Abstract
Objective:
Hypertrophic cardiomyopathy (HCM) as a common genetic heart disease characterized by ventricular hypertrophy and myocardial fibrosis is significantly associated with a higher risk of fatal ventricular arrhythmic events (VAEs). We aimed to assess the interval between the peak and the end of the electrocardiographic T wave (Tp–e) and Tp–e/corrected QT (QTc) ratio as candidate markers of ventricular arrhythmias in patients with HCM.
Methods:
In this single-center, prospective study, a total of 66 patients with HCM and 88 controls were enrolled. The patients were divided into two groups: those with VAEs (n=26) and those without VAEs (n=40). Tp–e interval and Tp–e/QTc ratio were measured using a 12-lead electrocardiogram.
Results:
Tp–e interval was significantly longer and Tp–e/QTc ratio were significantly higher in HCM patients than in the controls. In correlation analysis, maximal left ventricular (LV) thickness also has a significant positive correlation with Tp–e interval (r=0.422, p<0.001) and Tp–e/QTc ratio (r=0.348, p<0.001). Finally, multivariable regression analysis showed that a history of syncope, Tp–e interval [OR (odds ratio): 1.060; 95% confidence interval (CI): 1.005–1.117); p=0.012], Tp–e/QTc ratio (OR:1.148; 95%CI:1.086–1.204); p=0.049], and maximal LV thickness were independent predictors of VAEs in patients with HCM.
Conclusion:
Our findings suggested that prolonged Tp–e interval and increased Tp–e/QTc ratio may be good surrogate markers for the prediction of VAEs in HCM.
Keywords: Tp–e interval, Tp–e/QTc ratio, ventricular arrhythmia, hypertrophic cardiomyopathy
Introduction
Hypertrophic cardiomyopathy (HCM) has a prevalence of 1:500 that makes this heart muscle disorder the most frequently inherited cardiac disease (1, 2). The responsible genetic mutations are found in sarcomere protein genes that cause left ventricular (LV) hypertrophy, especially in the septal myocardium, and arrhythmias. In addition, myocardial fibrosis is a well-defined common maladaptive response to impaired diastolic properties (3). Although a large proportion of patients with HCM is asymptomatic, sudden cardiac death (SCD) can be the first presentation of the disease (4). Particularly in the young population, SCD from HCM is a common cause of death (4). Current data support that the presence of fibrosis on cardiac magnetic resonance imaging (MRI) is related to worse clinical status, increased risk of arrhythmic deaths, and any death from HCM among patients without high-risk criteria (5, 6). Although CMR is a useful tool for assessing myocardial fibrosis, its cost and accessibility are significant limitations. A 12-lead electrocardiogram (ECG) has been used for the assessment of electrophysiological abnormalities in HCM, and some of the ECG parameters including fragmented QRS complexes and QT duration were found to be well correlated with myocardial fibrosis and arrhythmic events (7, 8). Recently, it was shown that total (transmural, apicobasal and global) dispersion of repolarization could be represented by the interval between the peak and the end of T wave on ECG (Tp–e interval) (9, 10). Moreover, both Tp–e interval and Tp–e/corrected QT (QTc) ratio were described to detect ventricular repolarization abnormalities in various diseases (11–13).
We aimed to evaluate the repolarization dispersion represented by Tp–e interval and Tp–e/QTc ratio in patients with HCM and assess if these indices are related to ventricular arrhythmic events (VAEs) in HCM.
Methods
Study population
The present study was a single-center, prospective study and comprised 66 patients with HCM (mean age: 54.4±10.1) and 88 control subjects (mean age: 55.6±9.1) between May 2015 and May 2016. Exclusion criteria were as follows: presence of coronary heart or significant valvular heart disease, decompensated heart failure, reduced LV function [LV ejection fraction (LVEF) <50%], complete or incomplete bundle branch block, ST–T abnormalities, paced rhythm, atrial fibrillation, use of any drugs that could affect Tp–e or QT interval, resistant or uncontrolled hypertension, LV concentric hypertrophy, and evidence of acute or chronic infection or inflammatory condition. Baseline demographic and clinical characteristics of the study population were reviewed. The study was complied with the principles outlined in the Declaration of Helsinki, and approval for the study was obtained from the Institutional Review Board and Ethics Committee of our hospital, and informed consent was obtained from each patient before enrollment.
48-hour Holter monitoring was performed at least once in all patients when they were included in the present study. Furthermore, prior Holter monitoring reports were obtained from the medical records of our hospital. VAEs were defined as nonsustained ventricular tachycardia (>3 consecutive premature ventricular beats) or sustained ventricular tachycardia (>30 s). Hypertrophic cardiomyopathy was defined as a nondilated LV with a maximal wall thickness of ≥15 mm in ≥1 myocardial segments measured with echocardiography in patients without any causes for the magnitude of LV hypertrophy (4). Two-dimensional and M-mode transthoracic echocardiography (Vivid 7 system, 2.5–3.5 MHz transducer, GE-Vingmed Ultrasound AS, Horten, Norway) were performed according to standard methods. Continuous-wave Doppler was used to measure maximal velocity across the LV outflow tract both during resting state and Valsalva maneuver. The pressure gradient was estimated using the simplified Bernoulli equation. A peak pressure gradient of >30 mm Hg was regarded as a significant LVOT obstruction. LVEF was calculated using modified Simpson method. Left ventricle end-diastolic diameter was measured in the parasternal long-axis view with M-mode echocardiography at end-diastole, on the frame after mitral closure.
Electrocardiography
A 12-lead electrocardiogram with standard chest and limb leads was used to evaluate Tp–e and QTc intervals. The 12-lead ECG was recorded at a paper speed of 50 mm/s in the supine position. All of the ECGs were scanned and transferred to a personal computer and then used for 400´ magnification by Adobe Photoshop software to decrease error measurements. Measurements of Tp–e intervals and QTc were performed by two cardiologists who were blinded to patient data. Subjects with U waves on their ECGs were excluded from the study (a total of three patients). An average value of three readings was calculated for each lead. The QT interval was measured from the beginning of the QRS complex to the end of the T wave and corrected for heart rate using the Bazett formula: cQT=QT√ (R–R interval). Tp–e interval was defined as the interval between the peak and the end of T wave. Measurements of Tp–e interval were performed from precordial leads. Tp–e/QTc ratio was calculated from these measurements.
Statistical analysis
SPSS 20.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Whether the parameters normally distributed or not was assessed using Kolmogorov–Smirnov test. Normally distributed variables were represented as mean±standard deviation, and categorical variables were shown as number and percentage values. We compared the groups using chi-square test for categorical variables and ANOVA test for continuous variables. When the p value from one-way ANOVA test is statistically significant, post-hoc Tukey HSD or Tamhane’s tests were used to compare intergroup differences. Pearson rank tests were used to indicate the correlation of maximal LV thickness with Tp–e interval and Tp–e/QTc ratio. The independent predictors of VAEs were analyzed using univariable and multivariable logistic regression analysis. A probability value of <0.05 was considered statistically significant.
Results
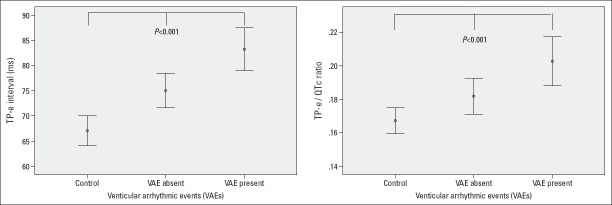
A total of 66 patients as HCM group (86.4% patients with asymmetric septal hypertrophy and 13.6% patients with apical hypertrophy) and 88 participants as control group were included in our study. The HCM group was subdivided into 40 patients without VAEs (VAE-absent group) and 26 patients with VAEs (VAE-present group). Baseline characteristics and electrocardiographic and echocardiographic parameters of the study groups are shown in Table 1. Baseline characteristics of the study groups were similar regarding age, sex, hypertension, diabetes mellitus, smoking, and resting heart rate (p>0.05). Tp–e interval and Tp–e/QTc ratio were significantly longer and higher, respectively, in the HCM group than in the control group (Fig. 1). Although QT interval was significantly longer in the HCM group, QTc interval was similar between the groups. With respect to the nature of the disease, maximal LV thickness (21.3±4.5 vs. 9.9±1.4), LVEF (68.3±3.7 vs. 64.5±3.5), and the presence of LVOT obstruction (37.9% vs. 0%) were significantly higher in the HCM group together with the use of beta-blockers (42.4% vs. 5.7%). Furthermore, in Pearson correlation analysis, maximal LV thickness showed a significant positive correlation with Tp–e interval (r=0.422, p<0.001) and Tp–e/QTc ratio (r=0.348, p<0.001). The results of post-hoc Tukey HSD or Tamhane’s tests for intergroup differences are given in Table 2.
Table 1.
Clinical, electrocardiographic, and echocardiographic findings of the study population
| Parameters | Control group (n=88) | HCM group (n=66) | P | |
|---|---|---|---|---|
| VEA absent (n=40) | VEA present (n=26) | |||
| Age, years | 55.6±9.1 | 55.0±9.7 | 53.4 ±10.9 | 0.579 |
| Male n (%) | 35 (39.8) | 18 (45.0) | 14 (53.8) | 0.435 |
| Hypertension, n (%) | 21 (23.9) | 12 (30.0) | 7 (26.9) | 0.758 |
| Diabetes mellitus, n (%) | 6 (6.8) | 3 (7.5) | 1 (3.8) | 0.826 |
| Smoking, n (%) | 12 (13.6) | 5 (12.5) | 4 (15.4) | 0.946 |
| Family history of SCD, n (%) | – | 7 (17.5) | 8 (30.8) | 0.209# |
| Syncopal history, n (%) | – | 5 (12.5) | 6 (23.1) | 0.260# |
| Medication, n (%) | ||||
| RAS blocker | 17 (19.3) | 8 (20.0) | 5 (19.2) | 0.995 |
| CCB | 10 (11.4) | 8 (20.0) | 4 (15.4) | 0.426 |
| β-blocker | 5 (5.7) | 15 (37.5) | 13 (50.0) | <0.001 |
| Tp-e interval, ms | 67.1±13.9 | 74.6±9.3 | 82.6±9.8 | <0.001 |
| QT interval, ms | 361±32 | 379±29 | 386±39 | 0.001 |
| QTc interval, ms | 404±40 | 416±33 | 414±34 | 0.152 |
| Tp-e/QTc ratio | 0.167±0.03 | 0.181±0.03 | 0.202±0.03 | <0.001 |
| Heart rate, beat/min | 75±11 | 73±10 | 70±9 | 0.136 |
| Maximal LV thickness, mm | 11.1±1.4 | 20.2±4.8 | 22.9±3.5 | 0.016# |
| LVOT obstruction, n (%) | – | 14 (35.0) | 11 (42.3) | 0.550# |
| LVED, mm | 45.6±2.9 | 45.5±4.2 | 46.8±4.6 | 0.348 |
| LVEF, % | 64.5±3.5 | 68.3±3.0 | 68.4±4.7 | <0.001 |
Data were given as mean±SD or %. CCC - calcium channel blocker; HCM - hypertrophic cardiomyopathy; LV - left ventricular; LVED - left ventricle end-diastolic diameter; LVEF - left ventricular ejection fraction; RAS - renin-angiotensin system; QTc - corrected QT; SCD - sudden cardiac death; Tp-e - T wave peak to end interval; VEA - ventricular arrhythmic event.
HCM (VEA absent and VEA present) groups were compared by using chi-square test or Student’s t-test
Figure 1.
Comparison of Tp–e interval and Tp–e/QTc ratio between the study groups
Table 2.
Electrocardiographic and echocardiographic of the study population compared using suitable post-hoc (multiple comparison) tests
| Parameters | Control group (n=88) | HCM group (n=66) | P1 value | P2 value | P3 value | |
|---|---|---|---|---|---|---|
| VEA absent (n=40) | VEA present (n=26) | |||||
| Tp-e interval | 67.1±13.9 | 74.6±9.3 | 82.6±9.8 | 0.001 | <0.001 | 0.005 |
| QT interval | 361±32 | 379±29 | 386±39 | 0.009 | 0.004 | 0.799 |
| QTc interval | 404±40 | 416±33 | 414±34 | 0.131 | 0.600 | 0.813 |
| Tp-e/QTc ratio | 0.167±0.03 | 0.181±0.03 | 0.202±0.03 | 0.128 | <0.001 | 0.024 |
| Heart rate | 75±11 | 73±10 | 70±9 | 0.657 | 0.091 | 0.449 |
| Max LVWT | 11.1±1.4 | 20.2±4.8 | 22.9±3.5 | <0.001 | <0.001 | 0.031 |
| LVEF | 64.5±3.5 | 68.3±3.0 | 68.4±4.7 | <0.001 | <0.001 | 0.992 |
HC - hypertrophic cardiomyopathy; LVEF - left ventricular ejection fraction; Max LVWT - maximal left ventricular wall thickness; RAS - renin-angiotensin system; QTc - corrected QT; Tp-e - T wave peak to end interval; VEA - ventricular arrhythmic event; P1: Comparison between control group and VEA absent; P2: Comparison between control group and VEA present; P3: Comparison between VEA absent and VEA present
Univariable and multivariable logistic regression analysis were performed to determine independent predictors of VAEs in HCM patients (Table 3). Finally, the multivariable regression analysis showed that the history of syncope [odds ratio (OR): 2.953; 95% confidence interval (CI): 1.652–3.379; p=0.047], Tp–e interval (OR: 1.060; 95% CI: 1.005–1.117; p=0.012), Tp–e/QTc ratio (OR: 1.148; 95% CI: 1.086–1.204; p=0.049), and maximal LV thickness (OR: 1.245; 95% CI: 1.131–1.370; p<0.001) were independent predictors of VAEs in HCM patients.
Table 3.
Univariable and multivariable logistic regression analysis for the assessment of independent predictors of ventricular arrhythmic events
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Age | 0.978 (0.935–1.022) | 0.322 | ||
| Male gender | 1.651 (0.707–3.853) | 0.246 | ||
| Family history of SCD | 3.368 (1.009–8.191) | 0.002 | ||
| Syncopal history | 5.380 (1.296–10.529) | 0.001 | 2.953 (1.652–3.379) | 0.047 |
| Tp-e interval | 1.195 (1.080–1.343) | <0.001 | 1.060 (1.005–1.117) | 0.012 |
| QT interval | 1.015 (1.003–1.028) | 0.016 | ||
| Tp-e/QTc ratio | 1.386 (1.037–1.952) | <0.001 | 1.148 (1.086–1.204) | 0.049 |
| Maximal LV thickness | 1.300 (1.181–1.431) | <0.001 | 1.245 (1.131–1.370) | <0.001 |
| LVOT obstruction | 2.971 (1.296–6.529) | <0.001 | ||
| LVEF | 1.191 (1.060–1.337) | 0.003 | ||
CI - confidence interval; LV - left ventricular; OR - odds ratio; SCD - sudden cardiac death
Discussion
This study showed that Tp–e interval and Tp–e/QTc ratio are significantly longer and higher, respectively, in patients with HCM than in healthy subjects. Furthermore, in patients with VAEs in the HCM group, Tp–e interval and Tp–e/QTc ratio were significantly greater than those in patients without VAEs, and there is a positive correlation between maximal LV thickness and Tp–e interval and Tp–e/QTc ratio. Moreover, in multivariate logistic regression analyses, these indices are independently associated with VAEs.
Although the annual risk of SCD is presumed to be low within the HCM population (1%), SCD is a devastating consequence of HCM (14). Therefore, selecting the appropriate candidate for implantable cardioverter–defibrillator (ICD) implantation is more than an academic exercise and concerns preventable deaths with ICDs. Surface 12-lead ECG simply provides the prediction of arrhythmic events in HCM (8, 15). Although fQRS is a well-studied ECG parameter in HCM, little is known if Tp–e interval and Tp–e/QTc ratio, which indirectly reflect ventricular repolarization abnormalities, are related to arrhythmic events in HCM (9, 10). Tp–e interval was described as an index of total dispersion of repolarization (9, 10), and longer Tp–e interval was found related to arrhythmias and mortality (16, 17). Although Tp–e interval is affected by heart rate and body surface area, Tp–e/QTc ratio is represented as a more accurate index of ventricular repolarization (18, 19). In our study, we found that there was a significant relation between VAEs and longer Tp–e interval and higher Tp–e/QTc ratio. In HCM, QTc prolongation is reported in most previous reports, and it was linked to the underlying LV hypertrophy and outflow obstruction, mutation in ion channels, and activated sympathetic tone (20, 21). The primary structural abnormalities in HCM are myocardial cell disarray, silent ischemia due to remodeling in intramyocardial arterioles, ongoing myocardial injury, premature cell death, and fibrosis (3, 22, 23). All these changes are not limited to hypertrophied myocardium and myocytes, fibroblasts and interstitium are affected as well (24). Consequently, altered properties of the myocardium may cause electrophysiological abnormalities represented by Tp–e and Tp–e/QTc ratio. In our study, we observed a significant positive correlation between maximal LV thickness and Tp–e interval and Tp–e/QTc ratio that the surface ECG may clue the underlying abnormal myocardial remodelings. Although b-blockers shorten the QT interval and suppress arrhythmic events, we found that in the VAE-present group, Tp–e interval was significantly longer and Tp–e/QTc ratio was significantly higher, despite the great amount of b-blockers used in this group. These results may suggest the limited effect of b-blockers in such population and the possible need of ICD implantation for SCD prevention (25). In previous reports, increased mortality was observed in patients with both Brugada syndrome and long QT syndrome if they had longer Tp–e intervals (18). Therefore, the prognostic value of Tp–e interval and Tp–e/QTc ratio in patients with HCM should be evaluated in prospective studies to understand if these indices should be added to a risk stratification model. Similar to fragmented QRS, longer Tp–e interval and higher Tp–e/QTc ratio may simply reflect the extension of the fibrosis in HCM and the progressive disease. Previously, among elderly Chinese patients, Lin et al. (26) showed that prolonged Tp–e interval was related to matrix metalloproteinases and tissue inhibitor of metalloproteinases that trigger the signal cascade of cardiac remodeling and fibrosis. Hence, studies evaluating the relation between myocardial fibrosis assessed by MRI and longer Tp–e interval and higher Tp–e/QTc ratio in HCM are also needed.
Study limitations
This study has several limitations. First, the number of the recruited patients is relatively small. Second, only a few patients have MRI that the relation between the former ECG indices and myocardial fibrosis was not studied for the study participants. Third, there were no detailed echocardiographic parameters, including LV mass and LV mass index, of the study population. Finally, because our hospital is a tertiary referral hospital, this may have caused a selection bias due to the recruitment of high-risk population.
Conclusion
Prolonged Tp–e interval and increased Tp–e/QTc ratio are independently associated with VAEs in HCM. In addition to conventional risk factors, simple ECG parameters may provide further information when assessing SCD risk in HCM population.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.K.A.; Design – M.K.A., K.G.B., S.A., A.G.E., Ç.Y., S.Ü.; Supervision – S.Y., M.M.B., Y.B., D.A., S.T.; Materials – M.K.A., K.G.B., S.Y., M.M.B., A.G.E., Ç.Y., S.Ü.; Data collection &/or processing – M.K.A., K.G.B., S.A., A.G.E., Ç.Y., S.Ü.; Analysis &/or interpretation – M.K.A., K.G.B., S.Y., S.A., M.M.B., A.G.E., Ç.Y., S.Ü., Y.B., D.A., S.T.; Literature search – M.K.A., K.G.B., S.Y., S.A., M.M.B.; Writing – M.K.A., K.G.B., S.Y., S.A., M.M.B., A.G.E., Ç.Y., S.Ü., Y.B., D.A., S.T.; Critical review – M.K.A., K.G.B., S.Y., S.A., M.M.B., A.G.E., Ç.Y., S.Ü., Y.B., D.A., S.T.

From Prof. Dr. Hasan Veysi Güneş’s collections
References
- 1.Maron BJ. Hypertrophic cardiomyopathy:an important global disease. Am J Med. 2004;116:63–5. doi: 10.1016/j.amjmed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults:Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–9. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 3.Konno T, Chen D, Wang L, Wakimoto H, Teekakirikul P, Nayor M, et al. Heterogeneous myocyte enhancer factor-2 (Mef2) activation in myocytes predicts focal scarring in hypertrophic cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:18097–102. doi: 10.1073/pnas.1012826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy:the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–79. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 5.Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG, et al. Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2012;60:922–9. doi: 10.1016/j.jacc.2012.03.076. [DOI] [PubMed] [Google Scholar]
- 6.Briasoulis A, Mallikethi-Reddy S, Palla M, Alesh I, Afonso L. Myocardial fibrosis on cardiac magnetic resonance and cardiac outcomes in hypertrophic cardiomyopathy:a meta-analysis. Heart. 2015;101:1406–11. doi: 10.1136/heartjnl-2015-307682. [DOI] [PubMed] [Google Scholar]
- 7.Konno T, Hayashi K, Fujino N, Oka R, Nomura A, Nagata Y, et al. Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:1081–7. doi: 10.1111/jce.12742. [DOI] [PubMed] [Google Scholar]
- 8.Femenía F, Arce M, Van Grieken J, Trucco E, Mont L, Abello M, et al. Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. J Interv Card Electrophysiol. 2013;38:159–65. doi: 10.1007/s10840-013-9829-z. [DOI] [PubMed] [Google Scholar]
- 9.Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41:575–80. doi: 10.1016/j.jelectrocard.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Antzelevitch C, Sicouri S, Di Diego JM, Burashnikov A, Viskin S, Shimizu W, et al. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4:1114–6. doi: 10.1016/j.hrthm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akboğa MK, Yüksel M, Balcı KG, Kaplan M, Çay S, Gökbulut V, et al. Tp-e Interval, Tp-e/QTc Ratio, and Fragmented QRS Are Correlated with the Severity of Liver Cirrhosis. Ann Noninvasive Electrocardiol. 2017;22:e12359. doi: 10.1111/anec.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yayla Ç, Bilgin M, Akboğa MK, Gayretli Yayla K, Canpolat U, DinçAsarcikli L, et al. Evaluation of Tp-e Interval and Tp-e/QT ratio in patients with aortic stenosis. Ann Noninvasive Electrocardiol. 2016;21:287–93. doi: 10.1111/anec.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Z, Yuan Z, Ji Y, Wu Y, Qi Y. Left ventricular hypertrophy amplifies the QT, and Tp-e intervals and the Tp-e/QT ratio of left chest ECG. J Biomed Res. 2010;24:69–72. doi: 10.1016/S1674-8301(10)60011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy:a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–831. doi: 10.1161/CIR.0b013e318223e2bd. [DOI] [PubMed] [Google Scholar]
- 15.Debonnaire P, Katsanos S, Joyce E, VAN DEN Brink OV, Atsma DE, Schalij MJ, et al. QRS fragmentation and QTc duration relate to malignant ventricular tachyarrhythmias and sudden cardiac death in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:547–55. doi: 10.1111/jce.12629. [DOI] [PubMed] [Google Scholar]
- 16.Smetana P, Schmidt A, Zabel M, Hnatkova K, Franz M, Huber K, et al. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease:Peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol. 2011;44:301–8. doi: 10.1016/j.jelectrocard.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Erikssen G, Liestol K, Gullestad L, Haugaa KH, Bendz B, Amlie JP. The terminal part of the QT interval (T peak to T end):A predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol. 2012;17:85–94. doi: 10.1111/j.1542-474X.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zehir R, Karabay CY, Kalaycı A, Akgün T, Kılıçgedik A, Kırma C. Evaluation of Tpe interval and Tpe/QT ratio in patients with slow coronary flow. Anatol J Cardiol. 2015;15:463–7. doi: 10.5152/akd.2014.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta P, Patel C, Patel H, Narayanaswamy S, Malhotra B, Green JT, et al. T(p-e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol. 2008;41:567–74. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JN, Grifoni C, Bos JM, Saber-Ayad M, Ommen SR, Nistri S, et al. Prevalence and clinical correlates of QT prolongation in patients with hypertrophic cardiomyopathy. Eur Heart J. 2011;32:1114–20. doi: 10.1093/eurheartj/ehr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouven X, Hagege A, Charron P, Carrier L, Dubourg O, Langlard JM, et al. Relation between QT duration and maximal wall thickness in familial hypertrophic cardiomyopathy. Heart. 2002;88:153–7. doi: 10.1136/heart.88.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poliac LC, Barron ME, Maron BJ. Hypertrophic cardiomyopathy. Anesthesiology. 2006;104:183–92. doi: 10.1097/00000542-200601000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Camici PG, Olivotto I, Rimoldi OE. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol. 2012;52:857–64. doi: 10.1016/j.yjmcc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Hensley N, Dietrich J, Nyhan D, Mitter N, Yee MS, Brady M. Hypertrophic cardiomyopathy:a review. Anesth Analg. 2015;120:554–69. doi: 10.1213/ANE.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 25.Algra A, Roelandt JR, Tijssen JG, Simoons ML, Pool J. Effect of beta-blockers on the relation between QT-interval and heart rate in exercise ECG. Eur Heart J. 1987;8(Suppl D):71–3. doi: 10.1093/eurheartj/8.suppl_d.71. [DOI] [PubMed] [Google Scholar]
- 26.Lin TH, Chiu HC, Lee YT, Su HM, Juo SH, Voon WC, et al. The C-allele of tissue inhibitor of metalloproteinases 2 is associated with increased magnitude of QT dispersion prolongation in elderly Chinese - 4-year follow-up study. Clin Chim Acta. 2007;386:87–93. doi: 10.1016/j.cca.2007.08.004. [DOI] [PubMed] [Google Scholar]