Abstract
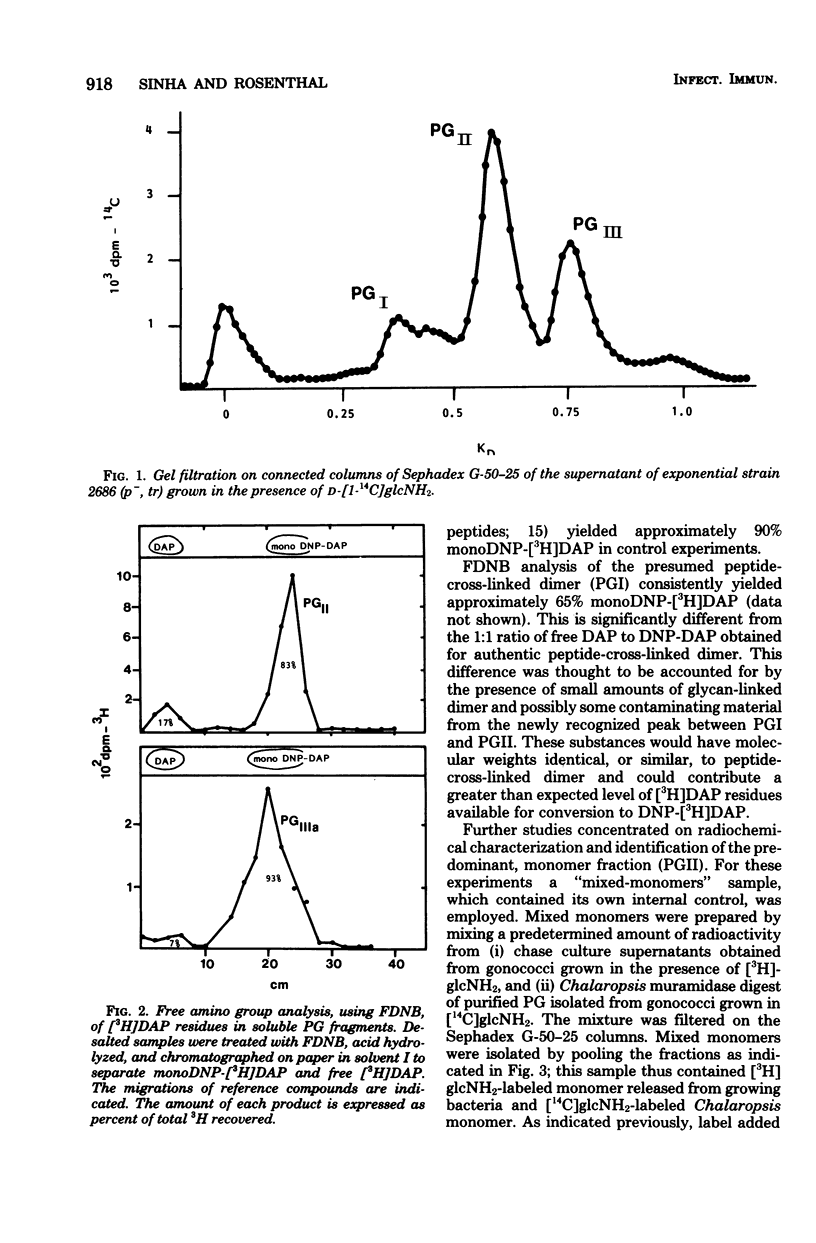
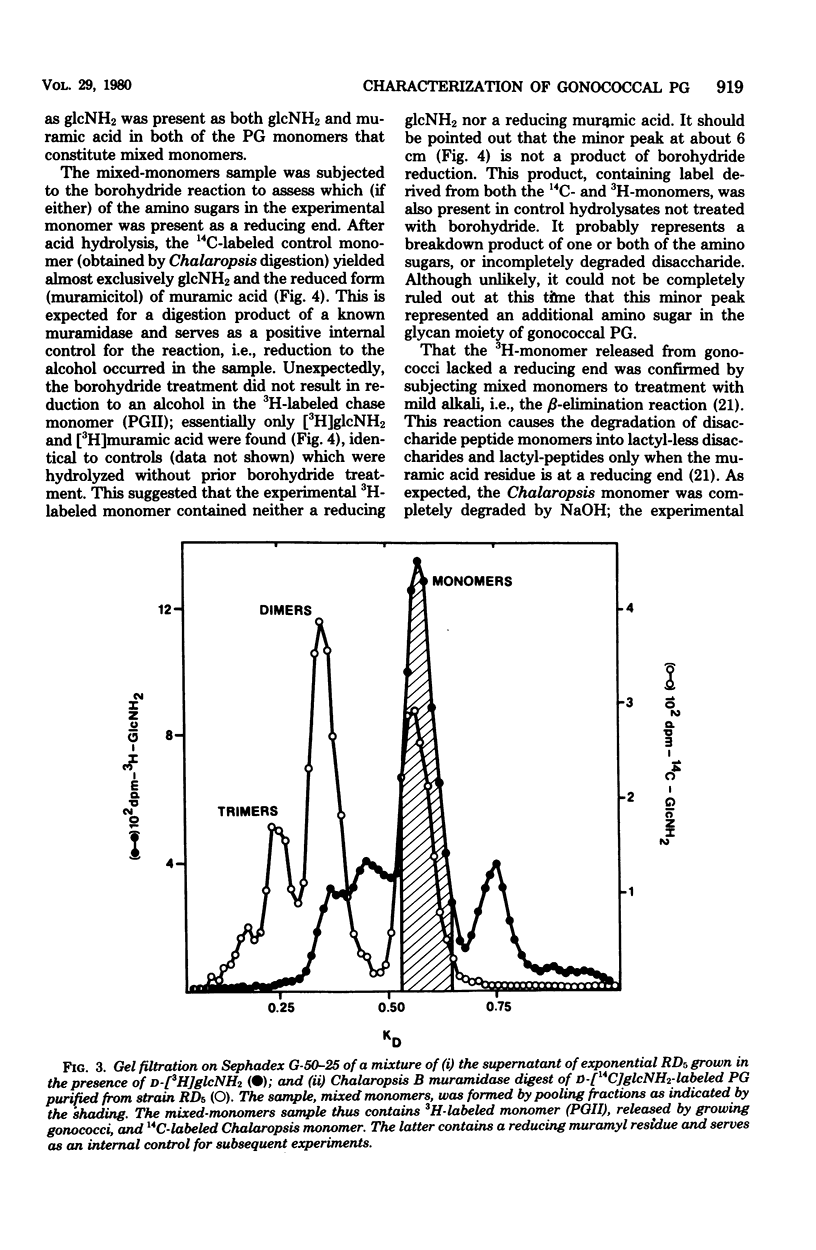
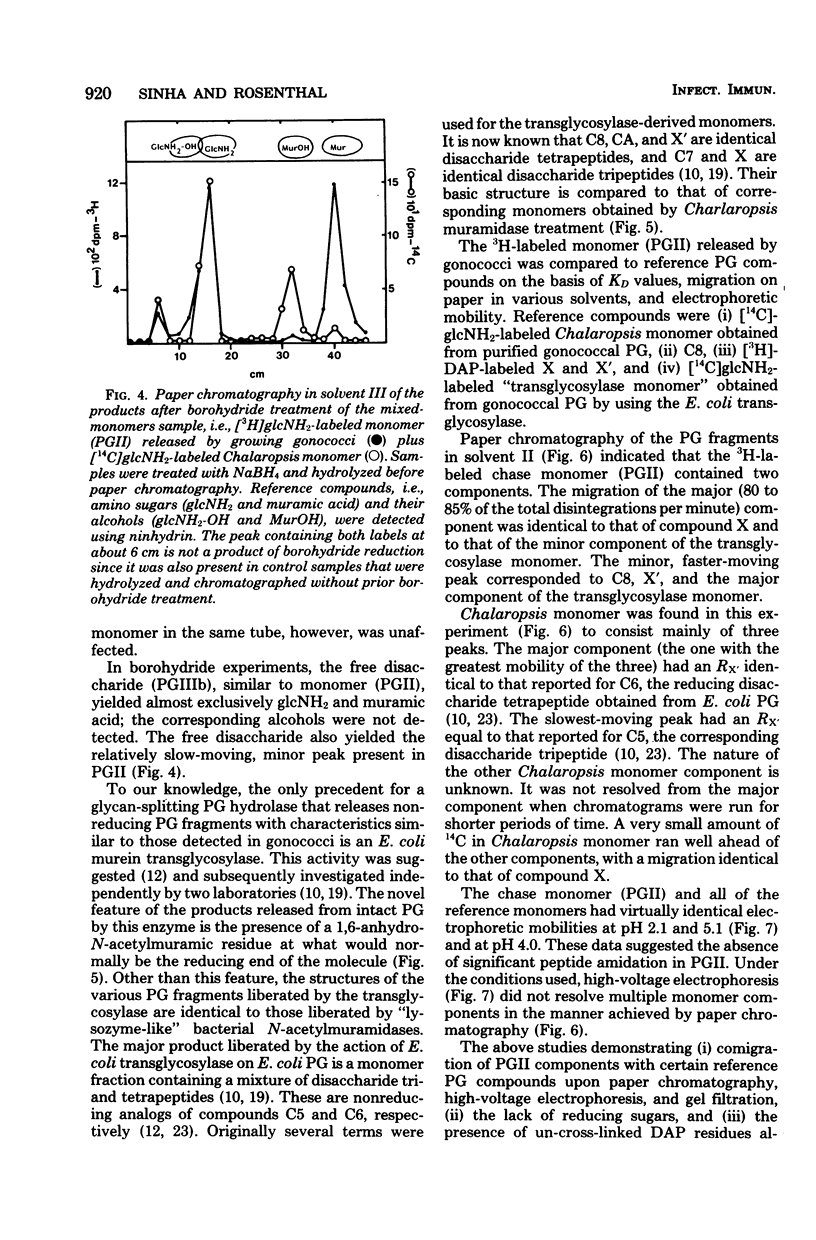
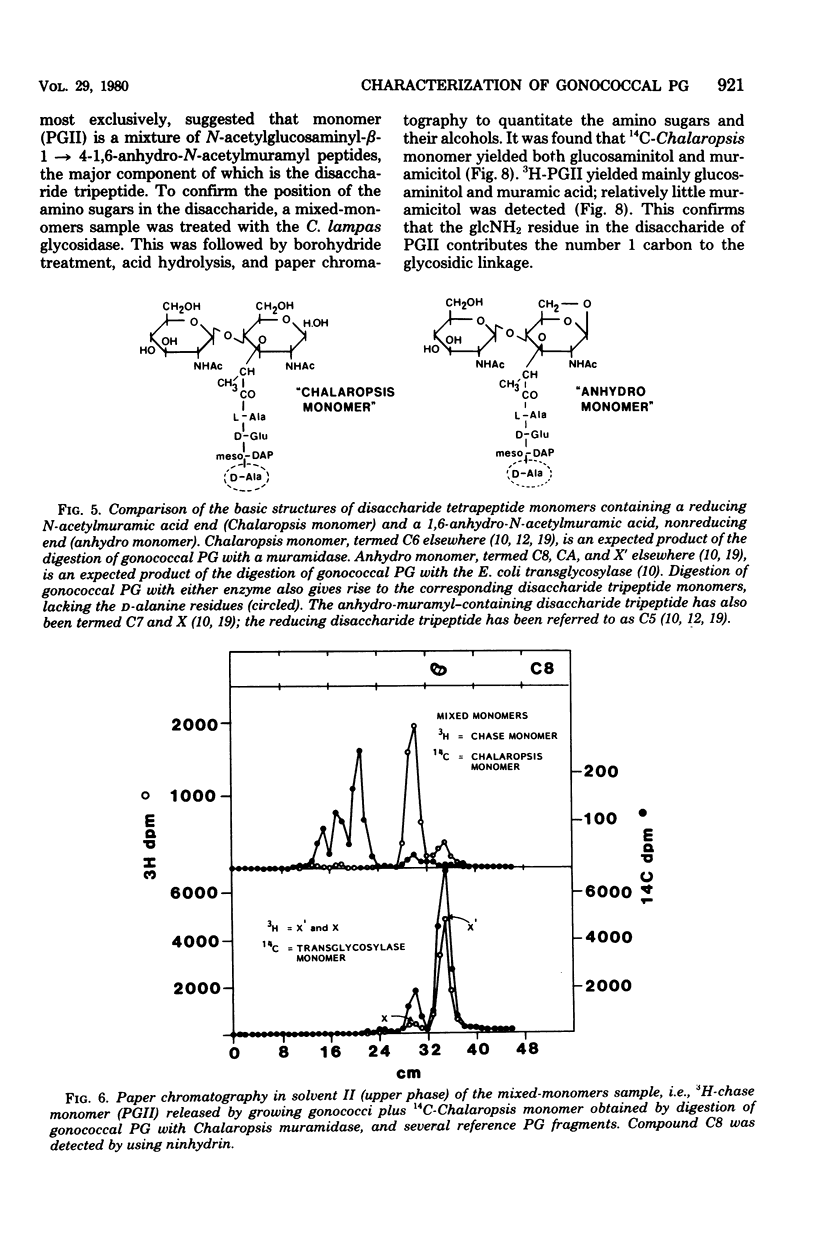
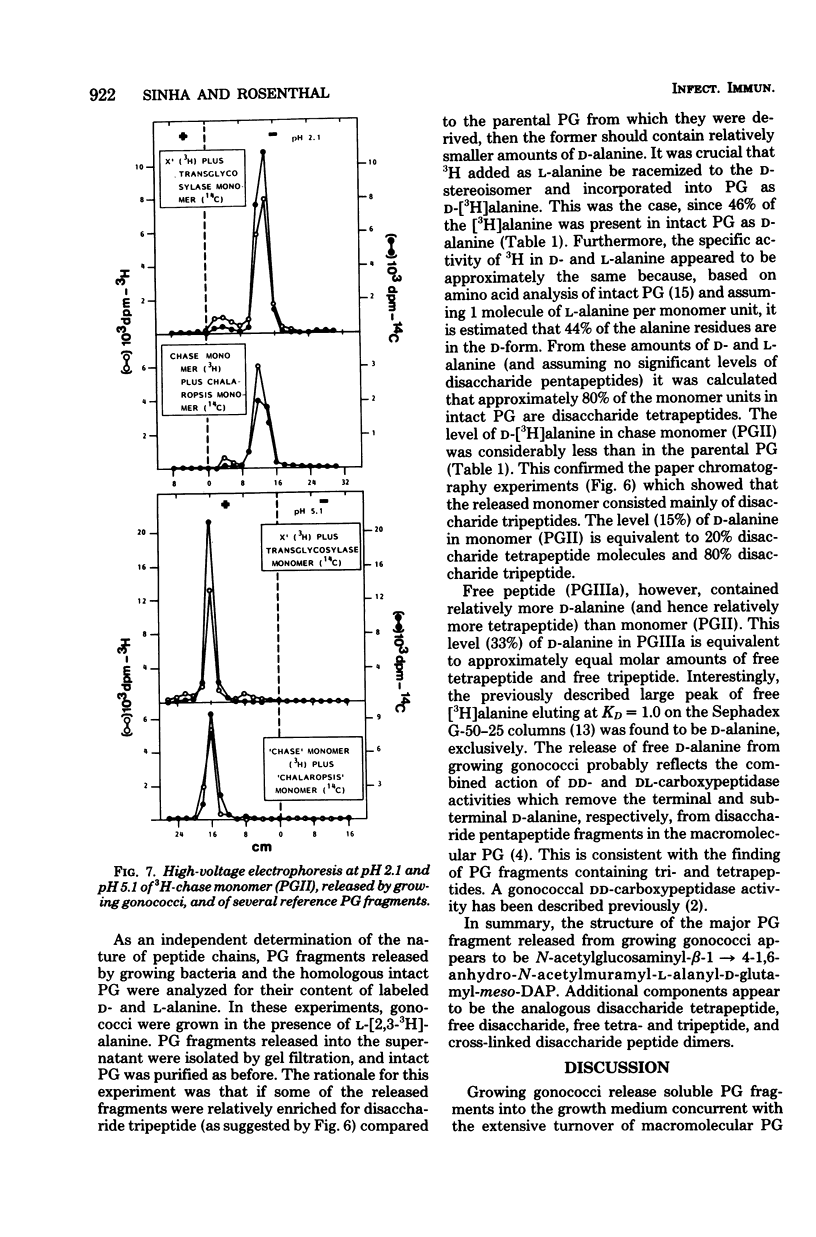
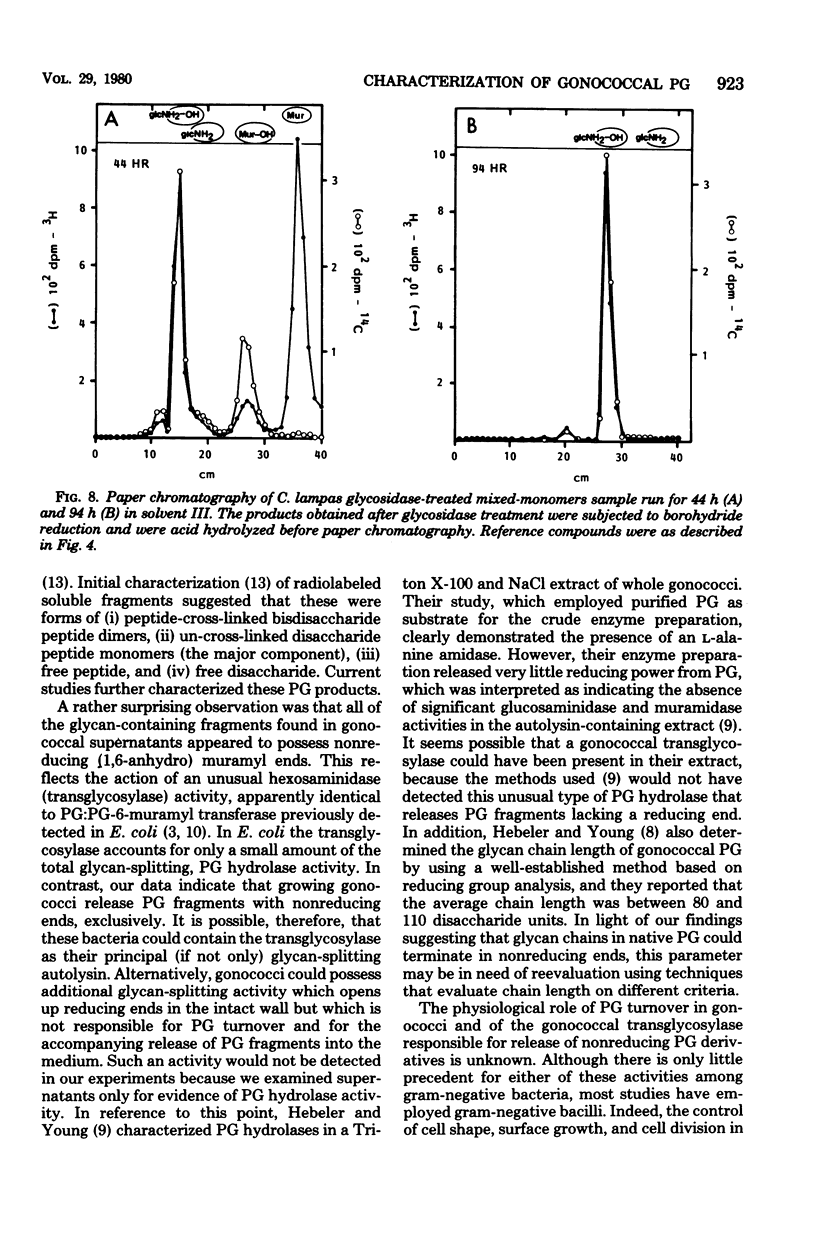
Previous analysis of soluble peptidoglycan (PG) fragments released by exponentially growing gonococci implicated the combined action of both hexosaminidase and amidase activities in PG turnover. Current studies further characterized PG fragments which were labeled in the glycan with D-glucosamine and in the peptide moiety with meso-diaminopimelic acid of L- and D-alanine. Labeled PG fragments were isolated by gel filtration and characterized on the bases of (i) KD values, (ii) free amino group analysis using fluorodinitrobenzene, (iii) borohydride reduction, (iv) alkali-catalyzed beta-elimination, (v) paper chromatography in various solvents, (vi) electrophoretic mobility at various pH values, (vii) digestibility by Charonia lampas glycosidases, and (viii) content of labeled D- and L-alanine. A set of well-characterized PG fragments was used as standards. The monomer fraction (the major extracellular product) was found to contain two components. Most (about 80%) appeared to be N-acetylglucosaminyl-beta-1 leads to 4-1,6-anhydro-N-acetylmuramyl-L-ala-D-glu-meso-diaminopimelic acid; the remainder was the corresponding disaccharide tetrapeptide containing a C-terminal D-alanine. An unusual feature of these products was the presence of the anhydro-muramyl (non-reducing) ends, reflecting the activity of a gonococcal transglycosylase, and the near absence of products containing detectable reducing ends. Otherwise, the structures of the monomer fragments were typical of those expected for a gram-negative bacterium (chemotype I). The corresponding peptide-cross-linked dimer and the free disaccharide also contained nonreducing ends, exclusively. Free peptides (products of amidase activity) consisted of both tripeptide and tetrapeptide. In summary, all gonococci examined appear to possess an unusual transglycosylase activity which contributes to the release of soluble PG fragments containing nonreducing, anhydro-muramyl ends. The release of these fragments in vivo might be a unique aspect of gonococci-host interactions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Davis R. H., Salton M. R. Some properties of a D-alanine carboxypeptidase in envelope fractions of Neisseria gonorrhoeae. Infect Immun. 1975 Nov;12(5):1065–1069. doi: 10.1128/iai.12.5.1065-1069.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell E. W., Fazio M., Tomasz A. Effect of benzylpenicillin on the synthesis and structure of the cell envelope of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Mar;13(3):514–526. doi: 10.1128/aac.13.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Chemical composition and turnover of peptidoglycan in Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1180–1185. doi: 10.1128/jb.126.3.1180-1185.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Mechanism of autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1186–1193. doi: 10.1128/jb.126.3.1186-1193.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S. Release of soluble peptidoglycan from growing gonococci: hexaminidase and amidase activities. Infect Immun. 1979 Jun;24(3):869–878. doi: 10.1128/iai.24.3.869-878.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. S., Wright R. M., Sinha R. K. Extent of peptide cross-linking in the peptidoglycan of Neisseria gonorrhoeae. Infect Immun. 1980 Jun;28(3):867–875. doi: 10.1128/iai.28.3.867-875.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Hebeler B. H., Morse S. A. Cell envelope of Neisseria gonorrhoeae: penicillin enhancement of peptidoglycan hydrolysis. Infect Immun. 1977 Dec;18(3):717–725. doi: 10.1128/iai.18.3.717-725.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


