Abstract
Advances in detection and supportive care strategies have led to improvements in cancer-specific and overall survival after a diagnosis of early-stage breast cancer. These improvements, however, are associated with an increase in competing forms of morbidity and mortality particularly cardiovascular disease (CVD). Indeed, in certain subpopulations of patients after early breast cancer, CVD is not only the leading cause of mortality but these women also have an increased risk of CVD-specific morbidity, including elevated incidence of coronary artery disease and heart failure (HF) compared to sex- and age-matched counterparts. Exercise treatment is established as the cornerstone of primary and secondary prevention of CVD in multiple clinical populations. The potential benefits of exercise treatment to modulate CVD or CVD risk factors before, immediately after, or in the months/years following adjuvant therapy for early-stage breast cancer has received limited attention. Here, we discuss the risk and extent of CVD in breast cancer patients, review the pathogenesis of CVD, and highlight existing evidence from select clinical trials investigating the efficacy of structured exercise treatment across the CVD continuum in early breast cancer.
Keywords: cardiovascular disease, exercise treatment, phenomapping
Introduction
Advances in early detection and adjuvant therapy have led to significant improvements in the five-year survival rate from 80% in 1950 to 98% today among women diagnosed with early-stage breast cancer.1,2 However, improvements in overall survival are at risk of being offset by an increased risk of late occurring cardiovascular toxicities.1,3 In comparison with age-matched counterparts, breast cancer patients have a 1.7 to1.8-fold increased risk of CVD-specific mortality,4,5 a 1.2 to 1.3-fold increased risk of CVD6 (e.g., coronary artery disease, cerebrovascular disease, and HF), and a 1.3 to 3.1-fold increased risk of CVD risk factors (e.g., hypertension, diabetes, and dyslipidemia).6 The excess CVD risk is likely a consequence of acute direct (i.e., direct cytotoxic/radiation-induced injury) as well as indirect (i.e., impacts secondary to therapy such as deconditioning) effects of breast cancer therapy.5,7–11 Given the growing clinical importance, a research agenda that systematically addresses CVD prevalence, pathogenesis, and treatment in early-stage breast cancer is important and timely.6,7
In recent work, our group adapted a classical cardiology paradigm (i.e., primordial, primary, secondary, tertiary prevention) to develop a continuum framework for cancer therapy-associated acute and long-term cardiovascular toxicities in early-stage breast cancer.8 This framework can be applied to better understand: (1) the role of tools and methodologies to identify those individuals with, or at high-risk of, cardiovascular toxicity, and, (2) the type and timing of strategies to prevent, mitigate, or treat cardiovascular toxicities.8 In terms of the latter, several research groups have started to investigate the efficacy of cardiovascular medications (e.g., angiotensin converting enzyme inhibitors, statins) as prophylactic strategies to prevent,9,10 as well as treat,11,12 cardiac-related effects (e.g. decreases in left ventricular ejection fraction (LVEF)) of known cardiotoxic agents such as anthracyclines or trastuzumab.
However, therapy-induced toxicity is not only limited to the heart, but can have deleterious effects across the cardiac/pulmonary/vascular/muscular axis.7,8,13 For example, both localized (e.g. radiotherapy) and systemic (e.g., chemotherapy or hormone therapy) treatment modalities can cause pulmonary dysfunction, anemia, arterial stiffness, and skeletal muscle dysfunction (e.g. reduced oxidative phosphorylation).7,14,15 The pleiotropic nature of these therapy-induced deficits creates a strong rationale for the design and testing of intervention strategies that can simultaneously improve function across these multiple organ systems, and consequently, the overall reserve capacity of the cardiovascular network.16 Exercise treatment has the unique capacity to improve the reserve function of multiple organs cumulating in marked augmentation of global cardiovascular reserve capacity in numerous clinical settings.17
Against this background, here we outline the extent of CVD in breast cancer patients, briefly overview the pathogenesis of CVD, and adopt our cancer - CVD continuum framework (Figure 1) to review existing evidence from investigations of structured exercise treatments.
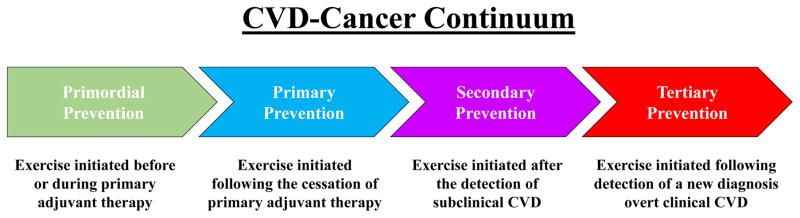
Figure 1.
Exercise-CVD Continuum in Oncology. (1) primordial prevention – exercise initiated before or during primary adjuvant therapy (i.e., definitive surgery followed by radiotherapy, chemotherapy, or trastuzumab) to mitigate and/or attenuate potential therapy-induced toxicity, (2) primary prevention – exercise initiated following the cessation of primary adjuvant therapy to reverse / attenuate subclinical cardiovascular impairments, (3) secondary prevention – exercise initiated after the detection of a LVEF decline of ≥10% from baseline (pre-treatment) or beyond the lower limit of normal (<53%), poor VO2peak (<15ml/kg/min), angina, transient ischemic attack, and (4) tertiary prevention – exercise initiated following detection of a new diagnosis of overt symptomatic HF, coronary artery disease, stroke, valve replacement, or serious arrhythmia following initiation of anticancer therapy.
Prevalence of CVD
The prevalence of CVD has been comprehensively reviewed previously12,25 and is summarized in Table 1. Importantly, evidence indicates that there is considerable long-term risk of CVD, particularly for women older than 65 years of age.18 For instance, data from the Surveillance, Epidemiology and End Results (SEER) indicated that after 12 years of follow-up, CVD was the leading cause of death (15.9%), closely followed by breast cancer (15.1%).19 Furthermore, Hooning et al.6 reported that compared to women from the general population, early breast cancer patients had a 30% increased standardized incidence ratio of CVD events. These findings demonstrate both an increased CVD prevalence and incidence in breast cancer patients and highlight the prematurity of its development compared to women matched in age and without a history of cancer. Unfortunately, in the absence of effective CVD screening and prevention strategies, there is no reason to expect that these rates will improve over time – especially against the backdrop of increasing breast cancer incidence, high survival rates, and rapidly evolving treatment regimes.13
Table 1.
Incidence of CVD risk factors and CVD in breast cancer patients.
Pathogenesis of CVD
As patients progress through treatment regimens they sustain subclinical and overt therapy-induced cardiovascular injuries which, when coupled with adverse lifestyle perturbations, may result in a significantly elevated incidence of CVD risk factors, overt CVD, and CVD-related mortality.4,6,20 We labeled this phenomenon the ‘multiple hit’ hypothesis;7 a brief overview is provided herein.
Therapy-Related Direct Cardiovascular Injury
For a comprehensive overview of the mechanisms of therapy-induced cardiovascular toxicity, the reader is referred to prior excellent reviews.21,22 In the following sections, we briefly outline the biological mechanisms that may underlie therapy-associated (i.e., anthracyclines, radiotherapy, trastuzumab, endocrine therapy) cardiovascular toxicity.
The adverse cardiovascular effects of anthracycline (e.g. doxorubicin) therapy are well-recognized.11,23 In brief, anthracycline-induced generation of reactive oxygen species influences multiple pathways of cardiotoxicity risk including the tumor suppressor protein and p53,24 and suppresses sarcomere protein synthesis through depletion of GATA-4 dependent gene expression25 and cardiac progenitor cells.26 Indeed, anthracycline-induced myocyte necrosis may elicit a maladaptive biomechanical effect, ultimately manifesting as a dilated cardiomyopathy, or HF with reduced ejection fraction in the years following therapy.27,28 The risk of HF increases with a cumulative dose of anthracycline, as such, the maximum lifetime cumulative dose for doxorubicin is 400 to 550 mg/m2.29
Radiation therapy has been demonstrated to be an independent risk factor for death from CVD up to 20 years after breast cancer.6,30,31 Ionizing radiation therapy can cause coronary vascular injury characterized by endothelial cell proliferation, intimal thickening, medial scarring, lipid deposits and adventitial fibrosis.32 Together with the perturbation of homoeostatic control of reactive oxygen species, this cascade of events ultimately contributes to CAD.6,30,31
Trastuzumab is approved by the U.S. Food and Drug Administration (FDA) for the treatment of early breast cancer.33 Trastuzumab prevents heterodimerization of ErbB4 and ErbB2 in cardiomyocytes,34 thus limiting myocardial cell growth, glucose uptake and protein regulation.35 These alterations may ultimately trigger the progression of HF.36 Evidence also indicates an additive, and possibly synergistic, adverse interaction between anthracyclines and trastuzumab, where trastuzumab may constitute sequential stress on an already compromised heart.37 Indeed, trastuzumab could prevent the myocyte’s adaptive response to, or repair of, the anthracycline injury – further compounding the damage.38
Endocrine therapy (e.g., tamoxifen, aromatase inhibitors) for women with hormone receptor-positive breast cancer has been associated with cardiotoxicity; however, recent data indicates that aromatase inhibitors and tamoxifen have different toxicity profiles.39 For example, in comparison with tamoxifen, aromatase inhibitors are associated with increased risk of CAD and HF.39,40 Furthermore, in 47 women receiving endocrine therapy, we reported those receiving aromatase inhibitors had a less favorable profile for several established (e.g. lipid profile) and emerging (e.g. C-reactive protein) risk factors for CVD.41 The marked reduction in serum estrogen associated with aromatase inhibitors therapy may contribute to the development of atherosclerosis. 42
Therapy-Related Indirect Cardiovascular Injury
In addition to the aforementioned direct treatment-related mechanisms of cardiovascular injury, preexisting and treatment-related moderators may also drive the observed increases in CVD risk.
Preexisting CVD risk factors such as hypertension, diabetes, hyperlipidemia, and obesity may be higher in breast cancer patients than in the general population, likely because there are common risk factors for both CVD and cancer.43 Importantly, preexisting CVD risk factors are strong predictors of anthracycline,18 trastuzumab,44 and radiation-related31 CVD. For example, Chapman et al.20 investigated the competing causes of death in a randomized trial of extended adjuvant endocrine therapy among 5,170 early breast cancer patients. After 3.9 years median follow-up, non-breast cancer-related deaths (primarily CVD) accounted for 60% of overall mortality. Moreover, patients with pre-existing CVD risk factors at baseline (i.e. self-report; yes vs. no) were 54% more likely to die from non-breast cancer-related causes. The development of de novo CVD risk factors following therapy also likely increases risk of CVD.41,45 Hooning et al.4,6 examined the long-term causes of mortality among 7,425 women treated for early breast cancer and found that, after a median follow-up of 13.8 years, patients diagnosed with one CVD risk factor (i.e., smoking, hypertension, diabetes mellitus, and hyperlipidemia) at any time during the study follow-up had 1.4 to 3.1-fold higher risk of CVD-related mortality relative to age-matched women in general population.
Additionally, preliminary evidence suggests that indirect treatment-related factors such as physical inactivity and weight gain, strong independent predictors of CVD and all-cause mortality in non-cancer populations,46 may also contribute to the observed increased CVD mortality risk in the breast cancer setting. In a meta-analysis of twelve studies involving 23,832 early-stage breast cancer patients, Playdon et al.47 reported that a weight gain of more than 5% from diagnosis to post-treatment (1 to 8 years) was associated with a 12% increase risk of all-cause mortality (HR = 1.12, 95% CI = 1.03 to 1.22) compared with weight maintenance. Similarly, Nichols and colleagues48 found that 3993 women with early-stage breast cancer (5.8 years post-diagnosis) with a body mass index greater than 30 kg/m2 (classified as obese) had a CVD mortality rate 1.65 times that of women with a normal body mass index (18.5–24.9 kg/m2); each 5 kg weight gain was associated with a 19% increase in CVD mortality (p = 0.04). This may be clinically important since weight gain during adjuvant therapy is common.49
Exercise Treatment Trials in Early-Stage Breast Cancer
Meta-analyses and systematic reviews conclude that structured exercise is a safe and well-tolerated adjunct strategy associated with significant improvements in a wide range of psychosocial (e.g., patient reported fatigue, quality of life, physical functioning) and physiological (e.g., peak oxygen consumption (VO2peak), muscle strength, body composition) outcomes among women with early-stage breast cancer.50–52 However, beyond investigation of changes in VO2peak, there is scant information on the effects on cardiovascular events as well as conventional and novel cardiovascular risk factors.50–52 The rationale for examining the effect and underlying mechanisms of exercise on CVD events and risk factors in women with early-stage breast cancer, as well as other cancer populations at high-risk of chronic and late-occurring cardiovascular toxicity, is supported by a growing number of observational studies indicating that post-diagnosis exercise is associated with reductions in all-cause mortality in women with non-metastatic breast cancer.53,54 In a recent meta-analysis, post-diagnosis exercise was associated with a significant summary effect size of 0.52 (0.43 to 0.64) for all-cause mortality.53 Of importance, all prior work has used broad clinical event classifications such as death from other causes or total deaths, and has not specifically assessed cardiovascular-specific mortality or non-fatal cardiovascular morbidity/events. To this end, our group recently reported that post-diagnosis exposure to exercise was associated with substantial graded reductions in a composite of newly diagnosed CVD events or CVD death in 2973 women with early-stage breast cancer. Adherence to national exercise guidelines for adult cancer patients (i.e., ≥9 MET hours/week) was associated with an adjusted 23% reduction in the risk of CVD events, in comparison with not meeting the guidelines (<9 MET hours/week; p=0.0002). The association with exercise did not differ according to age, CVD risk factors, menopausal status, or anticancer treatment.
To provide insight into the potential effects of structured exercise on cardiovascular outcomes, we selected exemplar clinical trials that included cardiovascular outcomes (e.g., LVEF, VO2peak, resting heart rate, systolic blood pressure) in women with early-stage breast cancer; if trials among women with breast cancer were not available, trials involving other cancer populations were included. Studies were reviewed adopting our prior CVD-cancer continuum as an organizing framework to facilitate data interpretation and clinical application. Specifically, studies are reviewed using the four following distinct categories as described in Figure 1:8 primordial, primary, secondary, and tertiary prevention.
Primordial setting
Characterization of global cardiovascular reserve with VO2peak is a powerful predictor of cardiovascular and all-cause mortality in a broad range of adult populations.55,56 Emerging evidence indicates that VO2peak is also predictive of all-cause mortality in cancer patients.56–58 For instance, we found that in breast cancer patients with metastatic disease (n=52), the adjusted HR for all-cause mortality was 0.59 (95% CI, 0.29 to 1.19) for patients achieving a VO2peak ≤15.4 mL/kg/min compared to patients achieving a VO2peak >15.4 mL/kg/min.59 No prospective, observational studies have examined longitudinal changes in VO2peak during and following adjuvant therapy in patients with solid tumors; however, in the context of patients assigned to the usual care condition in randomized controlled trials of exercise training, it is possible to glean information. For example, Courneya et al.60 reported that 12 weeks of standard neoadjuvant chemotherapy was associated with a 9.5% decline in VO2peak from pre-chemotherapy to post-chemotherapy. Given that VO2peak typically declines 10% every decade in healthy women, these findings suggest there may be accelerated ‘physiological aging’ during short-term chemotherapy.59
A growing number of studies have sought to assess the efficacy of exercise training interventions to improve VO2peak in breast cancer patients. In a recent meta-analysis of six exercise training randomized controlled trials (involving 571 patients) that assessed the effects of exercise training in adults with cancer, exercise training led to significant improvement in VO2peak (mean weighted difference =+2.90 mL/kg/min, 95% CI: 1.16 to 4.64), compared to non-exercising sedentary control participants.52 However, the data from individual studies is more heterogeneous. For instance, in 242 operable breast cancer patients receiving standard adjuvant chemotherapy, Courneya et al.60 reported that aerobic training was associated with a non-significant improvement in VO2peak (+0.5 mL/kg/min) but completely abrogated the VO2peak decline observed in the usual care group. Our group conducted a phase II randomized trial to determine the efficacy of 12 weeks of aerobic training in attenuating anthracycline-induced changes in VO2peak (n = 10 exercise; 10 usual care).61 Intention-to-treat analysis indicated that VO2peak increased by 2.6 ± 3.5 mL/kg/min (+ 13.3%) in the exercise group and decreased by 1.5 ± 2.2 mL/kg/min (−8.6%) in the usual care group (between group difference, p = 0.001). Thus, aerobic training not only mitigated the detrimental impact of chemotherapy but also resulted in significant improvements in VO2peak during therapy. The discrepancy in exercise-induced improvement in VO2peak between these studies may be attributable to the differences in adjuvant therapy (taxanes62 vs. no taxanes61), exercise prescription (i.e. linear62 vs. non-linear61), or exercise intensity (i.e. <75% VO2peak62 vs. >90% VO2peak61).
Autonomic function may be another marker of central importance for CVD risk. For instance, in a 2012 study of 112,680 men and women without a history of cancer pooled from 12 cohort studies, higher resting heart rate (≥80 beats/min compared to <65 beats/min) was associated with an increased risk of both CVD events (HR, 1.44, 95% CI: 1.29–1.60) and all-cause mortality (HR 1.54, 1.43–1.66).63 In early stage breast cancer, several investigations have reported that following the completion of primary adjuvant therapy resting heart rate is, on average, 9% to 16% (7–16 beats/min) higher compared to age-matched controls.41,45,59 Initial exercise intervention studies in breast cancer patients have shown that following traditional exercise prescription guidelines (i.e., 3 days/week for 30 min at 60–70% of peak heart rate for 8 to 16 weeks) there is a reduction in resting heart rate64,65 of ~2 beat/min. 89,90 The prognostic relevance of these changes in breast cancer patients is unknown at present; however, prior work suggests that exercise training can shift autonomic balance and improve survival among CVD patients.66
Only one study to date, to our knowledge, has evaluated the efficacy of aerobic exercise on cardiac function and morphology in any cancer population. Haykowsky et al.67 conducted a single-arm study to examine the efficacy of 4 months of supervised aerobic training (3 days/week for 30–60 min at 60–90% peak heart rate) on cardiac function in women receiving trastuzumab for HER2 positive early breast cancer in 17 women (53 ± 7 years). Cardiac function was evaluated using magnetic resonance imaging to evaluate resting and dobutamine-induced peak LV volumes, mass, and LVEF. From baseline to post-intervention, resting and peak end diastolic and end systolic volumes significantly increased, while resting and peak LVEF significantly decreased. There was no change in VO2peak from baseline to post-intervention (19.8 ± 4.2 vs. 21.7 ± 6.6, p=0.2). These intriguing findings may provide initial insight into the integrative reserve of the oxygen cascade that contributes to VO2peak. Specifically, given that VO2peak is equal to the product of cardiac output and the arteriovenous O2 content difference (a-VO2), standard exercise training prescriptions may improve a-VO2 in women receiving trastuzumab, but may not counteract the mechanisms underlying trastuzumab-induced LV remodeling. However, whether exercise training improves peripheral vascular, microvascular function, and/or skeletal muscle oxygen extraction resulting in increased a-VO2 remains to be elucidated.
Primary setting
The therapy-induced decline in VO2peak may not recover even years following the cessation of primary therapy, For example, Jones et al.59 found that despite ‘normal’ resting cardiac function (i.e., LVEF ≥50%), VO2peak was, on average, 22% below that of age-matched sedentary women in 140 early breast cancer patients, a mean of 27 months following the completion of primary adjuvant therapy. Similarly, Khouri et al.68 found that VO2peak was, on average, 20% below that of age-matched sedentary women in 57 early breast cancer patients a mean of 26 months following the completion of primary therapy. These findings suggesting VO2peak will remain low or become even further impaired without exercise training highlight the need for intervention strategies to offset the direct and indirect effects of anticancer therapy.
Several groups have examined whether exercise training can improve VO2peak after the completion of breast cancer therapy. For instance, Courneya et al.69 examined the effects of 15 weeks of aerobic training (3 days/week for 30–60 min at power output that elicited ventilatory threshold) on VO2peak in 53 postmenopausal breast cancer survivors (15 months post-therapy surgery, radiotherapy, and/or chemotherapy with or without current hormone therapy use) randomly assigned to an exercise (n = 25) or control (n = 28) group. Peak oxygen consumption increased by 2.7 mL/kg/min in the exercise group, whereas it decreased by 0.6 mL/kg/min in the control group (mean difference, 3.4 mL/min; 95% confidence interval, 2.1 to 4.6; P <.001). Dolan et al.70 conducted a pilot study to examine the effects of supervised aerobic interval training and continuous moderate exercise training (6 weeks) against an unsupervised control group in 33 postmenopausal breast cancer survivors (15 months post-therapy). VO2peak improved in both exercise groups by 12 % (p < 0.001) with no significant difference between exercise groups. The prognostic value of these exercise training-induced improvements in VO2peak in early breast cancer survivors is unknown; large-scale prospective studies are now warranted.
Secondary/tertiary settings
No studies have examined the efficacy of exercise treatment in secondary or tertiary settings among women with early-stage breast cancer. However, in the tertiary setting, an ancillary, unplanned retrospective analysis of the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was conducted to investigate the safety and efficacy of exercise in patients with multiple tumor types with stable HF.71 Patients were randomly assigned to 4 months of aerobic training (3 days/week for 20–45 min at 60–70% heart rate reserve; n = 47) or usual care control (n = 43).71 There were no differences between exercisers and controls (exercise: +0.6 mL/kg/min; control: +0.8 mL/kg/min) in VO2peak; however, post hoc analyses as a function of protocol-specified exercise adherence indicated that higher exercise dose (i.e. >90 minutes per week, n=25) was associated with an improvement in VO2peak. After a mean of 4 year follow-up, there was no significant difference in the combined end point of cardiovascular mortality and HF hospitalization in the exercise group compared with the usual care group (32% vs. 20%; adjusted hazard ratio, 1.77; 95% confidence interval, 0.85 to 3.70). However, the rate of all-cause mortality or hospitalization was lower in adherent patients (66.1%) compared with non-adherent patients (i.e., < 90 minutes per week; 83.9%). The generalizability of these findings to breast cancer is not known, but results highlight the critical need to develop individualized pre-exercise screening and personalized exercise prescriptions to both identify patients at risk for adverse response patterns and optimize the efficacy of exercise therapy in the tertiary setting.
Research Gaps
The current evidence base across the cancer-CVD continuum is emergent with many fundamental questions still to be answered. Some, but certainly not all, of the major questions are as follows.
Primordial/Primary Settings
The time course of therapy-induced subclinical cardiovascular alterations and manifestation of CVD risk factors (e.g., hypertension, dyslipidemia), CVD events (e.g., myocardial infarction, unstable angina, arrhythmias, stroke, HF), and CVD mortality remains poorly defined. Novel assessment techniques incorporating exercise testing, blood and imaging markers could enable the detection of subclinical CVD in breast cancer survivors, and thereby potentially lead to earlier and more accurate identification of patients at high risk for overt CVD. For example, speckle tracking assessment of systolic function with strain revealed impaired myocardial function prior to LVEF decline 72,73 and HF symptoms 74 in patients treated with anthracycline-containing therapy. This, in turn, may enable selection of survivors most likely to benefit from exercise treatment.
Secondary/Tertiary Settings
No studies have been conducted in the secondary or tertiary settings; accordingly, there is a clear need for research into the efficacy of exercise treatment in patients with asymptomatic (i.e. secondary setting) and symptomatic (i.e. tertiary setting) CVD. Indeed, exercise treatment has been shown to improve VO2peak with concomitant improvements in LVEF in non-cancer HF patients;75 whether exercise treatment confers similar benefits in women with breast cancer and asymptomatic or symptomatic CVD is unknown. Cross sectional studies are needed so that medical, demographic, and physiological variables in high risk patients can be extensively characterized, and the central versus peripheral mechanisms underlying reduced VO2peak and symptoms among patients with HF can be understood. These studies will provide the basis for future prospective trials investigating the safety and efficacy of exercise training in patients with anticancer therapy-induced CVD.
All Settings
First, aside from a growing number of studies examining VO2peak, no trial to date has been specifically designed to test the efficacy of exercise treatment to modulate CVD risk factors or events. In this regard, exercise trials in primordial and primary settings will likely need to focus on CVD risk factors and surrogates since event rates will be low. Second, the optimal exercise therapy dose (i.e., frequency, intensity, time and type) to modulate CVD is unknown. The majority of studies followed traditional guidelines consisting of aerobic training or aerobic training combined with resistance training (2 to 3 sessions per week, for 10 to 60 min per exercise session at 50–75% of a predetermined physiological parameter (typically age-predicted heart rate maximum or reserve) for 12 to 15 weeks). In the primordial setting, these traditional guidelines failed to prevent declines in VO2peak60,62 suggesting that novel prescriptions may be required. Moreover, recent findings suggest there is considerable patient heterogeneity in physiological adaptability to exercise treatment - some patients demonstrate robust improvements in cardiovascular outcomes (e.g., VO2peak, systolic blood pressure) while others seem not to respond at all.76 Consequently, a more targeted approach matching the exercise prescription to the clinical/treatment characteristics of an individual patient or patient groups is likely required to optimally mitigate CVD-related morbidity and mortality in the breast cancer setting.77
Future Directions: Phenogrouping and Precision Exercise Treatment
An interesting development in HF is the use of phenogrouping, an approach that incorporates extensive patient characterization and identification of novel disease subtypes on the basis of patient physiological, medical, and/or demographic variables.78–80 For example, Shah et al.81 performed phenogrouping in 397 HF with preserved EF patients using 67 continuous phenotypic variables and established 3 mutually exclusive phenogroups with unique CVD and mortality risk profiles. Similarly, Ahmad and colleagues82 conducted phenogrouping analysis using 45 phenotypic variables in 1619 HF with reduced EF patients (HF-ACTION) and found that significant improvements in VO2peak levels and reductions in CVD death and/or CVD hospitalization were remarkably phenogroup specific following 3 months of exercise treatment. Such studies demonstrate that by looking beyond traditional classifications using phenogrouping, it may be possible to better characterize exclusive groups of individuals with related CVD pathophysiologies. To this end, phenogrouping may well offer similar advantages in cardio-oncology settings where pathophysiologically similar individuals with similar CVD risk profiles are grouped together for improved prognostication. Such work could then, in turn, guide the development of phenogroup-specific exercise-treatment prescriptions and optimize the safety and efficacy of exercise training in women with early-stage breast cancer.
To illustrate the potential utility of phenogrouping to improve the safety and efficacy of exercise treatment in the breast cancer setting, Figure 1 depicts hypothetical phenogroups and the mechanistically-driven precision exercise treatment that could be used to prevent/treat phenogroup-specific CVD sequelae. Importantly, each prescription would be individualized based on cardiovascular reserve testing (CPET) (for aerobic training-centric prescriptions) and/or strength testing (for resistance-centric prescriptions), and the intensity and duration of training sessions would be sequenced in such a fashion that training volume is continually increased across the entire program.77 Moreover, each phenogroup would have a unique mechanistically driven exercise treatment focus (e.g. high intensity or resistance exercise treatment). For example, a precision exercise treatment prescription for one phenogroup may incorporate more high intensity aerobic exercise training (e.g. 4 x 4 minutes at ≥90% of VO2peak) given the evidence that high intensity exercise has been shown to reverse eccentric LV remodeling, and improve LV contraction.75 Another precision exercise treatment prescription may incorporate more continuous aerobic (e.g. 30 minutes at 55–70% VO2peak) and resistance exercise (e.g., 3 x 8 repetition maximum) training -- given the evidence that moderate intensity continuous exercise and resistance exercise have been shown to improve vascular dysfunction, reverse LV concentric remodeling, and increase arterial-venous oxygen content difference, thus improving cardiorespiratory fitness.83 Such efforts, in conjunction with existing tools used in the oncology setting, could guide the design and implementation of preventive and early-intervention strategies to modulate therapy-induced CVD morbidity and mortality.
Conclusions
With the dramatic improvements in cancer-specific survival, breast cancer patients now have sufficient survival to also be at risk for the late effects of adjuvant therapy, particularly CVD morbidity and mortality. Preliminary evidence indicates that general exercise prescription guidelines may be associated with cardiovascular benefits in primordial and primary settings; however, it is important to stress that the current evidence base is emergent with a small number of studies. As reviewed here, much work is needed to optimize exercise treatment in order to attenuate breast cancer-associated CVD. These have been noted throughout the text, and a summary of future research is provided in Table 2. Now, hypothesis-driven studies are needed to define the nature and magnitude of the cardioprotective effects of exercise treatment. Such research will lead to mechanistically-driven clinical trials which, in turn, will inform frequency, intensity, duration, and modality of precision exercise treatment for breast cancer patients.
Table 2.
Future Directions in Modulation of CVD with Precision Medicine.
Phenogrouping
|
Precision Exercise Therapy
|
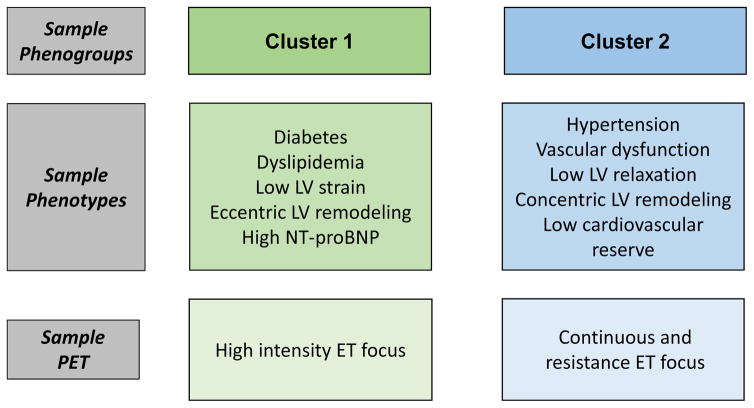
Figure 2.
Hypothetical phenogroups and precision exercise treatment that could be used to prevent/treat phenogroup-specific CVD sequelae. Cluster 1 patients have a prevalence of diabetes, dyslipidemia, decreased LV strain, eccentric remodeling, and elevated NT-proBNP. Cluster 2 patients have a prevalence of hypertension, vascular dysfunction, impaired LV relaxation, concentric LV remodeling, and low cardiovascular reserve. Each exercise treatment prescription would be individualized based on cardiovascular reserve testing (CPET) and/or strength testing, and the intensity and duration of training sessions would be sequenced in such a fashion that training volume is continually increased across the entire program.77 However, each patient cluster may have a unique exercise treatment focus. In cluster 1 patients, a sample precision exercise treatment prescription would incorporate more high intensity exercise training given the evidence that high intensity exercise has been shown to lower proBNP, reverse eccentric remodeling, and improve LV contraction.75 In cluster 2 patients, a sample precision exercise treatment prescription would incorporate more continuous and resistance exercise training given the evidence that moderate intensity continuous exercise and resistance exercise have been shown to improve vascular dysfunction, reverse concentric remodeling, and increase arterial-venous oxygen content difference (A-VO2 Diff), thus improving cardiorespiratory fitness.83
Acknowledgments
Funding / Support
LWJ is supported by research grants from the National Cancer Institute and from AKTIV Against Cancer Foundation.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–1690. doi: 10.1200/JCO.2006.09.2106. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer causes & control : CCC. 2008;19(5):537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 3.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular Disease Mortality Among Breast Cancer Survivors. Epidemiology. 2016;27(1):6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99(5):365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 10.Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2011;58(9):988–989. doi: 10.1016/j.jacc.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13(5):699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 12.Cardinale D, Colombo A, Bacchiani G, et al. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 13.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012;30(36):4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaosuwannakit N, D’Agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J Clin Oncol. 2010;28(1):166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ederer AK, Didier KD, Reiter LK, et al. Influence of Adjuvant Therapy in Cancer Survivors on Endothelial Function and Skeletal Muscle Deoxygenation. PloS one. 2016;11(1):e0147691. doi: 10.1371/journal.pone.0147691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nature reviews. Clinical oncology. 2012;9(5):288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23(34):8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 19.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100(4):252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Chen MH, Kerkela R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 24.Lim CC, Zuppinger C, Guo X, et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279(9):8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 25.Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101(18):6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Angelis A, Piegari E, Cappetta D, et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121(2):276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol. 2012;60(24):2465–2472. doi: 10.1016/j.jacc.2012.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JM, Khakoo A, Mackey JR, Haykowsky MJ, Douglas PS, Jones LW. Modulation of anthracycline-induced cardiotoxicity by aerobic exercise in breast cancer: current evidence and underlying mechanisms. Circulation. 2011;124(5):642–650. doi: 10.1161/CIRCULATIONAHA.111.021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. British journal of haematology. 2005;131(5):561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 30.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 31.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 32.Senkus-Konefka E, Jassem J. Cardiovascular effects of breast cancer radiotherapy. Cancer Treat Rev. 2007;33(6):578–593. doi: 10.1016/j.ctrv.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat Rev Drug Discov. 2011;10(2):111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
- 34.Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120(4):310–317. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pentassuglia L, Sawyer DB. The role of Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res. 2009;315(4):627–637. doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Gu X, Li Z, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48(7):1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Scott JM, Lakoski S, Mackey JR, Douglas PS, Haykowsky MJ, Jones LW. The potential role of aerobic exercise to modulate cardiotoxicity of molecularly targeted cancer therapeutics. Oncologist. 2013;18(2):221–231. doi: 10.1634/theoncologist.2012-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 39.Amir E, Seruga B, Niraula S, Carlsson L, Ocana A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103(17):1299–1309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 40.Howell A. Adjuvant aromatase inhibitors for breast cancer. Lancet. 2005;366(9484):431–433. doi: 10.1016/S0140-6736(05)67036-5. [DOI] [PubMed] [Google Scholar]
- 41.Jones LW, Haykowsky M, Pituskin EN, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12(10):1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 42.Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98(6):3589–3593. doi: 10.1073/pnas.051003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott JM, Koelwyn GJ, Hornsby WE, et al. Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin Oncol. 2013;40(2):218–228. doi: 10.1053/j.seminoncol.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clinic proceedings. Mayo Clinic. 2008;83(6):679–686. doi: 10.4065/83.6.679. [DOI] [PubMed] [Google Scholar]
- 45.Jones LW, Haykowsky M, Peddle CJ, et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16(5):1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 46.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341(6):427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 47.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight Gain After Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2015;107(12):djv275. doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin ML, McTiernan A, Bernstein L, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of cancer survivorship : research and practice. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 51.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2006;175(1):34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. The oncologist. 2011;16(1):112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 54.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. Journal of the National Cancer Institute. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42(12):2139–2143. doi: 10.1016/j.jacc.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 56.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakoski SG, Willis BL, Barlow CE, et al. Midlife Cardiorespiratory Fitness, Incident Cancer, and Survival After Cancer in Men: The Cooper Center Longitudinal Study. JAMA oncology. 2015;1(2):231–237. doi: 10.1001/jamaoncol.2015.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelsey CR, Scott JM, Lane A, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone marrow transplantation. 2014 doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 61.Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 62.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 63.Woodward M, Webster R, Murakami Y, et al. The association between resting heart rate, cardiovascular disease and mortality: evidence from 112,680 men and women in 12 cohorts. European journal of preventive cardiology. 2012;21(6):719–726. doi: 10.1177/2047487312452501. [DOI] [PubMed] [Google Scholar]
- 64.Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer nursing. 2006;29(2):156–165. doi: 10.1097/00002820-200603000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Kolden GG, Strauman TJ, Ward A, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psycho-oncology. 2002;11(5):447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 66.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106(8):945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 67.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15(15):4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 68.Khouri MG, Hornsby WE, Risum N, et al. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast cancer research and treatment. 2014;143(3):531–539. doi: 10.1007/s10549-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 70.Dolan LB, Campbell K, Gelmon K, Neil-Sztramko S, Holmes D, McKenzie DC. Interval versus continuous aerobic exercise training in breast cancer survivors--a pilot RCT. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2016;24(1):119–127. doi: 10.1007/s00520-015-2749-y. [DOI] [PubMed] [Google Scholar]
- 71.Jones LW, Douglas PS, Khouri MG, et al. Safety and Efficacy of Aerobic Training in Patients With Cancer Who Have Heart Failure: An Analysis of the HF-ACTION Randomized Trial. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.53.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jurcut R, Wildiers H, Ganame J, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21(12):1283–1289. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 73.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am Heart J. 2009;158(2):294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 74.Mercuro G, Cadeddu C, Piras A, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler echocardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007;12(9):1124–1133. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 75.Wisloff U, Stoylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 76.Jones LW, Hornsby W, Freedland SJ, et al. Effects of Non-Linear Aerobic Training on Erectile Function and Cardiovascular Function in Men Following Prostatectomy for Clinically-Localized Prostate Cancer. European urology. 2013 doi: 10.1016/j.eururo.2013.11.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW. A framework for prescription in exercise-oncology research. Journal of cachexia, sarcopenia and muscle. 2015;6(2):115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindman BR, Davila-Roman VG, Mann DL, et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J Am Coll Cardiol. 2014;64(6):541–549. doi: 10.1016/j.jacc.2014.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. J Appl Physiol (1985) 2014 doi: 10.1152/japplphysiol.00518.2014. jap 00518 02014. [DOI] [PubMed] [Google Scholar]
- 80.Wang J, Fang F, Yip GW, et al. Quantification of left ventricular performance in different heart failure phenotypes by comprehensive ergometry stress echocardiography. Int J Cardiol. 2013;169(4):311–315. doi: 10.1016/j.ijcard.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad T, Pencina MJ, Schulte PJ, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64(17):1765–1774. doi: 10.1016/j.jacc.2014.07.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J, Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61(1):112–119. doi: 10.1161/HYPERTENSIONAHA.111.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klepin HD, Pitcher BN, Ballman KV, et al. Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance) Journal of oncology practice / American Society of Clinical Oncology. 2014;10(5):e285–292. doi: 10.1200/JOP.2014.001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nechuta S, Lu W, Zheng Y, et al. Comorbidities and breast cancer survival: a report from the Shanghai Breast Cancer Survival Study. Breast cancer research and treatment. 2013;139(1):227–235. doi: 10.1007/s10549-013-2521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 87.Al-Kindi SG, Oliveira GH. Prevalence of Preexisting Cardiovascular Disease in Patients With Different Types of Cancer: The Unmet Need for Onco-Cardiology. Mayo Clinic proceedings. Mayo Clinic. 2016;91(1):81–83. doi: 10.1016/j.mayocp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60(24):2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 89.Redig AJ, Janne PA. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J Clin Oncol. 2015;33(9):975–977. doi: 10.1200/JCO.2014.59.8433. [DOI] [PubMed] [Google Scholar]