Uric acid and life on Earth
Uric acid is a key product of the metabolism of purines, the backbone of Desoxyribonucleic acid (DNA). Being a fundamental component of every living cell, the total Earth’s DNA base pairs is estimated to weigh 50 billion tons [1], which translates into a huge abundance of uric acid in our planet. It would be naive to think of all these virtual heaps of uric acid as useless, inert waste produces. Indeed, they are not. Uric acid in the environment is a rich source of nitrogen (33% of its weight) to plant life, hence its important role in the universal food chain. Most animals get rid of uric acid, thereby replenishing the ecosystem with a precious nutritional ingredient. Interestingly, primates have opted to keep some uric acid for their own internal environment, obviously reflecting an evolutionarily acquired physiological role that seems un-necessary for lower forms of animal life. Yet, like with many other molecules of physiological benefit, there is a risk of retaining too much. The role of increased extracellular uric acid pool in the pathogenesis of gout, urolithiasis, tumor lysis syndrome and rhabdomyolysis has been well known for decades. More recently, several animal and clinical observations have suggested a potential role of excess uric acid in the pathogenesis of hypertension, obesity and cardiovascular disorders, chronic kidney disease, and others. However, a conclusive cause-and-effect relationship has not been established, so far. The broad diversity of uric acid metabolism in different forms of life, its physiological role in plants and primates, and the debate on its significance in many common diseases in humans constitute the rationale for putting together this special issue of the Journal.
Uric acid metabolism
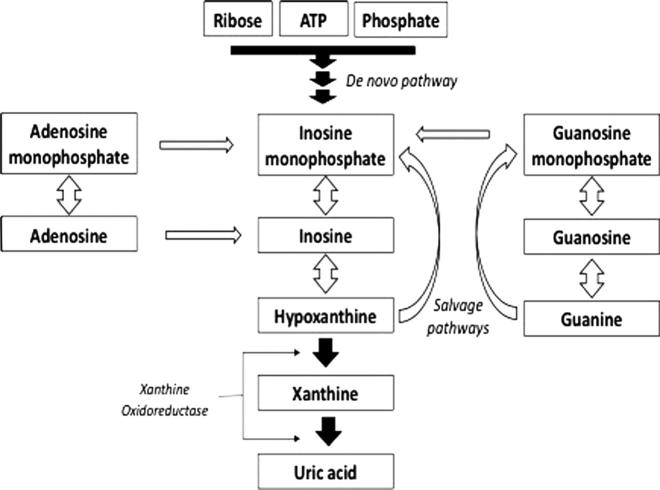
While earlier products of purine catabolism can be recycled directly through the salvage pathway (Fig. 1), uric acid cannot.
Fig. 1.
Broad lines of purine metabolism showing the de novo and salvage synthetic pathways.
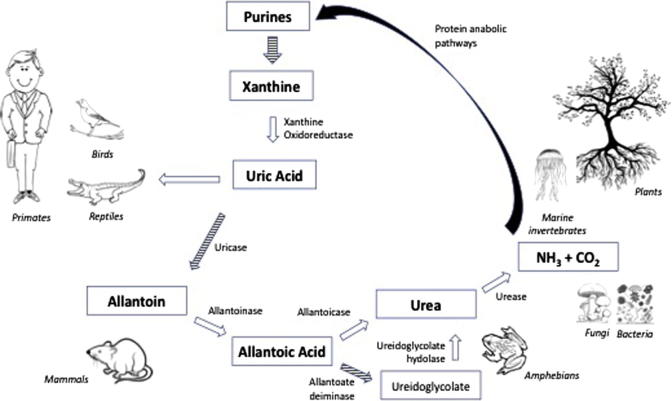
In order to be utilized for protein generation, including purine re-synthesis, it has to be broken down into ammonia and carbon dioxide. As explained by Hafez et al. in this issue of the Journal, this “complete” dissimilation requires several enzymes, encoded by multiple genes, which are available in bacteria, fungi, and indeed the entire plant kingdom. On the other hand, no member of the animal kingdom (with the exception of marine invertebrates), can do the same, owing to loss of functionality of one or more relevant genes. Accordingly, uric acid dissimilation is arrested at one step or another (Fig. 2), and the animal has to find a way for getting rid of the final metabolites that cannot by broken down any further.
Fig. 2.
Degradation steps of uric acid into ammonia and carbon dioxide, showing the key enzymes available to different living organisms.
One way of meeting this challenge is to colonize bacteria that can provide or enhance the missing enzymes. This is well known in the plant kingdom, where soil bacteria can help breaking down uric acid or its products introduced into the environment by animal excreta or fertilizers. Uric acid-splitting bacteria have also been reported in the gut of humans, and were observed to be altered in patients with gout [2], which opens the door for new speculations on the pathogenesis of the disease and to new therapeutic potentials.
The principal problem in getting rid of uric acid is its poor solubility in water. Various members of the animal kingdom adopt different strategies to overcome this difficulty. Birds, reptiles and desert dwelling animals excrete uric acid as a semi-solid material in their gut excreta, by a complicated, high energy-demanding process. Yet this has the advantage of conserving much-needed water. Interestingly, birds’ manure known as “guano” is known as high-quality plant fertilizers [3]. Large heaps of guano are deposited near the costs of the Pacific and Atlantic oceans, where seabirds search out for fish. Best known Guano islands are those near Peru, Namibia, Oman, Patagonia, and Baja California [4]. Guano trading has become a major source of income in those islands to the extent of triggering the Chincha Islands War (1864–1866) between Spain and a Peruvian-Chilean alliance. In this context the United States had passed the Guano Islands Act in 1856, which regulates the legal rights of citizens who discover guano in its territories [5].
Animals that cannot afford the energy requirement for intestinal excretion of uric acid prefer to convert it to a more soluble compound, being blessed by a functional uricase enzyme (Fig. 2). Mammals other than primates, and carnivorous dipteras, can thus metabolize uric acid into allantoin, which goes with urine. Amphibians and telecosts can take allantoin further down the road to urea, which is even more soluble and readily excreted in urine.
The mentioned methods of uric acid disposal are competent enough to keep its blood level as low as <0.5–1 mg/dl. Exceptionally, primates are far less efficient in this respect, leading to almost 10-fold higher blood level. This is attributed to: (a) loss of functionality of the uricase gene; and (b) inability to appreciably expel uric acid via the gastrointestinal tract. Uric acid is thus excreted as an intact molecule in urine. About 90% of uric acid in the glomerular filtrate is reclaimed by the proximal tubules in a tightly controlled manner that ensures maintenance of the blood level within the physiological range. For many decades, this process was thought to involve 4 steps, namely reabsorption and secretion in the proximal as well as the distal convoluted tubules. Only recently have we learnt that the whole process is completed in the proximal tubules, in three steps comprising reabsorption, secretion, then further reabsorption for final fine tuning. We also know that the main players are members of the organic anion transporter (OAT) family, principally the Urate Transporter 1 (URAT1), as well as the hexose transporter GLUT9. The interaction of the OAT family members in the process of reabsorption and secretion of uric acid, their genetic control, and their significance as drug targets are discussed in this issue by El-Ridi and Tallima and by Ragab et al.
Exceeding the tubular threshold for uric acid reabsorption in acute hyperuricemic states (as in the tumor lysis syndrome or rhabdomyolysis) can lead to acute kidney injury due to precipitation of sodium urate crystals in the distal nephron, as well as to tubular toxicity, as explained in this issue by Hahn et al.
Physiological role of uric acid in humans
The relative retention of uric acid in primates must have happened for a good reason. It has even been linked with the longer life span of homosapiens compared to other mammals. According to an interesting hypothesis, this was probably meant to compensate for the evolutionary loss of the ability to synthesize ascorbic acid in primates [6]. Mutation of l-gulonolactone oxidase (GULO) gene, responsible for the last step in ascorbic acid synthesis from glucose, was followed by several mutations in the uricase gene, thereby introducing uric acid as a circulating anti-oxidant substitute, though it still requires the permissive role of ascorbic acid, hence becoming a vitamin [7]. Epigenetic factors seem to emphasize the paradoxical relationship in-between serum uric acid and ascorbic acid, as vitamin C administration has been shown in a metanalysis of 13 Randomized Clinical Trials (RCTs), to significantly reduce serum uric acid concentration [8].
As far as we know, the major physiological role of uric acid is related to its paradoxical Redox effects [9], with a major extracellular anti-oxidant capacity and a dominant intracellular net pro-oxidant effect. It accounts for 50% of the anti-oxidant activity of the hydrophilic intravascular compartment [10], through scavenging reactive radicals released by autoxidation of hemoglobin, peroxide generation by macrophages and similar reactions. This seems to protect against oxidative damage of the blood cell membranes, as well as organs of ectodermal origin, i.e. skin and nervous system. This is shown in experimental models [11] and clinical association studies in several neurodegenerative disorders as Multiple Sclerosis [12], and skin diseases as pemphigus [13]. Animal models suggest additional protective effects in other organs, though clinical relevance remains unclear.
On the other hand, uric acid’s anti-oxidant activity is quite restricted in the intracellular lipophilic environment [14]. Ironically, while scavenging reactive radicals as O2-, uric acid generates other reactive radicals as peroxinitrate (ONOO-) that increase the overall oxidant stress [15] which is helpful in combating infection and cancer, as explained in this issue by El-Ridi and Tallima.
The pro-inflammatory role of uric acid is not limited to its Redox activity. It also upregulates leucocytic pro-inflammatory cytokines and autacoids. It provokes an innate immune response, by activating the NOD-like receptor P3 (NLRP3), the best known inflammasome, which is ultimately responsible for proteolytic cleavage of pro-interleukin (IL)-1β. Uric acid also provokes a TH2 immune response, which has an important protective role in certain infections as schistosomiasis, as well as in malignant conditions as explained in this issue by El-Ridi and Tallima.
Uric acid pathogenicity in humans
For many decades, urate crystal deposition has been the only known pathogenic impact of hyperuricemia. Typical crystal-induced disorders include gout (addressed in this issue by Ragab et al.), urolithiasis (discussed by Abul-Ela) and tumor lysis syndrome or rhabdomyolysis (by Hahn et al.). The latter authors, as well as Sharaf-Eldin et al. allude, in this issue of the Journal, to a recently described fatal condition where uric acid is claimed to play a significant pathogenic role, conventionally called “chronic kidney disease of unknown etiology (CKDu)”. This rapidly progressive disease has been initially observed in young field workers in central America who typically spend long hours exposed to extra-ordinarily hot climates. Similar cases were also described in other geographical regions with a matching climate as India and North Africa. It has been postulated that the association of repeated dehydration and rhabdomyolysis may lead to uric acid deposition in the kidneys, eventually causing chronic tubulo-interstitial fibrosis. While the latter histopathological pattern has been consistently observed in renal biopsies, urate deposits have not. Could this be due to delay in obtaining the biopsy along the course of the disease? We are yet to know.
It seems that uric acid-related morbidity is not only attributed to the mechanical effects of crystallization, since uric acid pathogenicity is confounded by its variable pro-inflammatory properties in different tissues, and its potential of inducing necrotic cell death [16]. The past decade has witnessed a plethora of observations which suggest a close association in-between hyperuricemia and several cardiovascular, metabolic and renal disorders, as discussed in this issue by Sharaf El-Din et al. and by Hahn et al. However, a significant pathogenic role of hyperuricemia in humans remains debatable, despite supportive experimental observations and established potential mechanisms that can explain them.
Therapeutic potential of uric acid-lowering strategies
A low purine diet is a time-honored prescription in gout and uric acid urolithiasis. Low animal purine diets, including meat, poultry, fish, liver and brain were typically restricted. Less well known are vegetables with high purine content, due to relatively high xanthine oxidase/uricase ratio. The role of dietary prescription in the control of hyperuricemia is comprehensively addressed in this issue by Hafez et al.
The Xanthine-Oxidase Inhibitor (XOI), allopurinol, has been the main uric acid lowering agent for many decades, being used for the treatment of gout and the prevention of urate stones and tumor lysis syndrome. Severe allergic reactions, albeit quite rare, have been of major concern when proposed for the treatment of asymptomatic hyperuricemia. However, with the recent introduction of non-XOI uric acid-lowering agents, this particular concern became alleviated, though at the expense of hypothetically losing the XOI anti-oxidant properties.
In the absence of conclusive randomized clinical trials (RCTs), and the inconsistent results of metanalysis of the available literature, the benefit of treating asymptomatic hyperuricemia remains questionable. In this issue of the Journal, Dousdampanis leads a debate “for” versus “against” treatment. Bargman and Ramirez defend treatment, while Eleftheriadis et al. see no good reason to exposing asymptomatic patients to un-necessary medication, while, perhaps depriving them from the physiological benefits of uric acid. Despite rigorous arguments on both sides, Dousdampanis concludes that there is no winner! We must await the outcomes of well-designed RCTs.
Editors declaration
The Guest Editors declare no conflict of interest.
Acknowledgements
We wish to acknowledge the professional support of Dr. Soha Fahmy, who has been instrumental in keeping track of the whole process of putting this special issue together.
Biographies

Rashad Barsoum

Mohammed El-Khatib
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Rashad Barsoum, Email: rashad.barsoum@gmail.com.
Mohammed El-Khatib, Email: elkhatibmmm66@gmail.com.
References
- 1.Nuwer R. “Counting All the DNA on Earth”. The New York Times. New York: The New York Times Company. ISSN 0362–4331. Retrieved 2015–07-18.
- 2.Guo Z., Zhang J., Wang Z., Ang K.Y., Huang S., Hou Q. Intestinal microbiota distinguish gout patients from healthy humans. Sci Rep. 2016;6:20602. doi: 10.1038/srep20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szpak P., Millaire J.F., White C.D., Longstaffe F.J. Influence of seabird guano and camelid dung fertilization on the nitrogen isotopic composition of field-grown maize (Zea mays) J Archaeol Sci. 2012;39(12):3721–3740. [Google Scholar]
- 4.Hutchinson G.E. Survey of existing knowledge of biogeochemistry: 3. The biogeochemistry of vertebrate excretion. Bullet Am Museum Natural History. 1950;96:1–554. [Google Scholar]
- 5.Skaggs J. St. Martin's; New York: 1994. The Great Guano rush: entrepreneurs and American overseas expansion. ISBN 0312103166. [Google Scholar]
- 6.Drouin G., Godin J., Pagé B. The genetics of vitamin C loss in vertebrates. Curr Genomics. 2011;12(5):371–378. doi: 10.2174/138920211796429736. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alvarez‐Lario B, Macarrón‐Vicente J. Evolution of Uric Acid Metabolism in Humans. In: eLS. John Wiley & Sons Ltd, Chichester, 2013. <http://www.els.net> http://dx.doi.org/10.1002/9780470015902.a0024618.
- 7.Frei B., Stocker R., Ames B.N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA. 1988;85:9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juraschek S.P., 1, Miller E.R., 3rd, Gelber A.C. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken) 2011;63(9):1295–1306. doi: 10.1002/acr.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sautin Y., Johnson R. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker B.F. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14(6):615–631. doi: 10.1016/0891-5849(93)90143-i. Review. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z.F., Bruce-Keller A.J., Goodman Y., Mattson M.P. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Pakpoor J., Seminog O., Ramagopalan S., Goldacre M. Clinical associations between gout and multiple sclerosis, Parkinson’s disease and motor neuron disease: record-linkage studies. BMC Neurol. 2015;15:16. doi: 10.1186/s12883-015-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yousefi M., Rahimi H., Barikbin B., Toossi P., Lotfi S., Hedayati M. Uric acid: a new antioxidant in patients with pemphigus vulgaris. Indian J Dermatol. 2011;56(3):278–281. doi: 10.4103/0019-5154.82480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraoka S., Miura T. Inhibition by uric acid of free radicals that damage biological molecules. Pharmacol Toxicol. 2003;93:284–289. doi: 10.1111/j.1600-0773.2003.pto930606.x. [DOI] [PubMed] [Google Scholar]
- 15.Neogi T., George J., Rekhraj S., Struthers A., Choi H., Terkeltaub R. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012;64(2):327–338. doi: 10.1002/art.33369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.F. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54(1):133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]