657–666. doi: 10.1016/0895-4356(94)00163-k. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism Research. 2012;5:160–179. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle PL, Black MM, Behrman JR, Cabral de Mello M, Gertler PJ, Kapiriri L, International Child Development Steering Group Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- Epidemiology & Disease Control Division Ministry of Health Singapore. Singapore burden of disease study 2010. 2014. Retrieved 20 October 2015 from https://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2014/singapore-burden-of-disease-study-2010.html.
- Eseigbe EE, Nuhu FT, Sheikh TL, Eseigbe P, Sanni KA, Olisah VO. Knowledge of childhood autism and challenges of management among medical doctors in Kaduna State, Northwest Nigeria. Autism Research and Treatment. 2015;2015:892301. doi: 10.1155/2015/892301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezegwui IR, Lawrence L, Aghaji AE, Okoye OI, Okoye O, Onwasigwe EN, Ebigbo PO. Refractive errors in children with autism in a developing country. Nigerian Journal of Clinical Practice. 2014;17:467–470. doi: 10.4103/1119-3077.134042. [DOI] [PubMed] [Google Scholar]
- Fewster DL, Gurayah T. First port of call: Facing the parents of autism spectrum disorder. South African Family Practice. 2015;57:31–34. [Google Scholar]
- Fletcher-Watson S, Apicella F, Auyeung B, Beranova S, Bonnet-Brilhault F, Canal-Bedia R, Yirmiya N. Attitudes of the autism community to early autism research. Autism. 2016;21:61–74. doi: 10.1177/1362361315626577. [DOI] [PubMed] [Google Scholar]
- Franz L, Guler J, Seris N, Shabalala N, de Vries PJ. Adapting caregiver-mediated early Autism interventions in South Africa: Contextual factors and acceptability of caregiver involvement; Paper presented at the IMFAR 2016; Baltimore, MD. 2016. Retrieved 26 July 2016 from https://imfar.confex.com/imfar/2016/webprogram/Paper21988.html. [Google Scholar]
- Garenne M, Gakusi E. Health transitions in sub-Saharan Africa: Overview of mortality trends in children under 5 years old (1950–2000) Bulletin of the World Health Organization. 2006;84:470–478. doi: 10.2471/blt.05.029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geils C, Knoetze J. Conversations with Barney: A conversation analysis of interactions with a child with autism. South African Journal of Psychology. 2008;38:200–224. [Google Scholar]
- Gersten R, Fuchs LS, Compton D, Coyne M, Greenwood C, Innocenti MS. Quality indicators for group experimental and quasi-experimental research in special education. Exceptional Children. 2005;71:149–164. [Google Scholar]
- Gona JK, Newton CR, Rimba K, Mapenzi R, Kihara M, Van de Vijver FJR, Abubakar A. Parents’ and professionals’ perceptions on causes and treatment options for Autism Spectrum Disorders (ASD) in a multicultural context on the Kenyan coast. PLoS One. 2015;10:8. doi: 10.1371/journal.pone.0132729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeff AP, van der Walt KJ. Resilience in families with an autistic child. Education and Training in Autism and Developmental Disabilities. 2010;45:347–355. [Google Scholar]
- Grynszpan O, Weiss PL, Perez-Diaz F, Gal E. Innovative technology-based interventions for autism spectrum disorders: A meta-analysis. Autism. 2014;18:346–361. doi: 10.1177/1362361313476767. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AJ, Slane MM, Hoang L, Campbell JM. An international review of autism knowledge assessment measures. Autism. 2016 doi: 10.1177/1362361316638786. [DOI] [PubMed] [Google Scholar]
- Harrison AJ, Zimak EH, Sheinkopf SJ, Manji KP, Morrow EM. Observation-centered approach to ASD assessment in Tanzania. Intellectual and Developmental Disabilities. 2014;52:330–347. doi: 10.1352/1934-9556-52.5.330. [DOI] [PubMed] [Google Scholar]
- Hedley D, Uljarevic M, Cameron L, Halder S, Richdale A, Dissanayake C. Employment programmes and interventions targeting adults with autism spectrum disorder: A systematic review of the literature. Autism. 2016 doi: 10.1177/1362361316661855. [DOI] [PubMed] [Google Scholar]
- Holford L. Asperger’s syndrome: A classification struggle. Southern African Journal of Child & Adolescent Psychiatry. 1994;6:47–56. [Google Scholar]
- Hoogenhout M, Malcolm-Smith S. Theory of mind in autism spectrum disorder: Does DSM classification predict development? Research in Autism Spectrum Disorders. 2014;8:597–607. [Google Scholar]
- Horner RH, Carr EG, Halle J, McGee G, Odom S, Wolery M. The use of single-subject research to identify evidence-based practice in special education. Exceptional Children. 2005;71:165–179. [Google Scholar]
- Igwe MN, Ahanotu AC, Bakare MO, Achor JU, Igwe C. Assessment of knowledge about childhood autism among paediatric and psychiatric nurses in Ebonyi state, Nigeria. Child and Adolescent Psychiatry and Mental Health. 2011;5:1. doi: 10.1186/1753-2000-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igwe MN, Bakare MO, Agomoh AO, Onyeama GM, Okonkwo KO. Factors influencing knowledge about childhood autism among final year undergraduate Medical, Nursing and Psychology students of University of Nigeria, Enugu State, Nigeria. Italian Journal of Pediatrics. 2010;36:44. doi: 10.1186/1824-7288-36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuwah DN, Okoh BA, Alikor EA. Clinical pattern of autism in Nigeria. Autism Research. 2015;9:376–381. doi: 10.1002/aur.1531. [DOI] [PubMed] [Google Scholar]
- Kakooza-Mwesige A, Ssebyala K, Karamagi C, Kiguli S, Smith K, Anderson MC, Grether JK. Adaptation of the “ten questions” to screen for autism and other neurodevelopmental disorders in Uganda. Autism. 2014;18:447–457. doi: 10.1177/1362361313475848. [DOI] [PubMed] [Google Scholar]
- Kapp L, Brown O. Resilience in families adapting to autism spectrum disorder. Journal of Psychology in Africa. 2011;21:459–463. [Google Scholar]
- Kasari C, Gulsrud A, Paparella T, Hellemann G, Berry K. Randomized comparative efficacy study of parent-mediated interventions for toddlers with autism. Journal of Consulting and Clinical Psychology. 2015;83:554–563. doi: 10.1037/a0039080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Hombarume J. Levels of autistic behaviour among the mentally handicapped children in Zimbabwe. Central African Journal of Medicine. 1996;42:36–39. [PubMed] [Google Scholar]
- Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, Rahman A. Child and adolescent mental health worldwide: Evidence for action. Lancet. 2011;378:1515–1525. doi: 10.1016/S0140-6736(11)60827-1. [DOI] [PubMed] [Google Scholar]
- Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, Grinker RR. Prevalence of autism spectrum disorders in a total population sample. American Journal of Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Koegel LK. Pivotal response treatments for autism: Communication, social & academic development. Baltimore, MD: Paul H. Brookes Publishing Co; 2006. [Google Scholar]
- Lachance J, Tishkoff SA. Population genomics of human adaptation. Annual Review of Ecology, Evolution, and Systematics. 2013;44:123–143. doi: 10.1146/annurev-ecolsys-110512-135833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunju IA, Bella-Awusah TT, Omigbodun OO. Autistic disorder in Nigeria: Profile and challenges to management. Epilepsy & Behavior. 2014;39:126–129. doi: 10.1016/j.yebeh.2014.08.020. [DOI] [PubMed] [Google Scholar]
- Lamb JA, Barnby G, Bonora E, Sykes N, Bacchelli E, Blasi F, Monaco AP, International Molecular Genetic Study of Autism Consortium Analysis of IMGSAC autism susceptibility loci: Evidence for sex limited and parent of origin specific effects. Journal of Medical Genetics. 2005;42:132–137. doi: 10.1136/jmg.2004.025668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugeson EA, Frankel F, Mogil C, Dillon AR. Parent-assisted social skills training to improve friendships in teens with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:596–606. doi: 10.1007/s10803-008-0664-5. [DOI] [PubMed] [Google Scholar]
- Levac D, Colquhoun H, O’brien KK. Scoping studies: Advancing the methodology. Implementation Science. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Jack H, Piette A, Mangezi W, Machando D, Rwafa C, Abas M. Mental health training for health workers in Africa: A systematic review. Lancet Psychiatry. 2016;3:65–76. doi: 10.1016/S2215-0366(15)00379-X. [DOI] [PubMed] [Google Scholar]
- Lord C, Bishop S, Anderson D. Developmental trajectories as autism phenotypes. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics. 2015;169:198–208. doi: 10.1002/ajmg.c.31440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop S. Autism diagnostic observation schedule, Second Edition. Torrance, CA: Western Psychological Services; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loth E, Spooren W, Ham LM, Isaac MB, Auriche-Benichou C, Banaschewski T, Murphy DG. Identification and validation of biomarkers for autism spectrum disorders. Nature Reviews. Drug Discovery. 2016;15:70–73. doi: 10.1038/nrd.2015.7. [DOI] [PubMed] [Google Scholar]
- Lotter V. Childhood autism in Africa. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1978;19:231–244. doi: 10.1111/j.1469-7610.1978.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Louw KA, Bentley J, Sorsdahl K, Adnams CM. Prevalence and patterns of medication use in children and adolescents with autism spectrum disorders in the Western Cape, South Africa. Journal of Child and Adolescent Mental Health. 2013;25:69–79. doi: 10.2989/17280583.2013.767265. [DOI] [PubMed] [Google Scholar]
- Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH. Social responsiveness, an autism endophenotype: Genomewide significant linkage to two regions on chromosome 8. American Journal of Psychiatry. 2015;172:266–275. doi: 10.1176/appi.ajp.2014.14050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund C, Tomlinson M, Patel V. Integration of mental health into primary care in low- and middle-income countries: The PRIME mental healthcare plans. British Journal of Psychiatry. 2016;208:s1–s3. doi: 10.1192/bjp.bp.114.153668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini E, IMGSAC Analysis of candidate genes for autism on chromosomes 7q, 2q and 16p. American Journal of Human Genetics. 2001;69:548–548. [Google Scholar]
- Mankoski RE, Collins M, Ndosi NK, Mgalla EH, Sarwatt VV, Folstein SE. Etiologies of autism in a case-series from Tanzania. Journal of Autism and Developmental Disorders. 2006;36:1039–1051. doi: 10.1007/s10803-006-0143-9. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Elphinstone RE, Kain KC. The impact of placental malaria on neurodevelopment of exposed infants: A role for the complement system? Trends in Parasitolology. 2013;29:213–219. doi: 10.1016/j.pt.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, Wu BL. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. Journal of Medical Genetetics. 2009;46:242–248. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic B, Carter Leno V, Simonoff E, Baird G, Pickles A, Jones CR, Happe F. Alexithymia in adolescents with autism spectrum disorder: Its relationship to internalising difficulties, sensory modulation and social cognition. Journal of Autism and Developmenal Disorders. 2016;46:1354–1367. doi: 10.1007/s10803-015-2670-8. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Holdt N. The search for a timely diagnosis: Parents’ experiences of their child being diagnosed with an Autistic Spectrum Disorder. Journal of Child and Adolescent Mental Health. 2014;26:49–62. doi: 10.2989/17280583.2013.849606. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Delgado C, Pomares Y, Van Hecke AV, Parlade MV. Individual differences and the development of joint attention in infancy. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Research. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Laakso T, Shibuya K, Hill K, Lopez AD. Can we achieve Millennium Development Goal 4? New analysis of country trends and forecasts of under-5 mortality to 2015. Lancet. 2007;370:1040–1054. doi: 10.1016/S0140-6736(07)61478-0. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. Autism spectrum disorder in under 19s: Recognition, referral and diagnosis. 2011 Retrieved 15 October 2016 from https://www.nice.org.uk/guidance/cg128. [PubMed]
- Noach M. Concept formation in the speaking autistic child. International Journal of Mental Health. 1971;3:100–109. [PubMed] [Google Scholar]
- Odom SL, Collet-Klingenberg L, Rogers S, Hatton DD. Evidence-based practices in interventions for children and youth with Autism Spectrum Disorders. Preventing School Failure. 2010;54:275–282. [Google Scholar]
- Olivier MA, Hing ADA. Autistic spectrum disorder (ASD): Parental challenges and strategies. Vulnerable Children and Youth Studies. 2009;4:58–66. [Google Scholar]
- Pansegrouw I, Alant E. Communication intervention in an adolescent with profound cognitive impairment and autistic features. South African Journal of Communication Disorders. 1996;43:63–75. [PubMed] [Google Scholar]
- Petersen I, Fairall L, Bhana A, Kathree T, Selohilwe O, Brooke-Sumner C, Patel V. Integrating mental health into chronic care in South Africa: The development of a district mental healthcare plan. British Journal of Psychiatry. 2016;208:s29–s39. doi: 10.1192/bjp.bp.114.153726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileggi LA, Malcolm-Smith S, Hoogenhout M, Thomas KGF, Solms M. Cradling bias is absent in children with autism spectrum disorders. Journal of Child and Adolescent Mental Health. 2013;25:55–60. doi: 10.2989/17280583.2013.767262. [DOI] [PubMed] [Google Scholar]
- Pileggi LA, Malcolm-Smith S, Solms M. Investigating the role of social-affective attachment processes in cradling bias: The absence of cradling bias in children with autism spectrum disorders. Laterality: Asymmetries of Body, Brain and Cognition. 2015;20:154–170. doi: 10.1080/1357650X.2014.948449. [DOI] [PubMed] [Google Scholar]
- Prizant BM, Wetherby AM, Rubin E, Laurent AC. The SCERTS model - A transactional, family-centered approach to enhancing communication and socio-emotional abilities of children with autism spectrum disorder. Infants and Young Children. 2003;16:296–316. [Google Scholar]
- Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, Hensley M. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Administration and Policy in Mental Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Divan G, Hamdani SU, Vajaratkar V, Taylor C, Leadbitter K, Green J. Effectiveness of the parent-mediated intervention for children with autism spectrum disorder in south Asia in India and Pakistan (PASS): A randomised controlled trial. Lancet Psychiatry. 2016;3:128–136. doi: 10.1016/S2215-0366(15)00388-0. [DOI] [PubMed] [Google Scholar]
- Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, Murray CJ. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: A systematic analysis of progress towards Millennium Development Goal 4. Lancet. 2010;375:1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental Health Perspectives. 2007;115:1482–1489. doi: 10.1289/ehp.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Casagrande K, Barton M, Chen CM, Dumont-Mathieu T, Fein D. Validation of the modified checklist for Autism in toddlers, revised with follow-up (M-CHAT-R/F) Pediatrics. 2014;133:37–45. doi: 10.1542/peds.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlwink UK, Donald K, Gavine B, Padayachy L, Wilmshurst JM, Fieggen GA, Figaji AA. Clinical characteristics and neurodevelopmental outcomes of children with tuberculous meningitis and hydrocephalus. Developmental Medicine and Child Neurolology. 2016;58:461–468. doi: 10.1111/dmcn.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA-Journal of the American Medical Association. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer AL, Chagan M, Kauchali S, Susser E. Global perspective on early diagnosis and intervention for children with developmental delays and disabilities. Developmental Medicine and Child Neurology. 2012;54:1079–1084. doi: 10.1111/j.1469-8749.2012.04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Van Bourgondein ME, Wellman GJ, Love SR. Childhood autism rating scale, second edition (CARS2) San Antonia, TX: Pearson; 2010. [Google Scholar]
- Schreibman L, Dawson G, Stahmer AC, Landa R, Rogers SJ, Mcgee GG, Halladay A. Naturalistic developmental behavioral interventions: Empirically validated treatments for autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45:2411–2428. doi: 10.1007/s10803-015-2407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): Preliminary development of a UK screen for mainstream primary-school-age children. Autism. 2002;6:9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- Sharma JR, Arieff Z, Gameeldien H, Davids M, Kaur M, van der Merwe L. Association analysis of two single-nucleotide polymorphisms of the RELN gene with autism in the South African population. Genetic Testing and Molecular Biomarkers. 2013;17:93–98. doi: 10.1089/gtmb.2012.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver EB. Operant conditioning of speech sounds in an autistic child. South African Journal of Psychology. 1970:3–12. [Google Scholar]
- Simons VIP Consortium. Simons Variation in Individuals Project (Simons VIP): A genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron. 2012;73:1063–1067. doi: 10.1016/j.neuron.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Smith L, Malcolm-Smith S, de Vries PJ. Translation and cultural appropriateness of the Autism Diagnostic Observation Schedule-2 in Afrikaans. Autism. 2016 doi: 10.1177/1362361316648469. [DOI] [PubMed] [Google Scholar]
- Spaulding CJ, Lerner MD, Gadow KD. Trajectories and correlates of special education supports for youth with autism spectrum disorder and psychiatric comparisons. Autism. 2016 doi: 10.1177/1362361316645428. [DOI] [PubMed] [Google Scholar]
- Springer PE, van Toorn R, Laughton B, Kidd M. Characteristics of children with pervasive developmental disorders attending a developmental clinic in the Western Cape Province, South Africa. SAJCH South African Journal of Child Health. 2013;7:95–99. [Google Scholar]
- Sykes NH, Toma C, Wilson N, Volpi EV, Sousa I, Pagnamenta AT, International Molecular Genetic Study of Autism Consortium Copy number variation and association analysis of SHANK3 as a candidate gene for autism in the IMGSAC collection. European Journal of Human Genetics. 2009;17:1347–1353. doi: 10.1038/ejhg.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo CP, Aber D. Asperger’s syndrome: A valid DSM IV diagnostic entitity? A case report. Southern African Journal of Child & Adolescent Psychiatry. 1992;4:3–7. [Google Scholar]
- The World Bank. Regions. 2015 Annual Report 2015. Retrieved 5 September 2016 from http://www.worldbank.org/en/about/annual-report/regions.
- Thomas MS, Davis R, Karmiloff-Smith A, Knowland VC, Charman T. The over-pruning hypothesis of autism. Developmental Science. 2016;19:284–305. doi: 10.1111/desc.12303. [DOI] [PubMed] [Google Scholar]
- Travis J, Geiger M. The effectiveness of the Picture Exchange Communication System (PECS) for children with autism spectrum disorder (ASD): A South African pilot study. Child Language Teaching & Therapy. 2010;26:39–59. [Google Scholar]
- UNICEF. Division of Data, Research, & and Policy. Generation 2030 Africa. 2014 Retrieved 5 September 2016 from http://www.unicef.org/publications/files/Generation_2030_Africa.pdf.
- United Nations. World and regional groupings Millennium Development Indicators. 2014 Retrieved 5 September 2016, from http://mdgs.un.org/unsd/mdg/Host.aspx?Content=Data/RegionalGroupings.
- United Nations. The Millennium Development Goals Report. World Health Organizationk; Geneva: 2015. Retrieved from. Retrieved 5 September 2016 from http://www.un.org/mil-lenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20%28July%201%29.pdf. [Google Scholar]
- Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. European Journal of Paediatric Neurology. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- van Schalkwyk GI, Beyer C, de Vries PJ. South Africa and autism. In: Volkmar FR, editor. Encyclopedia of autism spectrum disorders. New York: Springer Science; 2016. [Google Scholar]
- van Wijngaarden E, Davidson PW, Smith TH, Evans K, Yost K, Love T, Myers GJ. Autism spectrum disorder phenotypes and prenatal exposure to methylmercury. Epidemiology. 2013;24:651–659. doi: 10.1097/EDE.0b013e31829d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl I, Alant E, Uys IC. Immediate echolalia and the interactive behaviour of autistic children. South African Journal of Communication Disorders. 1985;32:25–31. [PubMed] [Google Scholar]
- Vivanti G, Paynter J, Duncan E, Fothergill H, Dissanayake C, Rogers SJ, Victorian AT. Effectiveness and feasibility of the early start Denver model implemented in a group-based community childcare setting. Journal of Autism and Developmental Disorders. 2014;44:3140–3153. doi: 10.1007/s10803-014-2168-9. [DOI] [PubMed] [Google Scholar]
- Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. Autism spectrum disorder: Interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology. 2014;25:44–47. doi: 10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels WH, Pompe van Meerdervoort M. Monozygotic twins with early infantile autism. A case report. South African Medical Journal. 1979;55:955–957. [PubMed] [Google Scholar]
- Western Psychological Services. WPS published translations. 2016 Retrieved 18 August 2016, http://www.wpspublish.com/app/OtherServices/PublishedTranslations.aspx.
- Wong AY, Hsia Y, Chan EW, Murphy DG, Simonoff E, Buitelaar JK, Wong IC. The variation of psychopharmacological prescription rates for people with autism spectrum disorder (ASD) in 30 countries. Autism Research. 2014;7:543–554. doi: 10.1002/aur.1391. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Autism spectrum disorders & other developmental disorders: From raising awarness to building capacity. Geneva, Switzerland: 2013. Retrieved 5 September 2015 from http://apps.who.int/iris/bitstream/10665/103312/1/9789241506618_eng.pdf. [Google Scholar]


