Abstract
Objective:
Atrial fibrillation (AF) is the most common rapid cardiac arrhythmia associated with high morbidity and mortality. Stimulation of the sympathetic nerve is involved in AF occurrence. The gap junction protein connexin 43 (Cx43) plays a key role in electrical conduction velocity in cardiac tissues, and under expression of Cx43 was linked with AF. The aim of this study was to investigate whether Cx43 was involved in sympathetic AF.
Methods:
Fifteen dogs were randomly divided into 3 groups (5 in each group). Sympathetic AF was induced in dogs and isolated canine atrial myocytes by isoproterenol (ISO) perfusion and rapid atrium pacing (RAP). The expression levels of nerve growth factor (NGF) and tyrosine hydroxylase (TH) in the atrial tissues were detected using immunohistochemical staining. The transcription and protein expression of Cx43 in the AF cell model was measured. Subsequently, Cx43 was blocked by short interfering (si) RNA in atrial myocytes and the gap junctional inter-cellular communication was detected using the scrape-loading and dye transfer assay.
Results:
Sympathetic AF was successfully induced by a combination of ISO perfusion and RAP. The expression levels of NGF and TH were increased in the RAP group, and further increased in the RAP + ISO group. Tissue samples from the AF dogs had a lower Cx43 level than those of the control group (p<0.05). The expressions of mRNA and protein of Cx43 in sympathetic AF cell model decreased by 26% and 28%, respectively, when compared with the control group, with p<0.05. Silencing Cx43 in cells by siRNA could also efficiently reduce Cx43 expression. The relative levels of Cx45 mRNA were decreased by 73% compared with unaffected cells. The scrape-loading and dye transfer assay showed that gap junctional intercellular communication was hampered in the sympathetic AF cell model and silencing Cx43 could impede channel conduction.
Conclusion:
The results suggested that low expression of Cx43 was involved in sympathetic AF by influencing intercellular channel conduction. Intervention of Cx43 expression might be an appealing therapy to sympathetic AF.
Keywords: sympathetic atrial fibrillation, connexin 43, Gap junction, rapid electric pacing
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia in clinical practice, is associated with an increased risk of cerebrovascular stroke and several other pathologies, including heart failure (1). The incidence of AF is increased with the aging of population, and AF can cause serious complications, such as artery thrombosis, stroke, and heart failure (2, 3). Currently, the treatment of AF is still a big challenge. Anti-arrhythmic drug therapy is limited by severe side effects; recurrence of atrial tachyarrhythmia is a major problem of catheter ablation (4, 5). The development of AF treatment procedures has been marked by a dynamic feedback between fundamental research and clinical observations. To improve the management of AF, better understanding of the underlying mechanisms is required.
Previous researches have shown that the stimulation of sympathetic nerve was involved in the occurrence of AF. Stimulating the cardiac autonomic nervous system can cause pulmonary vein ectopic beats and then trigger AF (6). In rheumatic heart disease patients, sympathetic density was significantly increased in patients with AF than in those with non-AF (7). Furukawa et al. (8) conducted autonomic nerve stimulation in dogs and found that sympathetic nerve stimulation might increase susceptibility to AF via electrical remodeling of the atria.
Intercellular communication via gap junctions plays a key role in electrical conduction velocity in cardiac tissue (9). Gap junction proteins, also known as connexins, mediate action potential propagation between cells, coordinate mechanical and electrical activity of heart muscle, and ensure simultaneous cardiac electro-mechanical activity (10, 11). The principle connexin protein in human atria, connexin 43 (Cx43), has been linked to AF in multiple studies. The expression of Cx43 was reduced in AF patients, and this low expression could be caused by mutations in the Cx43 gene (12, 13). The G60S Cx43 mutant mouse had sustained AF, and the expression of Cx43 was reduced in the atria (14). Cx43 gene transfer could improve conduction velocity and prevent AF in the swine model (15, 16).
Based on the previous studies, we hypothesized that Cx43 might be involved in the stimulation of sympathetic nerve and induce AF. To better understand the mechanism of Cx43 in the occurrence of AF, the expression of Cx43 was investigated in a canine model of sympathetic AF. Meanwhile, RNA interference technology was used to silence the Cx43 gene in a canine atrial myocyte model. The participation of Cx43 in channel conduction was also investigated.
Methods
Sympathetic AF canine model
Animal studies were approved by the Animal Use Committee of Guangxi Medical University. All the dogs were provided by the Experimental Animal Center of the Guangxi Medical University. Fifteen Chinese mongrel dogs weighing about 28 kg were randomly divided into 3 groups (5 in each group). Dogs in the control group were intraperitoneally injected with 3% sodium pentobarbital for anesthesia. A median sternotomy was made to expose the heart. The left ventricular was injected with 8000 U of heparin. The heart was removed and placed in a pre-cooled Tyrode’s solution. After extrusion of residual blood, the heart was perfused with a modified Tyrode’s solution (250 r/min) under 37.5°C; the heart was pressed at the same time to maintain the beat rate of 100/min. Electrode catheters were attached to both atriums and apexes to allow recording electrocardiogram (ECG) and rapid atrium pacing (RAP). The atrial tissue was harvested after perfusion of 1 h and maintained in liquid nitrogen for further use. The dogs in the RAP group were electrically stimulated during the perfusion at frequency of 800 beats/min for 30 s, a total of 30 times. The dogs in the RAP and isoproterenol (ISO) groups were perfused with the modified Tyrode’s solution containing 0.1 µM ISO and electrically stimulated as those in the RAP group.
Electrophysiological measurements
Atrial effective refractory period (AERP) was measured using the pre-period stimulation (S1S2) increment method. The S1S2 stimulation frequency was 8:1, with step as 10 ms. The longest S1S2 interval, which could not be passed down to the atrium, was defined as AERP. AF was defined as irregular atrial rates lasting longer than 2 s.
Immunohistochemical staining
The expression of nerve growth factor (NGF) and tyrosine hydroxylase (TH) in atrial tissue was detected using immunohistochemical staining. The atrial tissue was rinsed with normal saline, fixed in 10% formaldehyde, embedded in paraffin, and cut into 5-µm-thick sections. Immunohistochemical staining was performed as previously described with some modification (17). Briefly, the dewaxed sections were incubated with 3% hydrogen peroxide (H2O2) for 10 min and followed by incubation in normal rabbit serum for 1 h. The primary antibodies used were polyclonal rabbit anti-NGF (Bioss Antibodies, Beijing, China; 1:500) and polyclonal rabbit anti-TH (Bioss Antibodies, Beijing, China; 1:300) at 4°C overnight. The next day, the sections were stained using the PV-6001 immunohistochemical staining kit (ZSGB-Bio, Beijing, China) as per manufacturer’s instructions. Stained samples were examined under a microscope with 400 ´ amplification.
Cell culture
Primary canine atrial myocytes were isolated from 1-week-old pups. The right and left atrial appendages were collected and cut into approximately 1 mm ´ 1 mm ´ 1 mm tissue blocks. The tissue was digested with twice volume of 0.06% trypsin buffer at 37°C with 100 r/min shaking for 10 minutes. Additional digestion buffer with 2.5 g/L trypsin and 2 g/L type II collagenase was added to the final volume of 15 times the myocardial tissue with continuous shaking. Digested cells were then cultured with high-glucose/Dulbecco’s Modified Eagle’s Medium (HG/DMEM; Hyclone, Logan, USA) with 5% CO2 at 37°C. After 24 hours, adherent cells were maintained with citrate minimal medium (CMM; Hyclone, Logan, USA).
Establishment of AF cell model
Atrial myocytes are cultured to approximately 80% confluence, and cells are perfused with 10 nmol/L ISO for 30 min. Electrical field stimulation was performed with BL-420E stimulator (Zhenghua Biological Equipement Co., Huaibei, China) over a range 500–650 beats/min (1.5 v/cm field strength) for 24 h (18).
Cell transfection
Transfection was performed using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The short interfering (si) RNA for Cx43 gene (LV-Cx43siRNA) was synthesized and chemically modified (sense: 5’-AAGACTGTGGATCTCCGAAA-3’; anti-sense: 5’-GCTCACTTGCTTGTTTGTT G-3’; synthesized by Takara, Dalian, China). Cells transfected with LV-Cx43siRNA was named as low expression (LE) group. Cells not transfected (NE group) and cells transfected with empty vector (NC group) were used as controls.
Real-time quantitative polymerase chain reaction analysis
RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) from canine atrial tissue and atrial myocytes. Extracted RNA was treated with DNase I (Promega, Madison, WI, USA). Reverse transcription was performed with 0.5 µg of RNA using PrimeScript RT Master Mix reagent kit (Takara, Dalian, China). Real-time quantitative polymerase chain reaction (PCR) was done using SYBR Premix Ex Taq kit (Takara, Dalian, China) at MasterCycler RealPlex4 system (Eppendorf, Hamburg, Germany). Beta-actin was used as a housekeeping control. The PCR reaction profile was as follows: initial 95°C for 2 min: 40 cycles of 95°C for 15 s and 60°C for 32 s. The primers used were: Cx43 sense, 5’-AAGACYGYGGAYCYCCGAAA-3’; Cx43 anti-sense, 5’-GCTCACTTGCTTGT TTGTTG-3’; b-actin sense, 5’-TGAGCGCAAGTACTGTTGT-3’; b-actin antisense, 5’-AACAGTCCGCCTAGAAGCAT-3’. Relative expression of mRNA was calculated using the 2-ΔΔCt method (19).
Western blot analysis
The atrial tissue was grinded and lysed with radioimmunoprecipitaion assay (RIPA) buffer containing 100 mg/L phenylmethylsulfonyl fluoride (PMSF) to extract total protein. The atrial myocytes were lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and sonicated. Protein samples were isolated on 12% SDS-PAGE gels. Approximately 40 µg of total protein was loaded each line. Proteins were then transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA) and blocked with 5% dry milk. The membranes were probed with primary mouse antibody against Cx43 (Santa Cruz Biotechnology, Santa Cruz, USA; 1:500) and secondary horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG antibody (SouthernBiotech, Birmingham, USA; 1:10000). Signals were detected using the enhanced chemiluminescence (ECL) method and quantified using densitometry. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control using the primary antibody of mouse anti-GAPDH (KangChen Bio-tech, Shanghai, China; 1:10000). The relative expression level of Cx43 was compared by normalizing the gray density of Cx43 to the gray density of GAPDH.
Immunofluorescence analysis
The cells were fixed with 4% paraformaldehyde on ice for 20 min and then permeabilized with phosphate-buffered saline (PBS)-Triton for 5 min. After blocking in 10% goat serum for 30 min, cells were probed with primary mouse antibody against Cx43 (Santa Cruz Biotechnology, 1:100). Fluorescein-labeled isothiocyanate (FITC) goat anti-mouse antibody (Santa Cruz Biotechnology, 1:1000) was used as secondary antibody. Nuclei were visualized by 4’, 6-diamidino-2-phenylindole (DAPI) staining method.
Scrape-loading and dye transfer analysis
The -loading and dye transfer (SLDT) method was previously described by Elfouly et al. (20). Briefly, cells were cultured with DMEM medium with addition of 55% the essential amino acids, 6% vitamins, 0.01 mmol/L non-essential amino acids, 1 mmol/L sodium pyruvate, and 10% fetal calf serum. Cells were grown to approximately 80% confluence. Each plate was scraped twice in the presence of 0.05% fluorescence dye Lucifer Yellow (457 Da) dissolved in PBS. After incubation for 3 min, cells were washed and fixed. The dye transfer results were examined using a fluorescence microscope.
Statistical analysis
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS) version 16.0 software (SPSS Inc., Chicago, IL). All data were presented as mean ± standard deviation (SD). The comparison between groups was analyzed using the Student t-test. All p values were two-tailed and p<0.05 was considered as statistically significant.
Results
Induction of sympathetic AF in canine
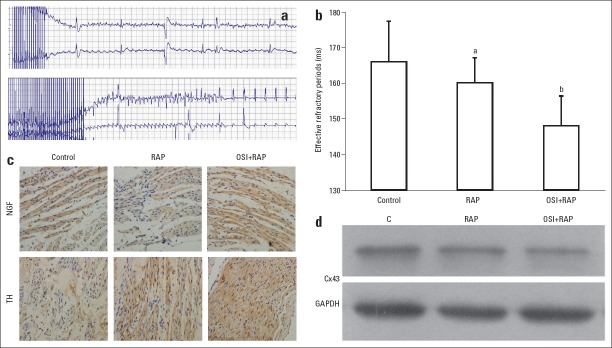
For dogs in the control and RAP groups, no sympathetic AF was induced. Sympathetic AF was only induced using a combination of ISO perfusion and RAP (success rate: 10%). A represent ECG of AF was shown in Figure 1a. Meanwhile, compared with the control group, the AERP was significantly shortened in the RAP + ISO group (Fig. 1b). The results indicated that ISO perfusion was required for induction of AF.
Figure 1.
Introduction of sympathetic atrial fibrillation (AF) in dogs. (a) a represent of electrocardiogram of AF induced with isoproterenol (ISO) perfusion and rapid atrium pacing (RAP). (b) Atrial effective refractory period (AERP) in different groups. Compared with the control (C) group, AERP was short in the RAP and RAP + ISO groups. (c) Detection of nerve growth factor (NGF) and tyrosine hydroxylase (TH) by immunohistochemical staining (light microscope, 400´). Brown particles represent positive expression. The protein levels of NGF and TH were increased in the RAP and RAP + ISO groups, indicating sympathetic AF. (d) Protein level of Cx43 in atrial tissues. Cx43 was down regulated in the RAP and the RAP + ISO group. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference. Error bars indicate standard deviation. ap>0.05 vs. control; bp<0.05 vs. control
To test whether sympathetic nerves were involved in the induction of AF, NGF and TH in the atrial tissues were detected as biomarkers. As shown in Figure 1c, the expression levels of NGF and TH were increased in the RAP group, and further increased in the RAP + ISO group. The results of immunohistochemical staining indicated that the AF was sympathetic related.
Protein samples were prepared from atrial tissues to determine whether Cx43 expression was changed. Figure 1d showed that the protein level of Cx43 was reduced by RAP stimulation and the level was the lowest in the RAP + ISO group. Therefore, down regulation of Cx43 was associated with sympathetic AF.
Expression of Cx43 in AF cell model
Isolated canine atrial myocytes were subjected to ISO perfusion and RAP to establish an AF cell model. The transcription and protein expression of Cx43 was then investigated (Figure 2). The expressions of mRNA and protein of Cx43 in sympathetic AF cell model decreased to 26% and 28%, respectively, when compared with the control group, with p<0.05. However, compared with the control group, ISO perfusion did not change the mRNA or the protein level of Cx43 (p>0.05). Meanwhile, RAP stimulation significantly reduced transcription level of Cx43 in approximately 20% (p<0.05). In parallel to the transcription change, RAP stimulation also lead to a 20% decrease in protein level of Cx43 (both p<0.05). Compared with RAP, combination with ISO perfusion and RAP did not further reduce the amount of Cx43 mRNA or Cx43 protein (p>0.05). The results suggested that the decrease of Cx43 during AF was mainly due to RAP stimulation rather than ISO perfusion.
Figure 2.
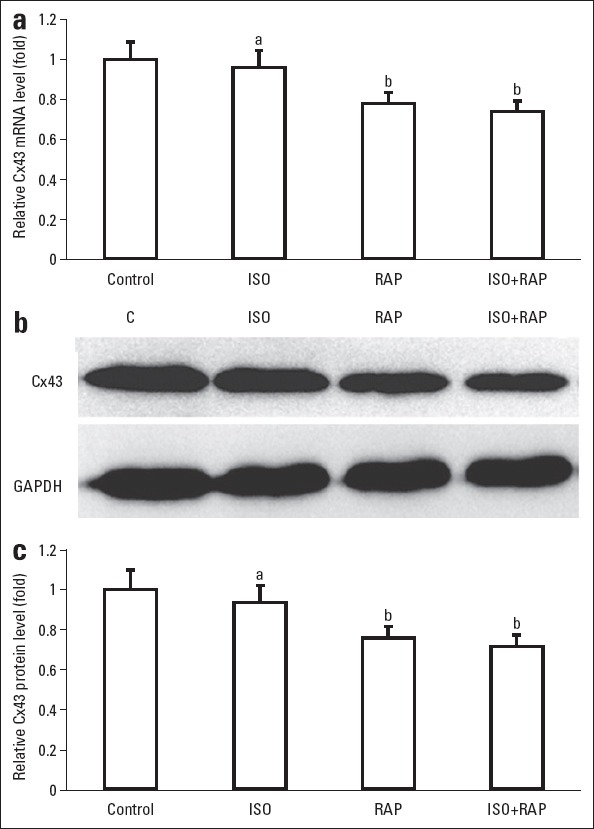
Expression of Cx43 in sympathetic atrial fibrillation (AF) cell model. Canine atrial myocytes were treated with isoproterenol (ISO) perfusion and rapid atrium pacing (RAP) to induce sympathetic AF. (a)Comparison of relative Cx43 mRNA level. (b) Western blot analysis of Cx43 in different groups. (c) Comparison of relative Cx43 protein level. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference. Error bars indicate standard deviation. aP>0.05 vs. control; bP<0.05 vs. control
Silencing of Cx43 by siRNA
To further confirm the role of Cx43 in sympathetic AF, siRNA technology was used to block the expression of Cx43 in canine atrial myocytes. A transfection rate of 90% was achieved. Compared with the unaffected cells (NE group) and the cells transfected with empty vector (NC group), cells transfected with LV-Cx43 siRNA (LE group) showed lower level of Cx43 mRNA (Fig. 3a, Fig. 3 p<0.05). The relative levels of Cx45 mRNA were decreased by 73% compared with unaffected cells. Western blot also showed that protein level of Cx43 was decreased significantly in the LE group (Fig. 3b, Fig. 3c, p<0.05). Meanwhile, the mRNA level or protein level of Cx43 did not change by transfecting only empty vector (NC group vs NE group, p>0.05). The results showed that LV-Cx43 siRNA successfully silenced the expression level of Cx43 in atrial myocytes.
Figure 3.
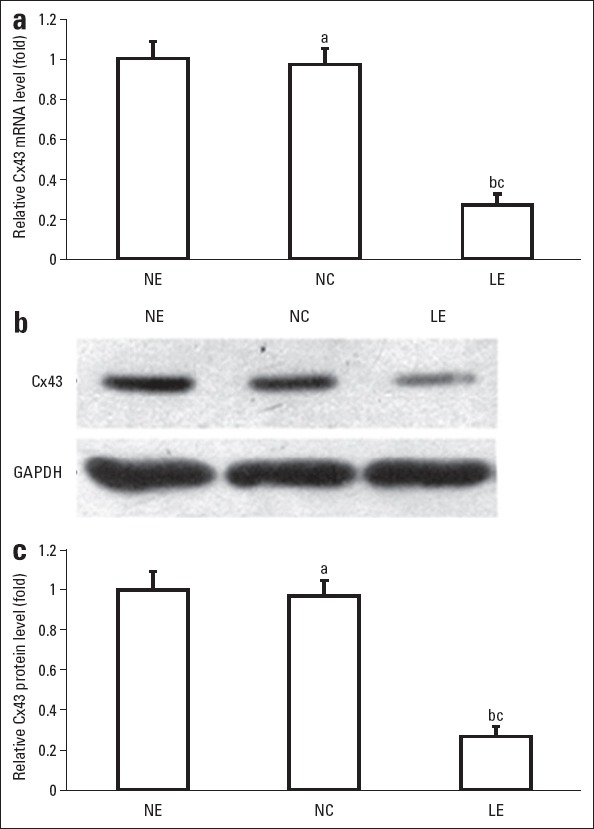
Expression of Cx43 in atrial myocytes transfected with LV-Cx43 siRNA (LE group). Myocytes unaffected (NE group) and myocytes transfected with empty vector (NC group) were used as controls. (a) Comparison of relative Cx43 mRNA level. (b) Western blot analysis of Cx43 in different groups. (c) Comparison of relative Cx43 protein level. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference. Error bars indicate standard deviation. aP>0.05 vs. NE group; bP<0.05 vs. NE Group; cP<0.05 vs. NC Group
Atrial myocytes in the NC and LE groups were next treated with ISO, RAP, or ISO + RAP. As shown in Figure 4a, the mRNA level of Cx43 was generally lower in the LE group than in the NC group (p<0.05). Consistent with our previous findings in normal atrial myocytes, ISO + RAP could obviously reduce the transcription level of Cx43 in the myocytes of NC group. Compared with the negative control myocytes treated with ISO + RAP, the myocytes transfected with siRNA alone showed significantly lower mRNA level of Cx43. For myocytes in the LE group, ISO and/or RAP could further reduce the Cx43 mRNA level. However, the difference within the LE group was not significant (p>0.05). The results suggest that siRNA transfection was more efficient to reduce Cx43 expression. The Cx43 protein level was also detected by immunofluorescence (IF). A similar trend of protein decrease was observed as the reduction of mRNA level (Fig. 4b).
Figure 4.
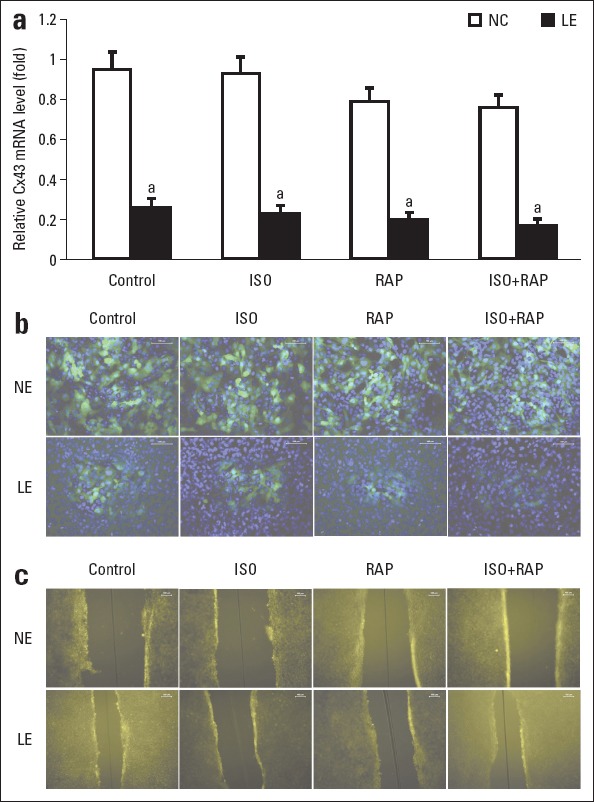
Induction of sympathetic atrial fibrillation (AF) in atrial myocytes unaffected (NE group) and atrial myocytes transfected with LV-Cx43 siRNA (LE group). (a) Comparison of relative Cx43 mRNA level. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as reference. Error bars indicate standard deviation. ap>0.05 vs. control in LE group. (b) Expression of Cx43 protein detected by immunofluorescence (200 ´). (c) Information transmission between cells under scrape-loading and dye transfer (100 ´). The white line segments in the upper right corner indicate 100 µm in length
Signal transduction between cells
The gap junctional intercellular communication in the atrial myocytes was subsequently detected using the SLDT test. The strength of signal transduction is correlated with the distance of the dye transfer. As shown in Figure 4c, the distance of dye travel from the scrape line was significantly shorter by RAP or ISO + RAP treatment in the NC group (p<0.05), indicating that signal conduction was blocked in the RAP or ISO + RAP groups. The results suggested that sympathetic AF was associated with hampered channel conduction. The distance of dye travel was also shortened in the LE groups compare with the control in the NC groups (p<0.05), indicating that Cx43 could impede channel conduction.
Discussion
In this study, the AF model in dogs and canine atrial myocytes by ISO perfusion and RAP was successfully established, and the role of Cx43 in AF was systematically studied. We found that low expression of Cx43 was involved in sympathetic AF by influencing inter-cellular channel conduction. Intervention of Cx43 could impede channel conduction, thus indicating that it might be an appealing therapy for sympathetic AF.
Cardiac remodeling is an important anatomical explanation of arrhythmia. Electrical remodeling is a major form of cardiac remodeling and can be caused by a functional insult (altered conductance) or structural alteration (altered content/distribution). These electrophysiological changes produce a substrate that is vulnerable to malignant ventricular arrhythmias (21, 22). Understanding the exact mechanism of electrical remodeling is important in elucidating potential therapeutic targets. Alterations in gap junction function contribute significantly to AF-induced remodeling, which could decrease conduction velocity and block unidirectional conduction block, thereby causing arrhythmias (23). Gap junction channels are composed of 4 transmembrane domain proteins, and Cx43 is the predominant connexin expressed by cardiomyocytes, occurring in abundance in adult working ventricular and atrial cardiomyocytes of all mammalian species, including humans (24). Therefore, in this article, we systematically studied the role of Cx43 in the occurrence of AF, which may contribute to further research on the treatment of AF.
As documented in literature, Cx43 is found to be down regulated during arrhythmogenic right ventricular (RV) cardiomyopathy and atrial arrhythmias (12, 13). Plotnikov et al. (25) demonstrated remodeling of the gap junction protein Cx43 following prolonged RV pacing. They found reduced Cx43 expression primarily in the early, activated myocardial segments. Tuomi et al. (14) induced Gx43 G60S mutation in mice, and the mutant mice had a 60% reduction in Cx43 as well as increased susceptibility to AF. Bikou et al. (15), for the first time, confirmed that the down regulation of Cx43 was required to trigger and maintain AF in large animal models. In this study, we further confirmed that Cx43 expression was reduced in both canine model and atrial myocyte model of AF. Previous studies have demonstrated that the expression of Cx40, 43, and 45 are not random variations but are in characteristic combinations and relative quantities in a chamber-related, myocyte-type-specific and developmentally regulated manner (26). As Cx40 and 45 were found to be increased during AF, it remains unclear whether an up regulation of Cx40 or Cx45 is a compensatory response to the decrease in Cx43 expression (27–29).
Current therapies for AF are targeted at reducing the risk of stroke (anti-coagulation) and tachycardia-induced cardiomyopathy (rate or rhythm control), which have limited efficacy and non-trivial potential to cause adverse effects (30, 31). Therapies targeting gap junctions are an attractive option for AF treatment. Although still in preclinical stage, previous studies have shown that Cx43 gene transfer could reduce susceptibility to AF and improve cardiac electrical conduction in a swine model (15, 16, 25). Furthermore, small-molecule drugs enhancing gap junction conductance, such as rotigaptide, have been developed as potential treatments for AF (30). Lene et al. (32) found that changes in gap junction function likely involve both phosphorylation and dephosphorylation of specific sites in Cx43, which may be possible downstream targets for rotigaptide signaling. In this study, the inter-cellular communication in the atrial myocytes was evaluated using the SLDT test. Figure 4c provides direct evidence that signal conduction was blocked by either ISO + RAP or transfection of LV-Cx43 siRNA. The results proved that an impeded gap junctional communication was involved in AF caused by decreased expression of Cx43. Therefore, gene therapy targeting Cx43 has sufficient theoretical basis, and treatments aimed to improve Cx43 expression levels exhibit new potentially anti-arrhythmic therapies during AF.
In this study, we investigated the influence of Cx43 down regulation by siRNA on gap junctional communication. In addition, Cx43 expression could also be regulated by dephosphorylation of Cx43 protein or mutation within the Cx43 gene. Studies of congestive heart failure have shown that Cx43 dephosphorylation caused Cx43 redistribution toward transverse cell-boundaries, while Cx43 mRNA and protein levels did not change (33). A single nucleotide deletion (c. 932delC) caused loss of function Cx43 and was associated with lone AF (34). These studies show that the in vivo regulation mechanism of Cx43 is quite complicated. Further researches are needed to fully understand the role of Cx43 in cardiac arrhythmia.
Study limitations
It is difficult to distinguish AF from atrial tachycardia. In this study, to identify AF in dogs, we used the standard clinical criteria, lack of regular P waves on ECGs and irregular ventricular responses. Although the atrial myocyte model was treated the same way as the canine model to induce AF, the cell model is most likely different from the real scenario in vivo. These limitations need to be considered when interpreting the results.
Conclusions
In conclusion, we developed an AF model in dogs and canine atrial myocytes by ISO perfusion and RAP. Cx43 was conformed to be down regulated in vitro and in vivo. Meanwhile gap junctional communication was impeded in the AF cell model, which could also be caused by blocking Cx43 expression. The results suggested that low expression of Cx43 was involved in sympathetic AF by influencing intercellular channel conduction. Intervention of Cx43 expression might be an appealing therapy to treat sympathetic AF.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (81260039), Guangxi Natural Science Foundation (2013GXNSFAA278005), Guangxi Medical and Healthcare Technology Research and Development Project (S201303-06), and the First Batch of Medical Cultivation of High-level Talents in Guangxi “139” Project Founding (to Yan He). The funding sources had no involvement on this study.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Y.H.; Design – Y.H.; Supervision – Y.H.; Fundings – Y.H.; Materials – C.S., W.H., B.L., H.L., J.L., J.X.; Data Collection and/or processing – C.S., W.H., Z.Z., B.L., H.L., J.L., J.X.; Analysis &/or interpretation – C.S., Z.Z., W.H.; Literature search – C.S., W.H.; Writing – C.S., WH.; Critical review – Y.H.
References
- 1.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke:a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–98. doi: 10.1378/chest.11-2888. [DOI] [PubMed] [Google Scholar]
- 2.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–7. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 3.Zoni-Berisso M, Filippi A, Landolina M, Brignoli O, D'Ambrosio G, Maglia G, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study) Am J Cardiol. 2013;111:705–11. doi: 10.1016/j.amjcard.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Mont L, Bisbal F, Hernández-Madrid A, Pérez-Castellano N, Viñolas X, Arenal A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation:a multicentre, randomized, controlled trial (SARA study) Eur Heart J. 2014;35:501–7. doi: 10.1093/eurheartj/eht457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation:translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;64:823–31. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- 6.Lim PB, Malcolme-Lawes LC, Stuber T, Wright I, Francis DP, Davies D, et al. Intrinsic cardiac autonomic stimulation induces pulmonary vein ectopy and triggers atrial fibrillation in humans. J Cardiovasc Elect. 2011;22:638–46. doi: 10.1111/j.1540-8167.2010.01992.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Lu Z, Tang Q, Jiang H, Huang C, He B, et al. The increase in sympathetic nerve density in the atrium facilitates atrial fibrillation in patients with rheumatic heart disease. Int J Cardiol. 2013;165:174–8. doi: 10.1016/j.ijcard.2011.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa T, Hirao K, Horikawa-Tanami T, Hachiya H, Isobe M. Influence of autonomic stimulation on the genesis of atrial fibrillation in remodeled canine atria not the same as in normal atria. Circ J. 2009;73:468–75. doi: 10.1253/circj.cj-08-0869. [DOI] [PubMed] [Google Scholar]
- 9.Levin M. Isolation and community:a review of the role of gap-junctional communication in embryonic patterning. J Membrane Biol. 2002;185:177–92. doi: 10.1007/s00232-001-0129-7. [DOI] [PubMed] [Google Scholar]
- 10.Yeager M. Structure of cardiac gap junction intercellular channels. J Struct Biol. 1998;121:231–45. doi: 10.1006/jsbi.1998.3972. [DOI] [PubMed] [Google Scholar]
- 11.Dhein S, Seidel T, Salameh A, Jozwiak J, Hagen A, Kostelka M, et al. Remodeling of cardiac passive electrical properties and susceptibility to ventricular and atrial arrhythmias. Front Physiol. 2014;5:424. doi: 10.3389/fphys.2014.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul M, Wichter T, Gerss J, Arps V, Schulze-Bahr E, Robenek H, et al. Connexin expression patterns in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013;111:1488–95. doi: 10.1016/j.amjcard.2013.01.299. [DOI] [PubMed] [Google Scholar]
- 13.Yan J, Kong W, Zhang Q, Beyer EC, Walcott G, Fast VG, et al. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc Res. 2013;97:589–97. doi: 10.1093/cvr/cvs366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomi JM, Tyml K, Jones DL. Atrial tachycardia/fibrillation in the connexin 43 G60S mutant (Oculodentodigital dysplasia) mouse. Am J Physiol Heart Circ Physiol. 2011;300:H1402–11. doi: 10.1152/ajpheart.01094.2010. [DOI] [PubMed] [Google Scholar]
- 15.Bikou O, Thomas D, Trappe K, Lugenbiel P, Kelemen K, Koch M, et al. Connexin 43 gene therapy prevents persistent atrial fibrillation in a porcine model. Cardiovasc Res. 2011;92:218–25. doi: 10.1093/cvr/cvr209. [DOI] [PubMed] [Google Scholar]
- 16.Igarashi T, Finet JE, Takeuchi A, Fujino Y, Strom M, Greener ID, et al. Connexin gene transfer preserves conduction velocity and prevents atrial fibrillation. Circulation. 2012;125:216–25. doi: 10.1161/CIRCULATIONAHA.111.053272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Wei C, Liu L, Lian AL, Qu XF, Yu G. Atrial fibrillation increases sympathetic and parasympathetic neurons in the intrinsic cardiac nervous system. Pacing Clin Electrophysiol. 2014;37:1462–9. doi: 10.1111/pace.12450. [DOI] [PubMed] [Google Scholar]
- 18.Brundel BJ, Kampinga HH, Henning RH. Calpain inhibition prevents pacing-induced cellular remodeling in a HL-1 myocyte model for atrial fibrillation. Cardiovasc Res. 2004;62:521–8. doi: 10.1016/j.cardiores.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Elfouly MH, Trosko JE, Chang C. Scrape-loading and dye transfer:A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987;168:422–30. doi: 10.1016/0014-4827(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol. 2014;29:20–7. doi: 10.1097/HCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 22.Nattel S, Harada M. Atrial remodeling and atrial fibrillation:recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–45. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 23.Magyar J, Banyasz T, Szentandrassy N, Kistamas K, Nanasi PP, Satin J. Role of gap junction channel in the development of beat-to-beat action potential repolarization variability and arrhythmias. Curr Pharm Design. 2015;21:1042–52. doi: 10.2174/1381612820666141029102443. [DOI] [PubMed] [Google Scholar]
- 24.Veeraraghavan R, Gourdie RG, Poelzing S. Mechanisms of cardiac conduction:a history of revisions. Am J Physiol Heart Circ Physiol. 2014;306:H619–27. doi: 10.1152/ajpheart.00760.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotnikov AN, Yu H, Geller JC, Gainullin RZ, Chandra P, Patberg KW, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 26.Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, et al. Remodelling of gap junctions and connexin expression in heart disease. Biochim Biophys Acta. 2004;1662:138–48. doi: 10.1016/j.bbamem.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 27.Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz J. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–12. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- 28.Betsuyaku T, Nnebe NS, Sundset R, Patibabdle S, Kruege CM, Yamada KA. Overexpression of cardiac connexin 45 increases susceptibility to ventricular tachyarrhythmias in vivo. Am J Physiol Heart Circ Physiol. 2006;290:163–71. doi: 10.1152/ajpheart.01308.2004. [DOI] [PubMed] [Google Scholar]
- 29.Imanaga I. Pathological remodeling of cardiac gap junction connexin 43-With special reference to arrhythmogenesis. Pathophysiology. 2010;17:73–81. doi: 10.1016/j.pathophys.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Grandi E, Maleckar MM. Anti-arrhythmic strategies for atrial fibrillation. The role of computational modeling in discovery, development, and optimization. Pharmacol Ther. 2016;168:126–42. doi: 10.1016/j.pharmthera.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nattel S, Harada M. Atrial remodeling and atrial fibrillation recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–45. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 32.Axelsen LN, Shabaz MS, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, et al. Identification of ischemia-regulated phosphorylation sites in connexin43:A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123) J Mol Cell Cardiol. 2006;40:790–8. doi: 10.1016/j.yjmcc.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, et al. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. 2009;105:1213–22. doi: 10.1161/CIRCRESAHA.108.183400. [DOI] [PubMed] [Google Scholar]
- 34.Thibodeau IL, Xu J, Li Q, Liu G, Lam K, Veinot JP, et al. Paradigm of genetic mosaicism and lone atrial fibrillation physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122:236–44. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]