Advances in the understanding of the genetic and biologic characteristics of thyroid cancer, coupled with the development of new molecular targeted therapeutics, have led to the improved diagnosis and treatment of patients with this cancer. In this review, we focus on the effect of these discoveries on all types of thyroid cancer and particularly on how they are transforming clinical care.
SPECTRUM OF THYROID CANCERS
The transformation of endodermal-derived thyroid follicular cells or neural crest–derived thyroid C cells leads to distinct types of cancer (Fig. 1). Follicular cells give rise to two main forms of differentiated thyroid cancer: papillary thyroid carcinoma and follicular thyroid carcinoma. Poorly differentiated and anaplastic thyroid carcinomas are comparatively rare tumors that also arise from follicular cells and are associated with aggressive disease. Medullary thyroid carcinoma is the canonical C-cell tumor and has distinct biologic features.
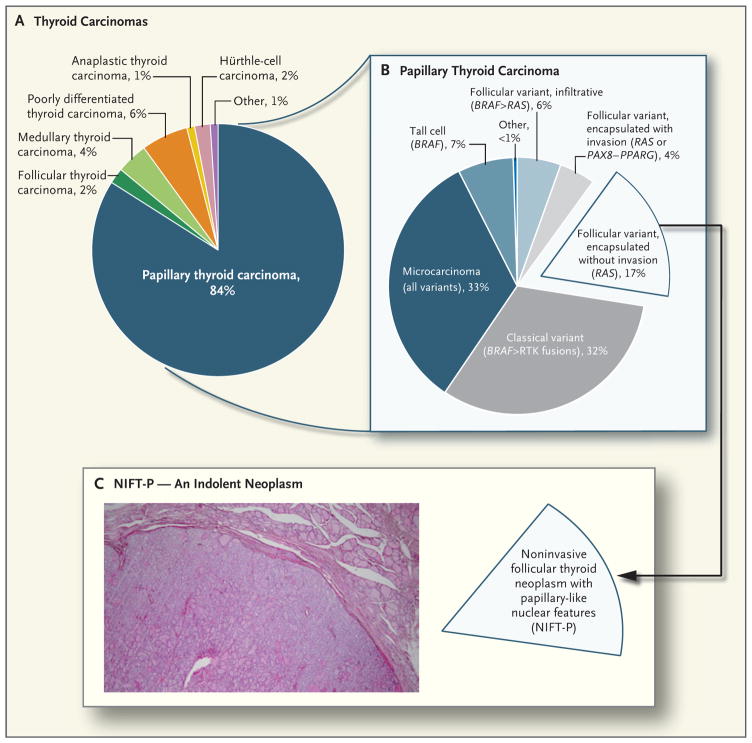
Figure 1. Pathologic Spectrum of Thyroid Cancers.
Panel A shows the relative incidence of the main types of thyroid cancer in the United States, and Panel B the relative frequency of pathologic variants of papillary thyroid carcinoma, with their corresponding main driver mutations shown in parentheses (the symbol > indicates more frequent than). RTK denotes receptor tyrosine kinase. Panel C shows the encapsulated follicular variant of papillary thyroid carcinoma without invasion, which until recently represented 17% of all papillary thyroid carcinomas. This cancer has recently been reclassified as a neoplasm of low malignant potential and is now termed “noninvasive follicular thyroid neoplasm with papillary−like nuclear features” (NIFT−P). This change will result in a corresponding reduction in the number of patients who are considered to have thyroid cancer. The hematoxylin and eosin–stained section in the inset shows the characteristic histologic appearance of an NIFT−P. The encapsulated tumor has a follicular growth pattern and papillary nuclear features, low mitotic rate, and absence of necrosis and capsular or vascular invasion.
DIFFERENTIATED THYROID CARCINOMA
DIAGNOSIS
Papillary thyroid carcinoma accounts for approximately 85% of thyroid cancers. From 1975 through 2009, the incidence of thyroid cancer tripled in the United States, primarily owing to the incidental detection of small-volume papillary carcinomas on imaging studies.1 Most papillary thyroid carcinomas are indolent clinically, consistent with their simple genome, which has few copy-number alterations. Papillary thyroid carcinoma has one of the lowest mutation densities of cancers that have been studied by means of whole-exome sequencing.2 Although formerly thought to be a single entity, papillary thyroid carcinoma encompasses several tumor types that have mutually exclusive mutations of genes encoding effectors that signal through the mitogen-activated protein kinase (MAPK) pathway.3,4 BRAF V600E accounts for 60% of these mutations, followed by RAS (15%) and chromosomal rearrangements that lead to illegitimate expression of the kinase domains of BRAF or of receptor tyrosine kinases, such as RET, NTRK, and ALK (12%). The remaining 13% mostly have no known driver mutations; a subgroup have copy-number abnormalities but no discrete recurrent genetic lesion. The different driver mutations are associated with different histologic variants of papillary thyroid carcinoma (Fig. 1) and confer distinct patterns of gene expression, signaling, and clinical characteristics.4
BRAF-mutated classical or tall-cell–variant papillary thyroid carcinomas have a high frequency of lymph-node metastases and recurrence after thyroidectomy; these carcinomas also have a poor response to radioiodine therapy.5 Their refractoriness to radioiodine appears to be due to the high MAPK-pathway output that is driven by the BRAF V600E oncoprotein, which suppresses the expression of genes required for iodide incorporation.6 RAS-mutated papillary thyroid carcinomas are associated with the follicular variant of papillary thyroid carcinoma. Follicular variant papillary carcinomas with vascular invasion spread infrequently to regional lymph nodes, retain the expression of iodine-metabolism genes, and are usually radioiodine-avid (Fig. 2).4,7–11 Encapsulated noninvasive follicular variants of papillary thyroid carcinoma have recently been reclassified as a benign entity and renamed as “noninvasive follicular thyroid neoplasms with papillary-like nuclear features,” thereby substantially reducing the number of patients who are considered to have thyroid cancer (Fig. 1).12
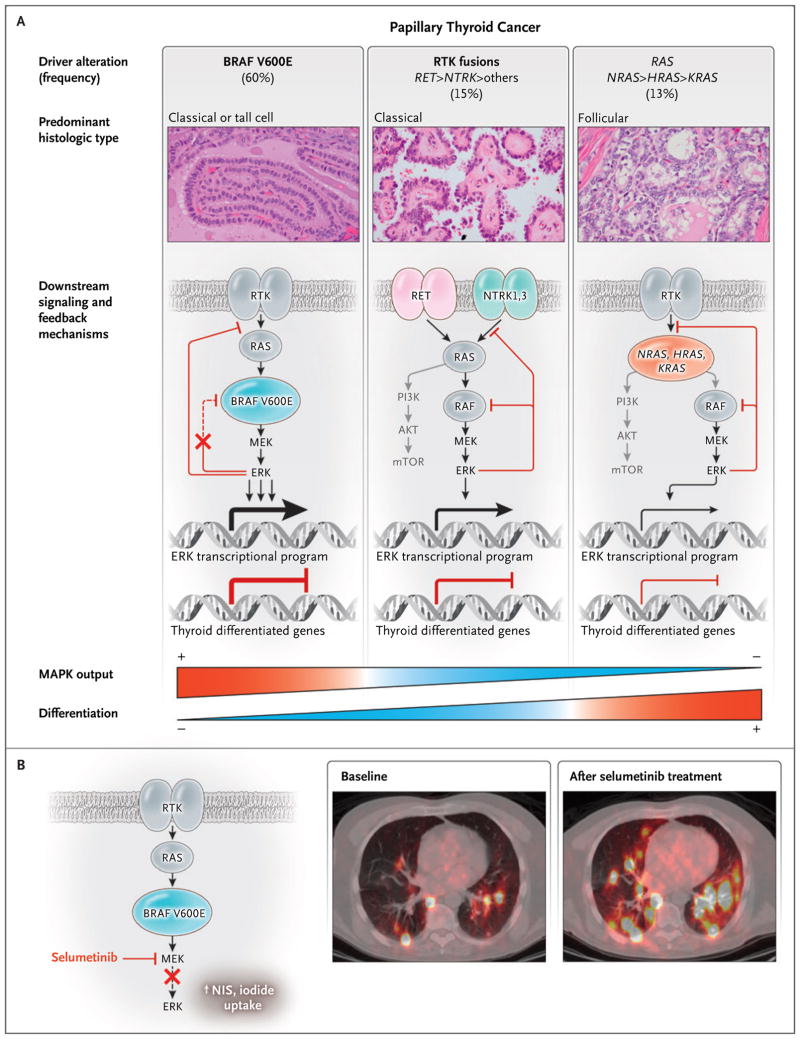
Figure 2. Functional Consequences of Driver Mutations in Papillary Thyroid Carcinomas.
Panel A shows that papillary thyroid carcinomas have mutually exclusive activating mutations in BRAF, RAS, and RTK. The photomicrographs show hematoxylin and eosin–stained slides of the indicated variants of papillary thyroid carcinoma. Mutant RTKs, RAS, and BRAF activate mitogen−activated protein kinase (MAPK) signaling but do so to different degrees. The symbol > indicates more frequent than. The signaling output driven by BRAF V600E is highest, because this oncoprotein signals as a monomer and is unresponsive to the negative−feedback effects of activated ERK on upstream inputs into the pathway.7–9 By contrast, the MAPK−signaling flux that is evoked by fusion RTK proteins or by mutated RAS is dampened by negative feedback. The expression of genes that is required for iodide uptake and metabolism, which are hallmarks of the differentiated state of thyroid follicular cells, is inhibited by MAPK signaling. This is consequential, because responsiveness to radioiodine therapy requires preservation of thyroid−differentiated function. The weight of the lines and arrows indicates the magnitude of the flux through the MAPK pathway and the transcriptional activities, respectively. The term mTOR denotes mammalian target of rapamycin, and PI3K phosphatidylinositol 3−kinase. Panel B (left side) shows that the MAPK kinase (MEK) inhibitor selumetinib decreases extracellular signal−regulated kinase (ERK) activation and restores expression of the sodium iodide transporter (NIS) and other thyroid differentiation genes in mice with Braf V600E–driven papillary thyroid carcinoma.6 The insets on the right side of Panel B are fused iodine−124 positron−emission tomographic–computed tomographic images showing the restoration of iodine−124 uptake with selumetinib treatment in a patient with radioiodine−refractory lung metastases of thyroid cancer. Adapted, with permission, from Ho et al.11
Follicular thyroid carcinomas represent 2 to 5% of thyroid cancers.13 Follicular thyroid carcinoma and follicular variants of papillary thyroid carcinoma are associated with mutually exclusive mutations of RAS or of the PAX8–PPARG fusion oncogene.14 The prognosis of patients with these cancers depends on the size of the tumor, the age of the patient, and the degree of angio-invasiveness, which predicts the risk of distant metastases. Hürthle-cell carcinomas, which are classified as a variant of follicular thyroid carcinoma, are genetically distinct.15 Widely invasive Hürthle-cell carcinomas, which are characterized by extensive capsular and vascular invasion, often metastasize to lung and bone and are particularly refractory to radioiodine.
Exposure to ionizing radiation is a risk factor for the development of papillary thyroid carcinoma. After the nuclear-reactor accident in Chernobyl in 1986, there was a sharp increase in the incidence of papillary thyroid carcinomas, primarily affecting very young children in iodide-deficient regions.16 Similar age-dependent trends were seen after the atomic-bomb explosions in Hiroshima and Nagasaki in 1945 and in persons receiving external radiotherapy for benign or malignant conditions of the head and neck. Radiation-induced papillary thyroid carcinomas have a high prevalence of fusion oncogenes, usually arising from intrachromosomal rearrangements that activate RET or, less frequently, the tyrosine kinase receptors encoded by NTRK.17 These translocations are favored by the spatial proximity of the participating genes during inter-phase in thyroid cells, which probably predisposes them to recombination after radiation-induced DNA damage.18 The disease-specific mortality is low, both among affected persons who have been followed for several decades and among children with sporadic papillary thyroid carcinoma.
Germline variants in chromosomes 9q22.33 and 14q13.3 are associated with a high risk of differentiated thyroid carcinoma.19 The genes encoding FOXE1 and NKX2-1, which are master regulators of thyroid development and differentiated function, are adjacent to these loci. A total of 3 to 9% of differentiated thyroid carcinomas are familial. These may arise as a component of cancer syndromes, such as Cowden’s disease, familial adenomatous polyposis, and Werner’s syndrome, which are caused by germline loss-of-function mutations in the respective genes PTEN, APC, and WRN. More commonly, the carcinomas occur as an isolated familial entity, defined as the presence of the disease in first-degree relatives. Recently, a germline variant of HABP2 was shown to be associated with papillary thyroid carcinoma in an extended kindred,20 although the validity of this finding has been questioned.21,22
Ultrasonography identifies lesions at high risk for cancer and is the best imaging method for the assessment of thyroid nodules. Papillary thyroid carcinomas that are less than 1 cm in the greatest dimension (papillary microcarcinomas) occur in up to 30% of adults in the general population, yet they rarely become clinically significant. Therefore, papillary microcarcinomas need not be biopsied unless there is extrathyroidal invasion, nodal metastases, or arguably, previous exposure to radiation or a family history of thyroid cancer.
Although cytopathological testing can discriminate between benign and malignant tumors, it is inconclusive in 20 to 30% of cases.23 Two molecular diagnostic methods can sharpen the differential diagnosis. Afirma, a proprietary gene-expression classifier with a high negative predictive value, is designed to identify benign nodules among those with inconclusive results on cytopathological testing.24,25 Alternatively, next-generation sequencing of a panel of oncogenes and tumor-suppressor genes identifies nodules with mutations that have been associated with thyroid cancer, with high positive and negative predictive values.26 These two tests appear to reduce the incidence of unnecessary surgery, although their reliability in various clinical-practice settings remains to be established.
SURGICAL MANAGEMENT
Prospective studies of prolonged surveillance show that most papillary microcarcinomas do not progress, and surgery may be avoided or deferred in selected cases.27 Lobectomy or total thyroidectomy is the treatment of choice for primary thyroid cancers that measure 1 to 4 cm in the greatest dimension.28 Thyroidectomy without prophylactic central neck dissection may be appropriate for noninvasive, node-negative papillary thyroid carcinomas of tumor stage T1 (tumor size ≤2 cm in the greatest dimension; intrathyroidal) or T2 (tumor size >2 cm and ≤4 cm; intrathyroidal) and for most follicular thyroid carcinomas. Clinically involved lymph-node compartments should be resected. Total thyroidectomy with resection of involved lymph-node compartments is the recommended treatment for tumors that are larger than 4 cm in the greatest dimension.
The 10-year disease-specific mortality that is associated with differentiated thyroid carcinoma is less than 5%. The American Joint Commission on Cancer (AJCC) staging system includes prognostic variables that include the age of the patient, tumor size, invasiveness, presence and location of nodal metastases, and the presence of distant metastases. The AJCC and similar staging systems identify only a fraction of patients who are at risk for death, probably because of failure to incorporate variables such as histologic characteristics, functional status (e.g., radioiodine avidity or positivity on 18F-fluorodeoxyglucose–positron-emission tomography [FDG-PET]) of distant metastases, key molecular markers, and initial response to therapy. Also, the AJCC classification does not predict the risk of recurrence, which is problematic because the method and intensity of surveillance and therapy are guided by individualized estimates of the risk of recurrence. Dynamic stratification of patients with differentiated thyroid carcinoma according to their response to initial therapy improves the prediction of the risk of recurrent or persistent disease as well as disease-specific mortality.29
Recent guidelines propose a more comprehensive set of variables to identify patients who are at low, intermediate, or high risk for recurrence.28 Among these variables, molecular markers show promise. Most groups have shown that the BRAF V600E mutation alone is of no practical value in risk stratification, even though it is associated with a greater likelihood of nodal recurrence than papillary cancers driven by other oncogenes.30 Somatic mutations of the telomerase gene (TERT) promoter are present in approximately 9% of papillary thyroid carcinomas.4,15,31,32 These mutations generate de novo binding motifs for the ETS (also called E26) family of transcription factors, resulting in inappropriate activation of telomerase expression.33 Such expression presumably leads to immortalization, a high likelihood of additional oncogenic events, and disease progression. Among patients with papillary thyroid carcinomas with both the BRAF V600E and TERT mutations, progression-free survival is markedly shorter than among those with BRAF V600E mutations alone.34 However, the risks and benefits of initiating intensive therapies that are based solely on genetic profiling need to be understood before their introduction into clinical practice.
RADIOIODINE THERAPY
Radioiodine therapy leverages the property of thyroid follicular cells to transport and incorporate iodide into thyroglobulin, a feature that is retained in a subgroup of differentiated thyroid carcinomas. Until recently, most patients with differentiated thyroid carcinoma received postoperative radioiodine therapy despite a lack of data from prospective clinical trials to support the practice. Radioiodine therapy is no longer recommended in patients with low-risk thyroid cancers, because the recurrence rate and mortality are low and large retrospective series have not shown improved outcomes.35,36 The data regarding radioiodine therapy in patients with intermediate-risk disease are not compelling; however, the treatment may be useful in a subgroup of patients who have high levels of thyroglobulin after surgery and persistent structural disease. Postoperative therapy with either 30 or 100 mCi (1.1 or 3.7 GBq) of iodine-131 is equally effective in ablating the remnant thyroid, regardless of whether injections of recombinant human thyrotropin or thyroid-hormone withdrawal is used to induce iodide accumulation.37
BRAF-mutated cancers and those that are positive on FDG-PET scans are often refractory to radioiodine.38 The expression of genes that are required for iodine transport and metabolism is low in most BRAF-mutated cancers, whereas they are comparatively preserved in RAS-mutated papillary thyroid carcinomas (Fig. 2).4 Accordingly, Braf V600E suppresses the expression of these genes in mouse models of papillary thyroid carcinoma and inhibits radioiodine uptake and response to radioiodine therapy, which can be partially restored by treatment with rapidly accelerating fibrosarcoma (RAF) or MAPK kinase (MEK) inhibitors.6 A pilot trial of the MEK inhibitor selumetinib in patients with radioiodine-refractory metastatic thyroid cancer showed the restoration of iodide uptake at metastatic sites in 14 of 20 patients. In 8 of the 14 patients, the uptake was sufficient to enable iodine-131 therapy with remarkable clinical responses (Fig. 2).11 Similar results have been shown with the BRAF inhibitor dabrafenib.39 An ongoing phase 3, placebo-controlled, double-blind, randomized trial (ClinicalTrials.gov number, NCT01843062) is evaluating the ability of selumetinib to enhance the response to adjuvant radioiodine therapy in patients at high risk for locoregional recurrence.
Most patients with differentiated thyroid carcinoma are treated with high doses of thyroid hormone, which are sufficient to suppress the secretion of thyrotropin. The intensity and duration of suppressive therapy can be affected by disease status. It is unclear whether this therapy benefits patients with BRAF-mutated papillary thyroid carcinoma, because most such tumors express low levels of the thyrotropin receptor.4
Patients with low-risk or intermediate-risk disease are followed by means of neck ultrasonography and measurements of serum thyroglobulin levels. Antithyroglobulin antibodies, which are present in patients with autoimmune thyroiditis, can interfere with the accuracy of thyroglobulin immunoassays; however, persistent or rising levels of antithyroglobulin antibody also indicate disease activity. Diagnostic radioiodine scans have low sensitivity and are unhelpful in routine surveillance unless there is structural or biochemical evidence of disease. Additional imaging studies, including FDG-PET scans, may help localize disease in patients with rising levels of thyroglobulin or antithyroglobulin antibody. Clinically apparent persistent or recurrent cervical nodal disease is found in approximately 10% of patients with thyroid cancer. Selected cases can be managed expectantly or by means of surgical resection, thermal destruction, or alcohol ablation.40–42
SYSTEMIC THERAPIES FOR METASTATIC RADIOIODINE-REFRACTORY THYROID CANCER
Thyroid cancers are often indolent, even when they have metastasized to distant sites. Most physicians reserve systemic therapy for patients who have metastatic disease that is progressing, symptomatic, or in a location that threatens vital structures and is not amenable to localized therapies. Palliative radiotherapy, either alone or concomitant with low-dose chemotherapy, or local therapies may control disease in patients with unresectable regional or metastatic disease.40,41,43 Treatment with bisphosphonates or anti–receptor activator of nuclear factor-κB (RANK) ligand antibody may benefit patients who have bone metastases, although the efficacy of the compounds has not been tested in prospective trials.44
The Food and Drug Administration (FDA) approved two multikinase inhibitors, sorafenib and lenvatinib, for the treatment of patients with radioiodine-refractory metastatic thyroid cancer on the basis of phase 3, prospective, double-blind, randomized, placebo-controlled trials that showed longer progression-free survival (Table 1).45,46 Although the two drugs have not been compared with each other, lenvatinib appears to have greater efficacy than sorafenib.49 Adverse effects of the two drugs make the maintenance of full-dose therapy a challenge. The effects of the drugs on quality of life and the long-term cumulative toxic effects remain to be fully explored.
Table 1.
Phase 3 Clinical Trials of Kinase Inhibitors in Patients with Differentiated or Medullary Thyroid Carcinoma.*
| Drug and Trial | No. of Patients
|
Tumor | Progression-free Survival
|
Dose-Related Events in Active-Drug Group | Deaths in Active-Drug Group | ||||
|---|---|---|---|---|---|---|---|---|---|
| Active Drug | Placebo | Active Drug | Placebo | Hazard Ratio (95% CI) † | P Value | ||||
| mo | % of patients | no. | |||||||
|
| |||||||||
| Sorafenib (DECISION)45 | 207 | 210 | DTC | 10.8 | 5.8 | 0.59 (0.45–0.76) | <0.001 | 64 had dose reduced, and 18 discontinued | 12, with 1 considered to be drug related |
|
| |||||||||
| Lenvatinib (SELECT)46 | 261 | 131 | DTC | 18.3 | 3.6 | 0.21 (0.14–0.31) | <0.001 | 68 had dose reduced, and 14 discontinued | 20, with 6 considered to be drug related |
|
| |||||||||
| Vandetanib (ZETA)47 | 231 | 100 | MTC | 30.5 | 19.3 | 0.46 (0.31–0.69) | <0.001 | 35 had dose reduced, and 12 discontinued | 5, with 1 considered to be drug related |
|
| |||||||||
| Cabozantinib (EXAM)48 | 219 | 111 | MTC | 11.2 | 4.0 | 0.28 (0.19–0.40) | <0.001 | 79 had dose reduced, and 22 discontinued | 12, with 9 considered to be drug related |
DECISION was a multicenter, randomized, double−blind, placebo−controlled phase 3 trial of sorafenib in patients with radioactive iodine–refractory locally advanced or metastatic, progressive, differentiated thyroid cancer,45 SELECT a phase 3, randomized, double−blind, multicenter trial of lenvatinib, as compared with placebo, in patients with progressive differentiated thyroid cancer that is refractory to iodine−131,46 ZETA a phase 3 prospective, randomized, double−blind, trial of vandetanib, as compared with placebo, in patients with locally advanced or metastatic medullary thyroid cancer,47 and EXAM a phase 3 prospective, randomized, double−blind, trial of cabozantinib, as compared with placebo, in patients with progressive advanced medullary thyroid cancer.48 Progression−free survival was the primary end point in each of the four trials. Outcome comparisons of these trials should be done with caution because there were differences in trial design and eligibility criteria. CI denotes confidence interval, DTC differentiated thyroid carcinoma, and MTC medullary thyroid carcinoma.
The hazard ratio is for disease progression or death. A 99% confidence interval was used for the hazard ratio in the SELECT trial.46
Phase 2 trials of other multikinase inhibitors that target vascular endothelial growth factor (VEGF) receptor signaling have shown efficacy in this disease.50–52 The mechanisms of action of these drugs are unknown, primarily because they inhibit multiple oncologic targets. Thyroid cells require contact with capillaries to function normally and secrete trophic signals for capillary endothelial cells, primarily VEGF.53 On transformation and loss of polarity, a disorganized tumor vasculature may result in cancer-cell hypoxia, loss of immune surveillance, increased VEGF-receptor activation, and a dependence on VEGF-receptor signaling that can be leveraged therapeutically.54 Sorafenib and lenvatinib are thought to act by suppressing angiogenesis, because they inhibit VEGF receptors 1, 2, and 3. They also have distinct activity profiles against other kinases. Consequently, the therapeutic window with which they inhibit their respective targets affects clinical outcomes in ways that are poorly understood.
Advanced thyroid cancers also have cell-autonomous oncogenic defects that generate vulnerabilities that can be exploited therapeutically.15 Chief among them is the BRAF V600E mutation, which is the most common driver along the entire spectrum of the disease.55 BRAF confers susceptibility to selective RAF kinase inhibitors in some, but not all, cancer lineages. Thus, patients with BRAF-mutated melanoma or hairy-cell leukemia have a high response rate to vemurafenib,56,57 whereas patients with colorectal cancer do not.58 The low sensitivity of colorectal cancer cells to growth inhibition by vemurafenib is due in part to the activation of epidermal growth factor receptor signaling.59,60 An analogous mode of adaptive resistance is also seen in cell lines of BRAF-mutated thyroid cancer and murine Braf-induced papillary thyroid carcinomas, which are refractory to vemurafenib by means of the activation of human epidermal growth factor receptor 3 (HER3) signaling. Accordingly, the response rate among patients with BRAF-mutated papillary thyroid carcinoma in a phase 2 trial of vemurafenib was 38.5%, which is considerably less than among patients with melanoma.61
Combination trials with RAF and MEK inhibitors, as well as RAF and HER3 inhibitors, are currently in development. Some advanced thyroid cancers have rearrangements of ALK, RET, NTRK1, NTRK3, or FGFR, which can be targeted by selective kinase inhibitors with proven efficacy in other tumor types. Because the prevalence of these mutations is low among thyroid cancers, patients can be enrolled in “basket trials,” in which the efficacy of a drug targeting a particular molecular abnormality is studied in cancers of different lineages.
Hence, the biologic underpinnings of meta-static, differentiated thyroid cancers offer two potential strategies for systemic treatment: disrupting the disorganized tumor vasculature and blocking the primary oncogenic driver. The ultimate application of these two approaches, either sequentially or in combination, remains to be defined but offers much promise.
POORLY DIFFERENTIATED AND ANAPLASTIC THYROID CARCINOMAS
Poorly differentiated thyroid carcinomas are aggressive and are defined histologically by a combination of architectural and high-grade features (high mitotic rate and presence of necrosis).62,63 Poorly differentiated thyroid carcinomas represent approximately 6% of thyroid cancers and are associated with a mean survival of 3.2 years. Radioiodine therapy is of limited benefit. Most patients require systemic therapies that are similar to those described for differentiated thyroid carcinomas.
Anaplastic thyroid carcinomas account for approximately 1% of thyroid cancers and are associated with a mean survival of 6 months. They are refractory to radioiodine, and traditional chemotherapy and radiotherapy are of limited benefit.64 Anaplastic thyroid carcinomas probably arise from preexisting differentiated or poorly differentiated thyroid carcinomas (Fig. 3) and have a high mutation burden.4,55 Although BRAF and RAS are the predominant drivers, anaplastic thyroid carcinomas are characterized by frequent mutations in TP53, the TERT promoter, effectors of the phosphatidylinositol 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway, and genes involved in epigenetic regulation, including components of the SWI/SNF complex and histone methyltransferases (Fig. 3).55 Mutations in EIF1AX, a component of the translational preinitiation complex, are markedly enriched in poorly differentiated and anaplastic thyroid carcinomas and have a striking pattern of co-occurrence with RAS.
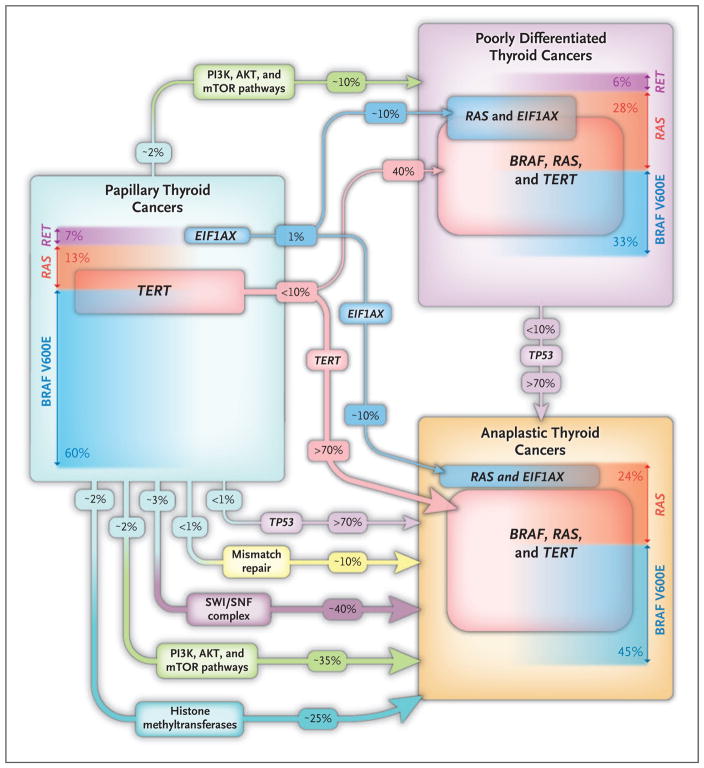
Figure 3. Genomic Hallmarks of Thyroid Cancer along the Spectrum of Disease Progression.
The frequency of the main somatic genetic defects in papillary, poorly differentiated, and anaplastic thyroid carcinoma is shown, based on the largest published series studied by next−generation sequencing.4,55 Because anaplastic thyroid carcinomas are extensively infiltrated by tumor−associated macrophages, deep sequencing is required to make reliable mutation calls. The prevalence of driver mutations (BRAF, RAS, and RET) in the histologic types of the three tumors is shown. In patients with advanced disease, tumors may have more than one mutation, so the overall mutation burden exceeds 100%. The frequency of the main drivers (BRAF, RAS, and RET) sums to less than 100% because in some cases the drivers are not known or they are lower−frequency events and are not listed here (e.g., NF1, PTEN). TERT promoter mutations appear to be key transitional steps in the microevolution of tumors. In papillary thyroid carcinoma, the TERT mutations are infrequent (in 10% of tumors) and usually subclonal. By contrast, their prevalence is substantially higher in poorly differentiated and anaplastic thyroid carcinomas, in which they are uniformly clonal. Mutations in TP53 are infrequent in all histologic types of thyroid cancer with the exception of anaplastic thyroid carcinomas, in which they occur in more than 70% of patients. Anaplastic thyroid carcinomas have mutations in genes encoding components of the PI3K–AKT–mTOR pathway and of proteins involved in epigenetic regulation, whereas poorly differentiated thyroid carcinomas have an intermediate frequency of these events (data not shown). Mutations of EIF1AX, a component of the translation preinitiation complex, are infrequent and are mutually exclusive with other driver mutations in papillary thyroid cancer. In poorly differentiated and anaplastic thyroid cancers, they are markedly enriched and are strongly associated with RAS−mutated tumors.
The genetic complexity of anaplastic thyroid carcinomas underscores their extreme virulence. When possible, these tumors should be resected and the patient treated with locoregional radiation therapy and chemotherapy with taxanes, either alone or in combination with carboplatin or doxorubicin.65 In patients with unresectable disease, preservation of the airway is critical, and palliative therapy is often the only option. Despite their genomic complexity, some anaplastic thyroid carcinomas retain dependence on the genetic drivers,66,67 and it is important to consider enrollment in experimental trials early in the course of disease. Candid discussions with patients and families about the extent and intensity of medical interventions and the option of home or institutional hospice care are important aspects of treatment.
MEDULLARY THYROID CARCINOMA
PATHOGENESIS
Medullary thyroid carcinoma accounts for 3 to 5% of thyroid cancers. In 75% of patients, the medullary thyroid carcinoma is sporadic, usually developing in the fourth to sixth decade of life. Less often, medullary thyroid carcinoma is the dominant component of the hereditary multiple endocrine neoplasia (MEN) type 2 syndromes, MEN2A and MEN2B (Table 2).68–77 MEN2A accounts for 95% of the cases of MEN type 2 and has four variants: classical MEN2A, MEN2A with Hirschsprung’s disease, MEN2A with cutaneous lichen amyloidosis, and isolated familial medullary thyroid carcinoma. MEN2B is characterized by a typical physical appearance and associated abnormalities.
Table 2.
Characteristics of Sporadic Medullary Thyroid Carcinoma, MEN2A, and MEN2B.*
| Disease | Associated_Phenotype | Mutations.† | Clinical_Characteristics |
|---|---|---|---|
| Sporadic MTC | None | RET (in approximately 50%), HRAS, NRAS, or KRAS (in 0 to 43%)68; rarely mutations in KIT or METor fusions of RET or ALK69,70 | RET M918T associated with more aggressive MTC than RAS71 |
| MEN2A | |||
| Classical | Pheochromocytoma (in 20 to 50%) and hyperparathyroidism (in 12 to 30%) | 95% of RET mutations occur in exon 10 (codon 609, 611, 618, or 620) or exon 11 (codon 634) | Pheochromocytoma occurs in 30 to 50% of patients with RET mutations in exon 1172 and in 15% of those with RET mutations in exon 10; hyperparathyroidism occurs in 30% of patients with RET mutations in exon 11 and in <12% of those with RET mutations in exons other than 1173 |
| With Hirschsprung’s disease | Hirschsprung’s disease | RET mutation in exon 10 at codon 620 (in 50%) and less often at codon 618, 609, or 61174 | MEN2A in 2 to 5% of patients with Hirschsprung’s disease75 |
| With cutaneous lichen amyloidosis | Cutaneous lichen amyloidosis | Usually RET mutation in codon 63476 | In approximately 30% of patients with MEN2A; may precede onset of medullary thyroid carcinoma76 |
| Familial MTC | None | Broad range of RET mutations | Appears to be less aggressive than the MTC associated with classical MEN2A |
| MEN2B | Typical facies, marfanoid habitus, medullated corneal nerves, and aerodigestive tract ganglioneuromatosis | RET M918T mutations in more than 95%, and RET A833F in the remainder | RET M918T associated with more aggressive MTC than RET A833F77 |
MEN2A denotes multiple endocrine neoplasia type 2A, and MEN2B multiple endocrine neoplasia type 2B.
Patients with sporadic MTC have somatic RET mutations, whereas patients with MEN2A or MEN2B have germline RET mutations.
RET, a gene encoding a receptor tyrosine kinase, is the dominant oncogene in medullary thyroid carcinoma. More than 100 gain-of-function RET mutations have been reported in patients with medullary thyroid carcinoma, including germline mutations in patients with hereditary disease and somatic mutations in patients with sporadic disease (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).78 There is a correlation between genotype and phenotype in hereditary medullary thyroid carcinoma. Thus, patients with MEN2A or MEN2B have multicentric disease, and other endocrine tumors or associated abnormalities may develop, depending on the specific RET mutation (Table 2). Somatic RET mutations are the most common drivers in sporadic medullary thyroid carcinoma, followed by RAS mutations and RET or ALK fusions.68–70 The clinical aggressiveness of hereditary or sporadic medullary thyroid carcinoma is related to the RET mutation.
Screening for RET germline mutations by direct DNA analysis is important in family members who are at risk for hereditary medullary thyroid carcinoma. Such screening is also important in patients with presumed sporadic medullary thyroid carcinoma, because approximately 7% of them will be found to have MEN2A.79 Medullary thyroid carcinoma cells secrete calcitonin and carcinoembryonic antigen (CEA). Serum levels of these markers are directly related to the parafollicular or C-cell mass and are useful in screening family members who are at risk for medullary thyroid carcinoma, in detecting persistent or recurrent medullary thyroid carcinoma after thyroidectomy, and in monitoring the response to local or systemic therapy.
DIAGNOSIS
Ultrasonography and cytologic testing of thyroid nodules by means of fine-needle aspiration are the preferred tests for the diagnosis of medullary thyroid carcinoma. If cytopathological testing is inconclusive, immunohistochemical testing for calcitonin in aspirated cells or the measurement of calcitonin in the washout fluid of the fine-needle aspiration may be diagnostic.80 Many centers in Europe measure serum calcitonin levels in all patients with thyroid nodules, and medullary thyroid carcinoma is detected in approximately 0.4% of them. However, this practice is controversial and has not been widely adopted.81,82
SURGICA MANAGEMENT
Surgery is the primary treatment for patients with sporadic or hereditary medullary thyroid carcinoma and ranges from thyroid lobectomy (in selected patients with sporadic disease), to total thyroidectomy with or without central neck dissection, to total thyroidectomy with central neck dissection and unilateral or bilateral lymph-node–compartment dissection. The type of operation depends on the age of the patient and the extent of disease as determined by means of physical examination, imaging of the neck, and measurement of serum calcitonin levels.78,83 In families with MEN2A or MEN2B, prophylactic thyroidectomy is indicated in clinically normal children who inherit a mutated RET allele. The age of onset depends to some extent on the specific RET mutation; however, given a specific RET mutation, the age of onset varies among and even within families.
In patients who have inherited a mutated RET allele, the reliable indicator for timing thyroidectomy is the serum calcitonin level rather than the specific RET mutation. The risk of nodal metastases is low among children younger than 10 years of age, and residual medullary thyroid carcinoma after thyroidectomy is uncommon in children younger than 8 years of age.84 Therefore, most children younger than 8 years of age can be treated by total thyroidectomy, without central neck dissection, which reduces the incidence of hypoparathyroidism. Medullary thyroid carcinoma is highly aggressive in MEN2B, and thyroidectomy should be performed when the diagnosis is made, even in the first year of life. In all patients with hereditary medullary thyroid carcinoma, it is imperative that the presence of a pheochromocytoma be ruled out before thyroidectomy.
After thyroidectomy, patients are evaluated at 6-month to yearly intervals by means of physical examination and measurement of serum calcitonin levels. An undetectable serum calcitonin level indicates the absence of C cells, whereas a detectable level, even in the normal range, indicates the presence of residual C cells in a thyroid remnant or at a locoregional or distant site, or the presence of a nonthyroid cancer that is secreting calcitonin.85 If the serum calcitonin level remains undetectable for 5 years after surgery, the patient is probably cured; however, patients with a measurable calcitonin level may remain asymptomatic for many years without clinical evidence of recurrence.
The most accurate measure of the aggressiveness of medullary thyroid carcinoma is the doubling time for levels of serum calcitonin or CEA. The prognosis of patients with an elevated level of serum calcitonin or CEA is directly related to the time it takes for the marker to double. Doubling times that are less than 6 months are especially ominous, whereas those that are greater than 2 years are associated with long-term survival.78
SYSTEMIC THERAPY
Although many patients with metastatic medullary thyroid carcinoma can be followed expectantly, it is important to treat those who have progressive or symptomatic disease with systemic therapy. Standard chemotherapy is characterized by low rates of response of short duration and is seldom used as the initial treatment. The FDA approved the multikinase inhibitors vandetanib and cabozantinib on the basis of prolongation of progression-free survival, as compared with placebo, in separate, randomized, phase 3 clinical trials involving patients with advanced medullary thyroid carcinoma (Table 1).47,48 The responses were partial, and although some were durable, progressive disease developed in the majority of patients. No survival advantage has been shown with either drug. Also, the drugs are costly and are associated with toxic effects, often leading to dose reduction or termination of treatment.
As with sorafenib and lenvatinib, the mechanisms of action of vandetanib and cabozantinib are unclear. The lack of specificity for RET diminishes their therapeutic window, because the inhibition of other kinases results in toxic effects at high doses. Current evidence indicates that the kinase activity of oncogenic drivers must be inhibited profoundly for maximal therapeutic benefit.86 Accordingly, there is growing interest in developing more selective RET kinase inhibitors, which may be more effective in patients with medullary thyroid carcinomas or other cancers that are driven by RET fusions, such as non–small-cell lung cancer,87 papillary thyroid carcinoma, and myelomonocytic leukemia.88
SUMMARY
Recent discoveries in molecular medicine, coupled with advances in biotechnology and medicinal chemistry, have led to enormous progress in the diagnosis and treatment of patients with thyroid cancer. We have no doubt that this progress will continue with the development of more effective therapies that are based on new compounds with greater specificity for oncogenic targets and combinatorial regimens that overcome resistance to single agents.
Supplementary Material
Acknowledgments
We thank Iňigo Landa, Ph.D., for help with the design of earlier versions of the figures and Ronald Ghossein, M.D., and Bin Xu, M.D., Ph.D., for information on the incidence of papillary thyroid carcinoma variants.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–7. [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–11. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their anti-tumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–33. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lito P, Pratilas CA, Joseph EW, et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell. 2012;22:668–82. doi: 10.1016/j.ccr.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabra MM, Dominguez JM, Grewal RK, et al. Clinical outcomes and molecular profile of differentiated thyroid cancers with radioiodine-avid distant metastases. J Clin Endocrinol Metab. 2013;98(5):E829–36. doi: 10.1210/jc.2012-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–9. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobrinho-Simões M, Eloy C, Magalhães J, Lobo C, Amaro T. Follicular thyroid carcinoma. Mod Pathol. 2011;24(Suppl 2):S10–8. doi: 10.1038/modpathol.2010.133. [DOI] [PubMed] [Google Scholar]
- 14.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–26. doi: 10.1210/jc.2002-021907. [DOI] [PubMed] [Google Scholar]
- 15.Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98(9):E1562–6. doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-Chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res. 2000;6:1093–103. [PubMed] [Google Scholar]
- 17.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–44. doi: 10.1172/JCI69766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;290:138–41. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 19.Gudmundsson J, Sulem P, Gudbjartsson DF, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet. 2009;41:460–4. doi: 10.1038/ng.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gara SK, Jia L, Merino MJ, et al. Germ-line HABP2 mutation causing familial non-medullary thyroid cancer. N Engl J Med. 2015;373:448–55. doi: 10.1056/NEJMoa1502449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahasrabudhe R, Estrada A, Lott P, et al. The 8q24 rs6983267G variant is associated with increased thyroid cancer risk. Endocr Relat Cancer. 2015;22:841–9. doi: 10.1530/ERC-15-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomsic J, Fultz R, Liyanarachchi S, He H, Senter L, de la Chapelle A. HABP2 G534E variant in papillary thyroid carcinoma. PLoS ONE. 2016;11(1):e0146315. doi: 10.1371/journal.pone.0146315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–65. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 24.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–15. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 25.Santhanam P, Khthir R, Gress T, et al. Gene expression classifier for the diagnosis of indeterminate thyroid nodules: a meta-analysis. Med Oncol. 2016;33:14. doi: 10.1007/s12032-015-0727-3. [DOI] [PubMed] [Google Scholar]
- 26.Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid. 2015;25:1217–23. doi: 10.1089/thy.2015.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda H, Miyauchi A, Ito Y, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–5. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuttle RM, Tala H, Shah J, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–9. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–10. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 33.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–26. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schvartz C, Bonnetain F, Dabakuyo S, et al. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97:1526–35. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 36.Hay ID, Hutchinson ME, Gonzalez-Losada T, et al. Papillary thyroid micro-carcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–7. doi: 10.1016/j.surg.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 37.Schlumberger M, Catargi B, Borget I, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–73. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Larson SM, Tuttle RM, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001;11:1169–75. doi: 10.1089/10507250152741028. [DOI] [PubMed] [Google Scholar]
- 39.Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028–35. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 40.De Bernardi IC, Floridi C, Muollo A, et al. Vascular and interventional radiology radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: literature review. Radiol Med. 2014;119:512–20. doi: 10.1007/s11547-014-0411-2. [DOI] [PubMed] [Google Scholar]
- 41.Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW. Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013;154:1448–54. doi: 10.1016/j.surg.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Tufano RP, Clayman G, Heller KS, et al. Management of recurrent/persistent nodal disease in patients with differentiated thyroid cancer: a critical review of the risks and benefits of surgical intervention versus active surveillance. Thyroid. 2015;25:15–27. doi: 10.1089/thy.2014.0098. [DOI] [PubMed] [Google Scholar]
- 43.Romesser PB, Sherman EJ, Shaha AR, et al. External beam radiotherapy with or without concurrent chemotherapy in advanced or recurrent non-anaplastic non-medullary thyroid cancer. J Surg Oncol. 2014;110:375–82. doi: 10.1002/jso.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21:31–5. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 45.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radio-iodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–30. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 47.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn L, Fagin JA. Therapy: lenvatinib and radioiodine-refractory thyroid cancers. Nat Rev Endocrinol. 2015;11:325–7. doi: 10.1038/nrendo.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 51.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–72. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–13. doi: 10.1200/JCO.2007.15.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hick AC, Delmarcelle AS, Bouquet M, et al. Reciprocal epithelial:endothelial paracrine interactions during thyroid development govern follicular organization and C-cells differentiation. Dev Biol. 2013;381:227–40. doi: 10.1016/j.ydbio.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26:605–22. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052–66. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373:1733–47. doi: 10.1056/NEJMoa1506583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kopetz S, Desai J, Chan E, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol. 2015;33:4032–8. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 60.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAFV600E-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016 Jul 22; doi: 10.1016/S1470-2045(16)30166-8. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–64. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 63.Hiltzik D, Carlson DL, Tuttle RM, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–95. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 64.Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bible KC. Treating advanced radio-resistant differentiated thyroid cancer. Lancet Oncol. 2012;13:854–5. doi: 10.1016/S1470-2045(12)70342-X. [DOI] [PubMed] [Google Scholar]
- 66.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–36. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Godbert Y, Henriques de Figueiredo B, Bonichon F, et al. Remarkable response to crizotinib in woman with anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma. J Clin Oncol. 2015;33(20):e84–7. doi: 10.1200/JCO.2013.49.6596. [DOI] [PubMed] [Google Scholar]
- 68.Moura MM, Cavaco BM, Leite V. RAS proto-oncogene in medullary thyroid carcinoma. Endocr Relat Cancer. 2015;22(5):R235–52. doi: 10.1530/ERC-15-0070. [DOI] [PubMed] [Google Scholar]
- 69.Ji JH, Oh YL, Hong M, et al. Identification of driving ALK fusion genes and genomic landscape of medullary thyroid cancer. PLoS Genet. 2015;11(8):e1005467. doi: 10.1371/journal.pgen.1005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grubbs EG, Ng PK, Bui J, et al. RET fusion as a novel driver of medullary thyroid carcinoma. J Clin Endocrinol Metab. 2015;100:788–93. doi: 10.1210/jc.2014-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–7. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 72.Machens A, Brauckhoff M, Holzhausen HJ, Thanh PN, Lehnert H, Dralle H. Codon-specific development of pheochromocytoma in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab. 2005;90:3999–4003. doi: 10.1210/jc.2005-0064. [DOI] [PubMed] [Google Scholar]
- 73.Frank-Raue K, Rybicki LA, Erlic Z, et al. Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum Mutat. 2011;32:51–8. doi: 10.1002/humu.21385. [DOI] [PubMed] [Google Scholar]
- 74.Decker RA, Peacock ML, Watson P. Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet. 1998;7:129–34. doi: 10.1093/hmg/7.1.129. [DOI] [PubMed] [Google Scholar]
- 75.Moore SW, Zaahl M. The Hirsch-sprung’s-multiple endocrine neoplasia connection. Clinics (Sao Paulo) 2012;67(Suppl 1):63–7. doi: 10.6061/clinics/2012(Sup01)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ceccherini I, Romei C, Barone V, et al. Identification of the Cys634 Tyr mutation of the RET proto-oncogene in a pedigree with multiple endocrine neoplasia type 2A and localized cutaneous lichen amyloidosis. J Endocrinol Invest. 1994;17:201–4. doi: 10.1007/BF03347719. [DOI] [PubMed] [Google Scholar]
- 77.Jasim S, Ying AK, Waguespack SG, et al. Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid. 2011;21:189–92. doi: 10.1089/thy.2010.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wells SA, Jr, Robinson BG, Santoro Pacini F. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013;98:3149–64. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elisei R, Romei C, Cosci B, et al. RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J Clin Endocrinol Metab. 2007;92:4725–9. doi: 10.1210/jc.2007-1005. [DOI] [PubMed] [Google Scholar]
- 80.Trimboli P, Cremonini N, Ceriani L, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: a retrospective multicentre study. Clin Endocrinol (Oxf) 2014;80:135–40. doi: 10.1111/cen.12234. [DOI] [PubMed] [Google Scholar]
- 81.Elisei R, Bottici V, Luchetti F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163–8. doi: 10.1210/jc.2003-030550. [DOI] [PubMed] [Google Scholar]
- 82.Daniels GH. Screening for medullary thyroid carcinoma with serum calcitonin measurements in patients with thyroid nodules in the United States and Canada. Thyroid. 2011;21:1199–207. doi: 10.1089/thy.2010.0297. [DOI] [PubMed] [Google Scholar]
- 83.Miyauchi A, Matsuzuka F, Hirai, et al. Prospective trial of unilateral surgery for nonhereditary medullary thyroid carcinoma in patients without germline RET mutations. World J Surg. 2002;26:1023–8. doi: 10.1007/s00268-002-6665-1. [DOI] [PubMed] [Google Scholar]
- 84.Skinner MA, Moley JA, Dilley WG, Owzar K, Debenedetti MK, Wells SA., Jr Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med. 2005;353:1105–13. doi: 10.1056/NEJMoa043999. [DOI] [PubMed] [Google Scholar]
- 85.Engelbach M, Görges R, Forst T, et al. Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab. 2000;85:1890–4. doi: 10.1210/jcem.85.5.6601. [DOI] [PubMed] [Google Scholar]
- 86.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gainor JF, Shaw AT. The new kid on the block: RET in lung cancer. Cancer Discov. 2013;3:604–6. doi: 10.1158/2159-8290.CD-13-0174. [DOI] [PubMed] [Google Scholar]
- 88.Ballerini P, Struski S, Cresson C, et al. RET fusion genes are associated with chronic myelomonocytic leukemia and enhance monocytic differentiation. Leukemia. 2012;26:2384–9. doi: 10.1038/leu.2012.109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.