Graphical abstract
Quoted from Urolithiasis – EAU Guidelines 2016 with adaptation.
Keywords: Urolithiasis, Calculi, Uric acid, Urinary stones, Uric acid stones, pH dissolution, Nephrolithiasis, Chemolysis
Abstract
An in-depth comprehension of the epidemiology as well as pathophysiology of uric acid urolithiasis is important for the identification, treatment, and prophylaxis of calculi in these patients. Persistently low urinary pH, hyperuricosuria, and low urinary volume are the most important factors in pathogenesis of uric acid urolithiasis. Other various causes of calculus formation comprises of chronic diarrhea, renal hyperuricosuria, insulin resistance, primary gout, extra purine in the diet, neoplastic syndromes, and congenital hyperuricemia. Non-contrast-enhanced computed tomography is the radiologic modality of choice for early assessment of patients with renal colic. Excluding situations where there is acute obstruction, rising blood chemistry, severe infection, or unresolved pain, the initial management ought to be medical dissolution by oral chemolysis since this method has proved to be effective in most of the cases.
Background
Uric acid calculi constitutes around 10% of calculi. These calculi are radiolucent and can be efficiently treated with chemolysis as well as endoscopic and surgical procedures. In developed countries the occurrence rates of urolithiasis has constantly increased over years. Calcareous calculi is responsible for the majority of urinary calculi cases followed by uric acid calculi [1]. The pathogenesis of uric acid urolithiasis is somewhat is still unclear. The risk factors include persistently low urinary pH, hyperuricosuria, and low urinary volume [2]. Diseases that causes hyperuricosuria and predispose to uric acid urolithiasis include uncontrolled diarrhea, myeloproliferative conditions, resistance to insulin encompassing diabetes mellitus, and monogenic metabolic conditions for instance Lesch-Nyhan condition. Researchers detected a gene linked to uric acid calculus formation; however, its purpose is yet to be well defined [3]. The clinical presentation of patients with calculi are usually the same irrelevant to the composition of the calculus. Among others, some of these signs and symptoms consists of; loin dull aching or colicky pain, nausea and vomiting, fatigue, lower urinary tract symptoms, and hematuria. Non-contrast computerized tomography of the urinary tract is the modality of choice in the diagnosis of uric acid calculi, and has the ability to detect calculi with a low attenuation coefficient value. Medical dissolution treatment approach is effective in most of the cases except in certain situations where there is rising blood chemistry, advanced uremia, sepsis, or constant pain. From that perspective, it can therefore be explained that uric acid calculi are without a doubt exceptional as they liquefy readily in an ideal urinary pH milieu, attainable with oral medical intervention.
Purine and uric acid metabolism
Uric acid (2,6,8-trioxypurine) is the final product of purine metabolism and has no known physiological function in humans. Uricase enzyme is lacking in humans and found in most mammals convert uric acid to allantoin (10–100 times more soluble). Urinary concentration of uric acid depends on urine pH, urine volume and excretion of uric acid. Urinary pH is the most important factor of uric acid solubility. Loss of a single proton from uric acid and hence dissociation of uric acid is controlled by two dissociation constants (pKa). The first pKa of pH 5.5, govern the conversion of uric acid to the more soluble anionic urate. The second pKa of pH 10.3 is not clinically significant sine the mean human urine pH is 5.9 and normally ranges from 4.8 to 7.4. At a urinary pH < 5.5 almost 100% of uric acid is undissociated and urine will be supersaturated with uric acid. Inversely, at a pH of >6.5 the majority of the uric acid in the form as anionic urate [4].
Endogenous sources
Under normal conditions, nearly 300–400 mg/dL is produced from de novo synthesis and tissue catabolism. Abnormally high synthesis of uric acid occur with gout, myeloproliferative disorders, certain congenital metabolic defects and patients receiving chemotherapy due to rapid cell turnover.
Exogenous sources
High purines diet e.g. meat, animal organs, fish, sweetbreads, and yeast.
In the intestinal tract, purine → free nucleic acids → inosinic acid → hypoxanthine → xanthine (by xanthine oxidase) → uric acid [5].
Kidney handling and elimination
The kidney excretes two-thirds of uric acid. Skin, nails, hair, saliva, and the gastrointestinal tract (GIT) eliminates the remaining third. In the GIT, bacteria convert part of the uric acid to ammonia and carbon dioxide, which is expelled as gas. Ammonia is either absorbed and excreted in the urine or utilized by bacteria as an energy source [6].
The majority of serum uric acid (95%) is in the form of monosodium urate and is freely filtered at in the glomeruli, while the remaining is protein bound. Ninety-nine percent of the filtered urate is reabsorbed in the proximal convoluted tubule (PCT) through complex successive reabsorption, secretion, and again reabsorption and 50% is then secreted back into the PCT. Post secretory absorption of 80% of this urate occurs in the distal PCT. Therefore, about 10% of the filtered urate is excreted in the urine. The fractional excretion of urate ranges from 60% in a premature neonate to 12% in a 3 children and 7% in the adults [7], [8].
Medications and factors affecting the renal handling of uric acid
The most important factors that affect the renal handling of uric acid include patient’s hydration status and urine output, serum urate concentration, medications and extra-cellular volume expansion that is inversely proportionate to serum urate concentration. Salicylates, sulfinpyrazone, and probenecid are uricosuric through blocking urate absorption in the PCT. The hyperuricosuria caused by of thiazides is by producing extra-cellular volume depletion and hence increases urate secretion in the PCT. Hyperuricosuria during pregnancy is due to fetal urate production and increased intravascular volume [9], [10].
Epidemiology
The incidence of uric acid calculi varies geographically, the worldwide incidence ranges from 5 to 40%. The frequency of nephrolithiasis in the US is approximated to be about 0.5% a year a prevalence rate that can be explained as been on the increase [10]. Indeed, when the data from US National Health and Nutrition Examination Survey II and III is summarized, it was reported that the calculus diseases occurrence rate has up surged from 3.8% in the year 1976 to 5.2% in the year 1980 to 1994 in most developed countries [11]. Similarly, the yearly economic expenses linked to the condition have also increased from a reported $1.3 billion in the year 1994 to a reported $2 billion in the year 2000 irrespective of the fact that various measures such as minimally invasive processes, decrease in periods of hospitalization, and changes in the care offered in outpatient clinics have been adopted [12].
Uric acid nephrolithiasis has been found to account for about 7–10 percent of all calculi. Calculi isolated from patients that were in the Administration System of the Veterans found that about 9.7% were made up only of uric acid. In another large series, it was reported that uric acid calculi was detected in the 7 percent of the calculi that were studied. Most authors consider this incidence is a miscalculation of the true frequency; however, it indicate the importance of this condition [13], [14].
The occurrence of uric acid calculi differs with; age, sex, demographics, and even the local environmental aspects. For instance, patients who are more than sixty-five years were reported to develop uric acid calculi twice the prevalence in youth patients in a retrospective research that has six thousand patients Males were found to be more to females approximately by three times [15], [16].
The variance in the ratio of uric acid calculi might also vary between various ethnic groups. Half of the Hmong patients that had kidney calculi had uric acid calculi while in non-Hmong patients; only 10% had the condition. The occurrence rate or uric acid calculus was 6 percent among the whites and 30 percent among the non-whites. The Frequency ratio in other nations is less than 1% in India, 440% in Israel, and less than 4% in Japan [17], [18].
Environment was found to be definitely affecting formation of uric acid calculus. Calculus formation occurrence rate was 9% for the factory laborers who worked in a hot environment while the occurrence rate of those who are working in a standard room environment was 0.9%. A drawback of this study was that calculus content was not reported in the research [18], [19].
Pathophysiology
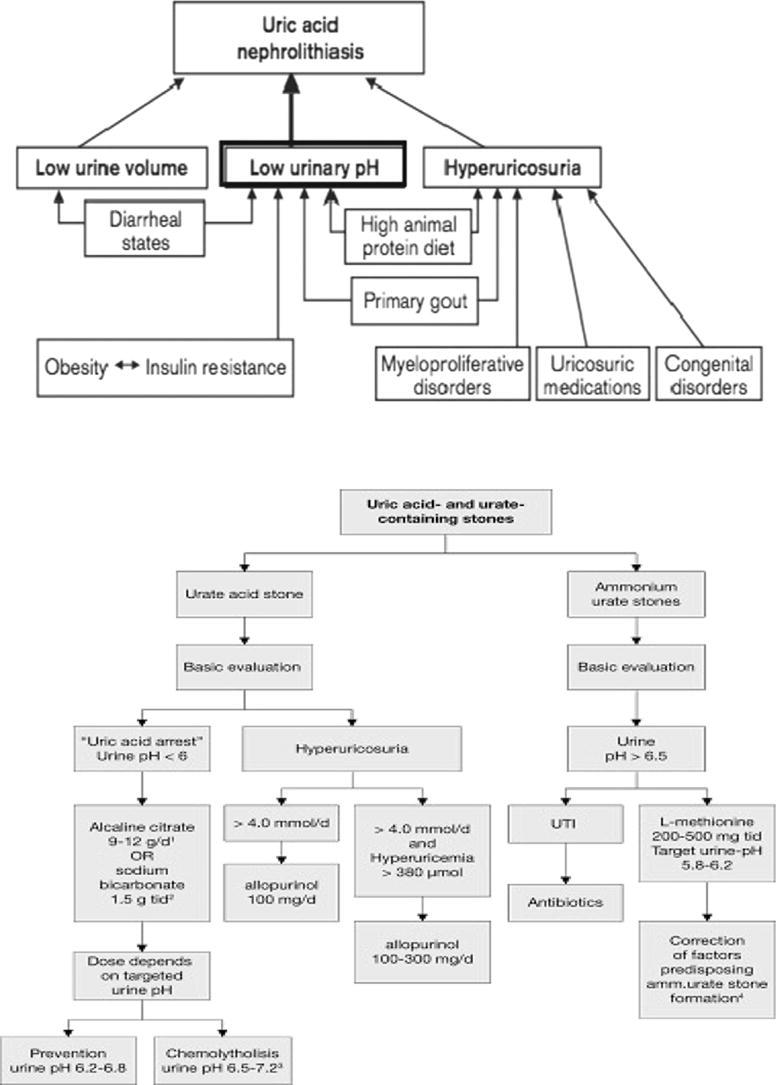
Calculus formation is a complex procedure that include biochemical disturbances of urine stimulating crystal nucleation, aggregation, and probably adhesion. Renal plaques of Randall were demonstrated to play a role in the formation of calcium oxalate but not uric acid calculi by different researches, who examined renal tissue gathered during percutaneous nephrolithotomy [20], [21]. Indeed, urinary irregularities that influence the development of uric acid calculi encompasses constantly low urinary pH (the main factor), hypovolemia and low urinary levels, and hyperuricosuria (explained as daily urinary uric acid exceeding 750 mg/d in females and 800 mg/d in males) [22], [23].
Persistently low urinary pH
Uric acid urolithiasis is usually associated with persistently low urine pH. Nearly all patients with uric acid calculi demonstrate constantly low urinary pH while the majority excrete normal amounts of urates.[24], [25] On the other hand, patients without congenital or attained conditions to that predispose to formation of uric acid calculi are supposed to have either idiopathic uric acid nephrolithiasis or “gouty diathesis [25], [26]. Both represents a syndrome of primary gout and exemplified by high serum uric acid, reduced fractional excretion of uric acid, and constantly low urinary pH. Low urinary pH is thought to induce uric acid calculi through basic acid-base chemistry and solubility of the uric acid [9], [27].
Patients with low urinary pH but a regular uric acid secretion may develop uric acid calculi, while others with a standard or increased urinary pH but additional urate secretion will not [28]. This fact may be demonstrated with the dissociation of uric acid in water. The nitrogen at position N-9 of urate, when dissolved in water, may receive a free proton to develop uric acid.
The first acid dissociation constant (pKa) of this reaction is 5.5 pH; the second pKa has no physiological significance. The solubility constant (Ksp) of uric acid is approximately 100 mg/L in aqueous solutions at 37 °C, while urate is 20 times more soluble. Urate and uric acid exist in equal proportions at a pH equal to the pKa (Henderson-Hasselbach equation) [10], [29]. Consequently, if 200 mg of urate were added to a 1-L aqueous solution with a pH of 5.5 at 37 °C, 100 mg will become uric acid and the remainder will continue to be urate. On the contrary, if 1200 mg of urate were added to an equal volume at a pH of 6.5, 1100 mg will remain in the soluble urate form. These interactions relay on the upward swing of the uric acid dissociation curve at this pH, which plateaus at a pH of nearly 7.2 [11], [30].
However, the precise mechanism of constantly acidified urine reported with uric acid calculi is still not clear. Despite that, a number of various hypotheses have been suggested. Participants that have idiopathic uric acid nephrolithiasis and ordinary subjects, both on controlled diets, were compared [31]. The comparison showed that uric acid calculus formers had persistent acidic urine as well as less excretion of their acid load in the form of ammonium. They depend instead on a higher amount of titratable acid secretion. Moreover, these patients also have a less effective reaction to ammonium chloride oral acid loading as confirmed by secreting urinary ammonium in volumes 7-fold lesser than those in the ordinary participants.
These findings hypothesized that these patients have a disorder of ammonium secretion, resulting in loss of a significant urinary buffer. Without this buffer, slight increases in the concentration of H could significantly decrease pH. Researchers have proposed that faults in the enzymes glutaminase and/or glutamate dehydrogenase, that metabolize glutamine into ammonia and ketoglutarate, could result to impaired ammonium secretion. Moreover, they have also theorized that low consumption of glutamine in the pathway could change it to other pathways that use glutamine resulting in hyperuricemia [8], [32], [33].
These two premises are aided by the findings of increased plasma levels of glutamate in participants that have uric acid nephrolithiasis and, when receiving 15 N-labeled glycine, integrated more 15 N into uric acid than ammonium contrasted with controls. Nevertheless, it should be pointed out that other researchers have not found distinct variation amongst the activity of renal glutaminase in participants with gout and those that do not have gout.
The precise function of renal glutamine catabolism in as a cause of inadequate urinary ammonium discharge is not yet clear. For uric acid calculi to be formed, pH need to remain persistently low and not only low. In noncalculus formers, the urine may occasionally develop acidity enough to precipitate crystals despite normal concentrations of uric acid; although it is thought that transient, alkalinisation of urine that occurs with meals halts the progression to bona fide calculi. Periodic urinary alkaline tides dissolve any uric acid crystals that have been created as a consequence of transiently acidic urine that supports this model. Conditions that may theoretically lead to absence of alkaline tides are: increased renal tubular reabsorption of bicarbonate, decreased glomerular filtration rate leading to decreased filtered load of bicarbonate, and defective gastric acid secretion. Available information suggest that an unrecognised renal defect is suspected to result in failure to produce the physiologic urinary alkaline tide rather than impaired gastric acid secretion [9], [34], [35].
Hyperuricosuria
Hyperuricosuria with regular urinary pH may also result in mixed calculi formation made up of urate and calcium oxalate. Even though urate is most of the times more soluble than uric acid, it can be noted that it is not considerably so. Monosodium urate at high levels precipitates out of solution and is conjectured to result in calcium oxalate crystallization through either; the attenuation of macromolecular inhibitors of lithogenesis, heterogeneous nucleation, and salting-out occurrence. Hyperuricosuria most of the times emanates from nutritional indiscretion, even though mutations in the URAT1 channel could result in congenital renal hypouricemic hyperuricosuria [7], [36], [37].
Low urinary volume
Diminished urinary output causes increased urinary concentrations of lithogenic solutes. The high concentrations of urate could result in uric acid and monosodium urate precipitation as a result of restricted solubility of uric acid. Consequently, uric acid calculi are prevalent in the tropics and hot environments [38], [39].
Macromolecular inhibitors of crystallization
Urine contains factors that inhibit crystal formation that modulate uric acid crystallization and calculus formation. Urinary surfactants, glycoproteins and glycosaminoglycans (GAGs) have inhibitory effect on uric acid crystallation [40]. Studies showed significantly lower levels of GAGs in urine of uric acid formers genetically and geographically isolated. It is not yet clear how the deficiency of such inhibitors may cause uric acid calculus formation [40], [41], [42].
Familial, genetic and environmental factors predispose to the formation of urinary calculi. The gene ZNF365 located on chromosome 10q21-q22 was reported to be linked with uric acid urolithiasis. Even though this DNA encodes for four various proteins through substitute splicing, only one prompts to the advancement of uric acid calculi [43]. The exact role of these genes is still unclear.
On the other hand, new gene of homologue for DNA which is not obvious in mice while normally present as an unexpressed gene in both old and new world monkeys appears to emerge in the Miocene era revolving in the time that the apes happened to lose the purpose of uricase. The product of this gene may possibly safeguards from the noxious impacts of hyperuricemia due to the silencing of the uricase gene while not losing its positive impacts [43], [44].
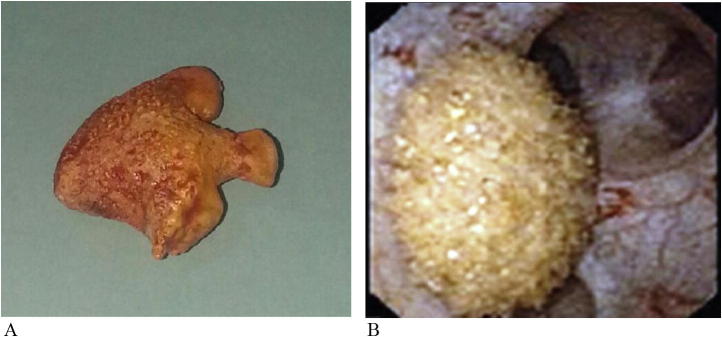
Future studies are needed to find out the actual role performed by this gene product in the body and the formation of uric acid calculi. Nevertheless, currently, any effort at explanation of the roles of this will be purely hypothetical (Fig. 1A,B).
Fig. 1.
(A and B) Gross appearance and endoscopic view of uric acid stones.
Associated conditions and possible causes
Primary gout
Primary gout either is due to defective renal excretion of uric acid resulting in hyperuricemia in the majority of cases or increased production in only a small percentage. Moreover, even though patients that have gout can also experience painful joints and urinary calculi, the occurrence of uric acid calculi among these gouty patients is around 10–20%. The causative factors for uric acid calculus formation in this group are assumed to be acidic urinary pH together with abnormalities in renal uric acid handling [1].
Idiopathic uric acid nephrolithiasis
Gouty diathesis or idiopathic uric acid nephrolithiasis are used to describe patients with no recognizable congenital or acquired error of metabolism that predispose to formation of uric acid calculi. Patients with hyperuricemia, decreased fractional secretion of uric acid, low urinary pH, and latent gout were historically categorized as having gouty diathesis [45]. Patients with solely low urinary pH associated with uric acid calculi are included in this classification. It is assumed that these patients have an early form of gout that may finally result in into gouty arthropathy.
Gastrointestinal conditions and chronic diarrhea
The formation of uric acid calculi in these patients is linked to loss of bicarbonate resulting in more acidic urine, dehydration and hypovolemia, which amplifies the supersaturation of these salts.
Patients with inflammatory bowel disease, ileostomy, or multiple bowel resections, especially involving the terminal ileum are predisposed to uric acid nephrolithiasis. The incidence of urolithiasis in patients with ulcerative colitis, ileostomy and Crohns disease is reported to be 0.5–3.2%, 50–70% and 80% respectively [45], [46]. These patients have a persistently low urinary pH but otherwise have normal serum and urine uric acid levels. They become dehydrated as a result of the ongoing water loss from the gastrointestinal tract. This also results in excessive bicarbonate losses with a resultant metabolic acidosis, hypocitraturia and low urinary pH. Such patients are predisposed to both uric acid and calcium oxalate lithiasis. Other situations that may lead to chronic dehydration such as heavy physical activity without fluid replacement, working in a hot environment, or living in an arid climate can result in increased uric acid calculus formation. These situations are met with in the Middle East and may account for the increased incidence of uric acid calculi.
Insulin resistance
Diabetic calculus formers have a 6 times more risk to form uric acid calculi compared to non-diabetic calculus formers. Insulin resistance is found in more than 50% of patients with uric acid calculi [47]. A research has shown that urinary pH inversely connects with the weight of the body. In comparison, the pH of urine is certainly linked to insulin resistance [48]. Physiologic studies have indicated that there are serious increase in insulin elevate urinary pH by stimulating proximal renal tubular ammoniagenesis through increasing catabolism of glutamine into two molecules of ammonia and ketoglutarate as well as the activity of the sodium/hydrogen ion exchanger 3 (NHE3) that secretes and traps ammonia in the urinary space as ammonium [49], [50], [51]. In certain animal models, lofty levels of liberated fatty acids raises levels of acetyl-CoA, that competes with ketoglutarate for admission into the Krebs cycle. Reduced metabolism ketoglutarate results to its build up and then slows down the catabolism of glutamine by the mass-law effect, successfully minimizing ammoniagenesis [52], [53]. In insulin-resistant states elevated levels of free fatty acids increase levels of acetyl-CoA, which competes with ketoglutarate for entry into the Krebs cycle. Decreased metabolism ketoglutarate leads to its accumulation and in turn impedes the catabolism of glutamine by the mass-law effect, effectively reducing ammoniagenesis [54], [55].
Increased purines in diet
The patients that consume high amounts of meat are at danger of developing uric acid calculi due to the increased purine load as well as acid-ash substance of animal protein. This encourages hyperuricosuria as well as a mild metabolic acidosis, which results to decreasing of urinary pH. Thus, dietary strategies could assist in averting development of uric acid calculus. Patients usually have normal serum uric acid levels [56].
Increased catabolism
Due to increase in the production and turnover of nucleic acids, around 40% of patients with myelo- or lympho-proliferative disorders develop uric acid calculi. In patients receiving chemotherapy, tissue necrosis result in increased endogenous purine pool may. This can lead to acute urinary obstruction because of severe crystalluria. Thalassemia, hemolytic anemia, polycythemia, and sickle cell disease are all benign disorders with high cell turnover that predispose to uric acid lithiasis [57], [58].
Renal hyperuricosuria
Renal wasting of uric acid, hyperuricosuria as well as uric acid nephrolithiasis occur in Fanconi disease, Hartnup syndrome, Wilson’s condition, and familial hypouricemic hyperuricosuria. The recognition of the uric acid carrier URAT1 was a major breakthrough in comprehension of urate management by the nephron. URAT1 carrier is abnormal in familial hypouricemic hyperuricosuria. Presently, various loss-of function alterations have been recognized in this DNA within this group [59].
Enzymatic defects causing congenital hyperuricemia
Enzymatic defects such as hypoxanthine guanine phosphoribosyl transferase (HGPRT) deficiency, type 1 collagen storage disease and other congenital errors of metabolism are accompanied with hyperuricemia and can predispose to uric acid calculi. Failure to save purines from cell break down due to HGPRT deficiency results in severe hyperuricemia. Lesch-Nyhan syndrome is the most severe form. It is X-linked recessive and is characterized by mental retardation, gout, uric acid nephrolithiasis, and self-mutilation [60]. HGPRT deficiency leads to failure to save purines from cell break down, resulting to clear hyperuricemia.
Type 1 collagen storage syndrome (von Gierke syndrome), is an autosomal recessive imperfection in glucose-6-phosphatase and impacted patients have been reported to have hypoglycemia, hyperlactacidemia, and hyperuricemia [61]. Phosphoribosyl pyrophosphate (PRPP) synthetase over activity is another X-linked disorder associated with uric acid lithiasis. PRPP synthetase is responsible for the formation of PRPP from ribose-5-phosphate and adenosine triphosphate. Increased PRPP synthetase activity results in hyperuricemia and hyperuricosuria [62] (Table 1).
Table 1.
Causative factors for uric acid stone formation.
| Low urinary volume | Low urinary pH | Hyperuricosuria | |
|---|---|---|---|
| Idiopathic or gouty diathesis | X | ||
| X | |||
| Obesity | X | ||
| Insulin resistance | X | X | |
| Animal protein in diet | X | X | X |
| Primary gout | X | X | |
| Chronic diarrhea | X | ||
| Dehydration | X | ||
| Lesch-Nyhan syndrome | X | ||
| Von Gierke disease | |||
| Disorders of high cell | |||
| Turnover | |||
| Neoplasias | |||
| Sickle cell disease | |||
| Hemolytic anemias | X | ||
| Polycythemia vera | |||
| Psoriasis | |||
| Renal hyperuricosuria | |||
| Familial hyperuricosuria | |||
| Fanconi syndrome | |||
| Hartnup disease | |||
| Wilson’s disease |
Recommendations in diagnosis of urolithiasis
Classification of urinary calculi
Urinary calculi may be categorized according to X-ray characteristics, size, location, aetiology of formation and composition [63], [64].
X-ray characteristics
Calculi can be classified according to their appearance in plain X-ray [kidney-ureter-bladder (KUB) radiography], according to their radio-opacity that differs according to mineral composition. Non-contrast-enhanced computed tomography of the urinary tract (NCCT-UT) is the radiologic study of choice to classify calculi according to density, inner structure and composition and consequently treatment decisions [65]. The density is measured in Hounsefield (HF) units.
Calculus size
For management purposes, calculi are classified into those measuring up to 5, 5–10, 10–20, and >20 mm in largest diameter. Measured in one or two dimensions.
Calculus location
Calculi can be classified according to their anatomical position into: renal pelvis; upper, middle or lower calyx; upper, middle or distal ureter; and urinary bladder.
Calculi classified by aetiology
Non-infection calculi
Calcium oxalate, calcium phosphate and uric acid.
Infection calculi
Magnesium ammonium phosphate, carbonate apatite and ammonium urate.
Genetic causes
Cystine, xanthine and 2,8-dihydroxyadenine.
Drug calculi (adverse reaction).
Diagnostic evaluation
Clinical evaluation should include a full history and physical examination. Patients usually present with loin pain either colicky or dull aching, nausea, vomiting, but may also be asymptomatic [66]. In the presence of infection, the patient my present with fever, rigors and malaise.
Imaging
Emergency measures and pain relief should start up and not delayed until imaging assessments. Immediate imaging is indicated in cases of fever, single kidney, and when diagnosis is uncertain.
Ultrasound (US)
US is readily available, bedside, safe (no risk of radiation), reproducible and inexpensive and is usually the primary diagnostic imaging tool. It can identify calculi located in the calices, pelvis, and pyeloureteric and vesicoureteric junctions. US also reveals upper urinary tract dilatation, renal parenchymal thickness, echogenic pattern and any abnormality in size, shape or position. US is sensitive in 45% and specific in 88% of cases of renal calculi and sensitive in 45% and specific in 94% of cases of ureteric calculi [67].
Non-contrast enhanced computed tomography of the urinary tract (NCCT-UT)
NCCT-UT is currently the standard for diagnosis of patients with acute urolithiasis. It is significantly more accurate and has supersede intravenous urography (IVU). NCCT-UT is capable of precisely revealing the calculus diameter and density. It may also reveal any associated abnormality and the cause of abdominal pain when calculi are absent [68], [69].
NCCT-UT can detect any type of calculi including uric acid and xanthine calculi, which are radiolucent on plain films [70]. NCCT-UT is useful in planning and outcome of future management of calculi especially if extracorporeal shock wave lithotripsy (ESWL) is used; since it can determine calculus density, inner structure of the calculus and surface-to-calculus distance. The drawbacks of NCCT-UT include higher radiation dose, loss of uptake and excretory function of the kidney and the anatomic configuration of urinary collecting system anatomy.
Low-dose CT is effective and reduces radiation risk. In patients with body mass index (BMI) <30 Low-dose, CT is reported to have a sensitivity of 86% for detecting ureteric calculi <3 mm and 100% for calculi >3 mm. Low-dose CT detected urolithiasis with a sensitivity of 96.6% (95% CI: 95.0–97.8) and specificity of 94.9% (95% CI: 92.0–97.0) in a meta-analysis of prospective studies [62]. Since NCCT-UT is superior to IVU, it should follow initial US assessment to confirm calculus diagnosis in patients with acute flank pain.
If endoscopic or surgical intervention is planned, a contrast study including IVU is usually requested to assess the anatomic configuration of the renal collecting system. Enhanced CT enables measurement of calculus density, surface-to-calculus distance and 3D reconstruction of the collecting system, and hence, it is preferable in complex cases.
KUB radiography
The sensitivity and specificity of KUB radiography is 44–77% and 80–87%, in detecting ureteric and renal stones respectively. If the calculus density is measured precisely by NCCT-UT, KUB radiography is unnecessary [68]. However, it is helpful in differentiating radiopaque from radiolucent stones.
Metabolism-related-diagnosis
All emergency patient with calcular disease whether high- or low-risk should undergo a metabolic work-up of urine and blood with imaging.
Urine
Dipstick test of spot urine sample.
RBCs, WBCs, nitrite, approximate urine pH and urine microscopy and/or culture.
Blood
Serum blood sample for creatinine, uric acid, calcium (ionised), C-reactive protein; INR = international normalised ratio and PTT = partial thromboplastin time.
Examination of sodium, potassium, CRP, and blood coagulation time can be omitted if no intervention is planned. Calculus-specific metabolic evaluation is indicated in patients at high-risk for calculus recurrence. The potential metabolic disorders can be identified by knowing mineral.
Analysis of calculus composition
All first-time calculus formers should undergo calculus analysis. In clinical practice, repeat Indications of repeated calculus analysis are recurrence under medical prophylaxis; early recurrence after EESWL, endoscopic and/or surgical complete calculus removal; and late recurrence after a prolonged calculus-free period.
Infrared spectroscopy (IRS) or X-ray diffraction (XRD) are the commonly used analytical procedures [64]. Polarisation microscopy is used in special centers. Chemical analysis (wet chemistry) is no more used [71].
Diagnosis of uric acid calculi
The clinical presentation of patients with uric acid calculi resemble those with other calculi of different composition and may include: flank and abdominal pain, loin or costovertebral tenderness, nausea, vomiting, change in appetite, lower urinary tract symptoms, hematuria (red blood cells 10 high-power fields), and referred pain to the genetalia. These symptoms and signs have a sensitivity of about 80% and specificity of 99% for identifying urolithiasis [1], [72].
A detailed medical, drug, and family history should be recorded focusing on conditions that predispose to uric acid calculus formation, for example situations of high cell turnover, such as myeloproliferative disorders, malignancy, congenital anomalies associated with hyperuricosuria and gastrointestinal problems, especially malabsorption and diarrhea and insulin resistance [73].
Laboratory investigations should include urine analysis. Constantly low urinary pH that lower than 5.5 should raise the suspicion of uric acid calculi and radiographic investigations should be done. Thus, urinalysis is crucial in the diagnosis. Renal functions, electrolytes, and uric acid should follow.
The radiologic investigation of choice in assessment of a suspected urinary calculus is noncontract-enhanced computed tomography. It is of special importance in the detection of uric acid calculi since they are normally radiolucent on plain radiographs. It is accurate with a 96% sensitivity, 99% specificity, 97% negative predictive value, and 98% positive predictive value in the diagnosis of calculi [71], [74], [75], [76].
The attenuation values of uric acid calculi is less than 400 Hounsfield units [77]. The differential diagnosis include those calculi composed of matrix, 2-8-dihydroxyade-nine, ammonium urate, xanthine and hypoxanthine, and calculi composed of certain drugs or their metabolites.
Both ultrasonography together with a radiolucent calculus in the plain urinary tract film confirm the diagnosis of uric acid calculus. Ultrasonography is particularly important in monitoring and follow up of patients under treatment (chemolysis). Calculus analysis should be performed once the calculus is extracted to confirm the diagnosis.
Forms of uric acid calculi
Anhydrous (the most common); dehydrate; monosodium urate and ammonium acid (Fig. 2).
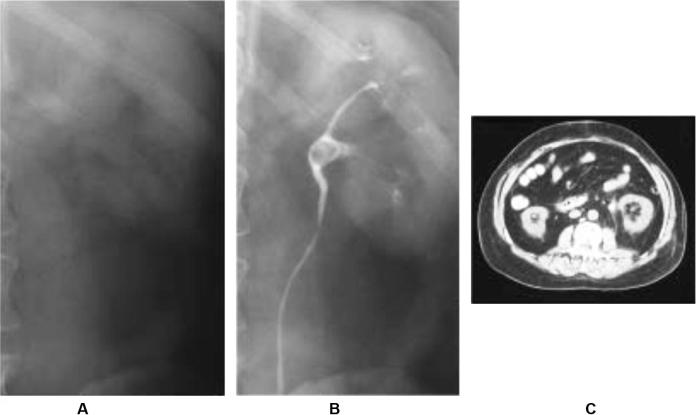
Fig. 2.
Radiographic imaging of uric acid stone. (A) KUB demonstrating lack of radiopaque stone. (B) IVP showing filling defect in left renal pelvis. (C) CT scan with corresponding stone demonstrated [79].
Treatment recommendations of patients with renal calculi
Treatment alternatives for renal calculi relay on multiple factors including calculus chemistry, size, location, symptoms, presence of backpressure changes and infection.
Acute episode (Renal colic)
Analgesia
Patient with acute calculus episode should be given an analgesic after exclusion of acute appendicitis and/or acute surgical abdomen. Non-steroidal anti-inflammatory drugs (NSAIDs) are superior to opioids since they have an anti-prostaglandin effect and are usually used alone as a single analgesic without requiring further analgesia in the short-term. On the contrary, opioids, particularly pethidine carries a higher risk of vomiting compared, and may require further analgesia [78].
Prevention of acute calculus episode
Analgesics
Except in patients with impaired renal function, patients with ureteral calculi that are likely to pass spontaneously, NSAID reduce inflammation and the risk of recurrent pain. In a double-blind, placebo-controlled trial, recurrent pain episodes of calculus colic were significantly fewer in patients treated with NSAIDs (as compared to no NSAIDs) during the first 7 days of treatment [79].
Alpha-blockers
Daily α-blockers may decrease recurrent attacks of pain and may relax the smooth fibers of the ureter to facilitate passage of the calculus.
Drainage/stone removal
Indications of stenting or percutaneous nephrostomy drainage, or calculus removal include symptomatic or complicated ureteral calculi as first-line treatment or if analgesia cannot be achieved medically.
Management of infected hydronephrosis
Infected hydronephrosis is a urological emergency. Prompt drainage must be performed to prevent additional complications.
Drainage
Urgent drainage of an obstructed kidney is achieved through either endoscopic insertion of a draining ureteral catheter/stent or percutaneous nephrostomy tube (PCN).
No statistically significant variation was reported in the efficacy or complications rate of nephrostomy and retrograde stenting for primary treatment of infected obstructed kidney in most studies. Definitive calculus removal must be postponed until the infection subside after a full course of antibiotics [80] (Fig. 3).
Fig. 3.
Urology system – X-ray and ultrasound stone localization for radiolucent (uric acid calculi).
Extracorporeal shock wave lithotripsy (ESWL)
Variants of success and outcome
Patient habitus, calculi size, location (ureteral, pelvic or calyceal) and composition (hardness), operator of lithotripter and efficacy of the lithotripter.
Contraindications
Contraindications of ESWL include bleeding diatheses, anticoagulants should stop at least 1 day and 2 days after treatment, untreated infection; severe skeletal malformations and severe obesity, which interfere with localization and focusing of the calculus; ipsilateral abdominal arterial aneurysm; anatomical obstruction distal to the calculus; and gestation (potential hazards on the fetus).
Placement of stents
Internal stents are not used routinely before ESWL. Ureteral stent decreases the risk of repeated colicky pains and backpressure but does not improve calculus free rate (SFR), reduce formation of impacted stone fragments (steinstrasse) or infective complications [81].
Rate of shock waves
The tissue damage is directly proportionate to the rate of shock waves. SFR is improved by reducing shock wave rate.
The ESWL technique
The model of lithotripter and shock wave intensity determine the total number of shock waves per session. The total number of shock waves is not agreed upon in different studies.
Starting on a lower energy setting with stepwise power (ESWL sequence) achieve vasoconstriction and prevent renal injury. Animal studies and a prospective randomized study reported better SFRs (96% vs. 72%) using stepwise power ramping, but no difference has been found for fragmentation or evidence of complications after ESWL, irrespective of whether ramping was used [82], [83]. The optimal shock wave frequency is 1.0–1.5 Hz. There are no conclusive data on the intervals required between repeated ESWL sessions. However, sessions can be repeated after 24 h for ureteral calculi.
Pain control during the session is necessary to reduce movements. The procedure is monitored with fluoroscopic and/or ultrasonography. Umbrella of antibiotic coverage should be commenced in case of infected calculi or bacteriuria.
Factors impairing success of ESWL
Hard calculi including brushite, calcium oxalate monohydrate and cystine (shockwave-resistant), long calyx, narrow infundibulum and steep infundibular-pelvic angle.
Endourology techniques in the management of renal calculi
Percutaneous nephrolithotomy (PCNL)
Wide ranges of rigid, semi-rigid and flexible urologic endoscopes are available for PCNL. PCNL is currently the gold standard procedure for large renal calculi. Variety of based on the surgeon’s own preference. The diameter of the standard access tracts are 24–30 F.
Many studies reported the use of mini pediatric access sheaths (18 French) in adults, and compared its efficacy to standard PCNL. The studies reported almost the same success rate, less bleeding complications but longer OR times [84].
Contraindications
Bleeding tendencies, unresolved UTI; renal tumor in the supposed access tract; potential malignant renal tumor and pregnancy.
Abnormal coagulation profile must be corrected before PCNL and patients must be observed carefully pre- and postoperatively.
Intracorporeal calculus disintegration (lithotripsy)
Methods are ultrasonic, electrohydraulic lithotripsy (EHL), pneumatic and Laser (Ho: YAG laser).
Laser lithotripsy is the gold standard in ureteroscopy, miniature PCNL and flexible endoscopes. Studies comparing different systems of lithotripsy reported that with Laser lithotripsy, the calculus migration rate is significantly less as compared with pneumatic lithotripsy and electrohydraulic lithotripsy (EHL). EHL is highly effective, but may cause collateral damage [85].
Preoperative imaging
Contrast studies including CT or IVP before PCNL facilitate the planning of the access, diagnose any calyceal and/or pelvic abnormalities and reveal the surrounding structures and organs that may interposition within the proposed percutaneous access such as pleura, lung, liver, and colon.
Urinary tract infection (UTI)
Following urgent drainage, urine samples should be obtained from the obstructed and infected urinary system and sent for culture and sensitivity. The new culture may differ from the preoperative urine culture due to the presence of obstruction. Prophylactic and maintenance antibiotic therapy should be given and the plan of treatment should be reconsidered accordingly. In spite of all measures septicemia may develop especially in immunocompromised patients and an intensive care bed should be available [86].
Technical considerations
Positioning of the patient:
| Prone | Supine |
| More access choices | Less access choices |
| Easier upper calyx or multiple punctures | Difficult upper calyx or multiple punctures |
| Higher calculus-free rate | Lower calculus-free rate |
| Longer OR time | Shorter OR time |
| Equally safe | Equally safe |
| Special X-ray devices and an operating table |
Puncture
The incidence of bowel and organ injury during PCNL puncture may be lowered by the use of intraoperative US.
Dilatation
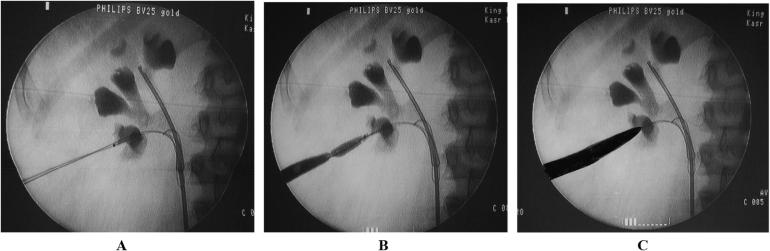
Depending on surgeon preference and experience, dilatation of the percutaneous access tract can be performed with metal (Alcan’s) telescopic dilators, Teflon serial dilators, or renal-balloon dilatators [87] (Fig. 4A–C).
Fig. 4.
(A–C) Fluoroscopy image showing steps of dilatation & disappearance of waist during dilatation using balloon dilatation set.
Nephrostomy and stents
Reports on tubeless PCNL (without postoperative nephrostomy tubes) are increasing now a day. The decision of whether to place or omit a nephrostomy tube following stone removal by PCNL procedure is determined by several parameters, including suspicion of residual calculi; possibility of a second procedure; significant bleeding; mucosal injury or perforation; ureteral obstruction; infected calculi and possibility of persistent infection; single kidney; bleeding diathesis and premeditated chemolysis.
Small-caliber nephrostomies have the advantages of less postoperative pain. When both a nephrostomy tube and a ureteral stent are omitted, the procedure is known as totally tubeless PCNL [88]. In uncomplicated cases, tubeless or totally tubeless PCNL procedures provide a safe alternative with a shorter hospital stay.
Ureterorenoscopy for renal calculi
Endoscopic mini-techniques, advances in deflection techniques, improved lenses, and high technology instruments are all technical improvements that revolutionized the use of URS and retrograde intrarenal surgery for both, ureteral and renal calculi. Although digital scopes have better image quality and shorter operation times, yet, they are less durable and costly.
Intracorporeal lithotripsy should be used for calculi that cannot be removed directly. Inaccessible calculi in lower renal calyx may be displaced into a more accessible calyx for disintegration [89].
Laparoscopic and open surgical nephrolithotomy
The indications for open or laparoscopic calculus surgery have significantly declined with the improvements in ESWL and endourological techniques. Moreover, combined (sandwich) techniques such as PCNL-ESWL-PCNL and combined PCNL-RIRS are efficient alternatives.
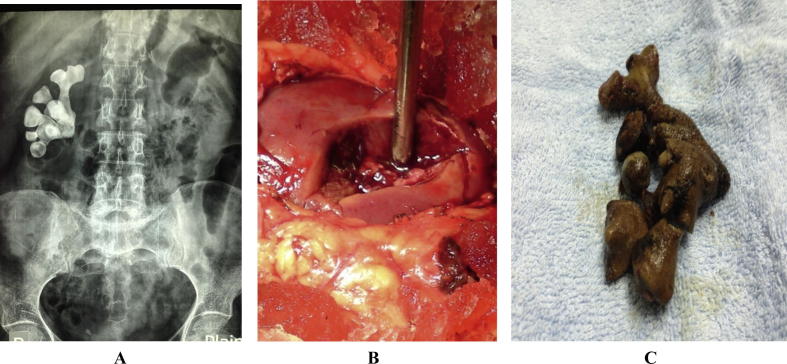
Open or laparoscopic surgery is indicated in cases of associated deformities that have to be repaired, an unreasonable number of punctures are needed, or failed endourologic approaches [90] (Fig. 5A–C).
Fig. 5.
(A–C) An X-ray showing a branching (stag-horn) stone, extracted with open surgery by anatrophic nephrolithotomy where the renal pedicle is clamped and kidney is cooled.
Indications for active intervention in renal calculi
Increased calculus size; calculi in patients with high risk for calculus development; unequivocal obstruction; sepsis; persistent symptoms; renal calculi >1.5 cm, calculi <1.5 cm if follow is not the best choice; patient preference; comorbidity and social situation of the patient (e.g. profession or travelling).
Specific calculus management in renal calculi
There is a controversy whether caliceal calculi should be treated however; there is consensus that the indications of treatment are calculus growth, development of obstruction, presence of infection, and acute or persistent pain [91].
Another controversy is the follow-up timing, duration, and choice of intervention in small, non-obstructing asymptomatic calculi that have unclear natural history and risk of progression. Available options are observation, chemolysis or active calculus removal.
Treatment alternatives
Conservative (watchful waiting)
Depending on their natural history, renal calyceal calculi may be observed.
Pharmacological treatment
Chemolysis through percutaneous irrigation
Percutaneous irrigation chemolysis for uric acid calculi is seldom used. Hemiacidrin 10%; pH 3.5–4 (Suby’s G solution) may be used to dissolute struvite calculi [92].
Oral chemolysis
The primary treatment of uric acid calculi except those formed of sodium or ammonium urate is dissolution by oral chemolysis. Even if renal backpressure is present, oral chemolysis is still an option after preliminary decompression. The calculus composition is confirmed by calculus analysis, urinary pH measurement and X-ray characteristics.
Oral alkaline citrate or sodium bicarbonate are used for chemolysis through alkalinisation of urine [93]. Although efficiency of chemolysis is directly proportionate to higher pH, the pH should be adjusted in the range of 7.0–7.2 to prevent formation of calcium phosphate calculus.
Ultrasound and less frequently repeat NCCT-UT are used to monitor and follow up radiolucent calculi therapy. A combination of alkalinisation with alpha-blocker may be used in cases of uric acid calculi in distal ureter with a high SFRs [94].
Selection of interventional procedures for renal calculi
Asymptomatic caliceal calculi
Whether yearly follow-up is enough for asymptomatic caliceal calculi that have remained stable for 6 months or it has to be treated, is still debatable.
The follow-up consists of periodic evaluation after 6 months and yearly of clinically and radiologically.
Calculi in renal pelvis or upper/middle calices
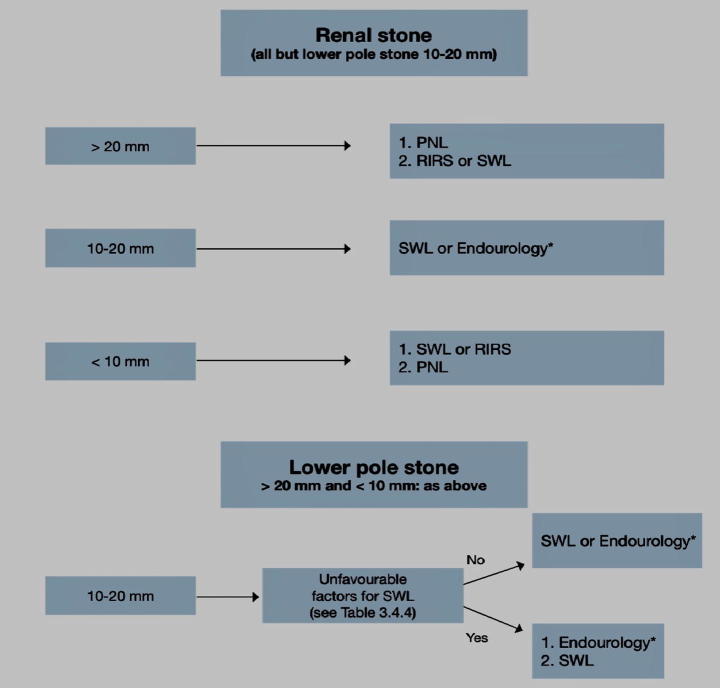
The commonly used treatments for renal calculi are ESWL, PCNL and RIRS. While the calculus size hardly affect the results of PCNL, it lowers the SFRs after ESWL or URS. There is general agreement that ESWL can be used for calculi less than 2 cm, except for those in the lower calyx, with a good outcome and satisfactory SFRs. Another option is endourology that is preferred by some urologists because it avoids the morbidity of multiple sessions and accordingly a shorter time to calculus clearance.
PCNL is the primary treatment of choice for calculi >2 cm. ESWL has the risk of ureteral obstruction with the fragmented calculus that may necessitate further procedures and usually requires repeated sessions. RIR may be a first-line of treatment when PCNL is not an option or contraindicated. Due to the low SFR and high rate of staging, RIR is not advised as a primary treatment for calculi >2 cm in uncomplicated cases [88], [95].
Calculi in the lower renal pole
Although the disintegration efficacy of ESWL is the same in different intrarenal locations, yet the SFR is lower for calculi in the lower renal calyx because the fragmented calculus usually settle in the calyx and predispose to recurrent calculus formation. The success rate of ESWL for lower calyceal calculi is 20–85% and accordingly, the preference of endoscopic maneuvers is still under study. PCNL and RIRS are advised for calculi >15 mm. and for smaller calculi if there are factors that render success of ESWL unlikely. Although RIRS are more invasive, their results are comparable and even with a higher success rate than ESWL in calculi up to 3 cm. However, staging of the procedure is usually required.
In complex calculus cases, open or laparoscopic approaches are possible alternatives.
Recommendations:
-
•
For calculi <20 mm within the renal pelvis and upper or middle calices, ESWL, PCNL and RIRS are treatment options.
-
•
Larger calculi >20 mm, PCNL should be the primary treatment.
-
•
Larger calculi (>2 mm) may be treated with flexible RIRS if PCNL is not an option, knowing that in this case, there is a higher risk for staging and leaving a ureteral stent may be necessary.
-
•
For the lower calyx calculi even >15 mm, endoscopic procedures are recommended because the success rate of ESWL is not encouraging (depending on affecting factors) (Fig. 6).
Fig. 6.
Treatment algorithm for renal calculi [67].
Specific calculus management
Ureteral calculi
Treatment types
Expectant treatment
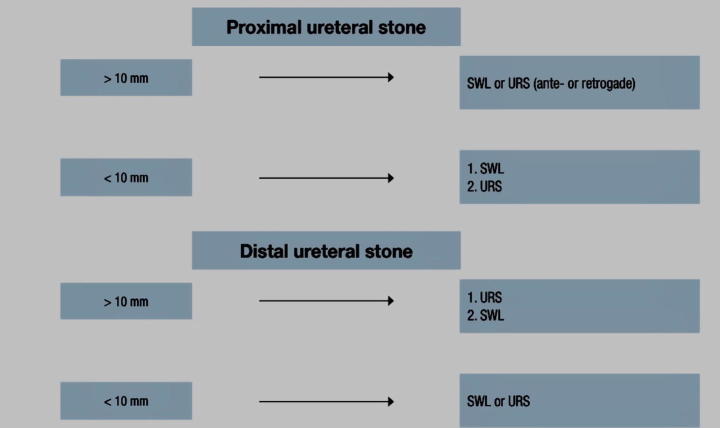
Since more than 90% of calculi less than 5 mm are expelled spontaneously, patients with newly diagnosed small ureteral calculi can be treated conservatively with medical therapy and regular follow up. Intervention is indicated if complicated patients with unresolved pain, infection, or deterioration of renal function.
The cut-off size for calculi that are likely to pass spontaneously is estimated to be <6 mm but the exact cut-off size is debatable. Spontaneous passage is inversely proportionate with increasing calculus size and is different among individual patients.
Pharmacological treatment
Medical expulsive therapy (MET)
Patients with ureteral calculi treated with α-blockers or nifedipine are more likely to pass calculi with fewer colic attacks than those receiving placebo as reported by meta-analyses. [96], [97] MET decreases pain and hasten spontaneous expulsion of ureteral calculi and calculi fragments following ESWL.
Medical agents
Alpha-1-adrenergic receptor antagonists including tamsulosin (the commonly used), terazosin, doxazosin, alfuzosin, naftopidil, and silodosin have been studied and are equally effective. Alpha-1 receptors are located in the human ureter, especially the distal ureter. Alpha-blockers have been reported to increase expulsion rates of distal ureteral stones, decrease time to expulsion, and decrease need for analgesia during stone passage. Alpha- blockers promote stone passage in patients receiving shock wave lithotripsy, and may be able to relieve ureteral stent–related symptoms. Nifedipine is the most studied calcium channel blocker used to treat ureteral spasm and promotes stone passage.
Combined administration of tamsulosin and nifedipine is safe and effective in patients with distal ureteral calculi with renal colic.
The possible drug side effects include postural hypotension and anejaculation. Renal functions should be normal and infection should be excluded. Patients should be given Proper analgesics are prescribed when needed. Close follow up is important to monitor calculus position and hydronephrosis [98].
Endourology techniques
Ureteroscopy (URS)
The current standard are thin semi-rigid ureteroscopes with a tip diameter of <7 F and flexible digital ureteroscopes that can be used for the whole ureter.
The procedure is commenced with ureteral dilatation with balloon or Teflon dilators under fluoroscopic guidance. If dilatation is difficult, a double J stent placement followed by ureteroscopy after 1 or 2 weeks.
The aim of URS should be complete calculus removal. Disintegration only or “Dust and go” procedure should be only used with large (renal) calculi. Endoscopic forceps or baskets are used to extract calculi [99].
Ultrasound, pneumatic and electrohydraulic intracorporeal lithotripters with success in rigid URS but the risk of calculus migration into the kidney was always there. However, special antimigration tools placed proximal of the calculus solved the problem. Currently, the gold standard intracorporeal lithotripter for varies endoscopies including flexible is the holmium: yttrium-aluminium-garnet (Ho: YAG) laser, the most effective lithotripsy for all calculus types.
Stenting
Pre-stenting (before URS)
Advantages include ureteroscopic manipulation of calculi are easier, higher SFR and less complications. It is not recommended for routine use.
Post-stenting (after URS)
Post stenting is not as a routine after uncomplicated URS and complete calculus removal and is necessary for patients with high likelihood of complications (e.g., mucosal injury, incomplete removal of calculi, extravasation, or infection). It carries a higher postoperative morbidity. Single-day ureteral catheter may be sufficient in uncomplicated cases.
The preferred time of stenting after URS range is 1–3 weeks.
Alpha-blockers increase tolerability and reduce the morbidity of ureteral stents [99], [100] (Fig. 7A,B).
Fig. 7.
(A and B) Ureteroscopy endoscopic view showing stone LASER intracorporeal lithotripsy and extraction with a Dormia basket.
Percutaneous antegrade ureteroscopy
Percutaneous antegrade URS is an alternative when the distal ureter cannot be manipulated or in patients with impacted large upper ureteral calculi in a dilated renal collecting system.
Laparoscopic/Open ureterolithotomy
Although these more invasive procedures results in high SFRs, yet, in the era of minimally invasive surgery; these procedures are usually kept for special cases and is advised for large impacted ureteric calculi when endoscopicy or ESWL are likely to fail or has failed.
Shock wave lithotripsy (Discussed before)
Indications for active intervention for ureteral calculi are:
Unresolved obstruction, renal impairment, bilateral obstruction, solitary kidney, calculi unlikely to pass spontaneous and persistent pain not responding to analgesics (Fig. 8).
Fig. 8.
Recommended treatment options (if indicated for active stone removal) [67].
Specific treatment of uric acid urinary calculi
Medical dissolution therapy is successful in the majority of patients with uric acid nephrolithiasis (70–80%) and is the primary treatment in these cases [101], [102]. The exclusions are patients with significant backpressure, renal impairment, sepsis, unresolved pain or intolerant to the prescribed medications.
Medical therapy should be directed to the 3-biochemical anomalies predisposing to calculus formation: low urinary pH, increases urinary uric acid, and low urinary output.
Acidic urinary pH
Alkalization of urine is the primary management for dissolving uric acid calculi. The pH should be kept in the range of 6.5–7.0. Below this level calcium phosphate calculi may develop. The dose may be modified according to clinical reaction and the urinary pH levels [103].
Oral potassium citrate is usually administered with an adult dosage of 15–30 mEq twice daily. Since the solubility of monopotassium urate is higher than monosodium urate, potassium salts are a better choice than sodium salts. Potassium salts refrain the reduction in citrate excretion and increase in calcium excretion accompanying with sodium loading. Nevertheless, potassium citrate therapy is contraindicated in patients with renal insufficiency or high serum potassium levels. Sodium citrate or sodium bicarbonate may substitute potassium citrate if gastrointestinal side effects of potassium citrate are intolerable [104], [105].
The normal adult prescription of sodium bicarbonate is normally 650–1000 mg thrice or four times a day. Commercial baking soda can be given to adults in a dose of 1–2 teaspoons to be taken either 3 or 4 times a day. Because acetazolamide minimizes citrate secretion and stimulates calcium secretion especially with diet rich in citrates may increase urinary pH; it is not recommended for regular use as it [106], [107].
The success of oral therapy and advances in mini endoscopic procedures abolished the use of local irrigation chemolysis [108].
Hyperuricosuria
Twenty-four hours urine analyses have to be carried out when hyperuricosuria is accused. Once hyperuricosuric state is established, the fundamental aetiology of this anomaly must be figured out. Since the most common source is purine consumption, patients need to be advised decrease their intake of foods rich in purine. Among others, these foods comprises of red meat, beer, poultry, and even fish. Indeed, it can be pointed that apart from that these foods results in transitory metabolic acidosis that minimizes the pH of urine; these increase the uric acid burden to the kidneys [108], [109].
It was recently reported that in subjects that consumed a balanced diet of vegetables as well as reasonable quantities of animal protein and purines; both the pH of urine and uric acid concentration were ominously decreased when contrasted with subjects using a normal Western diet. To monitor patient compliance with the balanced diet regulations, urinary urea and sulfate indexed to creatinine are used [108], [109], [110].
Allopurinol is competitive inhibitor of xanthine oxidase enzyme that catalyzes the conversion of hypoxanthine into xanthine and xanthine into urate. Allopurinol and its active metabolite oxypurinol act as purine analogues and reduce de novo purine synthesis by enhancing salvage by HGPRT.
Indications of allopurinol administration
Patients in whom dietary control alone fails, patients in whom a rapid a decrease in uric acid load is needed (symptomatic hyperuricemia), gout, hyperuricosuric calcium urolithiasis and urate nephropathy.
The usual dose in adults is 100–300 mg/d and should be adjusted to estimated creatinine clearance in the patient with renal insufficiency [111].
Reported side effects include gastrointestinal upset, precipitating acute gout attacks, Stevens-Johnson syndrome, and the allopurinol hypersensitivity syndrome (fever, rash, hepatitis, eosinophilia, and acute renal failure). Patients with myeloproliferative disorders, tumor lysis, and HGPRT deficiency may develop xanthinuria and the formation of xanthine calculi.
Recombinant PEGylated uricase can be used in the treatment of tumor lysis when allopurinol is ineffective or contraindicated [112], [113].
Low urinary output
To avoid increased levels of uric acid, it is vital to sustain a urinary output of at least 2–3 L per day with adequate fluid intake. Periods of high calculus-forming potential such as after meals, during physical activity, or during sleep should be compensated by fluid intake. Fluid therapy may be monitored by Dipstick testing for specific gravity.
ESWL, endoscopic and surgical measures
The indications of uric acid calculus-removing procedure whether endoscopic, lithotripsy or surgical are the same as with other stones and include persistent urinary obstruction, unresolved infection, continuous pain or failure of oral chemolysis. It is reported that uric acid calculi are responsive to lithotripsy and that the calculus free rate three months after shockwave lithotripsy and post ESWL chemolysis were achieved in 88.5 percent of the patients. Since uric acid calculi are radiolucent, ultrasound-based lithotripters are usually used to allow localization of the calculus. The success rate is higher in calculi less than 2 cm, situated in the renal pelvis if proper localization is achieved.
If X-ray-based lithotripter is used, intravenous contrast material or installation of contrast material through a nephrostomy tube or ureteral catheter can be administered to guide shock wave localization.
Ureteroscopy and percutaneous nephrolithotomy are effective alternatives for calculus removal. Calculus volume and location, presence of obstruction and/or infection and patient factors including BMI and preference affects the choice of treatment. Uric acid calculi can be efficiently disintegrated with all types of intracorporeal lithotripsy including Holmium yttrium-aluminium-garnet (Holmium: YAG) laser, elecrohydrolic, pneumatic and ultrasound [113].
Follow up of patients
Patients with uric acid calculi under dissolution with oral chemolysis should be followed up for calculus reduction and development of obstruction by ultrasonography and repeated NCCT-UT. Ultrasonography is preferred for long-term follow-up because it is free of ionizing radiation, quite sensitive for calculus detection and is highly sensitive in the diagnosis of hydronephrosis.
Moreover, renal functions and serum electrolytes, blood urea nitrogen, and creatinine needs to be examined with pH chemolysis. When allopurinol is prescribed, liver enzymes should be checked. There is no need for maintenance therapy after successful calculus dissolution or removal unless the underlying cause for uric acid calculus formation have been eliminated.
Conclusions and future perspectives
Uric acid stones represent 5–10% of stone cases. Risk factors include chronic diarrhea and hyperuricosuria. Uric acid stones occur in patients with a very low urine pH (<5.0), which reduces uric acid solubility. Therapy should include alkalinisation of the urine pH to higher than 6.5 with potassium citrate. Uricosuric patients can benefit from allopurinol.
These radiolucent stones are of particular interest because of their ability to be successfully managed with both medical therapy and surgical intervention. The pathophysiology is unique and important to understand when treatment is being planned.
The advent of the urate transporter URAT1, the discovery of the ZNF365 gene associated with uric acid nephrolithiasis and the better understanding the mechanisms of the persistently low urinary pH revolutionized the treatment of uric acid nephrolithiasis. However, much remains to be uncovered. Defining the molecular defects that cause insufficient ammoniagenesis or ammonium secretion in these patients and a search for anatomic correlates within the kidney may add precious insights and more advanced treatment options for uric acid urolithiasis.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biography

Ashraf Abou-Elela, Professor of Urology, Faculty of Medicine, Cairo University. Head of Urology department and the renal transplantation team, As-Salam International Hospital, Maadi, Cairo, Egypt. Consultant of Urology and member of the renal transplantation team, Al-Salam hospital, Mohandseen and Cairo Kidney Centre. Member of the Egyptian, American, European Association of Urology and Endourological Society. He authored many articles and chapters in international journals and books covering varies fields of Urology. He is a reviewer of many international journals of Urology including Journal of Urology, Journal of Endourology, Current Urology, European Journal of Surgical Oncology and International Braz J Urol.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ngo Tin C., Assimos Dean G. Uric acid nephrolithiasis: recent progress and future directions. Rev Urol. 2007;9(1):17–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Moe O.W. Uric acid nephrolithiasis: proton titration of an essential molecule? Curr Opin Nephrol Hypertens. 2006;15:366–373. doi: 10.1097/01.mnh.0000232876.04975.33. [DOI] [PubMed] [Google Scholar]
- 3.Gianfrancesco F., Esposito T., Ombra M.N., Forabosco P., Maninchedda G., Fattorini M. Identification of a novel gene and a common variant associated with uric acid nephrolithiasis in a Sardinian genetic isolate. Am J Hum Genet. 2003;72:1479–1491. doi: 10.1086/375628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daudon M, Frochot V. Crystalluria. Clinical chemistry and laboratory medicine 2015;53(Suppl 2):s1479–87. [DOI] [PubMed]
- 5.Gul Z., Monga M. Medical and dietary therapy for kidney calculus prevention. Kor J Urol. 2014;55(12):775–779. doi: 10.4111/kju.2014.55.12.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heilberg I.P. Treatment of patients with uric acid calculi. Urolithiasis. 2016;44(1):57–63. doi: 10.1007/s00240-015-0843-8. Martillo MA, Nazzal L, Crittenden DB. The crystallization of monosodium urate. Curr Rheumatol Rep 2014;16(2):400. [DOI] [PubMed] [Google Scholar]
- 7.Monti E, Trinchieri A, Magri V, Cleves A, Perletti G. Herbal medicines for urinary calculus treatment. A systematic review. Arch Ital Urol Androl 2016;88(1):38–46. [DOI] [PubMed]
- 8.Penniston K.L., Nakada S.Y. Diet and alternative therapies in the management of calculus disease. Urol Clin North America. 2013;40(1):31–46. doi: 10.1016/j.ucl.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Siener R. Can the manipulation of urinary pH by beverages assist with the prevention of calculus recurrence? Urolithiasis 2016;44(1):51–6. [DOI] [PubMed]
- 10.Woodward O.M. ABCG2: the molecular mechanisms of urate secretion and gout. Am J Physiol Renal Physiol. 2015;309(6):F485–F488. doi: 10.1152/ajprenal.00242.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe O.W. Kidney calculi: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 12.Stamatelou K.K., Francis M.E., Jones C.A., Nyberg L.M., Curhan G.C. Time trends in reported prevalence of kidney calculi in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearle M.S., Calhoun E.A., Curhan G.C. Urologic diseases of America project: urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. Gutman AB, Yu TF. Uric acid nephrolithiasis. Am J Med 1968;45:756–79. [DOI] [PubMed] [Google Scholar]
- 14.Mandel N.S., Mandel G.S. Urinary tract calculus disease in the United States veteran population. I. Geographical frequency of occurrence. J Urol. 1989;142:1513–1515. doi: 10.1016/s0022-5347(17)39144-9. [DOI] [PubMed] [Google Scholar]
- 15.Mandel N.S., Mandel G.S. Urinary tract calculus disease in the United States veteran population. II. Geographical analysis of variations in composition. J Urol. 1989;142:1516–1521. doi: 10.1016/s0022-5347(17)39145-0. [DOI] [PubMed] [Google Scholar]
- 16.Gault M.H., Chafe L. Relationship of frequency, age, sex, calculus weight, and composition in 15,624 calculi: comparison of results for 1980 to 1983 and 1995 to 1998. J Urol. 2000;164:302–307. [PubMed] [Google Scholar]
- 17.Gentle D.L., Stoller M.L., Bruce J.E., Leslie S.W. Geriatric urolithiasis. J Urol. 1997;158:2221–2224. doi: 10.1016/s0022-5347(01)68203-x. [DOI] [PubMed] [Google Scholar]
- 18.Henneman P.H., Wallach S., Dempsey E.F. The metabolic defect responsible for uric acid calculus formation. J Clin Invest. 1962;3:537–542. doi: 10.1172/JCI104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portis A.J., Hermans K., Culhane-Pera K.A., Curhan G.C. Calculus disease in the Hmong of Minnesota: initial description of a high-risk population. J Endourol. 2004;18:853–857. doi: 10.1089/end.2004.18.853. [DOI] [PubMed] [Google Scholar]
- 20.Maloney M.E., Springhart W.P., Ekeruo W.O., Enemchukwu C.U., Preminger G.M. Ethnic background has minimal impact on the etiology of nephrolithiasis. J Urol. 2005;173:2001–2004. doi: 10.1097/01.ju.0000159076.70638.1e. [DOI] [PubMed] [Google Scholar]
- 21.Ansari M.S., Gupta N.P., Hemal A.K., Gupta N.P., Wadhwa S.N., Goel A. Spectrum of calculus composition: structural analysis of 1050 upper urinary tract calculi from northern India. Int J Urol. 2005;12:12–16. doi: 10.1111/j.1442-2042.2004.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Grenabo L., Hedelin H., Pettersson S. The severity of infection calculi compared to other calculi in the upper urinary tract. Scand J Urol Nephrol. 1985;19:285–289. doi: 10.3109/00365598509180271. [DOI] [PubMed] [Google Scholar]
- 23.Hossain R.Z., Ogawa Y., Hokama S., Morozumi M., Hatano T. Urolithiasis in Okinawa, Japan: a relatively high prevalence of uric acid calculi. Int J Urol. 2003;10:411–415. doi: 10.1046/j.1442-2042.2003.00656.x. [DOI] [PubMed] [Google Scholar]
- 24.Hesse A., Schneider H.J., Berg W., Hienzsch E. Uric acid dihydrate as urinary calculus component. Invest Urol. 1975;12:405–409. [PubMed] [Google Scholar]
- 25.Atsmon A., deVries A., Frank M. Elsevier; Amsterdam: 1963. Uric acid lithiasis. [Google Scholar]
- 26.Atan L, Andreoni C, Ortiz V, Silva EK, Pitta R, Atan F, Srougi M, et al. High kidney calculus risk in men working in steel industry at hot temperatures. Urology 2005;65:858–61. [DOI] [PubMed]
- 27.Evan A.P., Lingeman J.E., Coe F.L., Shao Y., Matlaga B.R., Kim S.C. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda M., Satta Y., Takenaka O., Takahata N. Loss of urate oxidase activity in hominoids and its evo-lutionary implications. Mol Biol Evol. 2002;19:640–653. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.C., Coe F.L., Tinmouth W.W., Kuo R.L., Paterson R.F., Parks J.H. Calculus formation is proportional to papillary surface coverage by Randall’s plaque. J Urol. 2005;173(117–119):38. doi: 10.1097/01.ju.0000147270.68481.ce. [DOI] [PubMed] [Google Scholar]
- 30.Evan A., Lingeman J., Coe F.L., Worcester E. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int. 2006;69(1313–1318):39. doi: 10.1038/sj.ki.5000238. [DOI] [PubMed] [Google Scholar]
- 31.Pak C.Y., Poindexter J.R., Peterson R.D., Heller H.J. Biochemical distinction between hyperuricosuric calcium urolithiasis and gouty diathesis. Urology. 2002;60:789–794. doi: 10.1016/s0090-4295(02)01908-8. [DOI] [PubMed] [Google Scholar]
- 32.Pak C.Y., Sakhaee K., Peterson R.D., Poindexter J.R., Frawley W.H. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60:757–761. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 33.Pak C.Y., Poindexter J.R., Adams-Huet B., Pearle M.S. Predictive value of kidney calculus composition in the detection of metabolic abnormalities. Am J Med. 2003;115(26–32):42. doi: 10.1016/s0002-9343(03)00201-8. [DOI] [PubMed] [Google Scholar]
- 34.Finlayson B., Smith A. Stability of first dissociable proton of uric acid. J Chem Eng Data. 1974;19:94–97. [Google Scholar]
- 35.Menon M., Resnick M.I. Urinary lithiasis: etiology, diagnosis, and medical management. In: Walsh P.C., editor. Campbell’s urology. 8th ed. Saunders; Philadelphia: 2002. pp. 3229–3305. [Google Scholar]
- 36.Kamel K.S., Cheema-Dhadli S., Halperin M.L. Studies on the pathophysiology of the low urine pH in patients with uric acid calculi. Kidney Int. 2002;61:988–994. doi: 10.1046/j.1523-1755.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakhaee K., Adams-Huet B., Moe O.W., Pak C.Y. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–979. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 38.Gutman A., Yue T.F. An abnormality of glutamine metabolism in primary gout. Am J Med. 1963;35:820–831. doi: 10.1016/0002-9343(63)90244-4. [DOI] [PubMed] [Google Scholar]
- 39.Pagliara A., Goodman A.D. Elevation of plasma glutamate in gout. N Engl J Med. 1969;281:767–770. doi: 10.1056/NEJM196910022811405. [DOI] [PubMed] [Google Scholar]
- 40.Pollak V., Mattenheimer H. Glutaminase activity in the kidney in gout. J Lab Clin Med. 1965;66(564–570):49. [PubMed] [Google Scholar]
- 41.Niv Y., Fraser G.M. The alkaline tide phenomenon. J Clin Gastroenterol. 2002;35(5–8):50. doi: 10.1097/00004836-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Moore-Ede M.C. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am J Physiol. 1986;250:R735–R738. doi: 10.1152/ajpregu.1986.250.5.R737. [DOI] [PubMed] [Google Scholar]
- 43.Maalouf N., Cameron M.A., Moe O.W., Sakhaee K. Novel insights into the pathogenesis of uric acid nephrolithiasis. Curr Opin Nephrol Hypertens. 2004;13:181–189. doi: 10.1097/00041552-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Vaziri N.D., Byrne C., Ryan G., Wilson A. Preservation of urinary postprandial alkaline tide despite inhibition of gastric acid secretion. Am J Gastroenterol. 1980;74(328–331):53. [PubMed] [Google Scholar]
- 45.Johnson C.D., Mole D.R., Pestridge A. Postprandial alkaline tide: does it exist? Digestion. 1995;56(100–106):54. doi: 10.1159/000201228. [DOI] [PubMed] [Google Scholar]
- 46.Bilobrov V.M., Chugaj A.V., Bessarabov V.I. Urine pH variation dynamics in healthy individuals and calculus formers. Urol Int. 1990;45:326–331. doi: 10.1159/000281730. [DOI] [PubMed] [Google Scholar]
- 47.Murayama T., Taguchi H. The role of the diurnal variation of urinary pH in determining calculus compositions. J Urol. 1993;150(1437–1439):56. doi: 10.1016/s0022-5347(17)35801-9. [DOI] [PubMed] [Google Scholar]
- 48.Murayama T., Sakai N., Yamada T., Takano T. Role of the diurnal variation of urinary pH and urinary calcium in urolithiasis: a study in outpatients. Int J Urol. 2001;8(525–531):57. [PubMed] [Google Scholar]
- 49.Khatchadourian J., Preminger G.M., Whitson P.A., Adams-Huet B., Pak C.Y. Clinical and biochemical presentation of gouty diathesis: comparison of uric acid versus pure calcium calculus formation. J Urol. 1995;154:1665–1669. doi: 10.1016/s0022-5347(01)66743-0. [DOI] [PubMed] [Google Scholar]
- 50.Zerwekh J.E., Holt K., Pak C.Y. Natural urinary macromolecular inhibitors: attenuation of inhibitory activity by urate salts. Kidney Int. 1983;23:838–841. doi: 10.1038/ki.1983.103. [DOI] [PubMed] [Google Scholar]
- 51.Robertson W.G. Renal calculi in the tropics. Semin Nephrol. 2003;23:77–87. doi: 10.1053/snep.2003.50007. [DOI] [PubMed] [Google Scholar]
- 52.El-Reshaid K., Mughal H., Kapoor M. Epidemiological profile, mineral metabolic pattern and crystallographic analysis of urolithiasis in Kuwait. Eur J Epidemiol. 1997;13:229–234. doi: 10.1023/a:1007346727944. [DOI] [PubMed] [Google Scholar]
- 53.Borghi L., Meschi T., Amato F., Briganti A., Novarini A., Giannini A. Hot occupation and nephrolithiasis. J Urol. 1993;150:1757–1760. doi: 10.1016/s0022-5347(17)35887-1. [DOI] [PubMed] [Google Scholar]
- 54.Grases F., Ramis M., Villacampa A.I., Costa-Bauza A. Uric acid urolithiasis and crystallization inhibitors. Urol Int. 1999;62:201–204. doi: 10.1159/000030395. [DOI] [PubMed] [Google Scholar]
- 55.Ombra M.N., Casula S., Biino G., Maestrale G., Cardia F., Melis P. Urinary glycosaminoglycans as risk factors for uric acid nephrolithiasis: case control study in a Sardinian genetic isolate. Urology. 2003;62:416–420. doi: 10.1016/s0090-4295(03)00473-4. [DOI] [PubMed] [Google Scholar]
- 56.Goldfarb D.S., Fischer M.E., Keich Y., Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int. 2005;67:1053–1061. doi: 10.1111/j.1523-1755.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 57.Shekarriz B., Stoller M.L. Uric acid nephrolithiasis: current concepts and controversies. J Urol. 2002;168:1307–1314. doi: 10.1016/S0022-5347(05)64439-4. [DOI] [PubMed] [Google Scholar]
- 58.Gelzayd E.A., Breuer R.I., Kirsner J.B. Nephrolithiasis in inflammatory bowel disease. Am J Dig Dis. 1968;13:1027–1034. doi: 10.1007/BF02233547. [DOI] [PubMed] [Google Scholar]
- 59.McLeod R.S., Churchill D.N. Urolithiasis complicating inflammatory bowel disease. J Urol. 1992;148:974–978. doi: 10.1016/s0022-5347(17)36794-0. [DOI] [PubMed] [Google Scholar]
- 60.Daudon M., Traxer O., Conort P., Lacour B., Jungers P. Type 2 diabetes increases the risk for uric acid calculi. J Am Soc Nephrol. 2006;17:20–26. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 61.Pak C.Y., Sakhaee K., Moe O., Pak C.Y. Biochemical profile of calculus-forming patients with diabetes mellitus. Urology. 2003;61:523–528. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 62.Maalouf N.M., Sakhaee K., Parks J.H., Coe F.L., Adams-Huet B., Pak C.Y. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 63.Wu D.S., Stoller M.L. Indinavir urolithiasis. Curr Opin Urol. 2000;10(6):557–561. doi: 10.1097/00042307-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 64.El-Nahas AR, El-Assmy AM, Mansour O, Sheir KZ. A prospective multivariate analysis of factors predicting calculus disintegration by extracorporeal shock wave lithotripsy: the value of high-resolution noncontrast computed tomography. Eur Urol 2007;51(6):1688–93 [discussion 93–4]. [DOI] [PubMed]
- 65.Patel T., Kozakowski K., Hruby G., Reisiger K., Venkatesh R., Yan Y. Skin to calculus distance is an independent predictor of calculus-free status following shockwave lithotripsy. J Endourol. 2009;23(9):1383–1385. doi: 10.1089/end.2009.0394. [DOI] [PubMed] [Google Scholar]
- 66.Zarse C.A., Hameed T.A., Jackson M.E., Pishchalnikov Y.A., Lingeman J.E. CT visible internal calculus structure, but not Hounsfield unit value, of calcium oxalate monohydrate (COM) calculi predicts lithotripsy fragility in vitro. Urol Res. 2007;35(4):201–206. doi: 10.1007/s00240-007-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jel, lison FC. Smith JC, Heldt JP, Spengler NM, Nicolay LI, Ruckle HC et al. Effect of low dose radiation computerized tomography protocols on distal ureteral calculus detection. J Urol 2009;182(6):2762–7. [DOI] [PubMed]
- 68.Poletti P.A., Platon A., Rutschmann O.T., Schmidlin F.R., Iselin C.E., Becker C.D. Low-dose versus standard-dose CT protocol in patients with clinically suspected renal colic. AJR Am J Roentgenol. 2007;188(4):927. doi: 10.2214/AJR.06.0793. [DOI] [PubMed] [Google Scholar]
- 69.Niemann T., Kollmann T., Bongartz G. Diagnostic performance of low-dose CT for the detection of urolithiasis: a meta-analysis. AJR Am J Roentgenol. 2008;191(2):396–401. doi: 10.2214/AJR.07.3414. [DOI] [PubMed] [Google Scholar]
- 70.Kluner C, Hein PA, Gralla O, Hein E, Hamm B, Romano V, et al. Does ultra-low-dose CT with a radiation dose equivalent to that of KUB suffice to detect renal and ureteral calculi? J Comput Assist Tomogr 2006;30(1):44–50. [DOI] [PubMed]
- 71.Hesse A., Geilenkeuser W.J., Kruse R. Quality control in urinary calculus analysis: results of 4 ring trials (1980–2001) Clin Chem Lab Med. 2005;43(3):298–303. doi: 10.1515/CCLM.2005.051. [DOI] [PubMed] [Google Scholar]
- 72.Sutor D.J., Scheidt S. Identification standards for human urinary calculus components, using crystallographic methods. Br J Urol. 1968;40(1):22–28. doi: 10.1111/j.1464-410x.1968.tb11808.x. [DOI] [PubMed] [Google Scholar]
- 73.Caoili E.M., Cohan R.H., Korobkin M., Francis I.R., Cohan R.H., Dunnick N.R. Urinary tract abnormalities: initial experience with multidetector row CT urography. Radiology. 2002;222(2):353–360. doi: 10.1148/radiol.2222010667. [DOI] [PubMed] [Google Scholar]
- 74.Türk C., Knoll T., Petrik A., Sarica K., Skolarikos A., Straub M. European Association of Urology; 2015. Guidelines on urolithiasis. [Google Scholar]
- 75.Pearle MS, Asplin JR, Coe FL, Rodgers A, Worcester EM (Committee 3). Medical management of urolithiasis. In: Denstedt J, Khoury S, editors, 2nd International consultation on Calculus Disease. Health Publications; 2008. p. 57–84, ISBN 0-9546956-7-4.
- 76.Mandel N., Mandel I., Fryjoff K., Rejniak T., Mandel G. Conversion of calcium oxalate to calcium phosphate with recurrent calculus episodes. J Urol. 2003;169(6):2026–2029. doi: 10.1097/01.ju.0000065592.55499.4e. [DOI] [PubMed] [Google Scholar]
- 77.Abdel-Halim R.E., Abdel-Halim M.R. A review of urinary calculus analysis techniques. Saudi Med J. 2006;27(10):1462–1467. [PubMed] [Google Scholar]
- 78.Swartz M.A., Lydon-Rochelle M.T., Simon D., Wright J.L., Porter M.P. Admission for nephrolithiasis in pregnancy and risk of adverse birth outcomes. Obstet Gynecol. 2007;109(5):1099–1104. doi: 10.1097/01.AOG.0000259941.90919.c0. [DOI] [PubMed] [Google Scholar]
- 79.Bodo EK, Darren TB, John DD. Uric acid urolithiasis. In: Stoller ML, Meng MV, editors, From: Current clinical urology, urinary calculus disease: a practical guide to medical and surgical management. Totowa, NJ:Humana Press Inc..
- 80.Patel SJ, Reede DL, Katz DS, Subramaniam R, Amorosa JK. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics 2007;27(6):1705–22. [DOI] [PubMed]
- 81.Asrat T., Roossin M.C., Miller E.I. Ultrasonographic detection of ureteral jets in normal pregnancy. Am J Obstet Gynecol. 1998;178(6):1194–1198. doi: 10.1016/s0002-9378(98)70322-9. [DOI] [PubMed] [Google Scholar]
- 82.Van Der Molen A.J., Cowan N.C., Mueller-Lisse U.G., Nolte-Ernsting C.C. CT urography: definition, indications and techniques. A guideline for clinical practice. Eur Radiol. 2008;18(1):4–17. doi: 10.1007/s00330-007-0792-x. [DOI] [PubMed] [Google Scholar]
- 83.Thomson J.M., Glocer J., Abbott C., Maling T.M., Mark S. Computed tomography versus intravenous urography in diagnosis of acute flank pain from urolithiasis: a randomized study comparing imaging costs and radiation dose. Australas Radiol. 2001;45(3):291–297. doi: 10.1046/j.1440-1673.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 84.Juan Y.S., Wu W.J., Chuang S.M., Wang C.J., Shen J.T., Long C.Y. Management of symptomatic urolithiasis during pregnancy. Kaohsiung J Med Sci. 2007;23(5):241–246. doi: 10.1016/S1607-551X(09)70404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsimberidou A.M., Keating M.J. Hyperuricemic syndromes in cancer patients. Contrib Nephrol. 2005;147:47–60. doi: 10.1159/000082541. [DOI] [PubMed] [Google Scholar]
- 86.Tuyen do G., Adachi M., Miyoshi T, Nonoguchi H, Oka T. Mutations in human urate transporter 1 gene in presecretory reabsorption defect type of familial renal hypouricemia. J Clin Endocrinol Metab. 2005;90:2169–2174. doi: 10.1210/jc.2004-1111. [DOI] [PubMed] [Google Scholar]
- 87.Talente G.M., Coleman R.A., Alter C., Baker L., Brown B.I., Cannon R.A. Glycogen storage disease in adults. Ann Intern Med. 1994;120:218–226. doi: 10.7326/0003-4819-120-3-199402010-00008. [DOI] [PubMed] [Google Scholar]
- 88.Abou-Elela A, Emran M. Abdel Mohsen, Bedair AS, Abdel Kader M. Safety & efficacy of tubeless percutaneous renal surgery. J Endourol 2007; 21(9):977–84. [Eskelinen M, Ikonen J, Lipponen P. Usefulness of history-taking, physical examination and diagnostic scoring in acute renal colic. Eur Urol 1998;34:467–73].
- 89.Smith R.C., Verga M., McCarthy S., Rosenfield A.T. Diagnosis of acute flank pain: value of unenhanced helical CT. AJR Am J Roentgenol. 1996;166:97–101. doi: 10.2214/ajr.166.1.8571915. [DOI] [PubMed] [Google Scholar]
- 90.Dalrymple N.C., Verga M., Anderson K.R., Bove P., Covey A.M., Rosenfield A.T. The value of unenhanced helical computerized tomography in the management of acute flank pain. J Urol. 1998;159:735–740. [PubMed] [Google Scholar]
- 91.Nakada S.Y., Hoff D.G., Attai S., Heisey D., Blankenbaker D., Pozniak M. Determination of calculus composition by noncontrast spiral computed tomography in the clinical setting. Urology. 2000;55:816–819. doi: 10.1016/s0090-4295(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 92.Kursh E.D., Resnick M.I. Dissolution of uric acid calculi with systemic alkalization. J Urol. 1984;132:286–287. doi: 10.1016/s0022-5347(17)49598-x. [DOI] [PubMed] [Google Scholar]
- 93.Moran M.E., Abrahams H.M., Burday D.E., Greene T.D. Utility of oral dissolution therapy in the management of referred patients with secondarily treated uric acid calculi. Urology. 2002;59:206–210. doi: 10.1016/s0090-4295(01)01499-6. [DOI] [PubMed] [Google Scholar]
- 94.Eknoyan G. Michelangelo: art, anatomy, and the kidney. Kidney Int. 2000;57:1190–1201. doi: 10.1046/j.1523-1755.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- 95.Preminger G.M. Pharmacologic treatment of uric acid calculi. Urol Clin North Am. 1987;14:335–338. [PubMed] [Google Scholar]
- 96.Davids M.R., Edoute Y., Jungas R.L., Cheema-Dhadli S., Halperin M.L. Facilitating an understanding of integrative physiology: emphasis on the composition of body fluid compartments. Can J Physiol Pharmacol. 2002;80:835–850. doi: 10.1139/y02-114. [DOI] [PubMed] [Google Scholar]
- 97.Nich P.T., Wurzel R.S. Dissolution of uric acid calculi with intravenous 1/6 molar lactate. Urology. 1985;26:129–134. doi: 10.1016/0090-4295(85)90044-5. [DOI] [PubMed] [Google Scholar]
- 98.Freed S.Z. The alternating use of an alkalinizing salt and acetazolamide in the management of cystine and uric acid calculi. J Urol. 1975;123:96–99. doi: 10.1016/s0022-5347(17)59417-3. [DOI] [PubMed] [Google Scholar]
- 99.Bernardo N.O., Smith A.D. Chemolysis of urinary calculi. Urol Clin North Am. 2000;27:355–365. doi: 10.1016/s0094-0143(05)70264-0. [DOI] [PubMed] [Google Scholar]
- 100.Wabner C.L., Pak C.Y. Effect of orange juice consumption on urinary calculus risk factors. J Urol. 1993;149:1405–1408. doi: 10.1016/s0022-5347(17)36401-7. [DOI] [PubMed] [Google Scholar]
- 101.Seltzer M.A., Low R.K., McDonald M., Shami G.S., Stoller M.L. Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol. 1996;156:907–909. [PubMed] [Google Scholar]
- 102.Lewis R.W., Roth J.K., Jr., Polanco E.J., Roberts J.A. Molar lactate in the management of uric acid renal obstruction. J Urol. 1981;125:87–90. doi: 10.1016/s0022-5347(17)54913-7. [DOI] [PubMed] [Google Scholar]
- 103.Kok D.J., Iestra J.A., Doorenbos C.J., Papapoulos S.E. The effects of dietary excesses in animal protein and in sodium on the composition and the crystallization kinetics of calcium oxalate monohydrate in urines of healthy men. J Clin Endocrinol Metab. 1990;71:861–867. doi: 10.1210/jcem-71-4-861. [DOI] [PubMed] [Google Scholar]
- 104.Siener R., Hesse A. The effect of a vegetarian and different omnivorous diets on urinary risk factors for uric acid calculus formation. Eur J Nutr. 2003;42(332–337):1. doi: 10.1007/s00394-003-0428-0. [DOI] [PubMed] [Google Scholar]
- 105.Middendorf D.F., Hebert L.A., Zager R.A., Hartman J., Bitzel D. Simple method for monitoring 24-hour urinary urea nitrogen excretion. J Lab Clin Med. 1986;108:577–580. [PubMed] [Google Scholar]
- 106.Perez-Ruiz F., Hernando I., Villar I., Nolla J.M. Correction of allopurinol dosing should be based on clearance of creatinine, but not plasma creatinine levels: another insight to allopurinol-related toxicity. J Clin Rheumatol. 2005;11:129–133. doi: 10.1097/01.rhu.0000164822.98163.22. [DOI] [PubMed] [Google Scholar]
- 107.Pais VM Jr, Lowe G, Lallas CD, Preminger GM, Assimos DG. Xanthine urolithiasis. Urology. 2006;67:1084.e9-11. [DOI] [PubMed]
- 108.Landgrebe A.R., Nyhan W.L., Coleman M. Urinarytract calculi resulting from the excretion of oxypurinol. N Engl J Med. 1975;292:626–627. doi: 10.1056/NEJM197503202921208. [DOI] [PubMed] [Google Scholar]
- 109.Bessmertny O., Robitaille L.M., Cairo M.S. Rasburicase: a new approach for preventing and/or treating tumor lysis syndrome. Curr Pharm Des. 2005;11:4177–4185. doi: 10.2174/138161205774913291. [DOI] [PubMed] [Google Scholar]
- 110.Sun X.Z., Zhang Z.W. Shock wave lithotripsy for uric acid calculi. Asian J Surg. 2006;29:36–39. doi: 10.1016/S1015-9584(09)60292-X. [DOI] [PubMed] [Google Scholar]
- 111.Teichman J.M., Vassar G.J., Bishoff J.T., Bellman G.C. Holmium:YAG lithotripsy yields smaller fragments than Lithoclast, pulsed dye laser, or electrohydraulic lithotripsy. J Urol. 1998;159:17–23. doi: 10.1016/s0022-5347(01)63998-3. [DOI] [PubMed] [Google Scholar]
- 112.Zagone R.L., Waldmann T.M., Conlin M.J. Fragmentation of uric acid calculi with the holmium: YAG laser produces cyanide. Lasers Surg Med. 2002;31:230–232. doi: 10.1002/lsm.10094. [DOI] [PubMed] [Google Scholar]
- 113.Teichman J.M., Champion P.C., Wollin T.A., Denstedt J.D. Holmium:YAG lithotripsy of uric acid calculi. J Urol. 1998;160:2130–2132. [PubMed] [Google Scholar]