Abstract
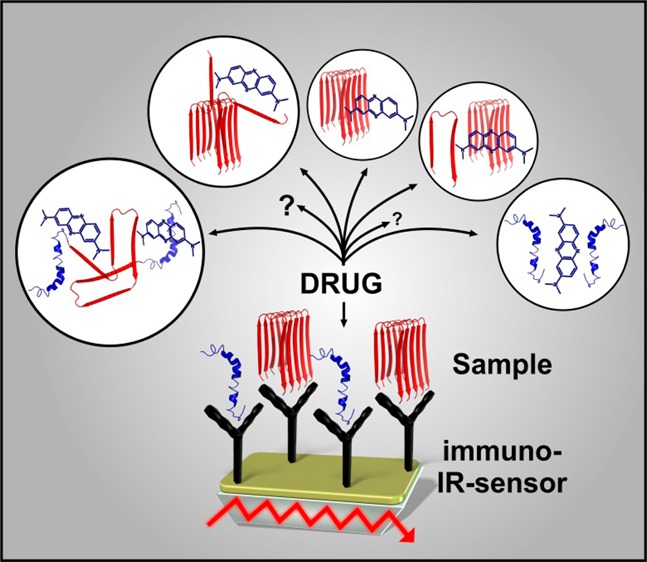
Alzheimer’s disease affects millions of human beings worldwide. The disease progression is characterized by the formation of plaques and neurofibrillary tangles in the brain, which are based on aggregation processes of the Aβ peptide and tau protein. Today there is no cure and even no in vitro assay available for the identification of drug candidates, which provides direct information concerning the protein secondary structure label-free. Therefore, we developed an attenuated total reflection Fourier transform infrared spectroscopy (ATR–FTIR) sensor, which uses surface bound antibodies to immobilize a desired target protein. The secondary structure of the protein can be evaluated based on the secondary structure sensitive frequency of the amide I band. Direct information about the effect of a drug candidate on the secondary structure distribution of the total target protein fraction within the respective body fluid can be detected by a frequency shift of the amide I band. Thereby, the extent of the amide I shift is indicative for the compound efficiency. The functionality of this approach was demonstrated by the quantification of the effect of the drug candidate methylene blue on the pathogenic misfolded tau protein as extracted from cerebrospinal fluid (CSF). Methylene blue induces a shift from pathogenic folded β-sheet dominated to the healthy monomeric state. A similar effect was observed for congo red on pathogenic Aβ isoforms from CSF. In addition, the effect of berberine on synthetic Aβ1–42 is studied. Berberine seems to decelerate the aggregation process of synthetic Aβ1–42 peptides.
Keywords: ATR−FTIR, immunoassay, protein drug intervention, methylene blue, berberine, Tau, amyloid beta
In 1886 Paul Ehrlich discovered the staining of nervous cells by methylthioninium chloride or methylene blue (MB).1 MB is a compound that is applied in many different scientific fields.2−5 The aggregation of the tau protein is a characteristic of several tauopathies such as Alzheimer’s disease (AD), Huntington’s disease (HD), or Pick’s disease (PiD).6−8 The analysis of repeat regions of tau and the disruption of fibril formation was frequently studied.9−11 Claude Wischik showed in 1996 the selective inhibition of tau protein aggregation by MB.12 In the last decades MB was investigated in several studies to delay the progression of cognitive decline in tauopathies and is nowadays analyzed in a clinical phase III trial.13−15 In 2013 the oxidation of the cysteine residues (C291/C322) was found to be a mechanistic reason for the inhibition of tau aggregation.16 Recently, the inhibition of microtubule affinity-regulating kinase (MARK4) by MB was reported, which describes an additional target and explanation how MB functions at molecular level.17
The fast and simple analysis of a potential drug interacting with a protein is an emerging field, especially in the research of neurodegenerative diseases.18−21 In the upcoming decades, medical treatment to prevent the pathological progression of these diseases will be one of the major challenges of mankind, most notably due to demographic change of society. To monitor such drug effect on a target protein we employed attenuated total reflection Fourier transform infrared spectroscopy (ATR–FTIR) difference spectroscopy. Our assay closes the gap between high throughput assays and animal models. The approach is universally applicable, also for human body fluids, easily accessible, and delivers data in real-time without requiring any label (Scheme 1).
Scheme 1. General Principle for the Developed Drug Sensor.
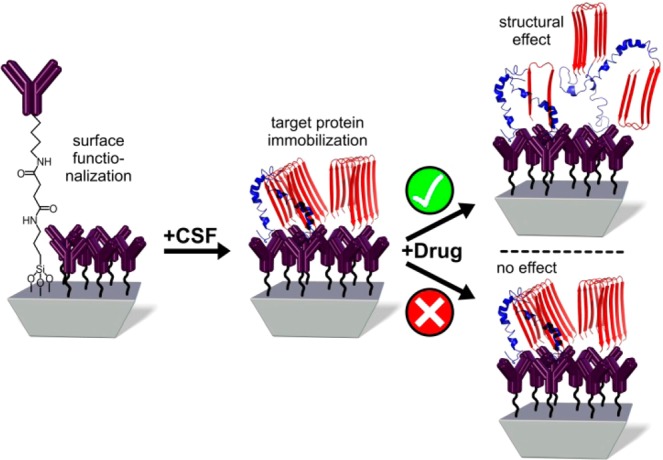
The desired antibody against a target protein (e.g., tau, Aβ) is covalently attached by silane chemistry.22 Subsequently, the secondary structural changes within the target protein upon application of a drug candidate are measured.
We previously demonstrated the development of an immuno–IR sensor with synthetic Aβ peptides,23 and recently, we were able to determine the secondary structure distribution of the extracted Aß fraction from body fluids and detect therewith AD with an 90% accuracy for CSF and 84% for blood plasma analyses.24 Based on this approach we focused on the analysis of drug interactions with the two major targets in AD, the tau protein and Aβ peptide. A detailed description of the sensor surface preparation principle can be found in the literature.22−24 Briefly, we employed silane chemistry to modify the surface of the germanium ATR crystal to covalently attach the desired monoclonal IgG antibody (tau-5 for tau).22 The immobilization is monitored within the ATR–FTIR spectrometer. After 2 h as presented in Figure S1A, the immobilization of the antibody is completed and an absorbance of 5 mOD is reached (Figure S1). After a blocking step,23 a complex sample such as cerebrospinal fluid (CSF) was flushed over the sensor and all isoforms of the tau protein were extracted from pooled CSF samples of AD patients (Figure S1B). Due to the fact that a representative mixture of all tau protein isoforms is extracted out of the CSF, the absorbance maximum of the amide I band can differ and was therefore also used as a marker band for the diagnosis of the disease.24 To analyze the effect of the potential drug MB, the immobilized tau protein fraction was incubated with a 50 μM solution of MB in PBS buffer (pH 7.4). As shown in Figure 1A a significant shift of the amide I peak position from 1640 to 1653 cm–1 was observed, which indicates a structural change from a β-sheet enriched secondary structure distribution to a mainly α-helical or random coil structure (Scheme 1). Similar spectral differences in the secondary structure composition of soluble monomeric and paired helical filaments of tau were also described in the literature.25 The drug intervention becomes even more obvious in the double difference spectrum of the treated (blue) minus the untreated (red) state (Figure 1B). Here, the distribution of the tau protein secondary structure shows a negative band at 1625 cm–1 before the drug intervention, indicating β-sheets or fibrils, whereas the corresponding positive band at 1655 cm–1 is typical for α-helices.26,27 Thus, with this assay it is possible to monitor the drug effect of methylene blue in vitro without any label. Thereby, the drug effect was analyzed in a concentration range of 5 to 50 μM (Figure S2). The shift of the amide I maximum position depends on the concentration of MB and a proportional shift for the lower concentrations and saturation for the higher concentrations was observed. To ensure that the observed effect originates from MB the following controls were performed: At first the immobilized antibody was incubated with MB, and no effect was observed (Figure S3). Furthermore, the immobilized tau was investigated under identical conditions with and without MB intervention, and the changes in the wavenumber were plotted against time (Figure 2A). The orange data points show a slight change in wavenumber about ±1, whereas with the MB a 10 wavenumber shift is detected (black squares, Figure 2A). To further strengthen the results a second small molecule berberine was investigated. Berberine comes from the traditional Chinese medicine and has shown to have positive effect on several diseases,28−30 and might also have a positive effect on AD as shown in animal models.28,31,32 Therefore, we incubated the immobilized tau protein extracted from human CSF with 100 μM berberine (PBS buffer, pH 7.4), but no shift of the amide I maximum was revealed; thus, berberine has no significant effect on the tau protein secondary structure (Figure 2B). Since the approach is universal, it can be applied to observe secondary structure changes of any target protein and the corresponding drug candidate.
Figure 1.
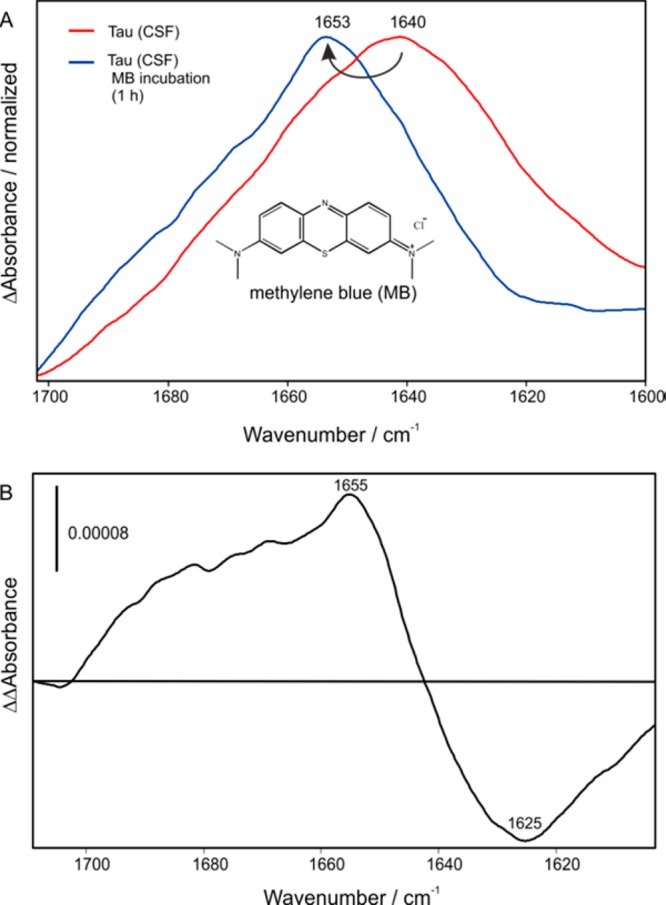
(A) Tau protein was immobilized on an antibody terminated germanium surface. The amide I of the tau protein from human CSF represents an increased level of β-sheet isoforms in the total tau fraction leading to an absorbance maximum of 1640 cm–1 (red). Upon treatment with the potential drug MB the amide I shifts to 1653 cm–1 indicating a structural change to α-helix and disordered conformations. (B) Double difference spectrum of the MB treated state (blue) minus the untreated baseline state (red), which indicates a change from a β-sheet to an α-helical/random secondary structure distribution of tau.
Figure 2.
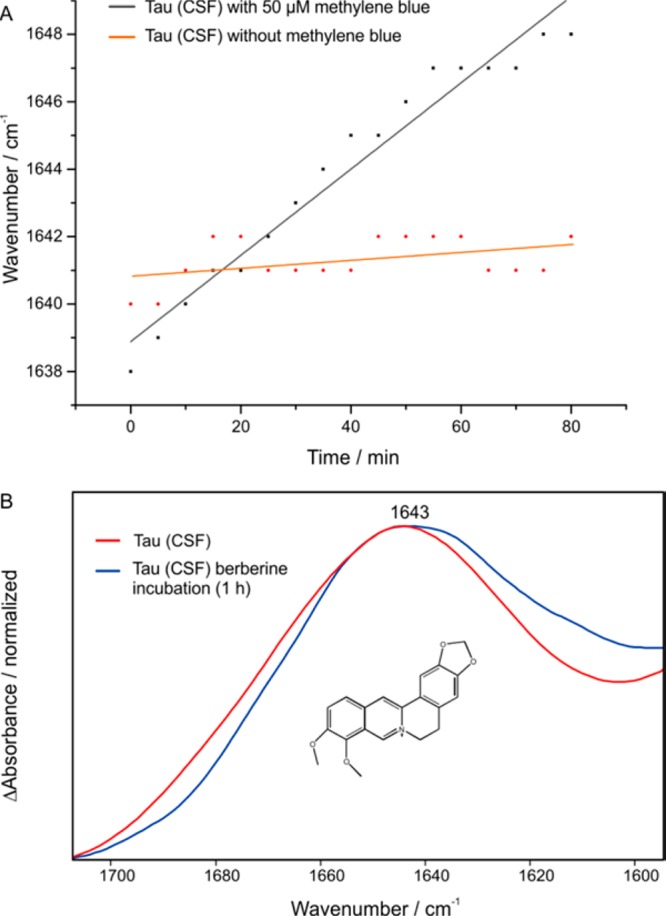
Control experiments regarding the effect of MB in vitro. (A) Without MB only a small change in the amide I maximum is observed (±1 cm–1), whereas in the presence on MB a clear shift of 10 wavenumbers is detected, indicating a secondary structural change from a β-sheet/fibril dominated state to a mainly monomeric state. (B) In contrast to the intervention with MB, the treatment with berberine has no significant effect on the amide I band of the tau protein.
In the next step, the possible intervention of MB and berberine on the synthetic Aβ1–42 peptide was investigated. The Aβ1–42 peptide is known for spontaneous fibrillization,33 thus we analyzed the spontaneous fibrillization of Aβ1–42 immobilized on the described sensor. For the immobilization the conformation insensitive antibody A8978 was chosen due to its known excellent properties.24 The synthetic Aβ1–42 was monomerized with hexafluoro–isopropanol23 and for the analysis 100 μg (22 μM) was flushed over the germanium surface and the fibrillization process was monitored for 18 h. The result is presented in Figure 3 (blue line) with an amide I maximum of 1634 cm–1 and an overall β–sheet dominated secondary structure distribution, which is consistent with the literature.34−36 The corresponding intermediate state of the process (160 min) has a maximum of 1643 cm–1 representative for mainly random or monomeric secondary structures but also portions of (self-) aggregated Aß species (light blue dashed line, Figure 3). The same experiment was now performed in the presence of 100 μM berberine. After the immobilization of Aβ1–42 (1 h) a solution of 100 μM berberine in PBS was added to the system and circulated for further 17 h (total 18 h as in the control). The measured amide I band shows an absorbance maximum of 1659 cm–1 with a small shoulder at 1634 cm–1 (red line, Figure 3) and a corresponding intermediate state (160 min) with a maximum of 1653 cm–1 (orange dashed line, Figure 3). This indicated that incubation with berberine prevented self-aggregation of synthetic Aß1–42 already within the first 100 min after immobilization. The effect of berberine on Aβ1–42 was further investigated by employing fibrillized Aβ1–42 for the immobilization experiment.23 As shown in Figure S4, neither the incubation with 100 μM nor 1 mM berberine affects the Aβ fibril since the IR spectra remain unchanged (Figure S4). This lead to the assumption that berberine seems to decelerate the fibrillization of Aβ1–42in vitro, but does not influence fibrils that already have been formed. Still, it could be an interesting drug candidate against the aggregation of Aβ1–42. In contrast to the effect of berberine on Aβ, intervention of the immobilized Aβ fraction from pooled AD CSF samples with congo red demonstrated a conformational change to predominantly monomeric isoforms. This effect was dependent on concentration and spectroscopically indicated by an amide I maximum shift to higher wavenumbers (Figure S6).
Figure 3.
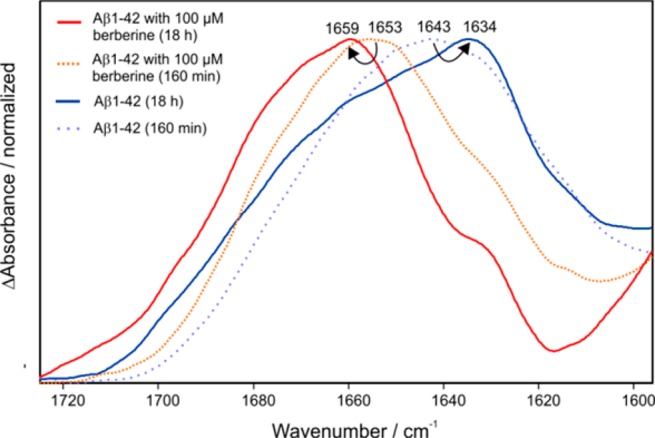
Aβ1–42 peptide was immobilized on an antibody (A8978) terminated germanium surface. The blue spectrum shows a secondary structure with an absorbance maximum at 1634 cm–1 characteristic for a distribution Aβ1–42 with a high amount of aggregated or fibrillized isoforms.24 Addition of the potential drug berberine (100 μM) resulted in a different secondary structure distribution dominated now by a mainly monomeric isoform of Aβ1–42 at 1659 cm–1 (red spectrum), however, with a significant shoulder at 1634 cm–1. The dashed spectra show intermediate states of the process after 160 min, with (dashed orange spectrum) and without berberine (dashed light blue spectrum). A lower amide I maximum of Aβ1–42 without berberine incubation was expected because of self-aggregation processes within the first 160 min.
Furthermore, the interaction of MB with synthetic Aβ1–42 was investigated with the developed drug sensor. The same protocol as described above for berberine was employed. The incubation of Aβ1–42 with 50 μM methylene blue resulted surprisingly in a typical fibril spectrum (for comparison a monomer and fibril spectrum of Aβ1–42 are shown in Figure S5), with absorbance bands at 1630 and 1690 cm–1 (green spectrum, Figure 4). For comparison spectra of the berberine treated (red spectrum, Figure 4) and untreated Aβ1–42 are shown (blue spectrum, Figure 4). The work of Necula et al. describes the promotion of fibrils by the inhibition of oligomerization in the presence of MB.37 This result is consistent with our findings and demonstrates the high potential of the established assay because it provides direct information about the effect of a drug candidate on the secondary structure of the target protein without requiring, for example, a fluorescent dye.
Figure 4.
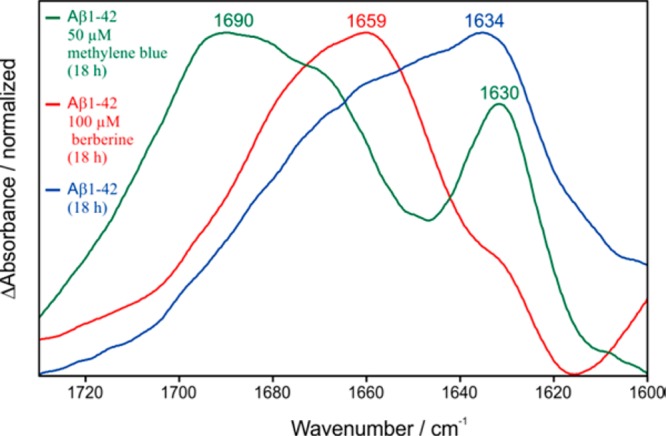
Comparison of the Aβ1–42 secondary structure distribution after the incubation with MB (green), berberine (red), and without any drug intervention (blue). Berberine shifts the distribution mainly to monomeric Aβ, whereas methylene blue induces β-sheet/fibril formation in agreement with the literature.37
In conclusion, we refined the ATR–FTIR system as a platform to analyze the influence of drug candidates on disease related proteins. The shift of the recorded amide I band maximum upon drug application is a tool to directly monitor the drug effect. Thereby, the extent of the amide I shift is indicative for the drug efficacy. Competing techniques such as surface plasmon resonance, surface acoustic waves, or quartz crystal microbalance are unable to obtain such detailed information because they are not sensitive to secondary structure changes.38 The presented approach is easily accessible and can be used with both, synthetic protein or protein extracted from complex human samples. In principle, it can be applied universally on any protein–drug pair paving the way for preselection of novel drugs.
Glossary
ABBREVIATIONS
- AD
Alzheimer’s disease
- ATR–FTIR
attenuated total reflection Fourier transform infrared spectroscopy
- CSF
cerebrospinal fluid
- MB
methylene blue
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.7b00079.
Immobilization of the tau-5 antibody, extraction of tau proteins from human CSF; intervention effect of MB on the tau protein fraction extracted from human CSF in different concentrations; control measurement of the immobilized antibody and MB; analysis of Aβ1–42 fibrils and berberine; typical ATR–FTIR difference spectra of immobilized monomeric and fibrillized Aβ1–42; intervention effect of CR on the Aβ peptide fraction extracted from human CSF in different concentrations; Materials and Methods (PDF)
Author Contributions
‡ J.S. and A.N. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This research was supported by the Protein Research Unit Ruhr within Europe (PURE), Ministry of Innovation, Science and Research of North-Rhine Westphalia, Germany. Prof. Jens Wiltfang is supported by an Ilídio Pinho professorship and iBiMED (UID/BIM/04501/2013), at the University of Aveiro, Portugal.
The authors declare no competing financial interest.
Supplementary Material
References
- Ehrlich P. Über Die Methylenblaureaction Der Lebenden Nervensubstanz. Dt. med. Wschr. Dtsch. Med. Wochenschr. 1886, 1, 49–52. 10.1055/s-0028-1139684. [DOI] [Google Scholar]
- Ramsay R. R.; Dunford C.; Gillman P. K. Methylene Blue and Serotonin Toxicity: Inhibition of Monoamine Oxidase A (MAO A) Confirms a Theoretical Prediction. Br. J. Pharmacol. 2007, 152 (6), 946–951. 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evora P. R. B. Methylene Blue Is a Guanylate Cyclase Inhibitor That Does Not Interfere with Nitric Oxide Synthesis. Tex Heart Inst J. 2016, 43 (1), 103. 10.14503/THIJ-15-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey-Funes M.; Larrayoz I. M.; Fernández J. C.; Contartese D. S.; Rolón F.; Inserra P. I. F.; Martínez-Murillo R.; López-Costa J. J.; Dorfman V. B.; Martínez A.; Loidl C. F. Methylene Blue Prevents Retinal Damage in an Experimental Model of Ischemic Proliferative Retinopathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1011. 10.1152/ajpregu.00266.2015. [DOI] [PubMed] [Google Scholar]
- Howland R. H. Methylene Blue: The Long and Winding Road From Stain to Brain: Part 2. J. Psychosoc Nurs Ment Health Serv 2016, 54 (10), 21–26. 10.3928/02793695-20160920-04. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Mandelkow E. Tau in Physiology and Pathology. Nat. Rev. Neurosci. 2016, 17 (1), 5–21. [DOI] [PubMed] [Google Scholar]
- Lee V. M.; Goedert M.; Trojanowski J. Q. Neurodegenerative Tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Alzheimer A.Über Eine Eigenartige Erkrankung Der Hirnrinde. Allg. Z. Psychiatrie Psychisch-gerichtl. Med. 1907, 64, 146–148 (in German). [Google Scholar]
- Rojas Quijano F. A.; Morrow D.; Wise B. M.; Brancia F. L.; Goux W. J. Prediction of Nucleating Sequences from Amyloidogenic Propensities of Tau-Related Peptides. Biochemistry 2006, 45 (14), 4638–4652. 10.1021/bi052226q. [DOI] [PubMed] [Google Scholar]
- Inoue M.; Konno T.; Tainaka K.; Nakata E.; Yoshida H.; Morii T. Positional Effects of Phosphorylation on the Stability and Morphology of Tau-Related Amyloid Fibrils. Biochemistry 2012, 51 (7), 1396–1406. 10.1021/bi201451z. [DOI] [PubMed] [Google Scholar]
- Lunven L.; Bonnet H.; Yahiaoui S.; Yi W.; Da Costa L.; Peuchmaur M.; Boumendjel A.; Chierici S. Disruption of Fibers from the Tau Model AcPHF6 by Naturally Occurring Aurones and Synthetic Analogues. ACS Chem. Neurosci. 2016, 7 (7), 995–1003. 10.1021/acschemneuro.6b00102. [DOI] [PubMed] [Google Scholar]
- Wischik C. M.; Edwards P. C.; Lai R. Y.; Roth M.; Harrington C. R. Selective Inhibition of Alzheimer Disease-like Tau Aggregation by Phenothiazines. Proc. Natl. Acad. Sci. U. S. A. 1996, 93 (20), 11213–11218. 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley T. C.; McCaffrey J.; Storey J. M. D.; Cheung J. K. S.; Melis V.; Horsley D.; Harrington C. R.; Wischik C. M. Complex Disposition of Methylthioninium Redox Forms Determines Efficacy in Tau Aggregation Inhibitor Therapy for Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2015, 352 (1), 110–118. 10.1124/jpet.114.219352. [DOI] [PubMed] [Google Scholar]
- Harrington C. R.; Storey J. M. D.; Clunas S.; Harrington K. A.; Horsley D.; Ishaq A.; Kemp S. J.; Larch C. P.; Marshall C.; Nicoll S. L.; Rickard J. E.; Simpson M.; Sinclair J. P.; Storey L. J.; Wischik C. M. Cellular Models of Aggregation-Dependent Template-Directed Proteolysis to Characterize Tau Aggregation Inhibitors for Treatment of Alzheimer Disease. J. Biol. Chem. 2015, 290 (17), 10862–10875. 10.1074/jbc.M114.616029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik C. M.; Staff R. T.; Wischik D. J.; Bentham P.; Murray A. D.; Storey J. M. D.; Kook K. A.; Harrington C. R. Tau Aggregation Inhibitor Therapy: An Exploratory Phase 2 Study in Mild or Moderate Alzheimer's Disease. Journal of Alzheimer's Disease 2015, No. 2, 705–720. [DOI] [PubMed] [Google Scholar]
- Akoury E.; Pickhardt M.; Gajda M.; Biernat J.; Mandelkow E.; Zweckstetter M. Mechanistic Basis of Phenothiazine-Driven Inhibition of Tau Aggregation. Angew. Chem., Int. Ed. 2013, 52 (12), 3511–3515. 10.1002/anie.201208290. [DOI] [PubMed] [Google Scholar]
- Sun W.; Lee S.; Huang X.; Liu S.; Inayathullah M.; Kim K.-M.; Tang H.; Ashford J. W.; Rajadas J. Attenuation of Synaptic Toxicity and MARK4/PAR1-Mediated Tau Phosphorylation by Methylene Blue for Alzheimer’s Disease Treatment. Sci. Rep. 2016, 6, 34784. 10.1038/srep34784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt M.; Neumann T.; Schwizer D.; Callaway K.; Vendruscolo M.; Schenk D. St; George-Hyslop P.; Mandelkow E. M.; Dobson C. M.; McConlogue L.; Mandelkow E.; Tóth G. Identification of Small Molecule Inhibitors of Tau Aggregation by Targeting Monomeric Tau As a Potential Therapeutic Approach for Tauopathies. Curr. Alzheimer Res. 2015, 12 (9), 814–828. 10.2174/156720501209151019104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulic B.; Pickhardt M.; Mandelkow E. Progress and Developments in Tau Aggregation Inhibitors for Alzheimer Disease. J. Med. Chem. 2013, 56 (11), 4135–4155. 10.1021/jm3017317. [DOI] [PubMed] [Google Scholar]
- Holtzman D. M.; Carrillo M. C.; Hendrix J. A.; Bain L. J.; Catafau A. M.; Gault L. M.; Goedert M.; Mandelkow E.; Mandelkow E.-M.; Miller D. S.; Ostrowitzki S.; Polydoro M.; Smith S.; Wittmann M.; Hutton M. Tau: From Research to Clinical Development. Alzheimer's Dementia 2016, 12 (10), 1033–1039. 10.1016/j.jalz.2016.03.018. [DOI] [PubMed] [Google Scholar]
- Iqbal K.; Liu F.; Gong C.-X. Tau and Neurodegenerative Disease: The Story so Far. Nat. Rev. Neurol. 2015, 12 (1), 15–27. 10.1038/nrneurol.2015.225. [DOI] [PubMed] [Google Scholar]
- Schartner J.; Güldenhaupt J.; Mei B.; Rögner M.; Muhler M.; Gerwert K.; Kötting C. Universal Method for Protein Immobilization on Chemically Functionalized Germanium Investigated by ATR–FTIR Difference Spectroscopy. J. Am. Chem. Soc. 2013, 135 (10), 4079–4087. 10.1021/ja400253p. [DOI] [PubMed] [Google Scholar]
- Nabers A.; Ollesch J.; Schartner J.; Kötting C.; Genius J.; Haußmann U.; Klafki H.; Wiltfang J.; Gerwert K. An Infrared Sensor Analysing Label-Free the Secondary Structure of the Abeta Peptide in Presence of Complex Fluids. Journal of Biophotonics 2016, 9 (3), 224–234. 10.1002/jbio.201400145. [DOI] [PubMed] [Google Scholar]
- Nabers A.; Ollesch J.; Schartner J.; Kötting C.; Genius J.; Hafermann H.; Klafki H.; Gerwert K.; Wiltfang J. Amyloid-β-Secondary Structure Distribution in Cerebrospinal Fluid and Blood Measured by an Immuno-Infrared-Sensor: A Biomarker Candidate for Alzheimer’s Disease. Anal. Chem. 2016, 88 (5), 2755–2762. 10.1021/acs.analchem.5b04286. [DOI] [PubMed] [Google Scholar]
- von Bergen M.; Barghorn S.; Jeganathan S.; Mandelkow E.-M.; Mandelkow E. Spectroscopic Approaches to the Conformation of Tau Protein in Solution and in Paired Helical Filaments. Neurodegener. Dis. 2006, 3 (4–5), 197–206. 10.1159/000095257. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E.; Cabiaux V.; Ruysschaert J. M. Secondary Structure and Dosage of Soluble and Membrane Proteins by Attenuated Total Reflection Fourier-Transform Infrared Spectroscopy on Hydrated Films. Eur. J. Biochem. 1990, 193 (2), 409–420. 10.1111/j.1432-1033.1990.tb19354.x. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E.; Cabiaux V.; Ruysschaert J. M. Determination of Soluble and Membrane Protein Structure by Fourier Transform Infrared Spectroscopy. I. Assignments and Model Compounds. Subcell. Biochem. 1994, 23, 329–362. 10.1007/978-1-4615-1863-1_8. [DOI] [PubMed] [Google Scholar]
- Ahmed T.; Gilani A.-U.-H.; Abdollahi M.; Daglia M.; Nabavi S. F.; Nabavi S. M. Berberine and Neurodegeneration: A Review of Literature. Pharmacol. Rep. 2015, 67 (5), 970–979. 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Campisi A.; Acquaviva R.; Mastrojeni S.; Raciti G.; Vanella A.; De Pasquale R.; Puglisi S.; Iauk L. Effect of Berberine and Berberis Aetnensis C. Presl. Alkaloid Extract on Glutamate-Evoked Tissue Transglutaminase up-Regulation in Astroglial Cell Cultures. Phytother. Res. 2011, 25 (6), 816–820. 10.1002/ptr.3340. [DOI] [PubMed] [Google Scholar]
- Yao J.; Kong W.; Jiang J. Learning from Berberine: Treating Chronic Diseases through Multiple Targets. Sci. China: Life Sci. 2015, 58 (9), 854–859. 10.1007/s11427-013-4568-z. [DOI] [PubMed] [Google Scholar]
- Lee B.; Sur B.; Shim I.; Lee H.; Hahm D.-H. Phellodendron Amurense and Its Major Alkaloid Compound, Berberine Ameliorates Scopolamine-Induced Neuronal Impairment and Memory Dysfunction in Rats. Korean J. Physiol. Pharmacol. 2012, 16 (2), 79–89. 10.4196/kjpp.2012.16.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.; Qian C. Berberine Chloride Can Ameliorate the Spatial Memory Impairment and Increase the Expression of Interleukin-1beta and Inducible Nitric Oxide Synthase in the Rat Model of Alzheimer’s Disease. BMC Neurosci. 2006, 7, 78. 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucker M.; Walker L. C. Self-Propagation of Pathogenic Protein Aggregates in Neurodegenerative Diseases. Nature 2013, 501 (7465), 45–51. 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandomeneghi G.; Krebs M. R. H.; McCammon M. G.; Fändrich M. FTIR Reveals Structural Differences between Native β-Sheet Proteins and Amyloid Fibrils. Protein Sci. 2004, 13 (12), 3314–3321. 10.1110/ps.041024904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf E.; Sarroukh R.; Tamamizu-Kato S.; Breydo L.; Derclaye S.; Dufrêne Y. F.; Narayanaswami V.; Goormaghtigh E.; Ruysschaert J.-M.; Raussens V. Antiparallel β-Sheet: A Signature Structure of the Oligomeric Amyloid β-Peptide. Biochem. J. 2009, 421 (3), 415–423. 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- Sachse C.; Fandrich M.; Grigorieff N. Paired -Sheet Structure of an A (1–40) Amyloid Fibril Revealed by Electron Microscopy. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (21), 7462–7466. 10.1073/pnas.0712290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necula M.; Breydo L.; Milton S.; Kayed R.; van der Veer W. E.; Tone P.; Glabe C. G. Methylene Blue Inhibits Amyloid Aβ Oligomerization by Promoting Fibrillization †. Biochemistry 2007, 46 (30), 8850–8860. 10.1021/bi700411k. [DOI] [PubMed] [Google Scholar]
- Konradi R.; Textor M.; Reimhult E. Using Complementary Acoustic and Optical Techniques for Quantitative Monitoring of Biomolecular Adsorption at Interfaces. Biosensors 2012, 2 (4), 341–376. 10.3390/bios2040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


