ABSTRACT
Host-microbe interactions are influenced by complex host genetics and environment. Studies across animal taxa have aided our understanding of how intestinal microbiota influence vertebrate development, disease, and physiology. However, traditional mammalian studies can be limited by the use of isogenic strains, husbandry constraints that result in small sample sizes and limited statistical power, reliance on indirect characterization of gut microbial communities from fecal samples, and concerns of whether observations in artificial conditions are actually reflective of what occurs in the wild. Fish models are able to overcome many of these limitations. The extensive variation in the physiology, ecology, and natural history of fish enriches studies of the evolution and ecology of host-microbe interactions. They share physiological and immunological features common among vertebrates, including humans, and harbor complex gut microbiota, which allows identification of the mechanisms driving microbial community assembly. Their accelerated life cycles and large clutch sizes and the ease of sampling both internal and external microbial communities make them particularly well suited for robust statistical studies of microbial diversity. Gnotobiotic techniques, genetic manipulation of the microbiota and host, and transparent juveniles enable novel insights into mechanisms underlying development of the digestive tract and disease states. Many diseases involve a complex combination of genes which are difficult to manipulate in homogeneous model organisms. By taking advantage of the natural genetic variation found in wild fish populations, as well as of the availability of powerful genetic tools, future studies should be able to identify conserved genes and pathways that contribute to human genetic diseases characterized by dysbiosis.
KEYWORDS: gnotobiotic, animal models, fish, host-microbe interactions, microbiota
INTRODUCTION
In vertebrates, the gut microbiome promotes the normal development of host physiology (1), skeletal systems (2, 3), and metabolism (4) while decreasing susceptibility to pathogens. It is sensitive to disruptions (5) that are often associated with short- and long-term consequences for host health and development (2, 6), such as inflammatory bowel disease, type II diabetes, colorectal cancer, autoimmune diseases, and autism (7, 8). Vertebrates harbor complex residential microbial communities that have been shaped by the host (9) and, unlike invertebrate models such as fruit flies and squid, have adaptive immune systems that recognize particular microbes and play vital roles in cultivating residential gut microbial communities (9). While studies of host-microbe interactions have provided novel insights into development, disease, and physiology, gaps remain in our understanding of the processes underlying microbial community assembly (10, 11), the mechanisms by which gut microbes influence host development and physiology (12–18), and the genetic and environmental factors that regulate gut microbial composition and diversity (19). Bridging these gaps requires the development of robust, versatile, and genetically tractable model systems (20).
COMPARISON OF MOUSE AND FISH MODELS
Inbred mouse models have traditionally been used to study host-microbe interactions. More than 450 strains have been described since the first inbred mice were created nearly 100 years ago. These strains are valuable not only because of their isogenicity, which allows the isolation of a particular genetic variant of interest, but also because phenotypic differences among strains have been described in great detail (21). The rich collection of knockout, knock-in, and mutant lines has greatly increased understanding of how host genetics contribute to microbial community composition, immune function, and metabolism (22).
However, studies using mouse models have been restricted in several ways. Use of inbred lines limits understanding of how complex genetic variation influences microbial community composition (23). For example, at least 163 genetic loci of small effect in the human genome have been linked to irritable bowel disorder (24), and many of them serve purposes with respect to immune system signaling and mucosal barrier integrity across vertebrates (25, 26). Disrupting these genes individually and/or in various combinations would require a staggering number of mouse lines. Genetic differences have often accumulated between mutant and wild-type colonies that had been separately maintained for multiple generations, leading to discordant results among strains reared at different laboratories (27). When genetically variable individuals are used, husbandry constraints can result in small sample sizes and limited statistical power (19).
In addition to genetic constraints, the inability to observe microbe interactions in live mice can prevent in-depth studies of host-microbe interactions. Most mouse studies rely upon indirect characterization of gut microbial communities from fecal samples, which are not consistently reliable indicators of gut microbial communities (28–34) and cannot be used to detect differences in microbial communities that are spatially separated along the gut (32). Additional concerns include whether observations made under artificial conditions are actually reflective of what occurs in the wild (20, 35). These limitations highlight the need for model systems that allow robust statistical examination of how microbial communities are shaped by complex natural host genetic variation (36) in both laboratory-reared and wild populations.
The 28,000 characterized fish species comprise nearly half of all vertebrate diversity and possess extensive variation in physiology, ecology, and natural history (37) that can facilitate our understanding of the evolution of host-microbe interactions (38). Relative to the contribution of fish species to overall vertebrate diversity, their microbial communities have remained underexplored (39), although they have been characterized in a range of fishes (see, e.g., references 40, 41, 42, and 43).
Teleosts possess physiological and immunological features common to all vertebrates as well as a complex gut microbiota. Both teleosts and mammals have a digestive tract consisting of a liver, gallbladder, pancreas, and intestine that develop in a similar trajectory, from the rostral gut to the hindgut and midgut. Guts are separated along the rostral-caudal axis and have an intestinal epithelium made up of absorptive enterocytes, secretory goblet cells, and enteroendocrine cells (44). Intestines initially form in a sterile environment and complete their development in the presence of microbes (14). In much the same way that mammalian newborns are first colonized by microbes at birth, fish initially acquire their gut microbes from the environment upon opening of the digestive tract, which typically occurs a couple of days after hatching (45). Gut microbes aid in fermentation of polysaccharides to short-chain fatty acids (46) and protect against pathogenic infection (47, 48). The genes involved in immune system signaling are highly conserved between mammals and teleosts, as well (26, 49, 50).
Teleost physiology and mammalian physiology also differ in several ways. Teleosts lack lymph nodes and bone marrow (51), although the head kidney is considered orthologous in function. The teleost innate immune system is more diverse than that of mammals, but their immunoglobulins have fewer antibodies (52–54). While a great diversity of gut microbes has been sampled across fish species, most communities have been dominated by the Proteobacteria (20, 38, 55–57). This is in contrast to healthy mammalian guts, which are dominated by Bacteroidetes and Firmicutes (22). An exception has been documented in herbivorous marine fishes, which closely resemble herbivorous mammalian guts, suggesting that their microbial communities share similar functions in gut fermentation (38).
Aside from physiological and microbial community differences between fishes and mammals, fish models also present some experimental constraints. The roles of early life exposures that have both short- and long-term consequences on gut microbial community structure in mammals, such as mode of delivery (vaginal versus cesarean) and breast milk (58), cannot be studied in teleosts. Humanized microbiome mice models (59) allow the transplantation of human microbes into mice to recapitulate some aspect of their host's phenotype and are a valuable tool for understanding the influence of the gut microbiome in disease and the role of diet in shaping the microbiome (60, 61). This technique has not been developed in fish.
ADVANTAGES OF ZEBRAFISH AND THREESPINE STICKLEBACK MODELS
Most of the host-microbe research using teleosts has focused on zebrafish (Danio rerio). However, threespine stickleback (Gasterosteus aculeatus), which is a widely used model organism in evolution, genetics, and ecology, has recently also been adapted for host-microbe interaction research. Advantages of these two systems lie in the powerful genetic tools that have been developed and their rich history of study, dating back to the 1800s (62) (Fig. 1). Single crosses produce a large number of offspring that can be housed in highly controlled environments (63) and permit statistically robust studies; their rapid development and small size have made them valuable resources for a wide range of genetic studies; and both their internal and external (environmental) microbial communities can be easily sampled and manipulated (20, 50, 56), unlike those of mammals. A powerful asset of these models lies in the ability to study the evolution of the relationship between the host and its microbiota due to the host's relatively short life span (1 to 2 years) and the extensive knowledge that we have of laboratory lines (zebrafish) and wild populations (stickleback). Coupled with annotated genomes and the ability to compare host and microbial DNA and transcriptomes, these teleosts have already begun to advance our understanding of host-microbe interactions.
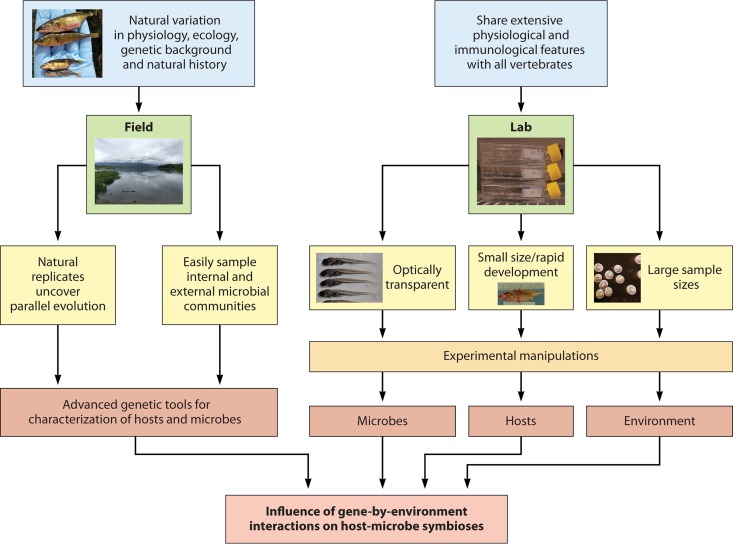
FIG 1.
Teleosts exhibit natural variation, and their physiology is remarkably similar to that of other vertebrates, including humans. These features have led to a rich history of study that has made teleosts strong model organisms for both field and laboratory studies of host-microbe interactions. The ease with which their internal and environmental microbial communities can be sampled, their adaptive radiations, and the availability of advanced genetic tools for characterizing hosts and microbes have made them ideal for empirical studies. In the laboratory, the optical transparency, large sample sizes, small size, and rapid development of fish have facilitated experimental manipulations of microbes and hosts and their environment. Combining field and laboratory studies allows identification of gene-environment interactions influencing host-microbe symbioses.
The transparency of zebrafish eggs and juveniles allowed the first successful examination of the colonization dynamics of bacteria within live, developing hosts (14). The ability to genetically manipulate both host cells and microbes to express fluorescent proteins allows real-time nondestructive observations of spatial and temporal variation in host-microbe interactions in developing zebrafish, which has granted insights into the distribution of bacterial populations along the gut (12), complex microbial behaviors (64), and population dynamics during colonization (65). However, this technique is limited to genetically modifiable microbes, which represent only a fraction of the community present in fish guts.
A primary advantage of using threespine stickleback as a model organism is the ability to study how natural genetic variation, which is of a magnitude similar to that found in the human population (Fig. 2), influences a range of phenotypes, including bone development (66), pigmentation (67, 68), and behavior (69). Many genetic regions, such as those associated with skeletal structures, also underlie variations in human populations (70). Stickleback therefore present a great potential to reveal genes important for driving microbial membership and the host response to microbes, including processes involved in metabolic changes, cell development, and cell-to-cell signaling.
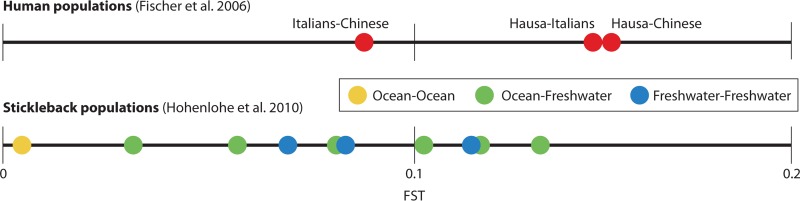
FIG 2.
The threespine stickleback is an appropriate model organism for studying the effects of host genetic background on microbial community because wild populations exhibit genetic variation that is comparable to that of human populations. FST, a measure of genetic divergence, among human populations ranges from about 0.08 to 0.15 (132) (top panel) and among stickleback populations from Alaska ranges from almost 0 to 0.13 (133) (bottom panel).
GNOTOBIOTIC STUDIES IN TELEOSTS
In gnotobiotic studies, animals are derived under germfree conditions and analyzed either in this sterile state or in association with specific microbes in comparison to conventionally reared animals with diverse microbial communities (71). Gnotobiotic techniques are straightforward in fishes since they develop ex utero and eggs can be surface sterilized shortly after fertilization (13). Gnotobiotic techniques were first developed in platyfish (Xiphophorus maculatus), followed by tilapia (Tilapia macrocephala), salmon (Salmo salar), sheepshead minnow (Cyprinidon vairegatus), Atlantic halibut (Hippoglossus hippoglossus), and turbot (Scopthalmus maximus) (72–77). However, the most detailed studies of host-microbe interactions have used gnotobiotic zebrafish (78, 79) and stickleback (80). Gnotobiotic studies in both mammals and teleosts allow the documentation of a broad array of host responses to gut microbiota (13), but zebrafish have revealed advantages over mice in identifying microbial signaling pathways influencing development (14, 56). However, while multigenerational gnotobiotic lines are able to be maintained in mice (22), this is not yet possible in fishes.
Gnotobiotic studies in zebrafish have revealed that the gut microbiota stimulates intestinal epithelial cell proliferation (13, 14) through MyD88 signaling pathways (15) and promotes shifts in epithelial glycan expression (14) as well as recruitment of gut-associated immune cells (13, 16, 81). Germfree zebrafish intestines have decreased secretory cell numbers and experience faster peristaltic contractions than conventionally reared individuals (14). Their guts are unable to fully develop and exhibit reduced function, but these deficiencies can be reversed after introduction of bacteria (14, 16). These studies reveal the varied roles that microbiota play in normal digestive development and function.
Gnotobiotic studies of laboratory-reared oceanic and resident freshwater stickleback have demonstrated that these two ecotypes have common gut microbial communities and similarities in intestinal development, despite their separation in the wild for at least 10,000 years (80). However, the two ecotypes differed in the intensity of their inflammatory responses to microbes, highlighting the potential for gene-environment interactions that influence host immune response (80).
INSIGHTS FROM WILD-CAUGHT VERSUS LABORATORY-REARED ZEBRAFISH
Wild-caught and laboratory-reared zebrafish populations have similar gut microbial communities, suggesting the existence of a core gut microbiota (20), which may also be true of mammals (82–84). However, neutral processes of drift and dispersal can generate a great deal of diversity within and among individuals. Bacterial taxa that deviate from neutral patterns and are more widespread than expected are likely adapted to, and selected by, the host (63). These examples highlight the utility of genetically variable model organisms that can be studied both in the wild and under controlled laboratory conditions to examine how gene-environment interactions drive microbial community dynamics.
INSIGHTS FROM WILD-STICKLEBACK POPULATIONS
The colonization of thousands of lakes throughout the Northern Hemisphere by oceanic ancestral stickleback resulted in an adaptive radiation of freshwater populations that are locally adapted to their environments. This “natural experiment” allows researchers to study the influences of environmental factors, such as water chemistry and predation regimes, on the evolution of a vertebrate host (134, 135). Host-microbe researchers are now beginning to use the natural variation found in wild populations to unravel interactions among diet, genetic background, and environmental microbial communities with respect to effects on gut microbiota composition. Such studies have revealed that microbial community structure appears to be more strongly driven by differences in host genotype than by differences in environment (85) and that food-associated microbes drive gut microbial community diversity to a greater extent than water-associated microbes (86). Inverse relationships between diversity in major histocompatibility complex class II (MHC-II) alleles and diversity in gut microbial community suggest that adaptive immunity could restrict the diversity of commensal bacteria. Sex also influences the degree and direction of influence of the MHC-II receptors as well as the magnitude of effects of diet on microbiota composition (100): males have higher phylogenetic diversity than females, and phylogenetic diversity increases with size more strongly in males than in females. While associations have also been found between MHC diversity and microbiota in mice (87), microbiota changes correlated more strongly with body size in females than in males (88). Sex differences across taxa are likely due to interactions among hormones, developmental rate, and/or gene expression, which are all mechanisms that can be readily examined in laboratory experiments.
INSIGHTS FROM OTHER FISH SPECIES
The changes in community composition that occur during development and migration in salmonids present the opportunity to explore how gene-environment interactions shape the microbiome (89). A study of wild Atlantic salmon (Salmo salar) revealed differences between environmental and gut microbial communities that were driven largely by ontogeny rather than geography (90). The intestinal microbiota of rainbow trout (Oncorhynchus mykiss) has been shown to be highly variable temporally, spatially, and interindividually (55, 91). Seasonal fluctuations in temperature were correlated with changes in gut microbiota (92) in both rainbow trout and gulf killifish (Fundulus grandis), with decreased bacterial counts in winter and increases in spring that were associated with rising temperature (93, 94). Seasonal differences have also been documented in wild-mouse populations (95).
Studies have also explored how antimicrobials change fish gut bacterial community composition (96). For example, low levels of triclosan exposure resulted in differences in microbial community structure in the fathead minnow (Pimephales promelas) (97). However, the communities recovered to baseline after 2 weeks in clean water, suggesting that short-term disruption to gut microbiota may be sufficient to harm a developing host but that there is an opportunity to recover normal bacterial diversity after disturbance.
CONCLUSIONS AND FUTURE DIRECTIONS
What have we learned from studying fish models?
Researchers have gained novel insights into mechanisms underlying development of the digestive tract and how microbiota contribute to disease states (13–18, 80, 81, 86, 90) (Table 1). We have learned from studies of fishes and other vertebrates that gut microbiota are dynamic and demonstrate complex successional patterns throughout development (58, 101–103). Differences in microbial communities between captive fishes and their wild counterparts argue for the use of model systems, such as threespine stickleback, that can be studied in the wild as well as under controlled laboratory conditions (98). Perhaps surprisingly, fish gut communities more closely resemble those of mammals than those of organisms found in their environment (38, 104), particularly with regard to abundances of Proteobacteria, Firmicutes, and Bacteroidetes (38, 99, 105, 106), which further promotes the idea of their utility as model organisms for human health research.
TABLE 1.
Studies using teleosts as model organisms have made major contributions to understanding host-microbe interactions
| Contribution | Reference(s) |
|---|---|
| Contributions of microbiota to host development | |
| Stimulation of intestinal epithelial cell proliferation through MyD88 signaling pathways | 13–15 |
| Promotion of a shift in epithelial glycan expression | 14 |
| Stimulation of recruitment of immune cells | 13, 16 |
| Promotion of gut development | 14 |
| Maintenance of normal levels of secretory cells and peristaltic contractions | 14, 16 |
| Aiding in host growth and development | 15, 17, 18 |
| Process of gut colonization | |
| Bacterial populations not uniformly distributed along gut | 12 |
| Establishment of bacteria during development | 13, 14, 56 |
| Quantification of bacterial population dynamics in a living host | 65 |
| Gene-environment interactions | |
| Core gut microbiota | 20, 80 |
| Taxa that deviate from neutral patterns are more likely adapted to, and selected by, host environment | 63 |
| Microbiota more strongly driven by differences in host genotype than environment | 85 |
| Diet and host genetics influence on microbiota | 18, 86, 98 |
| Microbiota influenced more by host developmental stage than geography | 90 |
| Sex influences magnitude of relationship to diet | 86 |
| Temporal, spatial, and interindividual variation | 20, 55, 91, 99 |
| Seasonal variation in microbiota | 92, 93 |
| Immune system-microbiota interactions | |
| Variation in strength of inflammatory response to microbes in genetically divergent populations | 80 |
| Correlations between MHC class II alleles and microbiota | 100 |
| Microbiota-induced neutrophil recruitment | 81 |
| Effects of antimicrobials: low levels of triclosan alter microbial community structure | 97 |
Where do we go from here?
Teleost systems can be used to identify selective pressures, including interactions among environment, diet, genetic background, and development, that influence gut microbial community assembly. For example, interactions among MHC diversity, sex, and diet raise the issue of how hormones, sexual dimorphisms, metabolism, and gene expression influence host-microbe interactions and susceptibility to disease. Epistatic interactions among a large number of genes can be difficult to characterize or manipulate in an inbred model, highlighting the utility of model organisms, such as threespine stickleback, that exhibit complex natural genetic variation. Taking advantage of the natural genetic variation found in wild fish populations as well as the availability of powerful genetic tools, future studies should be able to identify conserved genes and pathways that contribute to human genetic diseases characterized by dysbiosis (107–129).
Studies examining the effects of exposure to antibiotics and other contaminants suggest that juvenile dysbiosis can impact long-term fitness in contaminated habitats (97). While previous work has focused on how clinical levels of antibiotics (130) and antimicrobials (97) affect the abundance of specific taxa, what remains largely unknown is how environmentally relevant levels of common contaminants may disrupt the microbiota, resulting in developmental abnormalities and/or disease. Stickleback are already common model organisms for understanding the effects of chronic exposure to aquatic contaminants on physiological development (136) and can therefore easily be used to understand the effects of exposure to environmentally relevant levels of aquatic pollutants on gut microbial community and host development. The conservation of physiological and genetic pathways among vertebrates will allow insights into the environmental factors that may trigger dysbiosis in humans, as well.
How many teleost models do we need?
Since fishes exhibit dramatic variations in physiology, natural history, and ecology, they can be used as model organisms to address a wide range of factors relevant to host-microbe interactions. For example, studies of fishes that are of economic and cultural significance, such as salmonids, have potential to improve aquaculture (131) and safe harvesting practices and to contribute to our understanding of how populations may respond to climate and anthropogenic changes. Focusing on widespread species that have undergone adaptive radiations, such as whitefish (Coregonus), will allow further insight into the relative influences of phylogeny and environment in shaping microbial communities. Fishes living in extreme environments, such as Death Valley pupfish (Cyprinodon salinus) and Antarctic icefish (Notothenioidei), can help us understand how microbes may enable vertebrates to adapt to extreme environments. Finally, live-bearing fishes have advantages in understanding colonization dynamics early in development. Now that so much is known about how microbial communities influence many aspects of a host's life, including its physiology, immune response, and behavior, fish models can help us better understand the effects of microbial community diversity and disruption on host development and adaptation to its environment.
ACKNOWLEDGMENTS
We thank B. Briggs, R. Isenberg, and F. A. von Hippel for their feedback, as well as three anonymous reviewers for constructive comments that greatly improved the quality of the manuscript.
Funding was provided by the University of Alaska Anchorage and by NSF PRFB 1611913 (awarded to E.A.L.). Research reported in this publication was also supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395.
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
REFERENCES
- 1.Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E. 2010. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107:20051–20056. doi: 10.1073/pnas.1009906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. 2012. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. 2012. The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. 2012. Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dethlefsen L, McFall-Ngai M, Relman DA. 2007. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spor A, Koren O, Ley R. 2011. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 9.McFall-Ngai M. 2007. Adaptive immunity: care for the community. Nature 445:153–153. doi: 10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- 10.McCafferty J, Mühlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Jobin C, Fodor AA. 2013. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J 7:2116–2125. doi: 10.1038/ismej.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maignien L, DeForce EA, Chafee ME, Eren AM, Simmons SL. 2014. Ecological succession and stochastic variation in the assembly of Arabidopsis thaliana phyllosphere communities. mBio 5:e00682-13. doi: 10.1128/mBio.00682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taormina MJ, Jemielita M, Stephens WZ, Burns AR, Troll JV, Parthasarathy R, Guillemin K. 2012. Investigating bacterial-animal symbioses with light sheet microscopy. Biol Bull 223:7–20. doi: 10.1086/BBLv223n1p7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawls JF, Samuel BS, Gordon JI. 2004. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A 101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. 2006. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol 297:374–386. doi: 10.1016/j.ydbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Cheesman SE, Neal JT, Mittge E, Seredick BM, Guillemin K. 2011. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl Acad Sci U S A 108(Suppl 1):4570–4577. doi: 10.1073/pnas.1000072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates JM, Akerlund J, Mittge E, Guillemin K. 2007. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2:371–382. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanther M, Sun X, Mühlbauer M, Mackey LC, Flynn EJ, Bagnat M, Jobin C, Rawls JF. 2011. Microbial colonization induces dynamic temporal and spatial patterns of NF-κB activation in the zebrafish digestive tract. Gastroenterology 141:197–207. doi: 10.1053/j.gastro.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. 2012. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, Zhang M, Oh PL, Nehrenberg D, Hua K, Kachman SD, Moriyama EN, Walter J, Peterson DA, Pomp D. 2010. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci U S A 107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. 2011. Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MFW, Fisher EMC. 2000. Genealogies of mouse inbred strains. Nat Genet 24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 22.Kostic AD, Howitt MR, Garrett WS. 2013. Exploring host-microbiota interactions in animal models and humans. Genes Dev 27:701–718. doi: 10.1101/gad.212522.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeVoss J, Diehl L. 2014. Murine models of inflammatory bowel disease (IBD). Toxicol Pathol 42:99–110. doi: 10.1177/0192623313509729. [DOI] [PubMed] [Google Scholar]
- 24.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar J-P, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Büning C, Cohain A, Cichon S, D'Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, et al. . 2012. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham DB, Xavier RJ. 2013. From genetics of inflammatory bowel disease towards mechanistic insights. Trends Immunol 34:371–378. doi: 10.1016/j.it.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renshaw SA, Trede NS. 2012. A model 450 million years in the making: zebrafish and vertebrate immunity. Dis Model Mech 5:38–47. doi: 10.1242/dmm.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. 2011. The antibacterial lectin RegIIIγ promotes the spatial segregation of microbiota and host in the intestine. Science 334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durbán A, Abellán JJ, Jiménez-Hernández N, Ponce M, Ponce J, Sala T, D'Auria G, Latorre A, Moya A. 2011. Assessing gut microbial diversity from feces and rectal mucosa. Microb Ecol 61:123–133. doi: 10.1007/s00248-010-9738-y. [DOI] [PubMed] [Google Scholar]
- 29.Stearns JC, Lynch MDJ, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD. 2011. Bacterial biogeography of the human digestive tract. Sci Rep 1:170. doi: 10.1038/srep00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouwehand AC, Salminen S, Arvola T, Ruuska T, Isolauri E. 2004. Microbiota composition of the intestinal mucosa: association with fecal microbiota? Microbiol Immunol 48:497–500. doi: 10.1111/j.1348-0421.2004.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 31.Ott SJ, Musfeldt M, Timmis KN, Hampe J, Wenderoth DF, Schreiber S. 2004. In vitro alterations of intestinal bacterial microbiota in fecal samples during storage. Diagn Microbiol Infect Dis 50:237–245. doi: 10.1016/j.diagmicrobio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kohl KD, Miller AW, Marvin JE, Mackie R, Dearing MD. 2014. Herbivorous rodents (Neotoma spp.) harbour abundant and active foregut microbiota. Environ Microbiol 16:2869–2878. doi: 10.1111/1462-2920.12376. [DOI] [PubMed] [Google Scholar]
- 33.Lu H-P, Lai Y-C, Huang S-W, Chen H-C, Hsieh C-H, Yu H-T. 2014. Spatial heterogeneity of gut microbiota reveals multiple bacterial communities with distinct characteristics. Sci Rep 4:6185. doi: 10.1038/srep06185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasuda K, Oh K, Ren B, Tickle TL, Franzosa EA, Wachtman LM, Miller AD, Westmoreland SV, Mansfield KG, Vallender EJ, Miller GM, Rowlett JK, Gevers D, Huttenhower C, Morgan XC. 2015. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host Microbe 17:385–391. doi: 10.1016/j.chom.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weldon L, Abolins S, Lenzi L, Bourne C, Riley EM, Viney M. 2015. The gut microbiota of wild mice. PLoS One 10:e0134643-15. doi: 10.1371/journal.pone.0134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stagaman K, Guillemin K, Milligan-Myhre K. 2014. Tending a complex microbiota requires major immune complexity. Mol Ecol 23:4679–4681. doi: 10.1111/mec.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson JS, Grande TC, Wilson M. 2016. Fishes of the World, 5th ed. Wiley Publishing, Hoboken, NJ. [Google Scholar]
- 38.Sullam KE, Essinger SD, Lozupone CA, O'Connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. 2012. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol 21:3363–3378. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements KD, Angert ER, Montgomery WL, Choat JH. 2014. Intestinal microbiota in fishes: what's known and what's not. Mol Ecol 23:1891–1898. doi: 10.1111/mec.12699. [DOI] [PubMed] [Google Scholar]
- 40.Ringø E, Strøm E, Tabachek JA. 1995. Intestinal microflora of salmonids: a review. Aquac Res 26:773–789. doi: 10.1111/j.1365-2109.1995.tb00870.x. [DOI] [Google Scholar]
- 41.Ringø E, Gatesoupe FJ. 1998. Lactic acid bacteria in fish: a review. Aquaculture 160:177–203. doi: 10.1016/S0044-8486(97)00299-8. [DOI] [Google Scholar]
- 42.Ringø E, Lødemel JB, Myklebust R, Kaino T, Mayhew TM, Olsen RE. 2001. Epithelium-associated bacteria in the gastrointestinal tract of Arctic charr (Salvelinus alpinus L.). An electron microscopical study. J Appl Microbiol 90:294–300. [DOI] [PubMed] [Google Scholar]
- 43.Ward NL, Steven B, Penn K, Methé BA, Detrich WH. 2009. Characterization of the intestinal microbiota of two Antarctic notothenioid fish species. Extremophiles 13:679–685. doi: 10.1007/s00792-009-0252-4. [DOI] [PubMed] [Google Scholar]
- 44.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. 2005. Intestinal growth and differentiation in zebrafish. Mech Dev 122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Hansen GH, Olafsen JA. 1999. Bacterial interactions in early life stages of marine cold water fish. Microb Ecol 38:1–26. doi: 10.1007/s002489900158. [DOI] [PubMed] [Google Scholar]
- 46.Hooper LV, Midtvedt T, Gordon JI. 2002. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 47.McCracken VJ, Lorenz RG. 2001. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol 3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 48.Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671. doi: 10.1128/MMBR.64.4.655-671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meeker ND, Trede NS. 2008. Immunology and zebrafish: spawning new models of human disease. Dev Comp Immunol 32:745–757. doi: 10.1016/j.dci.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Kanther M, Rawls JF. 2010. Host–microbe interactions in the developing zebrafish. Curr Opin Immunol 22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zapata A, Amemiya CT. 2000. Phylogeny of lower vertebrates and their immunological structures, p 67–107. In Origin and evolution of the vertebrate immune system. Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 52.Vasta GR, Nita-Lazar M, Giomarelli B, Ahmed H, Du S, Cammarata M, Parrinello N, Bianchet MA, Amzel LM. 2011. Structural and functional diversity of the lectin repertoire in teleost fish: relevance to innate and adaptive immunity. Dev Comp Immunol 35:1388–1399. doi: 10.1016/j.dci.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoder JA, Litman GW. 2011. The phylogenetic origins of natural killer receptors and recognition: relationships, possibilities, and realities. Immunogenetics 63:123–141. doi: 10.1007/s00251-010-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunyer JO, Zarkadis IK, Lambris JD. 1998. Complement diversity: a mechanism for generating immune diversity? Immunol Today 19:519–523. doi: 10.1016/S0167-5699(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 55.Huber I, Spanggaard B, Appel KF, Rossen L, Nielsen T, Gram L. 2004. Phylogenetic analysis and in situ identification of the intestinal microbial community of rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol 96:117–132. doi: 10.1046/j.1365-2672.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- 56.Rawls JF, Mahowald MA, Ley RE, Gordon JI. 2006. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song W, Li L, Huang H, Jiang K, Zhang F, Chen X, Zhao M, Ma L. 2016. The gut microbial community of Antarctic fish detected by 16S rRNA gene sequence analysis. BioMed Res Int 2016:3241529. doi: 10.1155/2016/3241529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol 5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faith JJ, Rey FE, O'Donnell D, Karlsson M, McNulty NP, Kallstrom G, Goodman AL, Gordon JI. 2010. Creating and characterizing communities of human gut microbes in gnotobiotic mice. ISME J 4:1094–1098. doi: 10.1038/ismej.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, González A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 62.Darwin C. 2003. On the origin of species by means of natural selection (J. Carroll, ed). Broadview Press, Toronto, Ontario, Canada. [Google Scholar]
- 63.Burns AR, Stephens WZ, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJ. 21 August 2015 Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J doi: 10.1038/ismej.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rawls JF, Mahowald MA, Goodman AL, Trent CM, Gordon JI. 2007. In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc Natl Acad Sci U S A 104:7622–7627. doi: 10.1073/pnas.0702386104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jemielita M, Taormina MJ, Burns AR, Hampton JS, Rolig AS, Guillemin K, Parthasarathy R. 2014. Spatial and temporal features of the growth of a bacterial species colonizing the zebrafish gut. mBio 5:e01751-14. doi: 10.1128/mBio.01751-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimmel CB, Ullmann B, Walker C, Wilson C, Currey M, Phillips PC, Bell MA, Postlethwait JH, Cresko WA. 2005. Evolution and development of facial bone morphology in threespine sticklebacks. Proc Natl Acad Sci U S A 102:5791–5796. doi: 10.1073/pnas.0408533102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller CT, Beleza S, Pollen AA, Schluter D, Kittles RA, Shriver MD, Kingsley DM. 2007. cis-Regulatory Changes in Kit ligand expression and parallel evolution of pigmentation in sticklebacks and humans. Cell 131:1179–1189. doi: 10.1016/j.cell.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones FC, Grabherr MG, Chan YF, Russell P, Mauceli E, Johnson J, Swofford R, Pirun M, Zody MC, White S, Birney E, Searle S, Schmutz J, Grimwood J, Dickson MC, Myers RM, Miller CT, Summers BR, Knecht AK, Brady SD, Zhang H, Pollen AA, Howes T, Amemiya C; Broad Institute Genome Sequencing Platform & Whole Genome Assembly Team, Baldwin J, Bloom T, Jaffe DB, Nicol R, Wilkinson J, Lander ES, Di Palma F, Lindblad-Toh K, Kingsley DM. 2012. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wark AR, Greenwood AK, Taylor EM, Yoshida K, Peichel CL. 2011. Heritable differences in schooling behavior among threespine stickleback populations revealed by a novel assay. PLoS One 6:e18316. doi: 10.1371/journal.pone.0018316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Indjeian VB, Kingman GA, Jones FC, Guenther CA, Grimwood J, Schmutz J, Myers RM, Kingsley DM. 2016. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell 164:45–56. doi: 10.1016/j.cell.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Falk PG, Hooper LV, Midtvedt T, Gordon JI. 1998. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev 62:1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker JA, Ferguson MS, TenBroeck C. 2016. Growth of platyfish (Platypoecilus maculatus) free from bacteria and other micro'organisms. Proc Soc Exp Biol Med 51:116–119. doi: 10.3181/00379727-51-13854. [DOI] [Google Scholar]
- 73.Shaw ES, Aronson LR. 1954. Oral incubation in tilapia-b microcephala. Bull Am Mus Nat Hist 103:381. [Google Scholar]
- 74.Battalora MSJ, Ellender RD, Martin BJ. 1985. Gnotobiotic maintenance of Sheepshead minnow larvae. Progress Fish Culturist 47:122–125. doi:. [DOI] [Google Scholar]
- 75.Trust TJ. 1974. Sterility of salmonid roe and practicality of hatching gnotobiotic salmonid fish. Appl Microbiol 28:340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munro PD, Barbour A, Birkbeck TH. 1995. Comparison of the growth and survival of larval turbot in the absence of culturable bacteria with those in the presence of Vibrio anguillarum, Vibrio alginolyticus, or a marine Aeromonas sp. Appl Environ Microbiol 61:4425–4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verner-Jeffreys DW, Shields RJ, Birkbeck TH. 2003. Bacterial influences on Atlantic halibut Hippoglossus hippoglossus yolk sac larval survival and start feed response. Dis Aquat Organ 56:105–113. doi: 10.3354/dao056105. [DOI] [PubMed] [Google Scholar]
- 78.Pham LN, Kanther M, Semova I, Rawls JF. 2008. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc 3:1862–1875. doi: 10.1038/nprot.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Milligan-Myhre K, Charette JR, Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, Kim CH. 2011. Study of host-microbe interactions in zebrafish. Methods Cell Biol 105:87–116. doi: 10.1016/B978-0-12-381320-6.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milligan-Myhre K, Small CM, Mittge EK, Agarwal M, Currey M, Cresko WA, Guillemin K. 2016. Innate immune responses to gut microbiota differ between oceanic and freshwater threespine stickleback populations. Dis Model Mech 9:187–198. doi: 10.1242/dmm.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanther M, Tomkovich S, Xiaolun S, Grosser MR, Koo J, Flynn EJ III, Jobin C, Rawls JF. 2014. Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cell Microbiol 16:1053–1067. doi: 10.1111/cmi.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbaugh PJ, Quince C, Faith JJ, McHardy AC, Yatsunenko T, Niazi F, Affourtit J, Egholm M, Henrissat B, Knight R, Gordon JI. 2010. Organismal, genetic, and transcriptional variation in the deeply sequenced gut microbiomes of identical twins. Proc Natl Acad Sci U S A 107:7503–7508. doi: 10.1073/pnas.1002355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto J-M, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, et al. . 2010. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith CC, Snowberg LK, Caporaso JG, Knight R, Bolnick DI. 24 April 2015 Dietary input of microbes and host genetic variation shape among-population differences in stickleback gut microbiota. ISME J doi: 10.1038/ismej.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Knight R, Caporaso JG, Svanbäck R. 2014. Individuals' diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecol Lett 17:979–987. doi: 10.1111/ele.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toivanen P, Vaahtovuo J, Eerola E. 2001. Influence of major histocompatibility complex on bacterial composition of fecal flora. Infect Immun 69:2372–2377. doi: 10.1128/IAI.69.4.2372-2377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKnite AM, Perez-Munoz ME, Lu L, Williams EG, Brewer S, Andreux PA, Bastiaansen JWM, Wang X, Kachman SD, Auwerx J, Williams RW, Benson AK, Peterson DA, Ciobanu DC. 2012. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS One 7:e39191. doi: 10.1371/journal.pone.0039191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt VT, Smith KF, Melvin DW, Amaral-Zettler LA. 2015. Community assembly of a euryhaline fish microbiome during salinity acclimation. Mol Ecol 24:2537–2550. doi: 10.1111/mec.13177. [DOI] [PubMed] [Google Scholar]
- 90.Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Creer S, Derome N. 2016. The biogeography of the Atlantic salmon (Salmo salar) gut microbiome. ISME J 10:1280–1284. doi: 10.1038/ismej.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spanggaard B, Huber I, Nielsen J, Nielsen T, Appel KF, Gram L. 2000. The microflora of rainbow trout intestine: a comparison of traditional and molecular identification. Aquaculture 182:1–15. doi: 10.1016/S0044-8486(99)00250-1. [DOI] [Google Scholar]
- 92.Hagi T, Tanaka D, Iwamura Y, Hoshino T. 2004. Diversity and seasonal changes in lactic acid bacteria in the intestinal tract of cultured freshwater fish. Aquaculture 234:335–346. doi: 10.1016/j.aquaculture.2004.01.018. [DOI] [Google Scholar]
- 93.Naviner M, Giraud E, Le Bris H, Armand F, Mangion C, Ganière J-P. 2006. Seasonal variability of intestinal microbiota in rainbow trout (Oncorhynchus mykiss), with a particular attention to Aeromonas spp. as candidate indicator of antimicrobial resistance. Rev Med (Paris) 12:599–604. [Google Scholar]
- 94.Larsen AM, Bullard SA, Womble M, Arias CR. 2015. Community structure of skin microbiome of gulf killifish, Fundulus grandis, is driven by seasonality and not exposure to oiled sediments in a Louisiana salt marsh. Microb Ecol 70:534–544. doi: 10.1007/s00248-015-0578-7. [DOI] [PubMed] [Google Scholar]
- 95.Maurice CF, Knowles SC, Ladau J, Pollard KS, Fenton A, Pedersen AB, Turnbaugh PJ. 29 May 2015 Marked seasonal variation in the wild mouse gut microbiota. ISME J doi: 10.1038/ismej.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Y, Zhou Z, Wu N, Tao Y, Xu L, Cao Y, Zhang Y, Bin Yao. 2012. Gibel carp Carassius auratus gut microbiota after oral administration of trimethoprim/sulfamethoxazole. Dis Aquat Organ 99:207–213. doi: 10.3354/dao02477. [DOI] [PubMed] [Google Scholar]
- 97.Narrowe AB, Albuthi-Lantz M, Smith EP, Bower KJ, Roane TM, Vajda AM, Miller CS. 2015. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome 3:6. doi: 10.1186/s40168-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wong S, Waldrop T, Summerfelt S, Davidson J, Barrows F, Kenney PB, Welch T, Wiens GD, Snekvik K, Rawls JF, Good C. 2013. Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl Environ Microbiol 79:4974–4984. doi: 10.1128/AEM.00924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smriga S, Sandin SA, Azam F. 2010. Abundance, diversity, and activity of microbial assemblages associated with coral reef fish guts and feces. FEMS Microbiol Ecol 73:31–42. [DOI] [PubMed] [Google Scholar]
- 100.Bolnick DI, Snowberg LK, Caporaso JG, Lauber C, Knight R, Stutz WE. 2014. Major histocompatibility complex class IIb polymorphism influences gut microbiota composition and diversity. Mol Ecol 23:4831–4845. doi: 10.1111/mec.12846. [DOI] [PubMed] [Google Scholar]
- 101.Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kohl KD, Cary TL, Karasov WH, Dearing MD. 2013. Restructuring of the amphibian gut microbiota through metamorphosis. Environ Microbiol Rep 5:899–903. doi: 10.1111/1758-2229.12092. [DOI] [PubMed] [Google Scholar]
- 103.Vadstein O, Bergh Ø Gatesoupe FJ, Galindo Villegas J, Mulero V, Picchietti S, Scapigliati G, Makridis P, Olsen Y, Dierckens K, Defoirdt T, Boon N, De Schryver P, Bossier P. 2013. Microbiology and immunology of fish larvae. Rev Aquac 5:S1–S25. doi: 10.1111/j.1753-5131.2012.01082.x. [DOI] [Google Scholar]
- 104.Arnolds KL, Lozupone CA. 2016. Striking a balance with help from our little friends – how the gut microbiota contributes to immune homeostasis. Yale J Biol Med 89:389–395. [PMC free article] [PubMed] [Google Scholar]
- 105.Clements KD, Pasch IBY, Moran D, Turner SJ. 2007. Clostridia dominate 16S rRNA gene libraries prepared from the hindgut of temperate marine herbivorous fishes. Mar Biol 150:1431–1440. doi: 10.1007/s00227-006-0443-9. [DOI] [Google Scholar]
- 106.Ye L, Amberg J, Chapman D, Gaikowski M, Liu WT. 17 October 2013 Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J doi: 10.1038/ismej.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xavier RJ, Podolsky DK. 2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 108.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet M-F, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. 1 December 2016 Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Obata Y, Pachnis V. 10 August 2016 The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE, Sisodia SS. 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep 6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piper HG, Fan D, Coughlin LA, Ho EX, McDaniel MM, Channabasappa N, Kim J, Kim M, Zhan X, Xie Y, Koh AY. 12 July 2016 Severe gut microbiota dysbiosis is associated with poor growth in patients with short bowel syndrome JPEN J Parenter Enteral Nutr 2016:pii: 0148607116658762. [DOI] [PubMed] [Google Scholar]
- 112.Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, Luckey DH, Marietta EV, Jeraldo PR, Chen X, Weinshenker BG, Rodriguez M, Kantarci OH, Nelson H, Murray JA, Mangalam AK. 2016. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q, Zhou JM. 2016. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 114.Wu X, He B, Liu J, Feng H, Ma Y, Li D, Guo B, Liang C, Dang L, Wang L, Tian J, Zhu H, Xiao L, Lu C, Lu A, Zhang G. 2016. Molecular insight into gut microbiota and rheumatoid arthritis. IJMS 17:431–411. doi: 10.3390/ijms17030431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sandhya P, Danda D, Sharma D, Scaria V. 2016. Does the buck stop with the bugs?: an overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis 19:8–20. doi: 10.1111/1756-185X.12728. [DOI] [PubMed] [Google Scholar]
- 116.Wu J, Zhang Y, Yang H, Rao Y, Miao J, Lu X. 2016. Intestinal microbiota as an alternative therapeutic target for epilepsy. Can J Infect Dis Med Microbiol 2016:9032809. doi: 10.1155/2016/9032809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. 2015. Gut-microbiota-brain Axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther 37:984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borre YE, O'Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20:509–518. doi: 10.1016/j.molmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 119.Huttenhower C, Kostic AD, Xavier RJ. 2014. Inflammatory bowel disease as a model for translating the microbiome. Immunity 40:843–854. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang Y, Kasper LH. 2014. The role of microbiome in central nervous system disorders. Brain Behav Immun 38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. 2013. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. 2014. The microbiome: stress, health and disease. Mamm Genome 25:49–74. doi: 10.1007/s00335-013-9488-5. [DOI] [PubMed] [Google Scholar]
- 123.Mårild K, Ye W, Lebwohl B, Green PH, Blaser MJ, Card T, Ludvigsson JF. 8 July 2013 Antibiotic exposure and the development of coeliac disease: a nationwide case-control study. BMC Gastroenterol doi: 10.1186/1471-230X-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foster JA, Neufeld K-AM. 2013. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 125.Cryan JF, Dinan TG. 12 September 2012 Mind-altering microorganisms: the impact of the gut microbiota on brain and behavior. Nat Rev Neurosci doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 126.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 127.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. 2011. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141:599–609, 609.e1–e3. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 129.Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S. 2006. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55:1760–1767. doi: 10.1136/gut.2005.078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jernberg C, Lofmark S, Edlund C, Jansson JK. 2010. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 131.Zarkasi KZ, Abell GCJ, Taylor RS, Neuman C, Hatje E, Tamplin ML, Katouli M, Bowman JP. 2014. Pyrosequencing-based characterization of gastrointestinal bacteria of Atlantic salmon (Salmo salar L.) within a commercial mariculture system. J Appl Microbiol 117:18–27. doi: 10.1111/jam.12514. [DOI] [PubMed] [Google Scholar]
- 132.Fischer A, Pollack J, Thalmann O, Nickel B, Pääbo S. 2006. Demographic history and genetic differentiation in apes. Curr Biol 16:1133–1138. doi: 10.1016/j.cub.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 133.Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. 2010. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet 6:e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bell MA, Foster SA (ed). 1994. The evolutionary biology of the threespine stickleback. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 135.von Hippel FA. (ed). 2010. Tinbergen's legacy in Behaviour: sixty years of landmark stickleback papers. Brill, Leiden, Netherlands. [Google Scholar]
- 136.von Hippel FA. 2010. Predators and parasites, p 347–352. In von Hippel FA. (ed), Tinbergen's legacy in Behaviour: sixty years of landmark stickleback papers. Brill, Leiden, Netherlands. [Google Scholar]