Abstract
Mitochondrial respiratory complexes become assembled into supercomplexes (SC) under physiological conditions. One of the functional roles of these entities is the limitation of reactive oxygen species (ROS) produced by complex I (CI) of the respiratory chain. We sought to determine whether the systemic antioxidant effect of exercise is mediated by the assembly of mitochondrial CIs into SCs in rats. Male Wistar rats were exercise trained or remained sedentary for ten weeks; then, blood samples were collected, and the gastrocnemius muscle was isolated. The assembly of mitochondrial SCs and the lipid peroxidation of the mitochondrial and plasmatic fractions were assessed. Our results demonstrate that exercise induced the assembly of CI into SCs in the gastrocnemius and induced a systemic decrease in lipid peroxidation. We also found an inverse association between the superassembly of CIs and mitochondrial lipid peroxidation (p < 0.01) and protein carbonyls (p < 0.05). We conclude that exercise induces the chronic assembly of CIs into SCs, which provide mitochondrial protection against oxidative damage, at least in the studied muscle. Given the relevant role that mitochondria play in health and disease, these findings should help to elucidate the role of exercise as a therapeutic approach for metabolic diseases.
Keywords: Exercise, Supercomplexes, Complex I, Reactive oxygen species, Antioxidants
Graphical abstract
Highlights
-
•
Exercise induces Complex I (CI) assembly into supercomplexes in skeletal muscle.
-
•
Superassembly of CI is inversely associated with mitochondrial oxidative damage.
-
•
Supercomplexes may contribute to the systemic antioxidant effects of exercise.
1. Introduction
Mitochondria are critical organelles that are involved in many aspects of cell activity. The best characterized function of mitochondria is ATP production through the oxidative phosphorylation system and the generation of reactive oxygen species (ROS), mainly by complexes I (CI) and III (CIII) [7], [12]. Respiratory complexes have received increased attention because they can undergo superassemblies into supercomplexes (SC) [22]. Rather than mere structural entities, these SCs also have functional roles in the respiratory chain [6], such as the prevention of excessive ROS production; this prevention is conducted by CIs when they are assembled into SCs [20].
Exercise is one of the best-known stimuli for mitochondrial function in skeletal muscle [11]. Accordingly, a recent study reported that exercise induces chronic SC assembly, which is related to mitochondrial respiration and whole-body oxygen consumption [10]. However, an association between SC assembly and mitochondrial redox homeostasis induced by exercise has not yet been tested.
Recent findings from our research group demonstrate that lifelong endurance exercise training induces a systemic decrease in lipid peroxidation [2], as does moderate hypoxic training [4]. Frequently, the antioxidant effect of exercise has been explained by an increased content and activity of antioxidant enzymes [3], [8]; however, this is not the case under our experimental conditions [2], [4]. Because ex vivo studies demonstrate that CI is the main source of mitochondrial ROS production under aerobic exercise conditions [9], our hypothesis states that the CI assembly into an SC may have a primary role in the antioxidant effects of endurance exercise.
We aimed to identify SC formation within the skeletal muscle of chronically exercised rats and evaluate the association between the formation of SC and the oxidative status of the muscle.
2. Materials and methods
2.1. Animals
All experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Granada (procedures Granada, Spain. n°: 28/06/2016/116) and in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (CETS # 123), directive 2010/63/EU on the protection of animals used for scientific purposes and the Spanish law (R.D. 53/2013). Male Wistar rats were purchased from Charles River (USA) at six weeks of age. The rats were acclimated to the experimental conditions for two weeks. Next, the rats were randomly allocated into a sedentary (n = 6) or an exercised (n = 6) group for ten weeks, were weighed weekly, and their food and water intakes were also recorded. Seventy-two hours after the last exercise was performed, the rats were fasted overnight, anaesthetised with pentobarbital and euthanized by bleeding.
2.2. Exercise procedures
The animals were acclimated to running on a motorized treadmill (Rat 5-lanes Touchcreen Treadmill for rats, LE8710RTS, PanLab, Spain) for 5 sessions throughout the two weeks of the acclimation period. The exercise programme was divided into two similar 5-wk mesocycles; the rats ran 5 days per week and rested during the weekends (Fig. S1). During the entire protocol, the animals ran at 75% of their maximal velocity as calculated from the maximal velocity test performed at the beginning of each mesocycle. Each mesocycle progressively increased the running volume, the rats began running for 20 min, and this period was increased by 5 min every two days, and when 65 min was achieved, this duration was maintained until the end of the mesocycle. The animals of the sedentary group were daily handled and run for 10 min every two weeks to maintain their acclimation to the treadmill.
The maximal velocity tests were performed before the training period and at week 10 of the training period. These tests started at a velocity of 22 cm/s, and the velocity was increased by 5 cm/s every minute until fatigue was achieved. Fatigue was defined as the point at which the rats remained at the back of the treadmill on an electric shock pad for 5 s.
2.3. Evaluation of supercomplex formation in the skeletal muscle by BNGE
Blue native gel electrophoresis (BNGE) was performed on crude mitochondrial fractions from the gastrocnemius muscles of rats. Mitochondrial isolation was performed as previously described [17]. One aliquot of the crude mitochondrial fraction was used for protein determination. The remaining samples were then centrifuged at 13,000 × g for 3 min at 4 °C. The mitochondrial pellets were suspended in an appropriate volume of medium C (1 M aminocaproic acid, 50 mM Bis-Tris-HCl [pH 7.0]) to create a protein concentration of 10 mg/ml, and the membrane proteins were solubilized with digitonin (4 g/g) and incubated for 10 min in ice. After 30 min of centrifugation at 13,000 × g (4 °C), the supernatants were collected, and 3 µL of 5% Brilliant Blue G dye prepared in 1 M aminocaproic acid was added. Mitochondrial proteins (100 µg) were then loaded and run on a 3–13% gradient native gel as previously described [18]. After electrophoresis, the complexes were electroblotted onto PVDF membranes and sequentially tested with specific antibodies against CI, anti-NDUFA9 (Abcam, ab14713), CIII, anti-ubiquinol-cytochrome c reductase core protein I (Abcam, ab110252) and Vdac1 (Abcam, ab14734).
2.4. Lipid peroxidation in the plasma and mitochondria
The concentration of hydroperoxides (HPx), a specific and direct biomarker of lipid peroxidation, was determined with a Sigma PD1 kit (St Louis, MO, USA). The absorbance changes at 560 nm were monitored by spectrophotometry.
Blood obtained from the bleeding procedure was centrifuged for 10 min at 3000 rpm to isolate the plasma. Then, 40 µL of plasma was used for the quantification of the HPx concentration in plasma. A total of 100 µg of protein from the mitochondrial fraction was used to determine the mitochondrial concentration of HPx.
2.5. Protein carbonyls in the plasma and mitochondria
The formation of protein carbonyl adducts, a marker of oxidative stress [24], was used as an index of oxidative modifications of mitochondrial and plasma proteins. Protein carbonyls were measured in duplicate using an ELISA-based assay according to manufacturer's instructions (OxiSelect Protein Carbonyl ELISA Kit; Cell Biolabs Inc., San Diego, USA).
2.6. Statistics
The statistical analyses were performed using SPSS (version 22 for Windows; IBM Corp., Armonk, NY, USA). The data are presented as the means ± the standard errors of the means (SEM), and p < 0.05 was considered significant. Unpaired t-tests were used to analyse differences between the exercised and sedentary rats. Pearson correlation analyses were used to elucidate the relationships of the concentrations of HPx and PC with the superassembly of CI.
3. Results
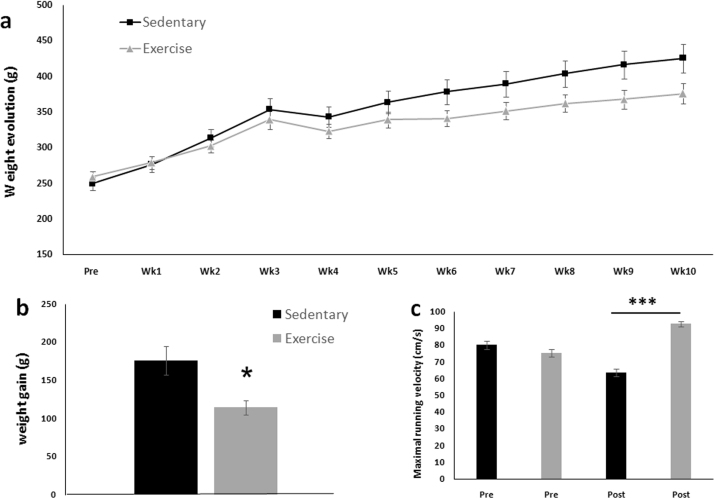
Fig. 1a illustrates the weekly weight changes of the sedentary rats and the exercised rats over the 10 weeks. The exercised rats exhibited less weight gain (p = 0.015) than the sedentary rats (Fig. 1b). A maximal velocity test (Fig. 1c) revealed that the sedentary rats decreased their performances by 20% from Test 1 (80 ± 6.1 cm/s) to Test 2 (64 ± 5.2 cm/s), while the exercised rats increased their performance by 24% from Test 1 (75 ± 5.2 cm/s) to Test 2 (93 ± 3.8 cm/s). The performance at Test 2 was significantly higher for the exercised animals (p < 0.001).
Fig. 1.
Weight responses and running performances. a) Weekly evolution of the animals’ weights. b) Weight gain was lower in the exercised rats than in the sedentary rats. c) The maximal running velocity was significantly higher in the exercised animals after the 10-week running protocol. *p < 0.05 and ***p < 0.001 vs. sedentary rats.
Fig. 2a shows that exercised rats (2.9 ± 0.19 nmol/ml) exhibited lower plasma concentrations of HPx (p < 0.05) than the sedentary animals (3.8 ± 0.90 nmol/ml). The mitochondrial concentration of HPx (Fig. 2b) decreased by 12% in the exercised (38 ± 5.0 nmol/mg) rats compared with the sedentary rats (43 ± 11.5 nmol/mg), but the difference was not statistically significant. PC analysis revealed no difference in the plasma concentration of HPx between the sedentary (0.39 ± 0.18 nmol/ml) and exercised rats (0.21 ± 0.06 nmol/ml). However, the mitochondrial PC (Fig. 2d) decreased (p < 0.05) in the exercised rats (0.80 ± 0.10 nmol/mg) compared with the sedentary animals (0.54 ± 0.07 nmol/mg).
Fig. 2.
Antioxidant responses to exercise. a) Endurance exercise decreased the circulating concentration of hydroperoxides. b) Mitochondrial hydroperoxide concentration. c) Circulating protein carbonyls. d) Exercise reduced mitochondrial protein carbonyls. * p < 0.05 and ** p = 0.01 vs. sedentary rats.
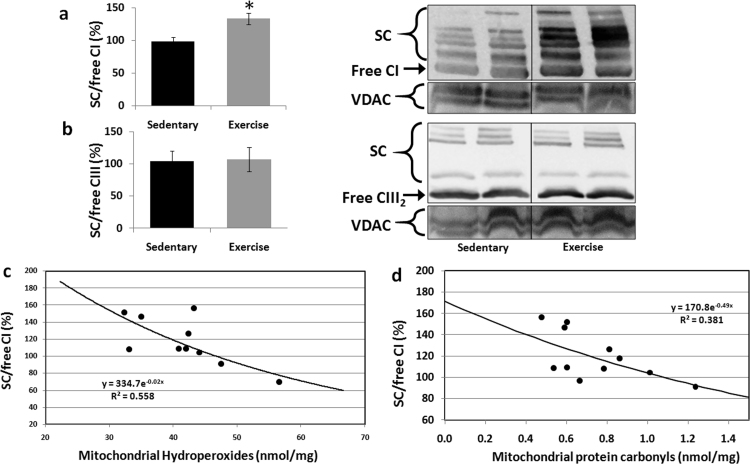
We next performed a BNGE analysis on the crude mitochondrial fraction of the gastrocnemius muscle. We found that the SC/free CI ratio was 26% higher (p = 0.01) in the exercised animals than in the sedentary animals (Fig. 3a). However, the SC/free CIII ratio was almost identical between the exercised and sedentary animals (Fig. 3b). Pearson correlation analysis (Fig. 3c) revealed a significant inverse relationship (p < 0.01) between the CI superassembly and the mitochondrial production of HPx. Additionally, we found a significant inverse relationship (p < 0.05) between CI superassembly and the mitochondrial protein carbonyls (Fig. 3d). No significant relationship was found between CI and plasma markers.
Fig. 3.
Exercise-induced complex I superassembly is related to mitochondrial oxidative damage. a) Exercise induced mitochondrial complex I assembly into SCs. b) Mitochondrial complex III assembly into SCs. c) The percentage of SCs containing complex I was related (p < 0.01) to mitochondrial hydroperoxide production. d) The percentage of SCs containing complex I was related (p < 0.05) to mitochondrial protein carbonyls. *p = 0.01 vs. sedentary rats.
4. Discussion
Endurance exercise as an antioxidant is extensively recognized. The mechanisms triggered by exercise induce effects that include the increased expressions and activities of key antioxidant enzymes [3], [8]. Moreover, exercise induces the mobilization of non-enzymatic antioxidants to mitochondrial membranes to prevent their oxidative damage [21]. However, subjects performing lifelong endurance exercise exhibit a systemic decrease in lipid peroxidation that is not related to the antioxidant system [2]. In the present study, we demonstrate that exercise-induced chronic superassemblies of CI protect against mitochondrial oxidative damage.
The antioxidant effects of the superassembly of mitochondrial complexes have been previously assessed in vitro. Maranzana et al. [20] demonstrated that SC assembly prevents excessive ROS generation by CI. More recently, observations based on cryo-electron microscopy suggest that the SC architecture might prevent excessive ROS production by limiting the maximal activity of the mitochondrial complexes [13]. Although CIs are fully functional both as free complexes and when assembled into SCs [19], the supramolecular organization of the CIs that is induced by exercise can exert a fine-tuned control of mitochondrial ROS production. This notion is consistent with the hypothesis that states that CI-containing SCs may physiologically occur with the aim of preventing excessive ROS production within living cells [16]. Additionally, under conditions of oxidative stress, the SC I1III2 is dissociated, which leads to a less efficient electron transfer [15]. Therefore, SCs can be functional entities that are optimized for preventing excessive ROS production and allow proper mitochondrial function during stressful stimuli such as exercise.
Nevertheless, our data revealed that only the CI is assembled into the SC. A plausible explanation for the absence of the superassembly of CIII comes from the stoichiometry of the mitochondrial respiratory complexes. Indeed, the ratio for complexes I:III:IV is 1.1:3.0:6.7 in mammals [23]. However, the main SC association formed in mammals, the so-called respirasome, is comprised of one copy of CI, two copies of CIII and several copies of CIV (I1III2IVn). Therefore, as previously suggested [14], one-third of the total CIII may not be bound to monomeric CI, which strengthens the observation that the CI assembly into the SC may be crucial for the control of mitochondrial ROS production.
In contrast, Greggio and colleagues [10] demonstrated that endurance exercise induces SC assembly of both CI and III in older subjects. There are several potential reasons for this inconsistency. First, the species or model-specific differences could underlie this difference. For example, supercomplex assembly factor I (SCAF1) is the protein responsible for CIII and IV superassembly; however, some murine models, such as C57BL/6 mice, only express a short isoform of this protein, which hampers the superassembly of CIII and IV [5]. Although no evidence has been reported for Wistar rats, the mechanisms and proteins involved in SC assembly are still poorly understood, and this possibility cannot be ruled out. Second, the study by Greggio [10] was conducted in older adults ranging from 60 to 80 years old. Because mitochondrial metabolism is impaired by the ageing of the skeletal muscle, exercise might lead to stronger stimuli for SC assembly within elderly skeletal muscle. Indeed, some age-related metabolic diseases are triggered by increased mitochondrial oxidative damage [1]. Thus, understanding the cellular mechanisms that regulate ROS production in health and disease is critical to improving our knowledge about the ageing process.
The specific contribution of SCs to the systemic antioxidant effects of exercise is unknown; this constitutes the main limitation of the present study. Indeed, the large number of antioxidant mechanisms triggered by exercise [3], [8], [12] may explain the lack of association found between the superassembly of CI and the systemic antioxidant effects of exercise. Notably, we did not find a significant decrease in plasma PC in the exercised animals due to the high variability. However, our results are within the range that has been previously described [24].
5. Conclusions
Exercise induces the chronic superassembly of mitochondrial CI to improve mitochondrial redox homeostasis. However, the contribution of SC to the antioxidant effects of exercise remains unknown. Further research to elucidate whether exercise-induced SC assembly can improve substrate channelling or whether it might prevent mitochondrial dysfunction is needed, given that this type of information would have clear implications in mitochondrial medicine and sport science.
Acknowledgements
LCL is supported by the "Ramón y Cajal" National Programme (RYC‐2011‐07643) and the grant SAF2015‐65786‐R from the Ministerio de Economía y Competitividad, Spain, and the ERDF. AHG is a “FPU fellow” from the Ministerio de Educación Cultura y Deporte, Spain. The present study will be a part of SAF's Ph.D. thesis which is being performed within the “Nutrition and Food Sciences Program” at the University of Granada. All the authors declare no conflicts of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.07.009.
Contributor Information
J.R. Huertas, Email: jhuertas@ugr.es.
R.A. Casuso, Email: casusopt@gmail.com.
Appendix A. Supplementary material
Supplementary material
References
- 1.Acin-Perez R., Enriquez J.A. The function of the respiratory supercomplexes: the plasticity model. Biochim. Biophys. Acta. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Barranco-Ruiz Y., Martínez-Amat A., Casals C., Aragón-Vela J., Rosillo S., Gomes S.N., Rivas-García A., Guisado R., Huertas J.R. A lifelong competitive training practice attenuates age-related lipid peroxidation. J. Physiol. Biochem. 2017;73:37. doi: 10.1007/s13105-016-0522-4. [DOI] [PubMed] [Google Scholar]
- 3.Brooks S.V., Vasilaki A., Larkin L.M., McArdle A., Jackson M.J. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J. Physiol. 2008;586:3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casuso R.A., Aragón-Vela J., López-contreras G., Gomes S.N., Casals C., Barranco-Ruiz Y., Mercadé J.J., Huertas J.R. Does swimming at a moderate altitude favor a lower oxidative stress in an intensity-dependent manner? Role of nonenzymatic antioxidants. High. Alt. Med. Biol. 2017;18:46–55. doi: 10.1089/ham.2016.0046. [DOI] [PubMed] [Google Scholar]
- 5.Cogliati S., Calvo E., Loureiro M., Guaras A.M., Nieto-Arellano R., Garcia-Poyatos C., Ezkurdia I., Mercader N., Vázquez J., Enriquez J.A. Mechanism of super-assembly of respiratory complexes III and IV. Nature. 2016;539:579–582. doi: 10.1038/nature20157. [DOI] [PubMed] [Google Scholar]
- 6.Enriquez J.A. Supramolecular organization of respiratory complexes. Annu. Rev. Physiol. 2016;78:533–561. doi: 10.1146/annurev-physiol-021115-105031. [DOI] [PubMed] [Google Scholar]
- 7.Genova M.L., Lenaz G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys. Acta. 2014;1837:427–443. doi: 10.1016/j.bbabio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Cabrera M.C., Domenech E., Romagnoli M., Arduini A., Borras C., Pallardo F.V., Sastre J., Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves R.L., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015;290:209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greggio C., Jha P., Kulkarni S.S., Lagarrigue S., Broskey N.T., Boutant M., Wang X., Conde Alonso S., Ofori E., Auwerx J., Cantó C., Amati F. Enhanced respiratory chain supercomplex formation in response to exercise in human skeletal muscle. Cell Metab. 2016;25:1–11. doi: 10.1016/j.cmet.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Holloszy J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 12.Jackson M.J. Redox regulation of muscle adaptations to contractile activity and aging. J. Appl. Physiol. 2015;119:163–171. doi: 10.1152/japplphysiol.00760.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letts J.A., Fiedorczuk K., Sazanov L.A. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 14.Lenaz G., Fato R., Genova M.L., Bergamini C., Bianchi C., Biondi A. Mitochondrial Complex I: structural and functional aspects. Biochim. Biophys. Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Lenaz G., Genova M.L. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am. J. Physiol. Cell Physiol. 2007;292:1221–1239. doi: 10.1152/ajpcell.00263.2006. [DOI] [PubMed] [Google Scholar]
- 16.Lenaz G., Tioli G., Falasca A.I., Genova M.L. Complex I function in mitochondrial supercomplexes. Biochim. Biophys. Acta. 2016;1857:991–1000. doi: 10.1016/j.bbabio.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Luna-Sánchez M., Hidalgo-Gutiérrez A., Hildebrandt T.M., Chaves-Serrano J., Barriocanal-Casado E., Santos-Fandila Á., Romero M., Sayed R.K., Duarte J., Prokisch H., Schuelke M., Distelmaier F., Escames G., Acuña-Castroviejo D., López L.C. CoQ deficiency causes disruption of mitochondrial sulfide oxidation, a new pathomechanism associated with this syndrome. EMBO Mol. Med. 2017;9:78–95. doi: 10.15252/emmm.201606345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luna-Sánchez M., Díaz-Casado E., Barca E., Tejada M.Á., Montilla-García Á., Cobos E.J., Escames G., Acuña-Castroviejo D., Quinzii C.M., López L.C. The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol. Med. 2015;7:670–687. doi: 10.15252/emmm.201404632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maas M.F., Krause F., Dencher N.A., Sainsard-Chanet A. Respiratory complexes III and IV are not essential for the assembly/stability of complex I in fungi. J. Mol. Biol. 2009;387:25969. doi: 10.1016/j.jmb.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Maranzana E., Barbero G., Falasca A.I., Lenaz G., Genova M.L. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid. Redox Signal. 2013;19:1469–1480. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiles J.L., Huertas J.R., Mañas M., Ochoa J.J., Battino M., Mataix J. Oxidative stress induced by exercise and dietary fat modulates the coenzyme Q and vitamin A balance between plasma and mitochondria. Int. J. Vitam. Nutr. Res. 1999;69:243–249. doi: 10.1024/0300-9831.69.4.243. [DOI] [PubMed] [Google Scholar]
- 22.Schägger H., Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19(1777):83. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schägger H., Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 2001;276:37861–37867. doi: 10.1074/jbc.M106474200. [DOI] [PubMed] [Google Scholar]
- 24.Weber D., Davies M.J., Grune T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: focus on sample preparation and derivatization conditions. Redox Biol. 2015;5:367–380. doi: 10.1016/j.redox.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material