Abstract
Here we report the identification of protein targets of chemopreventive phenethyl isothiocyanate (PEITC) via “click” chemistry in the A549 human lung cancer cell line, using a novel alkyne-tagged PEITC which was also found to show potent in vitro anticancer activity.
Isothiocyanates (ITCs) derived from cruciferous vegetables1 are shown to inhibit carcinogenesis in animal models.2-5 Inverse associations between cruciferous vegetable/ITCs intake and cancers have been demonstrated in epidemiological studies.6 Inducing apoptosis7-9 is considered as an important mechanism for their cancer preventive effects because it may eliminate the initiated cells.10 However, the molecular target(s) for inducing apoptosis by ITCs is still under investigation.11 It is known that ITCs can conjugate with glutathione (GSH) and induce cellular oxidative stress; ITCs can also bind to intracellular proteins (e.g. tubulin) which can induce apoptosis.12 We reported previously that phenethyl isothiocyanate (PEITC), an ITC rich in watercress, induced apoptosis underlies a mechanism of inhibiting tumorigenesis, and direct covalent binding to cellular proteins which may constitute an important early event in the induction of apoptosis by PEITC in A549 human lung cancer cells.13 As electrophiles, ITCs can form covalent bond with the thiol groups in proteins.14 To fully understand the cellular responses to PETIC, it is crucial to develop an unbiased method that can rapidly identify their protein targets. Compared with the two-dimensional gel electrophoresis method using radiolabeled PEITC reported previously,12 the major advantage offered by the click chemistry method are: (1) convenient and simple to perform; (2) easy to enrich targeted protein(s); (3) more cost-effective; (4) higher specificity; and (5) safer for the researchers.
“Click” chemistry15 was introduced in 2001 by K.B. Sharpless and associates. It has emerged as a powerful tool in drug discovery,16, 17 chemical biology7 and proteomic applications.18, 19 In this study, a click chemistry-based method, which is pioneered by Cravatt et al,16 was developed to identify the direct protein targets of PEITC in A549 human lung cancer cells. In order to perform the “click” chemistry, the tagged ITC (N-(2-(4-methoxyalkyne)phenethyl)isothiocyanate (NPEITC) (Scheme 1) was synthesized containing an alkyne moiety for copper-catalyzed Huisgen 1,3-dipolar cycloaddition reaction (click chemistry). In order to demonstrate the effect of ITC functional group, the amide analogue of NPEITC was also synthesized: N-(2-(4-methoxyalkyne)phenylethyl)acetamide (NPA) (Fig. 1). NPA serves as a negative control because it does not contain the ITC moiety to form conjugations with proteins, but the alkyne group was maintained for “click” chemistry.
Scheme 1.
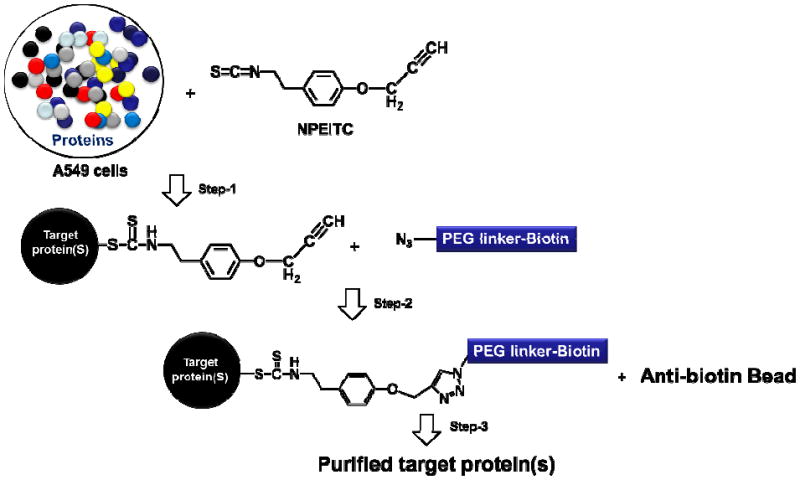
Proposed mechanism of using “click” chemistry to isolate proteins targeted by NPEITC. Step 1. Cancer cells are treated with NPEITC and NPEITC conjugated proteins are formed; Step 2. “Click” chemistry reaction of copper-catalyzed azide-alkyne cycloaddtion (CuAAC) of NPEITC with biotin-PEG azide, NPEITC conjugated proteins are tagged with biotin; Step 3. Streptavidin magnetic beads are used to pull down the NPEITC targeted proteins tagged with biotin through NPEITC conjugation.
Figure 1.
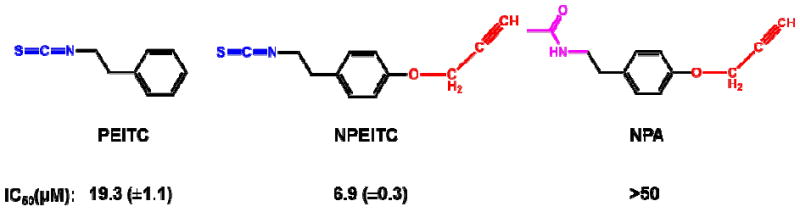
IC50 values of NPEITC, NPA and PEITC against A549 human lung cancer cell line.
The three-step reaction of using NPEITC to identify the direct protein targets is summarized in Scheme 1. After the treatment with NPEITC, the cell lysates were applied to “click” chemistry involving the reaction between an alkyne and an azide, with a bio-recognizable motif (biotin) for affinity chromatography. The facile and reversible interaction between biotin and streptavidin enables highly specific protein purification. The proteins were then analysed by mass spectrometry for the identification of targeted proteins. Using NPA as the negative control will rule out potential false positive proteins introduced by click chemistry.
To validate the alkyne tagged-ITC compound (NPEITC), the anticancer activities of NPETIC, NPA and PEITC were determined in A549 cells by WST-1 assay. NPEITC (IC50 = 6.9 μM) showed stronger anticancer activity than PEITC (IC50 = 19.3 μM) after 24 h treatment, this result may be attributed to the increased hydrophobicity of NPEITC compared to PEITC which could facilitate its cellular uptake. In contrast, NPA did not show anticancer activity with an IC50 value larger than 50 μM (Fig.1 and S1). The IC50 of PEITC is consistent with our previous report.12 The flow cytometry data also showed that NPEITC had a significantly higher potency to induce apoptosis than PEITC, but NPA did not induce apoptosis even at the highest concentration of 20 μM. The apoptosis data is consistent with the viability result. In summary, NPEITC showed potent growth inhibitory activity, similar to PEITC, and NPA is devoid of activity, indicating that the electrophilic ITC functional group is critical for the anticancer activity of NPEITC (Fig. 2A).
Figure 2.
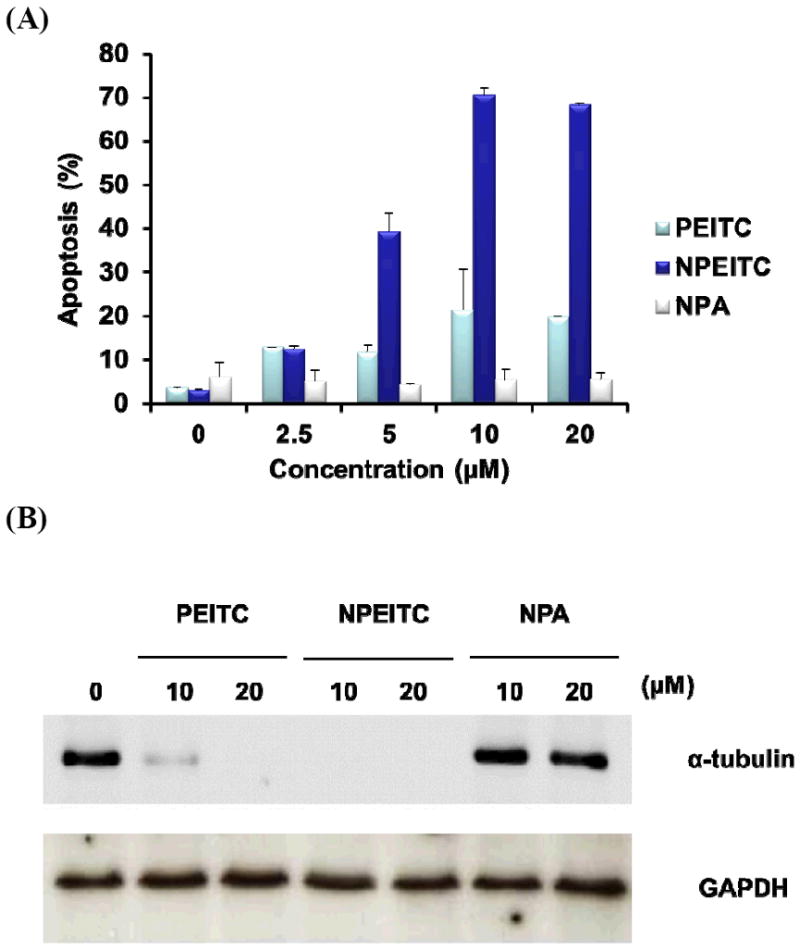
(A) Apoptosis induced by incubation with NPEITC, PEITC and NPA in A549 human lung cancer cells at different concentrations for 24 h at 37°C. (B) Tubulin degradation effects by NPEITC, PEITC and NPA in A549 human lung cancer cell line.
Previously, we reported PEITC was able to bind and subsequently degrade α-tubulin in vitro.12 To further confirm NPEITC behaves similarly to PEITC, the degradation of tubulin in A549 cells was examined. The results showed NPEITC could also degrade α-tubulin, suggesting that NPEITC is functionally similar to PEITC (Fig 2B). Therefore, NPEITC may serve as a valid and useful tool for identifying binding proteins of PEITC using the click chemistry approach. On the contrary, no tubulin degradation was observed after the treatment with NPA. Similar effects of PEITC and NPEITC on the α-tubulin and poly(ADP-ribose) polymerase (PARP) cleavage were also observed in HT29 human colon cancer cells further support NPEITC to be a viable compound to study the protein targets of PEITC (Fig S2). The binding of NPEITC, PEITC and NPA with tubulin was also studied using the Ellman assay, which quantitatively measures the sulfhydryl groups on tubulin.20 After incubation for 1 h at 37°C of NPEITC and PEITC (180, 360 and 720 μM) with tubulin (ca. 16 μM), a dose-dependent binding to sulfhydryl groups on tubulin proteins was observed, with ca. 40% of modification achieved for both NPEITC and PEITC at 720 μM (Fig S3).
Standard “click” chemistry reactions were performed to optimize the conditions for NPEITC and NPA with azide-TEG biotin:21 sodium ascorbate is used to reduce CuII to CuI, and the ligand tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) was used to stabilize the CuI catalyst. The details of the click reactions are listed in table S1. In brief: the azide-alkyne cycloaddition between NPEITC or NPA with biotin-TEG azide was performed in an aqueous solution with 20% ethanol to facilitate the solubility of NPEITC and NPA. The reactions were kept at 25°C for 2 h, producing a yield of larger than 99% for both reactions. The click chemistry reaction conditions of copper-catalyzed azide-alkyne cycloaddtion (CuAAC) for NPEITC and NPA with azide-TEG biotin were optimized and the reaction products were isolated after the reactions and then analysed by mass spectrometry (Fig. S3): for the click reaction product of NPEITC and azide-TEG biotin, the m/z = 662.3326 was found, (calculated m/z = 662.3336), for the product of NPA and azide-TEG biotin, the m/z = 662.2784 was found (calculated m/z = 662.2794). These results confirmed the formation of the conjugated products for both NPA and NPEITC with azide-TEG biotin.
Evidence of using NPEITC to label protein targets with biotin moiety through click reactions was obtained by a model reaction of NPPETIC with tubulin. NPEITC was incubated with tubulin at 37°C for 1 h, and then click reaction was performed under the same conditions as described above (details listed in Table S1). The western blot results showed that tubulin was labelled with biotin after incubation with NPEITC (Fig 3, lane 2). PEITC was found to show a competitive effect to the binding between NPEITC and tubulin (lane 4). On the contrary, there was no detectable biotin signal for tubulin samples treated with PEITC or NPA. Because PEITC does not have the alkyne group for click chemistry and NPA lacks the ITC group to bind to tubulin.
Figure 3.
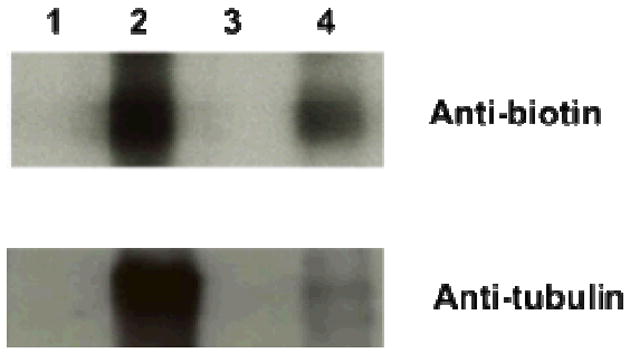
Tubulin labelled with biotin through NPEITC conjugation. (1) PEITC (2) NPEITC (3) NPA (4) PEITC + NPEITC (1:1).
We then used the cell lysate from A549 cells to react with increased concentrations of NPEITC for 1 h at 37°C. After click reactions with azide-TEG biotin, the western blot results showed the proteins were labelled with biotin in a concentration dependent manner when there was a barely detectable signal in the DMSO control (Fig. 4A). Finally, we treated A549 cells with ITCs (8 μM) for 4 h. The protein lysates obtained were used for click reactions with azide-TEG biotin, then purified with magnetic streptavidin beads. The protein bands from a stain-free gel are shown the Figure 4B: clear protein bands were shown after the treatment with NPEITC (lane 4); however, no observable bands of proteins were observed in the treatment with PEITC (lane 1), NPA (lane 2) or with PEITC (8 μM) competition (lane 3); indicating the specificity of this method. The proteins from lane 4 were then analysed by proteomic assay using MS/MS. The details of the proteins identified are listed in Table S3; however, no detectable proteins were found after the treatment of PEITC or NPA by mass spectrometry which is consistent with the results of stain-free gel.
Figure 4.
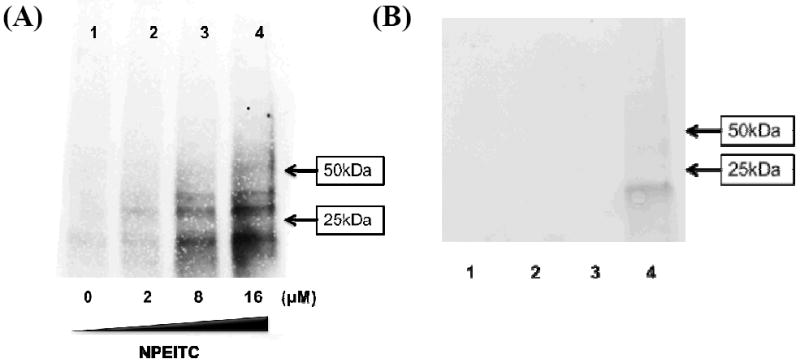
Western blot of protein labelled with biotin (A) and proteins obtained after click chemistry (B). (A) NPEITC at different concentrations (0, 2, 8 and 16 μM) were used to incubate with cell lysate from A549 cells at 37°C for 2 h; (B) A549 cells were treated with PEITC (lane 1, 8 μM), NPA (lane 2, 8 μM), PEITC (lane 3, 8 μM) + NPEITC (lane 3, 8 μM) and NPEITC (lane 4, 8 μM) for 4 h.
Among the seven protein targets of NPEITC identified by click chemistry, actin, vimentin and tubulin were already known as the targets for PEITC22 (Table 1); It is also known that actin,23 tubulin,23 vimentin,24 Hsp9025 are potential anticancer targets, It requires future work to identify which target(s) plays a central role in the biological function of NPEITC and PEITC.
Table 1.
Protein Targets identified through click chemistry method of NPEITC.
| Proteins identified by click chemistry | Targets known for PEITC |
|---|---|
| Actin | Yes |
| Annexin A2 | No |
| Elongation factor 1-alpha 1 | No |
| Vimentin | Yes |
| Glyceraldehyde-3-phosphate dehydrogenase | No |
| Heat shock protein HSP 90-alpha | No |
| Tubulin | Yes |
In summary, a novel alkyne-tagged ITC (NPEITC) was synthesized for the development of a “click” chemistry-based method for identifying the protein targets for PEITC. A short list of potential protein targets was identified, which may pave the road for understanding the biological functions of ITCs as chemopreventive agents.
Supplementary Material
Acknowledgments
This work is supported by NCI (grant CA100853 and RO1-CA-100083-7), National Institutes of Health grant RO1CA135069 and Lombardi Cancer Center Support Grant P30 CA51008. We thank Dr. Lawrence Marnett (Vanderbilt University) and Dr. Qiang Zhang (University of Warwick) for the suggestions on “click” chemistry, Mr. Jishen Pan and Dr. Marcin Dyba for the help of HPLC purification, Drs. Ercheng Li, Amrita Cheema and Georgetown Lombardi Shared Resources for NMR and MS analysis, Dr. Karen Creswell and Ms Marcy Chen (both are from core facilities of Lombardi Comprehensive Cancer Centre at Georgetown University) for flow cytometry analysis.
Footnotes
Electronic Supplementary Information (ESI) available: [experimental part, table S1-S2, figure S1-S4 and NMR, HR-MS data for NPEITC and NPA]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Song L, Morrison JJ, Botting NP, Thornalley PJ. Anal Biochem. 2005;347:234–243. doi: 10.1016/j.ab.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Powolny AA, Bommareddy A, Hahm E-R, Normolle DP, Beumer JH, Nelson JB, Singh SV. JNCI J Natl Cancer Inst. 2011;103:571–584. doi: 10.1093/jnci/djr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao CV. Cancer Prev Res. 2013;6:760–763. doi: 10.1158/1940-6207.CAPR-13-0242. [DOI] [PubMed] [Google Scholar]
- 4.Singh SV, Singh K. Carcinogenesis. 2012;33:1833–1842. doi: 10.1093/carcin/bgs216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pezzuto JM, Suh N, Dinkova-Kostova A. Natural Products in Cancer Prevention and Therapy. Vol. 329. Springer; Berlin Heidelberg: 2013. pp. 179–201. [Google Scholar]
- 6.London SJ, Yuan J-M, Chung F-L, Gao Y-T, Coetzee GA, Ross RK, Yu MC. The Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 7.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont M-A, Chevolleau S, Gasc N, Tulliez J, Tercé F. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 8.Yu R, Mandlekar S, Harvey KJ, Ucker DS, Kong ANT. Cancer Res. 1998;58:402–408. [PubMed] [Google Scholar]
- 9.Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Cancer Res. 1997;57:1301–1305. [PubMed] [Google Scholar]
- 10.Zhang Y, Talalay P. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 11.Cheung K, Kong A-N. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi L, Xiao Z, Hood BL, Dakshanamurthy S, Wang X, Govind S, Conrads TP, Veenstra TD, Chung F-L. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung F-L. Cancer Res. 2007;67:6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 14.Mi L, Di Pasqua AJ, Chung F-L. Carcinogenesis. 2011;32:1405–1413. doi: 10.1093/carcin/bgr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 16.Speers AE, Cravatt BF. J Am Chem Soc. 2005;127:10018–10019. doi: 10.1021/ja0532842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb HC, Sharpless KB. Drug Discov Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 18.Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Chem Res Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szapacs ME, Kim H-YH, Porter NA, Liebler DC. J Proteome Res. 2008;7:4237–4246. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellman GL. Arch Biochem Biophys. 1958;74:443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- 21.Meldal M, Wenzel Christian. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 22.Mi L, Hood BL, Stewart NA, Xiao Z, Govind S, Wang X, Conrads TP, Veenstra TD, Chung F-L. Chem Res Toxicol. 2011;24:1735–1743. doi: 10.1021/tx2002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan MA, Wilson L. Curr Opin Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 24.Lahat G, Zhu Q-S, Huang K-L, Wang S, Bolshakov S, Liu J, Torres K, Langley RR, Lazar AJ, Hung MC, Lev D. PLoS ONE. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Li Y, Zhang T, Schwartz SJ, Sun D. Drug Resist Update. 2009;12:17–27. doi: 10.1016/j.drup.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


