Graphical abstract
Keywords: Uric acid, Chronic kidney disease, Hyperuricemia, Allopurinol
Abstract
Asymptomatic hyperuricemia is increasing in prevalence. There is a growing body of literature suggesting that uric acid has deleterious effects on vascular health and renal histological integrity. Several trials, reviewed herein, suggest that lowering the serum uric acid level is associated with a slowing in the rate of renal deterioration in those with chronic kidney disease. Given that there is little available in the general armamentarium to slow the rate of kidney deterioration, strong consideration could be given to the administration of agents or lifestyle changes that decrease uric acid production in hyperuricemic patients with deteriorating kidney function.
Introduction
The prevalence of asymptomatic hyperuricemia has been increasing over the past decades, and can be as high as 20–25% in adult males [1]. Multiple explanations, including changes in diet, an aging population as well as earlier screening [2], [3] have been suggested as possible causes of this finding. However, the benefit of treating this common abnormality remains unclear.
Pathophysiology of uric acid metabolism
Uric acid is a weak acid that is a poorly soluble end product of endogenous and dietary purine metabolism. At a physiologic pH of 7.4, 98% of uric acid is in the urate anion form. Urate production is dependent on the balance between purine ingestion, de novo synthesis in cells, recycling and the degradation function of xanthine oxidase at the end of the purine pathway. Xanthine oxidase transforms xanthine to uric acid. In most animals, uric acid is further metabolized to highly water-soluble allantoin via the enzyme uricase. Humans and higher primates have inactivated the gene for uricase, thus the concentration of urate in humans is close to the limit of solubility [4].
Renal clearance of uric acid is greater in the presence of estrogenic compounds [5]. Studies have found that males younger than 65 years of age have a prevalence of hyperuricemia four times higher than that of females of the same age. After menopause, serum urate values increase in women to the same values as their male counterparts.
Urate levels have also been found to be increased in chronic kidney disease. The kidneys excrete two-thirds of uric acid produced daily and impaired excretion of uric acid is present in 90% of individuals with hyperuricemia [6]. The gut eliminates a third of the urate produced daily through colonic bacteria, which almost completely degrades the uric acid with very little left in the stool. This mechanism increases marginally in the presence of kidney failure.
Ninety percent of filtered uric acid is reabsorbed in the S1 segment of the proximal tubule [7]. Multiple urate transporters have been found, such as the urate transporter 1 (URAT1) which is expressed in the apical membrane of the proximal tubule cell and the urate transporter SLC2A9 (also known as glucose transporter 9), expressed on the basolateral side of the proximal tubule and on the apical membrane in the collecting duct [8].
Uric acid is secreted rather than reabsorbed in the S2 segment of the proximal tubule and post-secretory reabsorption occurs at a more distal site of the proximal tubule, with 10% of the filtered uric acid appearing in the urine [9].
Reviewing basic data on hyperuricemia and chronic kidney disease
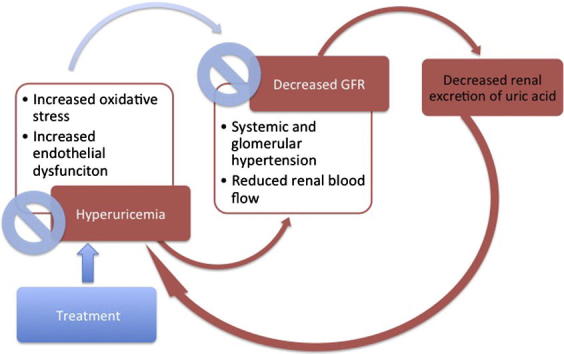
In 1960, Talbott and Terplan found that nearly all subjects with gout had arteriosclerosis, glomerulosclerosis and interstitial fibrosis in their kidneys. As many of these subjects also had urate crystals in their tubules and interstitium, the disease was termed “gouty nephropathy” [10]. Unfortunately for this hypothesis, urate crystal deposition in the kidneys was also found in patients without renal disease. In addition, the diffuse renal scarring and the coexistent conditions of hypertension and vascular disease in many of the autopsy subjects led some to suggest that the renal injury in gout was secondary to these latter conditions rather than to hyperuricemia [11]. The common association of CKD and hyperuricemia was attributed to the uric acid retention due to impaired renal excretion for many decades until the seminal work of Kang et al. in 2002. In this study, hyperuricemia was induced in experimental rats and was associated with increased renal renin and COX-2 expression, especially in the preglomerular arterial vessels. The study concluded that hyperuricemia itself could mediate progression of renal disease through accelerated hypertension and vascular disease. This was the first experimental study to provide direct evidence that uric acid may be a key factor in renal disease and progression [12]. Thereafter, multiple studies showed that increasing the uric acid level could induce oxidative stress and endothelial dysfunction. Hyperuricemia was associated with the development of systemic and glomerular hypertension with increased vascular resistance and reduced renal blood flow [13], [14]. In the tubular cells, uric acid was found to induce epithelial to mesenchymal transition, which had been widely accepted as a key contributor to the development of renal fibrosis in CKD [15].
Additional studies showed that lowering uric acid levels in diabetic mice led to a slowing in renal disease progression [16], [17].
In another important preclinical study by Mazzali et al., hyperuricemic rats were found to develop hypertension as well as mild tubulointerstitial injury. Lowering uric acid levels was associated with prevention of the development of hypertension as well as a decrease in the incidence and the progression of renal injury. The mechanism also involved the renin-angiotensin system and down-regulation of nitric oxide expression in the macula densa [15].
Thus in laboratory studies, hyperuricemia has been found to induce renal injury, as well as to accelerate progression of renal disease. In addition, lowering the serum uric acid level was associated with amelioration of this effect.
Reviewing clinical data on hyperuricemia and CKD
One of the greatest advances in recent decades has been the advent of renal angiotensin aldosterone system (RAAS) blockade. With respect to uric acid metabolism, it is interesting to note is that not all RAAS blockade works in the same way. A review comparing the effect of angiotensin II receptor blockers (ARBs) on hyperuricemia showed that losartan was the only ARB that reduces serum uric acid levels [18]. A post hoc analysis of the trial on Reduction of Endpoints in Non-Insulin-Dependent Diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) showed that the uric acid-lowering effect of losartan was associated with long-term renal risk reduction [19].
Currently, small trials have been undertaken showing that treatment of hyperuricemia in CKD retarded progression of renal disease (see Table 1).
Table 1.
Randomized controlled trials lowering serum uric acid and its effect on renal function.
| Study (Primary author and year) | Population | Intervention | Results |
|---|---|---|---|
| Gibson et al. (1982) [29] | 59 patients with primary gout | Colchicine and allopurinol versus colchicine alone | Retarded an apparent decline of renal function over 2 years |
| Chanard et al. (2003) [30] | 48 renal transplant patients with hypertension, on cyclosporine | Amlodipine or tertatolol | Amlodipine decreased serum uric acid levels and increased glomerular filtration rate as compared with tertatolol |
| Siu et al. (2006) [20] | 54 hyperuricemic patients with CKD | Allopurinol versus standard therapy | No significant differences but a trend toward a lower serum creatinine level in the treatment group compared with controls after 12 months of therapy |
| Liu and Sheng (2007) [31] | 47 hyperuricemic patients with CKD | Allopurinol versus standard therapy | Serum creatinine was lower in the allopurinol group and the rate of renal function deterioration was significantly decreased over 12 months |
| Kanbay et al. (2007) [32] | 59 patients | Allopurinol given to the hyperuricemic patients and no uric acid lowering therapy for the normouricemic patients | Allopurinol therapy significantly improved GFR but proteinuria was unchanged |
| Malaguarnera et al. (2009) [33] | 38 elderly patients with hyperuricemia | Rasburicase versus placebo | Significant reduction in creatinine and an increase in creatinine clearance over 2 months |
| Goicoechea et al. (2010) [22] | 113 patients with estimated GFR <60 mL/min | Allopurinol versus standard therapy (no uric acid lowering therapy) | Allopurinol treatment slowed down renal disease progression independent of age, gender, diabetes, C-reactive protein, albuminuria and renin-angiotensin blocker use over 24 months |
| Momeni et al. (2010) [34] | 40 patients with type 2 diabetes mellitus and diabetic nephropathy (proteinuria of 500 mg/day and serum creatinine level <3 mg/dL) | Allopurinol versus placebo | Allopurinol reduced severity of proteinuria after 4 months of drug administration. No change in creatinine was noted |
| Whelton et al. (2011) [35] | 116 hyperuricemic patients (post hoc) | Febuxostat in 40, 80 or 120 mg doses | Improvement or maintenance of estimated GFR was inversely correlated with the quantitative reduction in serum uric acid from baseline over 5 years |
| Shi et al. (2012) [36] | 40 hyperuricemic patients with IgA nephropathy | Allopurinol versus standard therapy | Hyperuricemia predicted progression of IgA nephropathy independently of baseline estimated GFR over 6 months. No change in renal progression or proteinuria was noted |
| Pai et al. (2013) [37] | 183 hyperuricemic patients with CKD | Allopurinol versus standard therapy (no uric acid lowering therapy) | Allopurinol was associated with decreased progression of renal disease in CKD |
| Sircar et al. (2015) [38] | 93 hyperuricemic patients with CKD 3 and 4 | Febuxostat versus placebo | Febuxostat slowed the decline in estimated GFR in CKD stages 3 and 4 compared to placebo |
In a prospective randomized controlled trial by Siu et al. [20] allopurinol safely decreased uric acid levels in patients with CKD 3 and showed a trend to slower progression to end stage renal disease (ESRD). There was no improvement in hypertension in these subjects over the 12 months of the study. A recent review and meta-analysis by Kanji et al. in 2015 summarized the randomized controlled trials that were undertaken to assess the effect of treating hyperuricemia in CKD. There were 19 studies analyzed and although all the trials had small sample sizes, there was a statistically significant improvement in renal function in the patients treated with allopurinol. There was also improvement in blood pressure and proteinuria [21] though it should be emphasized that hypertension may or may not be affected by treatment of hyperuricemia as found in the studies by Goicoechea et al. [22], Kao et al. [23], and through the comprehensive review by Bose et al. in 2014 [24]. We would like to highlight some of these studies.
Goicoechea et al. conducted one of the largest trials in 2010 in Madrid. One hundred and thirteen patients were randomly assigned to receive control treatment or allopurinol. After approximately 24 months, the use of allopurinol was associated with slower renal disease progression, decreased number of hospitalizations and reduced cardiovascular risk [22]. Unfortunately while the study by Kao et al. [23] in 2011 showed that there was improvement in left ventricular mass in patients with CKD, the mechanism was not fully understood as there was no improvement in hypertension in this study and we may infer that improvement in hypertension is unlikely to be the mechanism to which control of hyperuricemia would minimize progression of renal disease.
Also, withdrawal of allopurinol therapy seemed to worsen renal disease progression [25]. A study by Talaat and elSheikh published in 2007 [25] followed 50 patients who had been using allopurinol for asymptomatic hyperuricemia. The patients were followed 12 months after allopurinol withdrawal and there was marked acceleration of renal disease progression.
Unfortunately, there has been no unified theory as to the mechanism of preventing renal disease progression through improvement of serum uric acid levels. A recent study by Jalal et al. showed that treatment of hyperuricemia in humans did not improve markers of oxidative stress or brachial-artery flow mediated dilation, a surrogate marker for endothelial dysfunction [26].
Despite the small numbers, the trials have consistently shown that hyperuricemia is strongly associated with progression of renal disease and that treatment is beneficial in slowing this progression and that stopping therapy may be deleterious. In the presence of these suggestive studies, it may be worthwhile to treat hyperuricemia in patients at risk for progression of CKD. Two large scale randomized controlled trials are currently underway to address this issue definitively. The FEATHER trial (Febuxostat versus placebo randomized controlled trial regarding reduced renal function in patients with hyperuricemia complicated by chronic kidney disease stage 3) and the CKD FIX (Controlled trial of slowing of kidney disease progression from the inhibition of xanthine oxidase) are currently ongoing in Japan and in Australia respectively. Both trials were undertaken in 2014 and are predicted to complete in 2017.
One other important trial of note is the ongoing Uric Acid Lowering to Prevent Kidney Function Loss in Diabetes: The Preventing Early Renal Function Loss (PERL) Allopurinol study which is spearheaded by Maahs, starting in 2013 [27]. The study focuses on patients with Type 1 Diabetes Mellitus with mild to moderate decrease in their estimated GFR as well as presence of albuminuria and more importantly, the presence of hyperuricemia, with intervention in the form of allopurinol versus placebo. This study is scheduled to complete in June 2019 and will hopefully provide further insight into the use of allopurinol against progression of diabetic kidney disease.
Lastly, emphasis on the non-pharmacologic therapy, such as decreased alcohol consumption, dietary reduction in high purine foods and moderate increase in exercise, has been proven to be as effective as pharmacologic therapy [28]. Lifestyle modifications in the treatment of hyperuricemia as well as use of well tolerated, easily accessible medication such as allopurinol will certainly not be too onerous to institute especially with these multiple studies which seem to lead to delay of renal disease progression.
Conclusions and future perspectives
In summary, there is ample evidence to suggest that the presence of elevated blood levels of uric acid is associated with decline in kidney function. Animal studies demonstrate deleterious effects of uric acid at the vascular and renal level and lend strong face validity to the human studies. However, the studies are admittedly limited in terms of size and some studies are equivocal in terms of outcomes. Treatment of hyperuricemia may be considered as an option for slowing progression of renal disease especially in light of the simple treatment such as use of a single uricosuric agent as well as lifestyle changes. The results of the three ongoing randomized controlled trials will certainly be of great clinical interest and perhaps provide us with a definitive answer to this longstanding question.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Maria Erika Ramirez MD is a graduate of the Faculty of Medicine and Surgery University of Santo Tomas, Manila, Philippines. She completed her Internal Medicine Residency in the same university in 2010, serving as the Undergraduate (Clerkship and Internship) Training Officer. Upon graduation, she completed 4 months of training as a Critical Care Fellow in The Medical City in Pasay Philippines in 2011. She proceeded to Singapore thereafter and worked as a Clinical Associate for the Department of Nephrology in Singapore General Hospital from 2012 to 2014. She is currently completing her Fellowship training in Adult Nephrology with the University of Toronto.

Joanne Bargman MD FRCPC is a staff nephrologist at the University Health Network and Professor of Medicine at the University of Toronto. She received her MD cum laude from the University of Toronto. She was an exchange fellow in Melbourne for her senior medical residency year, and then pursued nephrology training at Stanford University. Her research focused on renal physiology and micropuncture. Upon returning to Toronto, she was recruited to the Toronto Western Hospital where she trained in peritoneal dialysis under Dimitrios Oreopoulos. She has more than 700 invited lectures internationally, on subjects as diverse as peritoneal dialysis, glomerulonephritis, and management of systemic lupus erythematosus. She is Director of Peritoneal Dialysis for the University Health Network in Toronto, President of the International Society of Peritoneal Dialysis 2012–2014, and co-director of the Combined Renal-Rheumatology Lupus Clinic for the University Health Network.She has won the “Silver Shovel”, given by the graduating medical class of the University of Toronto to the best lecturer in the undergraduate years. She has also won the University of Toronto Faculty of Medicine Postgraduate Teaching Award, given to the best teacher in the postgraduate program. She was chosen as the 12th Robert Collins Visiting Lecturer in Dialysis at the University of Colorado in Denver. In 2013 she was the recipient of both the Donald Seldin Award for excellence in nephrology at the National Kidney Foundation (US) and the award for teaching excellence from the Canadian Society of Nephrology. She was the 2015 Recipient of the Lifetime Achievement Award at the Annual Dialysis Conference in New Orleans, and received the International Distinguished Medal at the Spring Clinical Meetings of the National Kidney Foundation in 2016. Dr. Bargman is co-author of the chapter “Chronic Kidney Disease” in the 17th, 18th and 19th editions of Harrison’s Principles of Internal Medicine.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Lin K.C., Lin H.Y., Chou P. Community based epidemiological study on hyperuricemia and gout in Kin-Hu, Kinmen. J Rheumatol. 2000;27(4):1045. [PubMed] [Google Scholar]
- 2.Saag K.G., Choi H. Epidemiology, risk factors, and lifestyle modifications for gout. Arthritis Res Ther. 2006;8(Suppl. 1):S2. doi: 10.1186/ar1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richette P., Bardin T. Gout Lancet. 2010;375(9711):318–328. doi: 10.1016/S0140-6736(09)60883-7. Epub 2009. [DOI] [PubMed] [Google Scholar]
- 4.McLean L. The pathogenesis of gout. In: Hochberg M., editor. Rheumatology. Mosby; Edinburgh: 2003. pp. 1903–1918. [Google Scholar]
- 5.Antón F.M., García Puig J., Ramos T., González P., Ordás J. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism. 1986;35(4):343. doi: 10.1016/0026-0495(86)90152-6. [DOI] [PubMed] [Google Scholar]
- 6.Becker B.F. Towards the physiological function of uric acid. Free Radic Biol Med. 1993;14(6):615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary K., Malhotra K., Sowers J., Aroor A. Uric acid — key ingredient in the recipe for cardiorenal metabolic syndrome. Cardiorenal Med. 2013:208–220. doi: 10.1159/000355405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mende C. Management of Chronic Kidney Disease: the relationship between serum uric acid and development of nephropathy. Adv Ther. 2015;32:1177–1191. doi: 10.1007/s12325-015-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 10.Talbot J.H., Terplan K.L. The kidney in gout. Medicine (Baltimore) 1960;39:405–467. [PubMed] [Google Scholar]
- 11.Yu T.F., Berger L. Impaired renal function gout: its association with hypertensive vascular disease and intrinsic renal disease. Am J Med. 1982;72:95–100. doi: 10.1016/0002-9343(82)90593-9. [DOI] [PubMed] [Google Scholar]
- 12.Kang D.H., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Lozada L.G., Soto V., Tapia E., Avila-Casado C., Sautin Y.Y., Nakagawa T. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol. 2008;295:F1134–F1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi Y.J., Yoon Y., Lee K.Y., Hien T.T., Kang K.W., Kim K.C. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis. FASEB J. 2014;28:3197–3204. doi: 10.1096/fj.13-247148. [DOI] [PubMed] [Google Scholar]
- 15.Mazzali M., Hughes J., Kim Y.G., Jefferson J.A., Kang D.H., Gordon K.L. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 16.Ryu E.-S., Kim M.J., Shin H.-S., Jang Y.H., Choi H.S., Jo I. Uric acid-induced phenotypic transition of renal tubular cells as a novel mechanism of chronic kidney disease. Am J Physiol. 2013;304:F471–F480. doi: 10.1152/ajprenal.00560.2012. [DOI] [PubMed] [Google Scholar]
- 17.Kosugi T., Nakayama T., Heinig M., Zhang L., Yuzawa Y., Sanchez-Lozada L.G. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff M.I., Cruz J.L., Vanderman A.J., Brown J.N. The effect of angiotensin II receptor blocker on hyperuricemia. Ther Adv Chronic Dis. 2015;6(6):339–346. doi: 10.1177/2040622315596119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao Y., Ottenbros S.A., Laverman G.D., Brenner B.M., Cooper M.E., Parving H.H. Effect of a reduction in uric acid on renal outcomes during losartan treatment. A post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II antagonist losartan trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 20.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Kanji T., Gandhi M., Clase C.M., Yang R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2015;16:58. doi: 10.1186/s12882-015-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goicoechea M., de Vinuesa S.G., Verdalles U., Ruiz-Caro C., Ampuero J., Rincon A. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5(8):1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao M.P., Ang D.S., Gandy S.J., Nadir M.A., Houston J.G., Lang C.C. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bose B., Bhadve F.V., Hiremath S.S., Boudville N., Brown F.G., Cass A. Effects of uric acid lowering therapy on renal outcomes: a systematic review and meta analysis. NDT. 2014;29:406–413. doi: 10.1093/ndt/gft378. [DOI] [PubMed] [Google Scholar]
- 25.Talaat K.M., El-Sheikh A.R. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27:435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 26.Jalal D.I., Decker E., Perrenoud L., Nowak K.L., Bispham N., Mehta T. Vascular function and uric acid lowering in Stage 3 CKD. J Am Soc Nephrol. 2017;28(3):943–952. doi: 10.1681/ASN.2016050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maahs D.M., Caramori M.L., Cherney D.Z.I. Uric acid lowering to prevent kidney function loss in diabetes: preventing early renal function loss (PERL) Allopurinol study. Curr Diab Rep. 2013;13(4):550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peixoto M.R.G., Monego E.T., Veiga Jardim P.C.B., Carvalho M.M., Sousa A.L.L., de Oliveira J.S. Diet and medication in the treatment of hyperuricemia in hypertensive patients. Arq Bras Cardiol. 2001;76(6):468–472. [PubMed] [Google Scholar]
- 29.Gibson T., Rodgers V., Potter C., HA Simmonds. Allopurinol treatment and its effect on renal function in gout: a controlled study. Ann Rheum Dise. 1982;41(1):59–65. doi: 10.1136/ard.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chanard J., Toupance O., Lavaud S., Hurault de Ligny B., Bernaud C., Moulin B. Amlodipine reduces cyclosporine-induced hyperuricemia in hypertensive renal transplant recipients. Nephrol Dial Transplant. 2003;18(10):2147–2153. doi: 10.1093/ndt/gfg341. [DOI] [PubMed] [Google Scholar]
- 31.Liu J., Sheng D. Allopurinol in lowering serum uric acid level for the delay of the progression of chronic renal disease. China Pharmacy. 2007;18(32):2524–2525. [Google Scholar]
- 32.Kanbay M., Ozkara A., Selcoki Y., Isik B., Turgut F., Bavbek N. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearance, and proteinuria in patients with normal renal function. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 33.Malaguarnera M., Vacante M., Russo C., Dipasquale G., Gargante M.P., Motta M. A single dose of rasburicase in elderly patients with hyperuricemia reduces serum uric acid levels and improves renal function. Expert Opn Pharmacother. 2009;10(5):737–742. doi: 10.1517/14656560902781972. [DOI] [PubMed] [Google Scholar]
- 34.Momeni A., Shahidi S., Seirafian S., Taheri S., Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4(2):128–132. [PubMed] [Google Scholar]
- 35.Whelton A., Macdonald P.A., Zhao L., Hunt B., Gunawardhana L. Renal function in gout: long term treatment effects of Febuxostat. J Clin Rheumatol. 2011;17(1):7–13. doi: 10.1097/RHU.0b013e318204aab4. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y., Chen W., Jalal D., Li Z., Chen W., Mao H. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35(3):153–160. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pai B.H.Santhosh, Swarnalatha G., Ram R., Dakshinamurty K.V. Allopurinol for prevention of progressive kidney disease with hyperuricemia. Indian J Nephrol. 2013;23(4):280–286. doi: 10.4103/0971-4065.114499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sircar D., Chatterjee S., Waikhom R., Golay V., Raychaudhury A., Chatterjee S. Efficacy of Febuxostat for slowing the GFR decline in patients with CKD and symptomatic hyperuricemia: a 6 month, double blind, randomized, placebo controlled trial. Am J Kidney Dis. 2015;66(6):945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]


