Several studies from Southern Africa report a high risk of tuberculosis among individuals who have been previously treated for the disease compared to those never treated before [1–5]. In high-burden settings, recurrent tuberculosis may affect large numbers of individuals even after successful treatment, with exogenous reinfection as an important underlying mechanism [2–4]. For example, in Cape Town, a city with a high incidence of tuberculosis in South Africa, previously treated individuals constitute one-third of the burden of notified tuberculosis [6].
The impact of recurrent disease on tuberculosis epidemics in Southern Africa is not well understood. In particular, there is limited knowledge about the extent to which previously treated people contribute to the pool of undiagnosed prevalent tuberculosis and transmission in high-burden settings. Two prevalence surveys in Zambia [7] and Zimbabwe [8] reported that previous treatment was strongly associated with prevalent tuberculosis among HIV-uninfected individuals. Ten out of 18 smear-positive tuberculosis cases detected in a prevalence survey in a South African suburban setting had a history of previous treatment [9], consistent with the hypothesis that previously treated people contribute considerably to tuberculosis prevalence and transmission in this setting.
Better quantification of prevalent tuberculosis by treatment history can inform estimates of the importance of previously treated individuals for the dynamics of tuberculosis epidemics and help determine if specific interventions targeted to this risk group could accelerate tuberculosis control. We therefore aimed to investigate, across 24 African communities, how common a history of previous treatment was, whether the prevalence of tuberculosis differed by history of previous treatment, and to what extent previously treated individuals contributed to the overall prevalent tuberculosis burden.
We analysed data from tuberculosis prevalence surveys conducted in 2010 as the primary outcome measure of the Zambia South Africa Tuberculosis and AIDS Reduction (ZAMSTAR) study, a large community-based intervention trial in 24 tuberculosis and HIV high-burden communities, 16 in Zambia and 8 in South Africa (Western Cape Province) [10, 11]. All adults aged 18 or above who had spent the previous night in the community were eligible to participate in the surveys. Prevalent tuberculosis was ascertained through liquid (mycobacterial growth indicator tube [MGIT]) culture of single sputum specimens collected on the spot and confirmed as Mycobacterium tuberculosis by 16SrRNA sequencing. Further details related to the prevalence survey design have been previously published [10]. Here, we distinguished prevalent tuberculosis among adults who reported a history of previous tuberculosis treatment (treatment-experienced) from that among adults who reported no previous treatment (treatment-naïve). This analysis was approved by the ethics committee of Stellenbosch University (Reference number: N04/10/173), and the Institutional Review Boards of Partners Healthcare (2014P001719/BWH) and Yale University (1409014625).
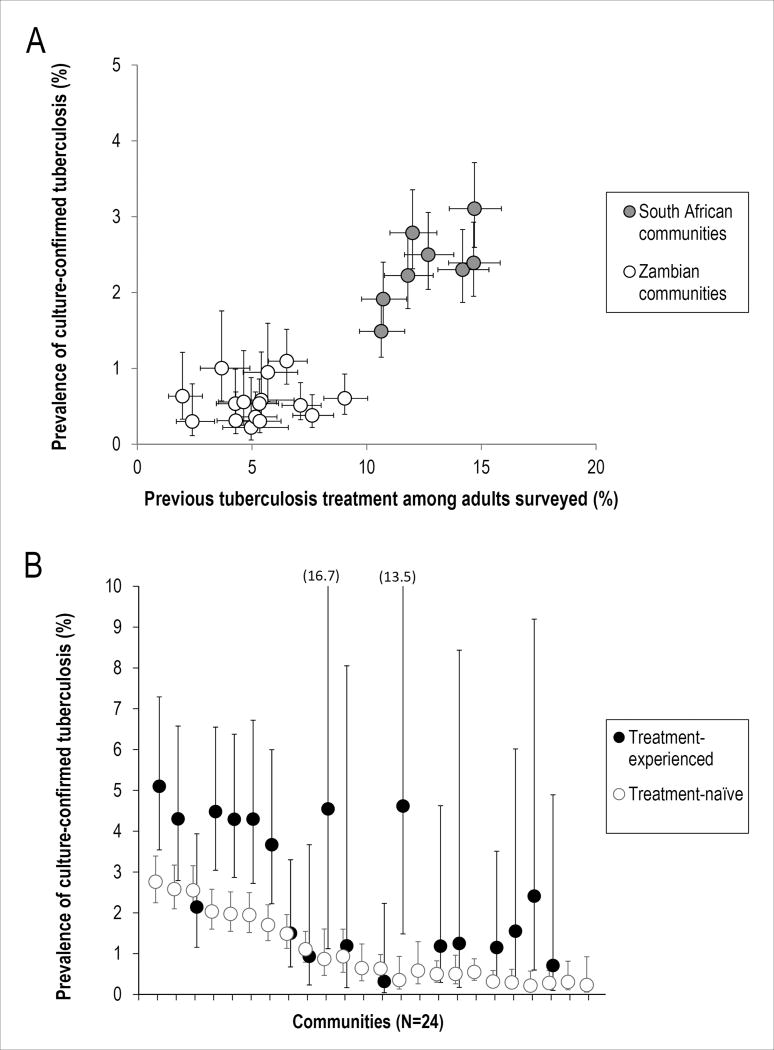
All but 15 of the 90,601 adults enrolled in the prevalence surveys provided information about history of previous tuberculosis treatment. Among these, 7,362 (8.1%) were treatment-experienced, and this proportion varied across the 24 communities between 2.0% and 14.9%. Previous treatment was more common in the South African communities, all of which had higher estimates of tuberculosis prevalence than the Zambian communities (Figure 1a). Treatment-experienced adults were older than treatment-naïve adults (median age: 38 vs. 29 years) and more often HIV-positive (45.1% vs. 14.3%).
Figure 1.
History of previous tuberculosis treatment and prevalent tuberculosis in 24 high tuberculosis burden communities in Zambia and the Western Cape Province of South Africa, 2010; Panel A: Correlation between the proportion of adults surveyed who reported a history of previous treatment and the prevalence of tuberculosis (regardless of treatment history); Panel B: Tuberculosis prevalence among treatment-experienced and treatment-naïve adults (communities are ordered by the overall tuberculosis prevalence in the communities; no treatment-experienced cases were found in 5 communities; error bars denote 95% confidence intervals)
Among 64,452 adults successfully evaluated for prevalent tuberculosis, 894 (1.39%) prevalent tuberculosis cases were detected. The mean prevalence of tuberculosis (weighted for numbers of adults evaluated) in the South African communities was 2.34 (95%CI: 2.17 - 2.52) per 100 adults overall, 3.81 (95%CI: 3.25 - 4.47) per 100 treatment-experienced adults and 2.13 (95%CI: 1.96 - 2.31) per 100 treatment-naïve adults. In the Zambian communities, it was 0.56 (95%CI: 0.48 - 0.64) per 100 adults overall, 1.01 (95%CI: 0.65 - 1.55) per 100 treatment-experienced adults and 0.53 (95%CI: 0.46 - 0.62) per 100 treatment-naïve adults. Prevalence was higher among treatment-experienced compared to treatment-naïve adults across most of the communities (Figure 1b). Stratifying by HIV status suggested that the observed difference in tuberculosis prevalence was restricted to HIV-negative adults. In the HIV-negative sub-population, TB prevalence was 3.32 (95%CI: 2.57 - 4.27) per 100 treatment-experienced adults vs. 1.78 (95%CI: 1.57 - 2.02) per 100 treatment-naïve adults in the South African, and 0.88 (95%CI: 0.42 - 1.84) per 100 treatment-experienced adults vs. 0.34 (95%CI: 0.27 - 0.42) per 100 treatment-naïve adults in the Zambian communities. Among HIV-positive adults, no significant difference by treatment history was found. TB prevalence among HIV-positive adults overall was 4.82 (95%CI: 4.11 - 5.66) per 100 in the South African and 1.61 (95%CI: 1.29 - 2.00) per 100 in the Zambian communities.
Among the 894 prevalent tuberculosis cases, 165 (18.5%) were previously treated. Previous treatment was also more common among these prevalent cases in the South African than in the Zambian communities (20.7% vs. 10.4%), though the proportion varied considerably and exceeded 20% in 9 communities. Treatment-experienced cases were more likely to be smear-microscopy positive (49.7% vs. 41.2%) and reported more current cough (43.0% vs. 34.0%) than treatment-naïve cases.
Our analysis of prevalence survey data from 24 African communities provides key insights into an important tuberculosis risk group. Individuals previously treated for tuberculosis represent a variably large fraction of the adult population, which is most sizeable in communities with the highest tuberculosis burden. Previously treated people may account for a considerable fraction of the overall prevalent tuberculosis burden and, among prevelant TB cases, those with previous treatment were more likely to be smear-positive and report active cough, suggesting substantial risk of onward transmission.
Our study is limited by its cross-sectional design which did not enable us to establish underlying causes of recurrent tuberculosis. History of previous treatment was self-reported, and no further information about the timing or outcome of previous treatment was available. Non-differential loss of specimens, attributable to a failure of positive mycobacterial controls in two laboratories, has been discussed previously, but is unlikely to have introduced bias into this analysis [10]. Finally, our results probably underestimate tuberculosis prevalence in the communities because the surveys did not include individuals within health care facilities and other institutions.
The results of our analysis emphasize that targeted interventions to prevent [12] or early identify recurrent tuberculosis among previously treated people might be a strategy worthwhile to consider for tuberculosis control in settings with a high prevalence of tuberculosis and HIV. While ensuring adherence to and the quality of anti-tuberculosis treatment within existing control programs remain essential priorities, such efforts may reduce relapse but will not directly prevent tuberculosis due to reinfection [2–5]. In areas where previously treated individuals are identifiable and reachable, new interventions targeted to this particular group could be practical to implement. For example, secondary preventive chemotherapy has been shown to substantially reduce the risk of recurrent tuberculosis [13, 14]. Active case finding [15] targeted to previously treated people may reduce morbidity and transmission as it may shorten the time that recurrent disease remains undiagnosed. While such targeted interventions are beneficial to individuals at high risk of recurrence, our results suggest that their benefits may extend to the community in settings where recurrent tuberculosis contributes to transmission. Future research, in which the costs of such targeted interventions and their effects on reducing recurrent tuberculosis and associated transmission are better quantified are needed to understand if they can be a cost-effective element of improved strategies to control tuberculosis in high-burden settings.
“Take home message”.
High TB prevalence among previously treated people suggests potential for targeted interventions in high-burden settings
Acknowledgments
Support statement
This work was supported by the German Research Foundation (DFG) through a scholarship grant [MA 5483/2-1] to FMM, and a grant provided by the National Institutes of Health (NIH) [R01 AI112438-01] to TC. The ZAMSTAR study was supported by a subcontract from Johns Hopkins University with funds provided by a grant from the Bill & Melinda Gates Foundation [19790.01]. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
We would like to thank the ZAMSTAR study team, in particular Ab Schaap, Rory Dunbar, and those involved in the prevalence surveys. We are grateful to those who agreed to participate in the surveys. We thank the City of Cape Town Health Department, and the Provincial and National Tuberculosis Programs of South Africa and Zambia for their support.
References
- 1.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. High rates of recurrence in HIV-infected and HIV-uninfected patients with tuberculosis. J Infect Dis. 2010;201:704–11. doi: 10.1086/650529. [DOI] [PubMed] [Google Scholar]
- 2.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers.[Erratum appears in Lancet 2002 Jun 15;359(9323):2120] Lancet. 2001;358:1687–93. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 3.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, Enarson DA, Behr MA, van Helden PD. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–5. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 4.Crampin AC, Mwaungulu JN, Mwaungulu FD, Mwafulirwa DT, Munthali K, Floyd S, Fine PE, Glynn JR. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS. 2010;24:417–26. doi: 10.1097/QAD.0b013e32832f51cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marx FM, Dunbar R, Enarson DA, Williams BG, Warren RM, van der Spuy GD, van Helden PD, Beyers N. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clinical Infectious Diseases. 2014 doi: 10.1093/cid/ciu186. [DOI] [PubMed] [Google Scholar]
- 6.Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6:e25098. doi: 10.1371/journal.pone.0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P, Muyoyeta M, Beyers N Peter Godfrey-Faussett for the ZST. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett EL, Bandason T, Cheung YB, Makamure B, Dauya E, Munyati SS, Churchyard GJ, Williams BG, Butterworth AE, Mungofa S, Hayes RJ, Mason PR. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis. 2009;13:1231–7. [PMC free article] [PubMed] [Google Scholar]
- 9.den Boon S, van Lill SW, Borgdorff MW, Enarson DA, Verver S, Bateman ED, Irusen E, Lombard CJ, White NW, de Villiers C, Beyers N. High prevalence of tuberculosis in previously treated patients, Cape Town, South Africa. Emerg Infect Dis. 2007;13:1189–94. doi: 10.3201/eid1308.051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, Simwinga M, Shanaube K, Chishinga N, Bond V, Dunbar R, De Haas P, James A, Gey van Pittius NC, Claassens M, Fielding K, Fenty J, Sismanidis C, Hayes RJ, Beyers N, Godfrey-Faussett P. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–94. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 11.Ayles HM, Sismanidis C, Beyers N, Hayes RJ, Godfrey-Faussett P. ZAMSTAR, The Zambia South Africa TB and HIV Reduction Study: design of a 2 × 2 factorial community randomized trial. Trials. 2008;9:63. doi: 10.1186/1745-6215-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harries AD, Chimzizi RB, Nyirenda TE, van Gorkom J, Salaniponi FM. Preventing recurrent tuberculosis in high HIV-prevalent areas in sub-Saharan Africa: what are the options for tuberculosis control programmes? Int J Tuberc Lung Dis. 2003;7:616–22. [PubMed] [Google Scholar]
- 13.Fitzgerald DW, Desvarieux M, Severe P, Joseph P, Johnson WD, Jr, Pape JW. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet. 2000;356:1470–4. doi: 10.1016/S0140-6736(00)02870-1. [DOI] [PubMed] [Google Scholar]
- 14.Churchyard GJ, Fielding K, Charalambous S, Day JH, Corbett EL, Hayes RJ, Chaisson RE, De Cock KM, Samb B, Grant AD. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS. 2003;17:2063–70. doi: 10.1097/00002030-200309260-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, Schaap A, Corbett EL, Lonnroth K, Glynn JR. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17:432–46. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]