Graphical abstract
Keywords: Chronic kidney disease, Hyperuricemia, Uric acid, Urate lowering treatment
Abstract
Today there is plausible evidence both on experimental and epidemiological basis, that hyperuricemia represents a risk factor for the development and progression of chronic kidney disease (CKD). Nevertheless, the role of serum uric acid lowering treatment in CKD is still a matter of serious controversy. Review of randomised controlled trials, suggests that there may be an improvement of renal function with allopurinol treatment in CKD stage 3–5. However, these studies have included a relatively limited number of participants and provide insufficient information on adverse events and on the incidence of the end stage renal disease. Therefore, before adequately powered randomised, placebo-controlled trials are completed we cannot recommend treating asymptomatic hyperuricemia in patients with CKD.
Introduction
Uric acid is in humans the end product of purine metabolism. In this pathway xanthine oxidase catalyzes the final oxidation of hypoxanthine and xanthine to uric acid [1], [2], [3]. In contrast to humans, most other mammals possess an additional enzyme in purine metabolism, namely uricase (urate oxidase). Uricase oxidizes uric acid to 5-hydroxyisourate, and to allantoin, a highly water soluble compound which is most efficiently excreted in urine. Early during the evolution, due to distinct gene mutations, primates have lost uricase activity and the ability to enzymatically produce allantoin. As a result, humans have much higher serum uric acid levels than other mammals and can easily develop hyperuricemia [4]. Uric acid is a poorly soluble weak organic acid, that circulates in blood (under physiologic pH of 7.40) as urate anion [1], [3], [4].
Hyperuricemia may result from an enhanced production or a reduced secretion of uric acid. There is no universally accepted definition of hyperuricemia. Preferably, it is defined physicochemically as a serum urate concentration exceeding its solubility point (6.8 mg/dL). Crystals of monosodium urate form at levels exceeding solubility, and they precipitate in joint tissues causing gout.
About two-thirds of uric acid load is derived from internal sources (liver, muscle, intestine) and one-third from dietary sources, including fructose, alcohol, and purine-rich foods like certain meats and seafood [2]. High fructose intake (e.g. corn-syrup or various soft-drinks) can cause intracellular adenosine triphosphate depletion with enhanced nucleotide turnover and uric acid formation [5]. Therefore, uric acid lowering treatment almost always includes changes in diet and lifestyle.
Kidneys are responsible for most of the daily uric acid excretion (65–75%), with the remaining (25–35%) being excreted through the gastrointestinal tract [6]. Urate is freely filtered by the glomerulus but, owing to a net proximal tubular reabsorption, its fractional excretion is <10%. Knowledge about tubular handling of uric acid is still evolving and was recently shown to include reabsorption and secretion in the proximal tubule [7], [8], These processes are mediated by certain organic acid transport proteins, namely URAT1 (urate transporter 1, encoded by SLC22A12) and GLUT9 (glucose transporter 9, encoded by SLC2A9) [7], [9]. It has been shown, that, in about 90% of cases, hyperuricemia is due to an impaired renal excretion [10]. In advanced stages of chronic kidney disease (CKD) prevalence of hyperuricemia exceeds 60% [11]. In addition, CKD is one of the most common independent risk factors for gout [12], [13].
CKD is defined as kidney structure or function abnormalities that persist for more than 3 months [14]. These abnormalities include decreased glomerular filtration rate (GFR < 60 mL/min/1.73 m2) or evidence of one or more markers of kidney damage (e.g. albuminuria or urine sediment abnormalities) [14]. Prevalence of CKD is increasingly recognized as a worldwide public health problem [15], which is present in about 14% of the US population [16]. CKD is, furthermore, associated with a significantly increased cardiovascular mortality [16] especially in patients with diabetes [17]. These facts highlight the need to adopt more effective treatment strategies for CKD [18].
There is no doubt about a strong relationship between hyperuricemia and CKD but the details of this relationship are still controversial. In particular, the putative causal relationship between hyperuricemia and CKD is a source of controversy. In the last two decades, hyperuricemia was accepted as a risk factor for incident or progressive CKD, but the causality remained uncertain. It was believed that urate nephropathy does not exist [19] and hyperuricemia was considered to cause merely uric acid nephrolithiasis or acute uric acid nephropathy in the tumor lysis syndrome [1], [20]. However, this historical belief was based on the incorrect assumption, that the mechanism of kidney damage should be mediated obligatorily by precipitation of monosodium urate crystals, similar to the pathogenesis of gout [1], [20]. However, recent epidemiological data in humans, and experimental evidence in an animal model with mild hyperuricemia, have reasonably implicated a direct involvement of soluble serum urate in the pathogenesis of CKD [1], [21], [22], [23].
In the following narrative review we present experimental findings, epidemiological studies and clinical trials about hyperuricemia as a risk factor for initiation and progression of CKD. We try to answer the question if currently treatment to control serum urate is invariably indicated in CKD.
Experimental findings associating hyperuricemia with CKD
It is really difficult to study the role of hyperuricemia in the pathogenesis of CKD in humans. Especially, taking into account that uric acid is excreted primarily by the kidneys [6] and hence in CKD serum uric acid levels are usually increased [10], [11]. Therefore, in vitro experimental and animal studies are critical to understanding the relationship of uric acid and hyperuricemia in the causation or progression of kidney disease.
In order to link uric acid with the processes leading to kidney damage and indirectly to CKD various potential pathogenic pathways were investigated in vitro. In an experimental study involving human endothelial cells, uric acid significantly increased the production of reactive oxygen species and angiotensin II, both known to be involved in pathomechanisms of endothelial dysfunction and in the development of hypertension and renal disease [24].
In laboratory animals serum uric acid can be easily modulated, either by raising it with an uricase inhibitor such as oxonic acid or by lowering it with xanthine oxidase inhibitors or uricosuric agents. Hyperuricemic rats, after addition of oxonic acid in the diet (50–100% increase in serum urate), developed significant increase in blood pressure, as compared to the control group [25]. Raising the uric acid level also induced oxidative stress and endothelial dysfunction, resulting in systemic and glomerular hypertension [26].
In rats with pre-existing renal disease the effects of increased uric acid levels on progression of renal disease were impressive despite the absence of crystals in the kidney [21]. Authors attributed these histological changes to hypertension with preglomerular arteriolosclerosis induced ischemia as well as to interstitial inflammation, induced by an activation of the renin-angiotensin system. However, soluble urate, which has been previously proven to act as a proinflammatory mediator [22], [23], [27], might also be involved in the process of tubulointerstitial fibrosis. Findings of both above studies [21], [25], were independent of oxonic acid administration, as they could readily be prevented by normalising uric acid either with a xanthine oxidase inhibitor or an uricosuric agent. In type 2 diabetic db/db mice with huperuricemia lowering previously elevated uric acid levels led to a significant improvement of kidney disease [28].
The above experimental studies clearly implicate that hyperuricemia probably causes kidney damage by a mechanism involving systemic and glomerular hypertension. Tubulointerstitial fibrosis, which might be readily associated to the direct proinflammatory effects of soluble urate, is independent from the precipitation of monosodium urate crystals in the kidney.
Epidemiological data associating hyperuricemia with CKD
Several epidemiological studies in the general population and in patients with CKD show that uric acid is a major independent risk factor for the development and progression of renal disease [29], [30], [31], [32], [33]. This association was seen in studies with patients with diabetes mellitus as well [29], [34], [35], [36]. In the recent meta-analysis involving 13 observational trials with more than 190,000 patients with normal renal function, the presence of hyperuricemia was an independent predictor for the development of CKD. In hyperuricemia the risk for new-onset CKD was twofold increased and this effect was seen with comparable magnitude in both patients with and without diabetes [29].
These findings establish a firm association of hyperuricemia with the development of nephropathy in healthy subjects. However, evidence about secondary prophylaxis (i.e. prophylaxis for progression) in patients with CKD still remains debatable. In this context, certain epidemiological studies showed no relationship between hyperuricemia and the progression of kidney disease [11], [37]. Namely, in the Mild to Moderate Kidney Disease study, patients with non-diabetic CKD (n = 177) were followed for a 7 year period and hyperuricemia (after adjustment for GFR and proteinuriaat baseline) was not an independent predictor of CKD progression [37]. Similarly, in the Modification of Diet in Renal Disease study 838 patients with CKD stage 3–4 were followed for 10 years and again hyperuricemia was not significantly associated with the development of end stage renal disease (ESRD) [11].
An explanation for this contradiction may lay on the specific pathomechanism. Uric acid leads to renal damage primarily by causing systemic and glomerular hypertension [25], [38]. In patients with renal disease, who commonly develop severe systemic hypertension mainly due to water and sodium retention, the contribution of the uric acid dependent pathomechanism may become less relevant.
Nevertheless, some studies have reported hyperuricemia to predict progression in established CKD, especially patients with IgA nephropathy [39], [40]. In a retrospective cohort of patients with IgA nephropathy (n = 353), hyperuricemia was a significant independent risk factor for the doubling of serum creatinine or progression to ESRD over a mean follow-up of 5 years [39]. In another retrospective cohort of 803 patients with CKD, hyperuricemia (>6 mg/dl) was significantly associated with progression to ESRD [40].
A similar relationship, as that in patients with IgA nephropathy, was found also between uric acid levels and the progression of diabetic nephropathy [41]. In subjects with type 1 diabetes, an elevated serum uric acid, even when within the normal range, is a strong predictor for the development of CKD [41]; uric acid also predicts the development and progression of CKD in subjects with type 2 diabetes [34], [42].
Interestingly, the presence of a functional polymorphism in the gene of the urate transporter GLUT9, which is associated with enhanced serum urate levels in healthy individuals, strongly predicted progression in a cohort of 755 patients with CKD [43]. In addition, an elevated serum uric acid level has been associated with intrarenal arteriolar lesions [44], [45], consistent with the vascular effects observed in laboratory animals with hyperuricemia [25], [38]. Concretely, a renal biopsy study in Japanese patients with CKD (eGFR < 60 mL/min/1.73 m2) found that increased serum uric acid levels (>7.2 mg/dL) were independently associated with histological evidence of renal arteriolosclerosis, characterised by arterial wall thickening and hyalinosis [44].
In conclusion, according to the above epidemiological findings it is clear that an elevated uric acid is strongly associated with the development of CKD, but not ubiquitously with the progression of CKD.
Clinical trials
In CKD the prevalence of hyperuricemia, gout and uric acid lithiasis is increased because the kidneys are the primary excretion root for uric acid [3], [11]. In addition, epidemiological findings implicate an additional connection of hyperuricemia with the development and progression of CKD, however, a respective treatment strategy has not been adopted yet. On the contrary, indications for treatment of hyperuricemia in CKD patients are limited to a prophylaxis of gout and lithiasis. In this context, several studies have been conducted, to investigate the impact of uric acid lowering treatment on renal outcomes [39], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66]. These were all single centre trials, which had only small number of participants and a limited duration of follow up and most of them were studying allopurinol [3], [67].
Today, apart from diet and life style modifications, the main available options for uric acid lowering therapy in CKD are the xanthine oxidase inhibitors allopurinol and febuxostat. Allopurinol is generally a safe drug, but about 2% of patients develop hypersensitivity reactions. It can also lead to fatal Stevens-Johnson syndrome [68]. Side effects of allopurinol can be dose-related (e.g. gastrointestinal intolerance and rashes). Therefore, side effects occur more often in CKD because allopurinol and its metabolite oxipurinol may accumulate in subjects with low GFR [8], [69].
The new xanthine oxidase inhibitor, febuxostat, is a non-purine, xanthine oxidase inhibitor with a chemical structure different from allopurinol. Febuxostat does not appear to be associated with Stevens-Johnson-syndrome to date, and its dosage does not need to be modified in CKD. The most commonly reported adverse drug reactions are liver function abnormalities, diarrhea, headache, nausea, and rash [69].
Results of two meta-analyses, which included most available randomized trials, were not conclusive. In the first, which involved 8 trials of patients with or without CKD at baseline [39], [46], [47], [48], [49], [50], [51], [52], allopurinol therapy had no effect on eGFR but showed a reduction of serum creatinine levels in some studies [31], [70]. In the second meta-analysis, which involved randomized trials with a total of 992 patients with CKD stage 3–5 [39], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], treatment with allopurinol was associated with significant reductions in serum uric acid levels and a favorable influence on blood pressure and on eGFR compared with untreated controls [71]. Expectedly, both meta-analyses reported significant heterogeneity among these allopurinol trials in respect with design, end-points and follow-up period.
Recently, treatment with febuxostat was also evaluated in a randomized, double blind, placebo-controlled trial, which included 93 patients with asymptomatic hyperuricemia and CKD stage 3 and 4. After 6 months, mean changes in eGFR were significantly more favourable in the febuxostat group compared with the placebo group [72]. Furthermore, topiroxostat, a xanthine oxidase inhibitor approved in Japan, was evaluated in a recent double-blind trial of 123 patients with CKD stage 3 and hyperuricemia. This study showed that treatment with topiroxostat compared with placebo significantly reduced serum uric acid and the levels of albuminuria [73]. These two trials are interesting but also accompanied with serious limitations, namely, the small sample, the short follow up period and the single centre design. Their results need to be confirmed in larger multicenter trials, with longer duration of follow up.
Losartan, an angiotensin receptor blocker (ARB), has significant renoprotectively effects in diabetic nephropathy [74]. Unlike other ARBs, however, losartan has the unique ability to lower serum uric acid levels by decreasing reabsorption, most probably by a direct URAT1 inhibition in the proximal tubule [75], [76]. There is now evidence, that this aspect of losartan treatment may provide additional benefits for renal disease [77]. In a post hoc analysis of the RENAAL (Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan) trial, the risk of renal events (a doubling of serum creatinine or development of ESRD) was decreased by 6% for every 0.5 mg/dL decrement in serum uric acid levels during losartan treatment [77].
Sodium glucose co-transporter 2 (SGLT2 encoded by SLC5A2) is the major glucose transporter in the kidney. It is found primarily in the proximal tubules and responsible for 90% of renal glucose reabsorption. Inhibition of SGLT2 increases urinary glucose excretion, thereby improving glycemic control [78]. In addition, SGLT2 inhibitors reduce serum uric acid levels, possibly by indirect (via glucosuria) activation of the GLUT9 mediated urate transport [79]. Indeed, in the pooled analysis of data from four phase 3 placebo controlled trials the effect of canagliflozin, a SGLT2 inhibitor, was shown to reduce serum uric acid levels [80]. A recently published randomized placebo controlled trial showed, that the SGLT2 inhibitor, empagliflozin slowed the progression of renal disease in patients with diabetes mellitus. Empagliflozin slowed the progression of renal disease and effectively reduced serum uric acid levels. The authors postulated a possible contribution of this latter effect on renal outcomes [80]. These beneficial effects of SGLT2 inhibition must be weighed against potential side effects [78].
In conclusion, before a uric acid lowering therapy of any form can be embraced for prophylaxis of CKD, there is a need to establish its efficacy by large randomised controlled trials. Also safety issues are to be adequately addressed.
Ongoing studies (discussion)
CKD has now become an increasing global public health problem with enhanced morbidity and mortality [15]. This fact underscores the urgent need for investigations of new and putatively more efficient treatment [18]. In this context, there is renewed interest in the relationship between uric acid and nephropathy, which has been considered to be a dead subject in the lasts decades [19]. New data in this field suggest that serum uric acid may be a risk factor for CKD. However, the influence of uric acid lowering therapies on renal outcomes is still largely unclear. Efficacy and safety of uric acid lowering therapies has to be further investigated and large randomised controlled trials have to be planned.
Concretely, the effects of allopurinol on the progression of IgA nephropathy (ClinicalTrials.gov identifier: NCT00793585) and diabetic nephropathy in type 1 diabetes mellitus [81] are currently evaluated in two ongoing nationally sponsored trials.
Furthermore, a prospective, double-blind, placebo-controlled study (FEATHER; UMIN identifier, UMIN000008343) is currently investigating the febuxostat effect on eGFR in adult Japanese patients (n = 400) with CKD stage 3 and asymptomatic hyperuricemia (without gout) for a follow up period of approximately two-years [82].
Apart from these large clinical trials, which are currently running with xanthine oxidase inhibitors, studies are also required to better understand the biological action of uric acid. In particular, the primary role of xanthine oxidase, as a cause of kidney damage independent from uric acid, should be also clarified. Xanthine oxidase, inhibited by allopurinol, produces apart from uric acid also reactive oxygen species (ROS); inhibition of ROS formation may have beneficial renal effects unrelated to serum uric acid levels [2], [69].
Preliminary data suggest that SGLT2 inhibitors can reduce serum uric acid levels, which may in turn contribute to the renoprotective effect shown in diabetic nephropathy. Ongoing clinical studies are prospectively evaluating the effect of SLGT2 inhibitors on serum uric acid levels, renal outcomes and CKD. The CREDENCE trial (ClinicalTrials.gov identifier, NCT02065791), is a phase 3 study evaluating canagliflozin as secondary prophylaxis in patients with type 2 diabetes mellitus and nephropathy (CKD stage 2 or 3 and severe albuminuria). The trial will assess whether canagliflozin has renal and vascular protective effects. The ongoing phase 4 CANVAS-R trial (ClinicalTrials.gov identifier: NCT01989754) will study the effects of canagliflozin on renal endpoints in adults with type 2 diabetes mellitus.
In respect to uric acid lowering treatment in CKD, there are still many unresolved points to address. Namely, the choice of the oxidase inhibitor with the right efficacy-safety profile in CKD, the role of uric acid reducing diet alterations, the limit of uric acid serum levels to be aimed at, the optimal medication dose. It is also unclear whether in subjects with CKD the combination with either ACE inhibitors or with ARBs abolishes the benefit of the uric acid lowering treatments. Finally, it is clear, that there are more questions than answers. The ongoing studies will probably add some more clarity to the field of uric acid lowering therapy in CKD.
Conclusions
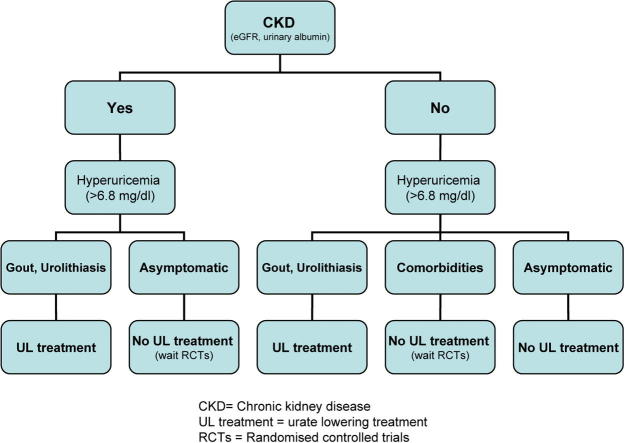
In conclusion, hyperuricemia is clinically significant in the setting of CKD, which is an established independent risk factor for hyperuricemia and for gout. On the other hand, the role of hyperuricemia as an independent risk factor for CKD, is still being debated. There is serious experimental and epidemiological evidence as well as a number of clinical trials to support a relationship of hyperuricemia to CKD, and that uric acid lowering treatment might forestall CKD progression. These trials were of limited duration and included only small number of patients. Only large randomised controlled trials (RCTs) would provide definitive answers about efficacy and safety of a pharmacological treatment for asymptomatic hyperuricemia in CKD. The dangers of inappropriately treating asymptomatic hyperuricemia are well documented [81], [83]. Large RCTs on treatment of hyperuricemia for primary or secondary CKD prophylaxis are under way in populations with hypertension or diabetic nephropathy. The unavailability of these RCTs – despite the serious evidence that uric acid lowering drugs could be suggested for asymptomatic hyperuricemia in the setting of CKD – makes a routine recommendation of these drugs unsubstantiated. Finally, in respect of hyperuricemia, only lifestyle and dietary modifications along with an appropriate treatment for gout and uric acid lithiasis are today the only recommended strategies for reducing the risk of developing or worsening CKD [84].
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Theodoros Eleftheriadis, MD, PhD, is currently Assistant Professor of Nephrology in the Faculty of Medicine, University Thessaly, Larissa, Greece. Beyond teaching and clinical duties he runs a research laboratory focused on molecular medicine. He graduated from the Faculty of Medicine, Aristotelian University of Thessaloniki, Greece and during his specialty in Nephrology he was awarded his PhD. Has been a visiting investigator at the Scripps Research Institute, La Jolla, CA.. He has published >120 studies in journals indexed in PUBMED, which were cited >1000 times. In most of these studies he is the first and corresponding author.

Spyridon Golphinopoulos, MD, graduated from the Faculty of Medicine, Ferrara, Italy in 2001. He obtained his Master’s degree in Administration of Health Units at the Greek Open University in 2008. His main clinical expertise is in Clinical Nephrology and Renal Replacement. Since 2012 he is holding a clinical position, as a consultant nephrologist, in the Clinic of Nephrology, University Hospital of Larissa, Greece. His main research interest resides in the field of cytokines in renal disease.

Georgios Pissas, geneticist holding an MSc in Human Molecular Genetics at Imperial College, London, UK. He earned his PhD from the University of Thessaly. He is currently a Senior Post-Doctoral researcher in the Faculty of Medicine at the University of Thessaly. He has earned various conference awards and two renowned Greek scholarships. His research is focused in the fields of nephrology, cell metabolism and immunology with 40 published papers in Medline-Pubmed database, over 200 citations and an h-index of 12.

Ioannis Stefanidis, Professor of Medicine/Nephrology, Medical School Larissa, University Thessaly, Greece and Visiting Professor (Privat Dozent), Internal Medicine, Medical School, Technical University (RWTH) Aachen, Germany. From 2010 to 2013 vice Dean and currently Dean of the Medical School of Larissa, since 2014. Head of the Clinic of Nephrology, University Hospital of Larissa and leading member of the Nephrology Research Group of the Medical School, since 2000. His research, focused in genetics of multifactorial disorders in Nephrology, physiology of mesothelial cells and in the systemic influence of renal disease, has been published in 200 papers with about 1900 citations (h-index 24).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Johnson R.J., Nakagawa T., Jalal D., Sanchez-Lozada L.G., Kang D.H., Ritz E. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplant. 2013;28:2221–2228. doi: 10.1093/ndt/gft029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang D.H., Chen W. Uric acid and chronic kidney disease: new understanding of an old problem. Semin Nephrol. 2011;31:447–452. doi: 10.1016/j.semnephrol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Dousdampanis P., Trigka K., Musso C.G., Fourtounas C. Hyperuricemia and chronic kidney disease: an enigma yet to be solved. Ren Fail. 2014;36:1351–1359. doi: 10.3109/0886022X.2014.947516. [DOI] [PubMed] [Google Scholar]
- 4.Wu X.W., Muzny D.M., Lee C.C., Caskey C.T. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 5.Johnson R.J., Nakagawa T., Sanchez-Lozada L.G., Shafiu M., Sundaram S., Le M. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannella A.C., Mikuls T.R. Understanding treatments for gout. Am J Manag Care. 2005;11:S451–S458. [PubMed] [Google Scholar]
- 7.Wright A.F., Rudan I., Hastie N.D., Campbell H. A ‘complexity’ of urate transporters. Kidney Int. 2010;78:446–452. doi: 10.1038/ki.2010.206. [DOI] [PubMed] [Google Scholar]
- 8.Bobulescu I.A., Moe O.W. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. 2012;19:358–371. doi: 10.1053/j.ackd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preitner F., Bonny O., Laverriere A., Rotman S., Firsov D., Da Costa A. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci USA. 2009;106:15501–15506. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richette P., Bardin T. Gout. Lancet. 2010;375:318–328. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 11.Madero M., Sarnak M.J., Wang X., Greene T., Beck G.J., Kusek J.W. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juraschek S.P., Kovell L.C., Miller E.R., III, Gelber A.C. Association of kidney disease with prevalent gout in the United States in 1988–1994 and 2007–2010. Semin Arthritis Rheum. 2013;42:551–561. doi: 10.1016/j.semarthrit.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan E. Chronic kidney disease and the risk of incident gout among middle-aged men: a seven-year prospective observational study. Arthritis Rheum. 2013;65:3271–3278. doi: 10.1002/art.38171. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. Chapter 1: Definition and classification of CKD. Kidney Int Suppl 3; 2011. 2013. pp. 19–62. [DOI] [PMC free article] [PubMed]
- 15.Couser W.G., Remuzzi G., Mendis S., Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 16.Coresh J., Selvin E., Stevens L.A., Manzi J., Kusek J.W., Eggers P. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 17.Saran R., Li Y., Robinson B., Abbott K.C., Agodoa L.Y., Ayanian J. US renal data system 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67 doi: 10.1053/j.ajkd.2015.12.014. Svii, S1-Svii, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillespie B.W., Morgenstern H., Hedgeman E., Tilea A., Scholz N., Shearon T. Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clin Kidney J. 2015;8:772–780. doi: 10.1093/ckj/sfv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck L.H. Requiem for gouty nephropathy. Kidney Int. 1986;30:280–287. doi: 10.1038/ki.1986.179. [DOI] [PubMed] [Google Scholar]
- 20.Hediger M.A., Johnson R.J., Miyazaki H., Endou H. Molecular physiology of urate transport. Physiology (Bethesda) 2005;20:125–133. doi: 10.1152/physiol.00039.2004. [DOI] [PubMed] [Google Scholar]
- 21.Kang D.H., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 22.Crisan T.O., Cleophas M.C., Oosting M., Lemmers H., Toenhake-Dijkstra H., Netea M.G. Soluble uric acid primes TLR-induced proinflammatory cytokine production by human primary cells via inhibition of IL-1Ra. Ann Rheum Dis. 2016;75:755–762. doi: 10.1136/annrheumdis-2014-206564. [DOI] [PubMed] [Google Scholar]
- 23.Xiao J., Fu C., Zhang X., Zhu D., Chen W., Lu Y. Soluble monosodium urate, but not its crystal, induces toll like receptor 4-dependent immune activation in renal mesangial cells. Mol Immunol. 2015;66:310–318. doi: 10.1016/j.molimm.2015.03.250. [DOI] [PubMed] [Google Scholar]
- 24.Yu M.A., Sanchez-Lozada L.G., Johnson R.J., Kang D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 25.Mazzali M., Hughes J., Kim Y.G., Jefferson J.A., Kang D.H., Gordon K.L. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Lozada L.G., Tapia E., Santamaria J., Avila-Casado C., Soto V., Nepomuceno T. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 27.Eleftheriadis T., Pissas G., Karioti A., Antoniadi G., Golfinopoulos S., Liakopoulos V. Uric acid induces caspase-1 activation, IL-1beta secretion and P2X7 receptor dependent proliferation in primary human lymphocytes. Hippokratia. 2013;17:141–145. [PMC free article] [PubMed] [Google Scholar]
- 28.Kosugi T., Nakayama T., Heinig M., Zhang L., Yuzawa Y., Sanchez-Lozada L.G. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Yang C., Zhao Y., Zeng X., Liu F., Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease? A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner D.E., Tighiouart H., Elsayed E.F., Griffith J.L., Salem D.N., Levey A.S. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellomo G., Venanzi S., Verdura C., Saronio P., Esposito A., Timio M. Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis. 2010;56:264–272. doi: 10.1053/j.ajkd.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Bakan A., Oral A., Elcioglu O.C., Takir M., Kostek O., Ozkok A. Hyperuricemia is associated with progression of IgA nephropathy. Int Urol Nephrol. 2015;47:673–678. doi: 10.1007/s11255-015-0939-7. [DOI] [PubMed] [Google Scholar]
- 33.Obermayr R.P., Temml C., Gutjahr G., Knechtelsdorfer M., Oberbauer R., Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–2413. doi: 10.1681/ASN.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoppini G., Targher G., Chonchol M., Ortalda V., Abaterusso C., Pichiri I. Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care. 2012;35:99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iseki K., Ikemiya Y., Kinjo K., Iseki C., Takishita S. Prevalence of high fasting plasma glucose and risk of developing end-stage renal disease in screened subjects in Okinawa, Japan. Clin Exp Nephrol. 2004;8:250–256. doi: 10.1007/s10157-004-0293-z. [DOI] [PubMed] [Google Scholar]
- 36.Rosolowsky E.T., Ficociello L.H., Maselli N.J., Niewczas M.A., Binns A.L., Roshan B. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturm G., Kollerits B., Neyer U., Ritz E., Kronenberg F. Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol. 2008;43:347–352. doi: 10.1016/j.exger.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Mazzali M., Kanellis J., Han L., Feng L., Xia Y.Y., Chen Q. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y., Chen W., Jalal D., Li Z., Chen W., Mao H. Clinical outcome of hyperuricemia in IgA nephropathy: a retrospective cohort study and randomized controlled trial. Kidney Blood Press Res. 2012;35:153–160. doi: 10.1159/000331453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchida S., Chang W.X., Ota T., Tamura Y., Shiraishi T., Kumagai T. Targeting uric acid and the inhibition of progression to end-stage renal disease–a propensity score analysis. PLoS One. 2015;10:e0145506. doi: 10.1371/journal.pone.0145506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ficociello L.H., Rosolowsky E.T., Niewczas M.A., Maselli N.J., Weinberg J.M., Aschengrau A. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care. 2010;33:1337–1343. doi: 10.2337/dc10-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altemtam N., Russell J., El Nahas M. A study of the natural history of diabetic kidney disease (DKD) Nephrol Dial Transplant. 2012;27:1847–1854. doi: 10.1093/ndt/gfr561. [DOI] [PubMed] [Google Scholar]
- 43.Testa A., Mallamaci F., Spoto B., Pisano A., Sanguedolce M.C., Tripepi G. Association of a polymorphism in a gene encoding a urate transporter with CKD progression. Clin J Am Soc Nephrol. 2014;9:1059–1065. doi: 10.2215/CJN.11041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohagura K., Kochi M., Miyagi T., Kinjyo T., Maehara Y., Nagahama K. An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res. 2013;36:43–49. doi: 10.1038/hr.2012.135. [DOI] [PubMed] [Google Scholar]
- 45.Wu J., Chen X., Xie Y., Yamanaka N., Shi S., Wu D. Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant. 2005;20:719–727. doi: 10.1093/ndt/gfh716. [DOI] [PubMed] [Google Scholar]
- 46.Gibson T., Rodgers V., Potter C., Simmonds H.A. Allopurinol treatment and its effect on renal function in gout: a controlled study. Ann Rheum Dis. 1982;41:59–65. doi: 10.1136/ard.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanbay M., Huddam B., Azak A., Solak Y., Kadioglu G.K., Kirbas I. A randomized study of allopurinol on endothelial function and estimated glomular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goicoechea M., de Vinuesa S.G., Verdalles U., Ruiz-Caro C., Ampuero J., Rincon A. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao M.P., Ang D.S., Gandy S.J., Nadir M.A., Houston J.G., Lang C.C. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Momeni A., Shahidi S., Seirafian S., Taheri S., Kheiri S. Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis. 2010;4:128–132. [PubMed] [Google Scholar]
- 51.Sarris E., Bagiatudi G., Stavrianaki D., Salpigidis K., Siakotos M. Use of allopurinol in slowing the progression of chronic renal disease. Nephrol Dial Transplant. 2007;22 vi61. [Google Scholar]
- 52.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Deng Y.H., Zhang P., Liu H., Jia Q. Observation on allopurinol in lowering blood uric acid for slowing the progression of chronic renal failure. J Pract Med. 2010;26:982–984. [Google Scholar]
- 54.Liu J., Sheng D. Allopurinol in lowering serum uric acid level for the delay of the progression of chronic renal disease. China Pharm. 2007;18:2524–2525. [Google Scholar]
- 55.Shen H., Liu D., Shen H., Liu D. Clinical research on allopurinol in lowering serum uric acid level for the delay of the progression of chronic renal disease. China Foreign Med Treat. 2010;12:88–89. [Google Scholar]
- 56.Tan Y., Fu J.Z., Liang M., Lin Z.X., Huang J. Clinical observation of the effect of allopurinol to protect renal function in patients with diabetic nephropathy. Mod Hosp. 2011;11:36–38. [Google Scholar]
- 57.Lei J., Li S.T. Clinical research on allopurinol lowering of uric acid level of chronic renal disease for the delay of the progression of renal disease. Shaanxi Med J. 2009;38:1191–1212. [Google Scholar]
- 58.Schmidt A., Gruber U., Bohmig G., Koller E., Mayer G. The effect of ACE inhibitor and angiotensin II receptor antagonist therapy on serum uric acid levels and potassium homeostasis in hypertensive renal transplant recipients treated with CsA. Nephrol Dial Transplant. 2001;16:1034–1037. doi: 10.1093/ndt/16.5.1034. [DOI] [PubMed] [Google Scholar]
- 59.Nouri-Majalan N., Ardakani E.F., Forouzannia K., Moshtaghian H. Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts. Vasc Health Risk Manage. 2009;5:489–494. doi: 10.2147/vhrm.s5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katholi R.E., Woods W.T., Jr., Taylor G.J., Deitrick C.L., Womack K.A., Katholi C.R. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64–71. doi: 10.1053/ajkd.1998.v32.pm9669426. [DOI] [PubMed] [Google Scholar]
- 61.Kamper A.L., Nielsen A.H. Uricosuric effect of losartan in patients with renal transplants. Transplantation. 2001;72:671–674. doi: 10.1097/00007890-200108270-00019. [DOI] [PubMed] [Google Scholar]
- 62.Chanard J., Toupance O., Lavaud S., Hurault d.L., Bernaud C., Moulin B. Amlodipine reduces cyclosporin-induced hyperuricaemia in hypertensive renal transplant recipients. Nephrol Dial Transplant. 2003;18:2147–2153. doi: 10.1093/ndt/gfg341. [DOI] [PubMed] [Google Scholar]
- 63.Malaguarnera M., Vacante M., Russo C., Dipasquale G., Gargante M.P., Motta M. A single dose of rasburicase in elderly patients with hyperuricaemia reduces serum uric acid levels and improves renal function. Expert Opin Pharmacother. 2009;10:737–742. doi: 10.1517/14656560902781972. [DOI] [PubMed] [Google Scholar]
- 64.Perez-Ruiz F., Calabozo M., Fernandez-Lopez M.J., Herrero-Beites A., Ruiz-Lucea E., Garcia-Erauskin G. Treatment of chronic gout in patients with renal function impairment: an open, randomized, actively controlled study. J Clin Rheumatol. 1999;5:49–55. doi: 10.1097/00124743-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Doehner W., Schoene N., Rauchhaus M., Leyva-Leon F., Pavitt D.V., Reaveley D.A. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 66.Goicoechea M., Garcia d.V., Verdalles U., Verde E., Macias N., Santos A. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis. 2015;65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Mende C. Management of chronic kidney disease: the relationship between serum uric acid and development of nephropathy. Adv Ther. 2015;32:1177–1191. doi: 10.1007/s12325-015-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung J.W., Song W.J., Kim Y.S., Joo K.W., Lee K.W., Kim S.H. HLA-B58 can help the clinical decision on starting allopurinol in patients with chronic renal insufficiency. Nephrol Dial Transplant. 2011;26:3567–3572. doi: 10.1093/ndt/gfr060. [DOI] [PubMed] [Google Scholar]
- 69.Jalal D.I., Chonchol M., Chen W., Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134–146. doi: 10.1053/j.ajkd.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bose B., Badve S.V., Hiremath S.S., Boudville N., Brown F.G., Cass A. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis. Nephrol Dial Transplant. 2014;29:406–413. doi: 10.1093/ndt/gft378. [DOI] [PubMed] [Google Scholar]
- 71.Kanji T., Gandhi M., Clase C.M., Yang R. Urate lowering therapy to improve renal outcomes in patients with chronic kidney disease: systematic review and meta-analysis. BMC Nephrol. 2015;16:58. doi: 10.1186/s12882-015-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sircar D., Chatterjee S., Waikhom R., Golay V., Raychaudhury A., Chatterjee S. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: a 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015;66:945–950. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Hosoya T., Ohno I., Nomura S., Hisatome I., Uchida S., Fujimori S. Effects of topiroxostat on the serum urate levels and urinary albumin excretion in hyperuricemic stage 3 chronic kidney disease patients with or without gout. Clin Exp Nephrol. 2014;18:876–884. doi: 10.1007/s10157-014-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Zeeuw D., Remuzzi G., Parving H.H., Keane W.F., Zhang Z., Shahinfar S. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int. 2004;65:2309–2320. doi: 10.1111/j.1523-1755.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 75.Daskalopoulou S.S., Tzovaras V., Mikhailidis D.P., Elisaf M. Effect on serum uric acid levels of drugs prescribed for indications other than treating hyperuricaemia. Curr Pharm Des. 2005;11:4161–4175. doi: 10.2174/138161205774913309. [DOI] [PubMed] [Google Scholar]
- 76.Hamada T., Ichida K., Hosoyamada M., Mizuta E., Yanagihara K., Sonoyama K. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients. Am J Hypertens. 2008;21:1157–1162. doi: 10.1038/ajh.2008.245. [DOI] [PubMed] [Google Scholar]
- 77.Miao Y., Ottenbros S.A., Laverman G.D., Brenner B.M., Cooper M.E., Parving H.H. Effect of a reduction in uric acid on renal outcomes during losartan treatment: a post hoc analysis of the reduction of endpoints in non-insulin-dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial. Hypertension. 2011;58:2–7. doi: 10.1161/HYPERTENSIONAHA.111.171488. [DOI] [PubMed] [Google Scholar]
- 78.Ferrannini E., Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 79.Davies M.J., Trujillo A., Vijapurkar U., Damaraju C.V., Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:426–429. doi: 10.1111/dom.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 81.Maahs D.M., Caramori L., Cherney D.Z., Galecki A.T., Gao C., Jalal D. Uric acid lowering to prevent kidney function loss in diabetes: the preventing early renal function loss (PERL) allopurinol study. Curr Diab Rep. 2013;13:550–559. doi: 10.1007/s11892-013-0381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hosoya T., Kimura K., Itoh S., Inaba M., Uchida S., Tomino Y. The effect of febuxostat to prevent a further reduction in renal function of patients with hyperuricemia who have never had gout and are complicated by chronic kidney disease stage 3: study protocol for a multicenter randomized controlled study. Trials. 2014;15:26. doi: 10.1186/1745-6215-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pasina L., Brucato A.L., Djade C.D., Di Corato P., Ghidoni S., Tettamanti M. Inappropriate prescription of allopurinol and febuxostat and risk of adverse events in the elderly: results from the REPOSI registry. Eur J Clin Pharmacol. 2014;70:1495–1503. doi: 10.1007/s00228-014-1752-4. [DOI] [PubMed] [Google Scholar]
- 84.Khanna D., Fitzgerald J.D., Khanna P.P., Bae S., Singh M.K., Neogi T. American college of rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]