Abstract
Objective:
Lead exposure has been associated with various cardiovascular disorders. It may also cause increased inflammation and fibrosis in the arterial system resulting in an increase in arterial stiffness. In this study, the ambulatory arterial stiffness index (AASI), which is a technique that measures arterial stiffness, was evaluated in occupationally lead-exposed workers.
Methods:
In this cross-sectional study, 68 lead-exposed workers without known cardiovascular risk factors and 68 healthy subjects were evaluated according to the 24-hour ambulatory blood pressure monitorization (ABPM) profiles and blood and 24-hour urine lead levels. A regression slope of diastolic over systolic blood pressure (BP) was computed in each participant. AASI was defined as 1 minus the regression slope.
Results:
There were no significant differences in terms of baseline demographic, clinical, echocardiographic characteristics, and ABPM profiles between the groups. In lead-exposed workers, the mean age was 34.7±8.1 years, and the median blood and urine lead levels were 40.5 µg/dL and 34.9 µg/L, respectively. AASI was 0.42±0.10 in lead-exposed workers and 0.37±0.10 in control subjects (p=0.007). In correlation analysis, AASI was correlated with both blood (r=0.417, p<0.001) and urine lead (r=0.242, p=0.047) levels. In regression analysis, blood lead level was found to be associated with AASI (b=0.086, p<0.001).
Conclusion:
AASI, which is an indicator of arterial stiffness, was found to be higher in lead-exposed workers than in healthy individuals. Increased AASI may be considered an early sign of arterial involvement in case of lead exposure.
Keywords: lead exposure, arterial stiffness, ambulatory blood pressure monitoring, dipping
Introduction
Lead exposure can lead to a wide range of cardiovascular diseases including atherosclerosis, ischemic and structural heart diseases, and conduction disturbances (1–3). Additionally, numerous studies have demonstrated that lead exposure can lead to increased diastolic and systolic blood pressure (BP) (4). It causes hypertension (HT) by promoting vascular inflammation via oxidative stress, decreased nitric oxide availability, and increased endothelin production (5, 6). Moreover, lead has been shown to cause endothelial dysfunction and stimulate vascular smooth muscle cell proliferation (5). All of these changes that seen in vasculature in case of lead exposure increase vascular fibrosis of large arteries, resulting in a decrease in arterial compliance. All of these factors result in increased arterial stiffness (7).
Arterial stiffness is recognized as an important measure of the functional properties of large arteries, and it has been demonstrated to be a predictor for increased risk of cardiovascular organ damage and future cardiovascular events (8, 9). As a non-invasive technique, the ambulatory arterial stiffness index (AASI) derived from ambulatory blood pressure monitoring (ABPM), obtained over 24 hours in a given individual, was introduced as an index that predicts cardiovascular risk (10, 11). AASI gives valuable information about arterial stiffness by analysis of the relationship between systolic and diastolic BP. In this study, arterial stiffness was evaluated using AASI in occupationally lead-exposed workers.
Methods
Study population
In this cross-sectional study, 144 occupationally lead-exposed workers admitted to our center between January 2015 and December 2015 were enrolled. Past medical histories, physical examinations, 12-lead surface electrocardiogram, results, and transthoracic echocardiography (TTE) were evaluated. Workers with coronary artery disease, systolic and/or diastolic dysfunction, valvular heart disease except trivial valvular regurgitation, diabetes mellitus, pre-existing HT defined as systolic BP >140 mm Hg and diastolic BP >90 mm Hg and/or taking anti-hypertensive medication, thyroid dysfunction, hypercholesterolemia, electrolyte imbalance, cigarette smokers, chronic lung disease and obesity [body mass index (BMI) >30 kg/m2] were excluded. After taking into account these exclusion criteria, 76 workers were excluded, and the study was carried out with a total of 68 workers.
Of the 68 workers, 49 (72.0%) were battery-production workers and 19 (27.9%) were metal-recycling workers. Sixty-eight healthy subjects who worked in our center as hospital staff without any known overt diseases served as the control group. The study population was male and aged between 18 and 65 years. Written informed consent was obtained from each subject, and the institutional ethics committee approved the study protocol.
Analysis methods
Lead levels were determined in whole blood and 24-hour urine samples using inductively coupled plasma mass spectrometry (Agilent 7700 series, Tokyo, Japan). Blood samples were digested by the microwave-induced acid digestion method. A standard solution of lead was prepared by dilution of certified standard solutions (High-Purity Standards, Charleston, SC, USA). Two-level quality control materials were used (Seronorm, Billingstad, Norway). The lead calibration curve ranged from 0 to 100 µg/dL. The limit of detection and limit of quantification were 0.02 and 0.1 µg/dL, respectively. The relative standard deviation of the measurements was 4.2. Subjects were asked to collect 24-hour urine samples. They were instructed not to collect urine from the first urination after waking in the morning of the day that they started to collect the urine. Urine samples were collected in sterile plastic pots during every urination thereafter, including the first urination after waking the following morning, and then diluted 1 in 10 with 5% nitric acid solution.
Ambulatory blood pressure monitorization
Ambulatory BP monitorization studies were carried out using the WatchBP O3 (Microlife AG, Widnau, Switzerland) monitoring device. The device was applied to the non-dominant arm for 24 hours. The first hour was discarded from analysis. BP readings were obtained automatically at 15-minute intervals during daytime and 30-minute intervals during nighttime. Recordings were accepted only if more than 85% of the raw data were valid. Time in bed was defined based on the patient-kept diary that documented the exact time of getting into and arising from bed. The average BP for this time in bed was calculated from the ambulatory monitoring data (termed nighttime BP). Daytime BP was defined as the average BP during the remainder of the 24-hour period. Mean BP was calculated as the diastolic pressure plus one-third of the pulse pressure. A regression slope of diastolic over systolic BP was computed for each participant (10, 12). A regression line was not forced through the origin (intercept 0), because during diastole, when the flow drops to 0, such a phenomenon does not occur for BP. We defined AASI as 1 minus the regression slope. The stiffer the arterial tree, the closer the regression slope and AASI are to 0 and 1, respectively.
Nocturnal dipping (%) was defined as the percentage decrease in nocturnal systolic BP compared with daytime systolic BP. When patients exhibited nocturnal dipping of less than 10%, they were defined as non-dippers.
Transthoracic echocardiography
Standard echocardiographic imaging was performed in the left lateral decubitus position with the Esaote MyLabTM 50 cardiac ultrasound scanner (Florence, Italy). Images were obtained using a 2.5–3.5 MHz transducer in the parasternal and apical views. The left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) were determined with M-mode echocardiography under two-dimensional guidance in the parasternal long-axis view, according to the recommendations of the American Society of Echocardiography (13). The left ventricular ejection fraction (LVEF) was calculated from the apical four-chamber view, according to the modified Simpson’s rule (13). In addition, the left atrial dimension in the parasternal long-axis view and the right ventricular (RV) end-diastolic diameter in the apical four-chamber view were also calculated. Pulmonary systolic arterial pressure (sPAP) was estimated by continuous-wave (CW) Doppler as peak regurgitation velocity plus an assumed right atrial pressure in relation to the size and respiratory excursion of the inferior cava vein visualized in the subcostal view. Measurements of mitral inflow included the peak early filling (E-wave) and late diastolic filling (A-wave) velocities, the E/A ratio, deceleration time (DT) of the early filling velocity, and the isovolumic relaxation time (IVRT), derived by placing the cursor of the CW Doppler in the left ventricular (LV) outflow tract to simultaneously display the end of aortic ejection and the onset of mitral inflow.
Statistical analysis
Statistical evaluation was performed using the Statistical Package for Social Sciences 20 (SPSS 20) for Windows (IBM SPSS Inc., Chicago, IL, USA) program. Variables with normal distribution were shown as mean±standard deviation while those without normal distribution were shown as median with minimum (min) and maximum (max) (range). Categorical variables were shown as numbers and percentage. The comparison between groups of continuous variables was performed with t-test for independent variables showing normal distribution and the Mann-Whitney U test for those not showing normal distribution. Pearson correlation analysis was used to test normal distribution while Spearman correlation analysis was used for variables not showing normal distribution. Stepwise linear regression analysis was used to multiple regression analysis. Before this analysis, logarithmic transformation was applied on parameters that did not show normal distribution. A p<0.05 was considered statistically significant.
Results
The baseline characteristics and echocardiographic parameters of the two groups are shown in Table 1. There was no statistically significant difference between the lead-exposed and control groups in terms of age, resting HR, BMI, smoking habits, and low-density lipoprotein levels. The mean age of the lead-exposed group was 34.7±8.1 years and that of the control group was 34.1±9.4 years. All lead-exposed workers and control group participants had a similar and normal LVEF (mean 64.5±3.7 vs. 64.5±3.1%, p=0.980). Additionally, the LVEDD, LVESD, left atrial diameter, RV diameter, sPAP, mitral E-wave, A-wave, DT, and IVRT were similar in the two groups. In the lead-exposed group, the median duration of exposure to lead was 45 months (6–360). The median blood lead level [40.5 µg/dL (11.4–90) vs. 0.5 (0.1–0.8), p<0.001] and median 24-hour urine lead level [34.9 µg/L (2.1–128) vs. 0.1 (0.1–0.9), p<0.001] were significantly higher in lead-exposed workers than in control subjects. In terms of 24-hour ABPM parameters, average day, average night, and total systolic, diastolic, and mean BP values between the groups did not show statistically differences (Table 2). While in the control group, 25 subjects (36.7%) showed non-dipper status, in the lead-exposed group 19 (27.9%) workers showed non-dipper status (p=0.273). In the lead-exposed group, AASI was significantly lower in subjects with dipper status than in subjects with non-dipper status (0.40±0.09 vs. 0.47±0.09, respectively, p=0.006).
Table 1.
Demographic characteristics and echocardiographic parameters of the lead-exposed and control groups
| Characteristics | Lead-exposed group (n=68) | Control group (n=68) | P |
|---|---|---|---|
| Age, year | 34.7±8.1 | 34.1±9.4 | 0.690 |
| Resting heart rate, bpm | 76.3±10.6 | 73.4±9.7 | 0.098 |
| Body mass index, kg/m2 | 24.7±3.6 | 23.6±3.8 | 0.085 |
| Smoking, n (%) | 29 (42.6) | 22 (32.3) | 0.217 |
| Low-density lipoprotein, mg/dL | 141.0±26.0 | 136.8±19.9 | 0.294 |
| LV ejection fraction, % | 64.5±3.7 | 64.5±3.1 | 0.980 |
| End-diastolic diameter, mm | 45.7±3.3 | 46.1±3.5 | 0.494 |
| End-systolic diameter, mm | 27.4±2.6 | 27.7±2.9 | 0.526 |
| Left atrial diameter, mm | 32.6±3.0 | 33.2±3.2 | 0.261 |
| RV diameter, mm | 25.1±2.4 | 25.8±2.7 | 0.112 |
| sPAP, mm Hg | 23.4±4.6 | 22.7±4.5 | 0.371 |
| E wave, cm/s | 74.5±18.6 | 80.1±19.8 | 0.093 |
| A wave, cm/s | 64.8±12.6 | 63.8±13.0 | 0.649 |
| DT, ms | 184.5±40.7 | 178.4±45.5 | 0.411 |
| IVRT, ms | 96.3±15.5 | 95.4±14.9 | 0.730 |
| Working duration, months | 45 [6 – 360] | – | – |
| Blood lead level, µg/dL | 40.5 [11.4 – 90] | 0.5 [0.1 – 0.8] | <0.001 |
| 24-hour urine lead level, µg/L | 34.9 [2.1 – 128] | 0.1 [0.1 – 0.9] | <0.001 |
DT - deceleration time; IVRT - isovolumic relaxation time; LV - left ventricle; RV - right ventricle; sPAP - systolic pulmonary arterial pressure. Numerical variables are expressed as mean±standard deviation or median (minimum–maximum)
Table 2.
24-hour ambulatory blood pressure profiles of the lead-exposed and control groups
| Variables | Lead-exposed group (n=68) | Control group (n=68) | P |
|---|---|---|---|
| Average day SBP, mm Hg | 116.5±15.4 | 118.4±13.3 | 0.442 |
| Average day DBP, mm Hg | 67.9±9.6 | 70.1±11.3 | 0.223 |
| Average day MBP, mm Hg | 85.2±8.2 | 87.4±9.1 | 0.141 |
| Average night SBP, mm Hg | 107.9±12.3 | 108.7±12.8 | 0.710 |
| Average night DBP, mm Hg | 60.8±7.8 | 62.1±8.1 | 0.342 |
| Average night MBP, mm Hg | 77.6±9.8 | 79.8±10.4 | 0.206 |
| Total Average SBP, mm Hg | 112.3±13.4 | 114.0±13.0 | 0.450 |
| Total Average DBP, mm Hg | 63.7±7.7 | 65.6±9.1 | 0.191 |
| Total Average MBP, mm Hg | 80.9±8.6 | 81.4±9.7 | 0.750 |
| Non-dipper, % | 19 (27.9) | 25 (36.7) | 0.273 |
| AASI | 0.42±0.10 | 0.37±0.10 | 0.007 |
AASI - ambulatory arterial stiffness index; DBP - diastolic blood pressure; MBP - mean blood pressure; SBP - systolic blood pressure. Numerical variables are expressed as mean±standard deviation or median (minimum to maximum)
AASI was found to be 0.39±0.10 in the whole population. AASI was 0.42±0.10 and 0.37±0.10 in lead-exposed workers and control subjects, respectively, and this difference was statistically significant (p=0.007) (Fig. 1). In correlation analysis, AASI was found to be significantly correlated with both blood (r=0.417, p<0.001, Fig. 2a) and 24-hour urine lead levels (r=0.242, p=0.047, Fig. 2b). On the other hand, AASI was not found to be correlated with working duration (r=0.124, p=0.312). Multiple regression analysis showed that blood lead level was found to be associated with AASI (b=0.086, p<0.001).
Figure 1.
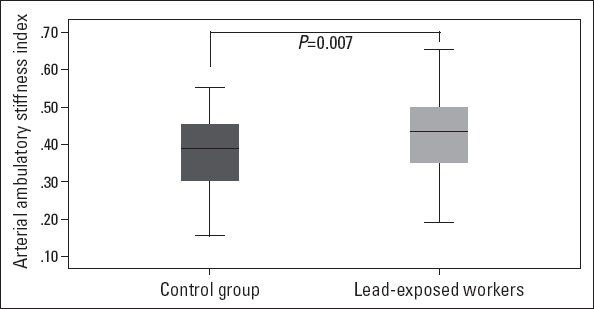
The ambulatory arterial stiffness index in lead-exposed and control groups
Figure 2.
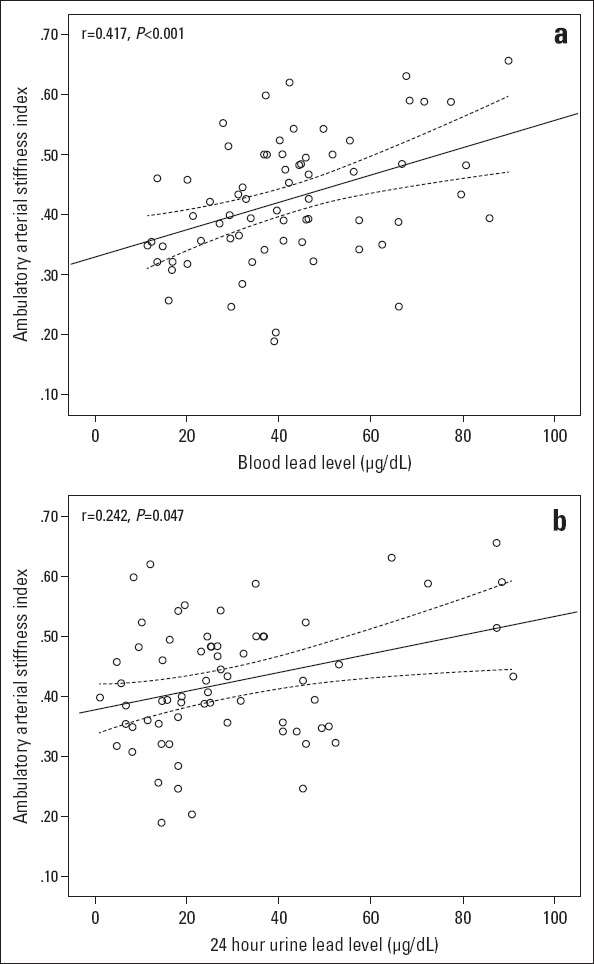
Correlation graphics between the ambulatory arterial stiffness index and blood lead (a) and (b) 24-hour urine lead level in the lead-exposed group. The dotted line represents a 95% confidence interval level in the lead-exposed group
Discussion
This study is the first to demonstrate that AASI, which is an indicator of arterial stiffness, is higher in occupationally lead-exposed workers than in healthy individuals. Although AASI was not found to be correlated with working duration, it was found to be significantly correlated with both blood and 24-hour urine lead levels. Moreover, AASI was significantly higher in non-dippers than in dippers in the lead-exposed population.
Functional and structural changes in the arterial tree occur inevitably with progressing age. One of these changes, increased arterial stiffness, results in a decrease in arterial compliance, which is an independent risk factor for stroke, coronary heart disease, and heart failure (14, 15). Exposure to heavy metals or toxic agents can accelerate this process. Wong et al. (16) have shown that nickel exposure was associated with increased arterial stiffness. In this study, arterial compliance was measured by the augmentation index. Our results are in concordance with this study, but we evaluated the effects of lead exposure on arterial stiffness by using AASI, which was found to be significantly higher than that of the control group. As an indicator of arterial stiffness, AASI has been associated with an increased risk of cardiovascular morbidity and mortality in various clinical settings (17, 18).
A growing number of epidemiological studies associate environmental and/or occupationally exposure to heavy metals with increased arterial BP (19, 20). One of these metals, exposure to lead results in increased systolic and diastolic BP, and chronic exposure may cause arterial HT (21). Moreover, cardiovascular complications such as LV hypertrophy and LV diastolic dysfunction were more frequently seen in hypertensive and lead-exposed patients than in hypertensive patients without lead exposure (22). In our study, both workers and control subjects were normotensive and had normal systolic and diastolic LV functions on TTE. In the lead-exposed group, blood and urine lead levels were correlated with AASI. Besides, blood lead level was found to be an independent predictor of AASI. These findings confirmed the previous study of Poreba et al. (23). They found a positive correlation between blood lead concentration and arterial stiffness measured by the augmentation index and pulse wave velocity analysis. Additionally, blood zinc protoporphyrin, which is used to monitor lead exposure, was found to be independently associated with arterial stiffness.
AASI was first introduced by Dolan et al. (10) in 2006; however, as the clinical studies were carried out, some debates have emerged about the validity of AASI in arterial stiffness. Kips et al. (24) studied the determinants of AASI by using a computer model and found that AASI was influenced by HR, such that an increase in HR caused AASI to decrease by 37%. In our study, AASI did not correlate with HR, and there was no difference between the lead-exposed group and the control group in terms of HR. However, it should be kept in mind that our study population consisted of an extremely homogeneous group, and this restricts to reflect the real world and reduces the generalizability of the findings. Another shortcoming for AASI is that it is influenced by the degree of nocturnal BP fall (25). It is suggested that the predictive capability attributed to AASI actually belongs to the presence of non-dipping status. In our study, although the dipping status of two groups was found to be similar, AASI was lower in subjects with dipper status than in subjects with non-dipper status in the lead-exposed group. This finding is consistent with the hypothesis mentioned, and dipping status should be considered when assessing the clinical importance of AASI in future studies. Finally, Benetos and Lacolley (26) reported that AASI may be a more sensitive predictor of stroke risk than cardiovascular risk in young and low-risk individuals, similar to our study population. This result may be a source of inspiration for further studies in which AASI is used to assess the risk of stroke in case of lead exposure.
Study limitations
Several limitations of the present study should be addressed. Nearly 95% of the total body lead burden is located in bone. In this study, we only analyzed blood and 24-hour urine lead levels. Bone lead levels were not measured. Lead in bone may better predict long-term lead toxicity than does the concurrent blood lead level, which reflects only relatively recent exposure. Another limitation is that, to sort out possible confounding factors, strict exclusion criteria were applied at the beginning of the study and only the effects of blood and 24-hour urine lead levels have tried to evaluate. Finally, this study was cross-sectional in design, and patients were not followed-up in terms of cardiovascular end-points; thus, a possible association between AASI and these end-points has not been evaluated. These limitations make it difficult to speculate about long-term cardiovascular complications. The findings of this study require confirmation in larger studies.
Conclusion
This study showed that AASI, which is an indicator of arterial stiffness, is higher in occupationally lead-exposed workers even without an elevated BP profile and other traditional cardiovascular risk factors as compared to healthy individuals. Increased AASI may be considered as an early sign of arterial involvement in case of lead exposure.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – U.N.K., Ö.H.Y., E.T.; Design – U.N.K., Ö.H.Y., E.T.; Supervision – Ö.H.Y., E.T., C.B.; Fundings – Ö.H.Y., E.T., C.B.; Materials – M.G., C.B.; Data Collection and/or processing – M.G., C.B.; Analysis &/or interpretation – U.N.K., Ö.H.Y., E.T.; Literature search – U.N.K., İ.A.; Writing – U.N.K., Ö.H.Y., E.T.; Critical review – Ö.H.Y., E.T.
References
- 1.Jain NB, Potula V, Schwartz J, Vokonas PS, Sparrow D, Wright RO, et al. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men:the VA Normative Aging Study. Environ Health Perspect. 2007;115:871–5. doi: 10.1289/ehp.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee TH, Tseng MC, Chen CJ, Lin JL. Association of high body lead store with severe intracranial carotid atherosclerosis. Neurotoxicology. 2009;30:876–80. doi: 10.1016/j.neuro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen CC, Yen HW, Lo YH, Chu YH, Chiu YW, Chuang HY. The association of prolonged QT interval on electrocardiography and chronic lead exposure. J Occup Environ Med. 2013;55:614–9. doi: 10.1097/JOM.0b013e318291787a. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Liu X, Deng Q, Duan Y, Dai H, Li Y, et al. Continuous lead exposure increases blood pressure but does not alter kidney function in adults 20-44 years of age in a lead-polluted region of China. Kidney Blood Press Res. 2015;40:207–14. doi: 10.1159/000368496. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2008;295:454–65. doi: 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dursun N, Arifoğlu C, Süer C, Keskinol L. Blood pressure relationship to nitric oxide, lipid peroxidation, renal function, and renal blood flow in rats exposed to low lead levels. Biol Trace Elem Res. 2005;104:141–9. doi: 10.1385/BTER:104:2:141. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53:258–61. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness:methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 9.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness:a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–70. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 11.Kikuya M, Staessen JA, Ohkubo T, Thijs L, Metoki H, Asayama K, et al. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke. 2007;38:1161–6. doi: 10.1161/01.STR.0000259604.67283.69. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Wang JG, Dolan E, Gao PJ, Guo HF, Nawrot T, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension. 2006;47:359–64. doi: 10.1161/01.HYP.0000200695.34024.4c. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification:a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 15.Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, et al. Relation of Central Arterial Stiffness to Incident Heart Failure in the Community. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong JY, Fang SC, Grashow R, Fan T, Christiani DC. The relationship between occupational metal exposure and arterial compliance. J Occup Environ Med. 2015;57:355–60. doi: 10.1097/JOM.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Li Y, Dolan E, et al. Ambulatory arterial stiffness index predicts stroke in a general population. J Hypertens. 2006;24:2247–53. doi: 10.1097/01.hjh.0000249703.57478.78. [DOI] [PubMed] [Google Scholar]
- 18.Kalaycıoğlu E, Gökdeniz T, Aykan AC, Hatem E, Gürsoy OM, Çavuşoğlu G, et al. Ambulatory arterial stiffness index is associated with impaired left atrial mechanical functions in hypertensive diabetic patients:A speckle tracking study. Anatol J Cardiol. 2015;15:807–13. doi: 10.5152/akd.2014.5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. 2012;120:98–104. doi: 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Deng F, Huang J, Wang H, Shima M, Wang X, et al. Blood pressure changes and chemical constituents of particulate air pollution:results from the healthy volunteer natural relocation (HVNR) study. Environ Health Perspect. 2013;121:66–72. doi: 10.1289/ehp.1104812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115:472–82. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poreba R, Gac P, Poreba M, Derkacz A, Pilecki W, Antonowicz-Juchniewicz J, et al. Relationship between chronic exposure to lead, cadmium and manganese, blood pressure values and incidence of arterial hypertension. Med Pr. 2010;61:5–14. [PubMed] [Google Scholar]
- 23.Poreba R, Gac P, Poreba M, Antonowicz-Juchniewicz J, Andrzejak R. Relationship between occupational exposure to lead and local arterial stiffness and left ventricular diastolic function in individuals with arterial hypertension. Toxicol Appl Pharmacol. 2011;254:342–8. [Google Scholar]
- 24.Kips JG, Vermeersch SJ, Reymond P, Boutouyrie P, Stergiopulos N, Laurent S, et al. Ambulatory arterial stiffness index does not accurately assess arterial stiffness. J Hypertens. 2012;30:574–80. doi: 10.1097/HJH.0b013e32834fca18. [DOI] [PubMed] [Google Scholar]
- 25.Schillaci G, Parati G, Pirro M, Pucci G, Mannarino MR, Sperandini L, et al. Ambulatory arterial stiffness index is not a specific marker of reduced arterial compliance. Hypertension. 2007;49:986–91. doi: 10.1161/HYPERTENSIONAHA.106.082248. [DOI] [PubMed] [Google Scholar]
- 26.Benetos A, Lacolley P. From 24-hour blood pressure measurements to arterial stiffness:a valid short cut? Hypertension. 2006;47:327–8. doi: 10.1161/01.HYP.0000200705.61571.95. [DOI] [PubMed] [Google Scholar]


