ABSTRACT
Mycoplasma hominis lacks a cell wall, and lipoproteins anchored to the extracellular side of the plasma membrane are in direct contact with the host components. A Triton X-114 extract of M. hominis enriched with lipoproteins was shown to stimulate the production of interleukin-23 (IL-23) by human dendritic cells (hDCs). The inflammasome activation of the host cell has never been reported upon M. hominis infection. We studied here the interaction between M. hominis PG21 and hDCs by analyzing both the inflammation-inducing mycoplasmal lipoproteins and the inflammasome activation of the host cell. IL-23-inducing lipoproteins were determined using a sequential extraction strategy with two nondenaturing detergents, Sarkosyl and Triton X-114, followed by SDS-PAGE separation and mass spectrometry identification. The activation of the hDC inflammasome was assessed using PCR array and enzyme-linked immunosorbent assay (ELISA). We defined a list of 24 lipoproteins that could induce the secretion of IL-23 by hDCs, 5 with a molecular mass between 20 and 35 kDa and 19 with a molecular mass between 40 and 100 kDa. Among them, lipoprotein MHO_4720 was identified as potentially bioactive, and a synthetic lipopeptide corresponding to the N-terminal part of the lipoprotein was subsequently shown to induce IL-23 release by hDCs. Regarding the hDC innate immune response, inflammasome activation with caspase-dependent production of IL-1β was observed. After 24 h of coincubation of hDCs with M. hominis, downregulation of the NLRP3-encoding gene and of the adaptor PYCARD-encoding gene was noticed. Overall, this study provides insight into both protagonists of the interaction of M. hominis and hDCs.
IMPORTANCE Mycoplasma hominis is a human urogenital pathogen involved in gynecologic and opportunistic infections. M. hominis lacks a cell wall, and its membrane contains many lipoproteins that are anchored to the extracellular side of the plasma membrane. In the present study, we focused on the interaction between M. hominis and human dendritic cells and examined both sides of the interaction, the mycoplasmal lipoproteins involved in the activation of the host cell and the immune response of the cell. On the mycoplasmal side, we showed for the first time that M. hominis lipoproteins with high molecular mass were potentially bioactive. On the cell side, we reported an activation of the inflammasome, which is involved in the innate immune response.
KEYWORDS: Mycoplasma hominis, human dendritic cells, lipoproteins, lipopeptide, inflammasome, PCR array, caspase, IL-1β, IL-23, interaction, innate response
INTRODUCTION
Mycoplasmas are small bacteria that lack a cell wall. Their membranes contain a large number of lipoproteins that are anchored to the extracellular side of the plasma membrane, in direct contact with the host components. Many mycoplasmal lipoproteins have been reported to be involved in adhesion to host cells but also in the recognition by the host immune system (1). Indeed, mycoplasmal lipoproteins can act as proinflammatory factors and can stimulate human macrophages and human dendritic cells (hDCs), induce cytokine production, activate NF-κB, and polarize the adaptive immune response (2–8). Nondenaturing detergent extracts, such as Triton X-114 (TX-114) extracts enriched with mycoplasmal lipoproteins, were shown to have modulatory capacities (2, 5, 6, 9, 10). In addition, synthetic lipopeptides derived from lipoproteins such as MALP-2 from Mycoplasma fermentans (11), FSL-1 from M. salivarium (12), and MPPL-1 from M. pneumoniae (13) were shown to have immunomodulatory properties.
In M. hominis, which is a human urogenital pathogen involved in gynecologic and opportunistic infections, Peltier et al. partially purified from a TX-114 extract a macrophage-stimulating factor of 29 kDa that could stimulate tumor necrosis factor alpha (TNF-α) production by the THP-1 macrophage cell line (3). Recently, Hasebe et al. isolated and purified a lipoprotein of 40 kDa from an octyl-glucopyranoside (OG) extract derived from M. hominis membranes (14). This lipoprotein, which is a truncated form of the Vaa adhesin, also induced TNF-α production by the THP-1 cell line. Additionally, we showed that a TX-114 extract of M. hominis membranes induced the maturation of human dendritic cells (hDCs) and stimulated the production of proinflammatory cytokines such as interleukin-12 (IL-12) and TNF-α by hDCs (8). This M. hominis extract also induced a predominant IL-23 secretion that promoted the development of an IL-17-producing CD4+ helper T cell subset and polarized the adaptive immune system toward the IL-23/Th-17 axis (8). Although inflammasome activation has not yet been reported in M. hominis, the activation of the NLRP3 and NLRP7 inflammasome has been described for other mycoplasmal species such as M. pneumoniae, M. hyorhinis, M. salivarium, and Acholeplasma laidlawii (15–18).
In the present study, to further examine both sides of the interaction between M. hominis and hDCs, we assessed (i) the M. hominis lipoproteins that could induce IL-23 production by hDCs using a sequential extraction strategy with two nondenaturing detergents and (ii) the activation of the hDC inflammasome using PCR array and enzyme-linked immunosorbent assay (ELISA).
RESULTS
Selective extraction of M. hominis lipoproteins that induce IL-23 production by hDCs.
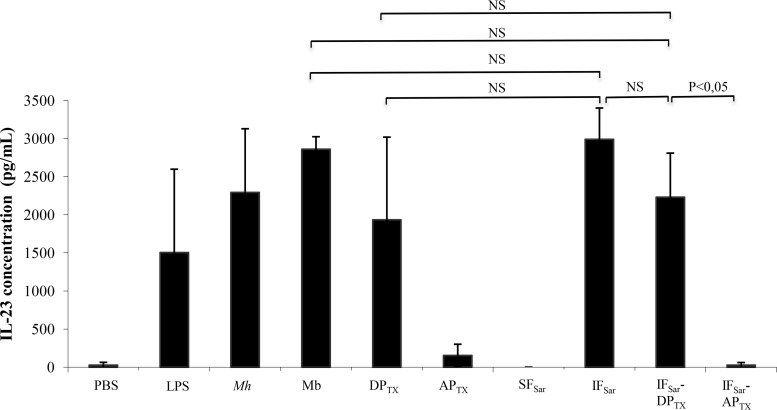
A simple TX-114 extract of M. hominis membranes contains a large number of proteins, including transmembrane proteins and lipoproteins (8). To obtain an M. hominis fraction enriched in bioactive lipoproteins, we developed a strategy based on the sequential use of two nondenaturing detergents. The goal here was to remove most nonbioactive proteins after the first extraction step and to selectively extract the bioactive molecules from the remaining fraction using TX-114. Five detergents were evaluated for their capacity to extract proteins from M. hominis membranes. Sarkosyl extracted a larger number of proteins than TX-100, OG, CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and deoxycholate (DOC) (see Fig. S2 in the supplemental material). Both Sarkosyl-insoluble fractions (IFSar) and Sarkosyl-soluble fractions (SFSar) were evaluated for their capacity to induce IL-23 production when added to hDCs. After 24 h of incubation, a significant IL-23 release was observed with the IFSar but not with SFSar (Fig. 1), indicating that the bioactive proteins were present in the IFSar.
FIG 1.
Release of IL-23 by hDCs after incubation with M. hominis PG21 and detergent extracts. hDCs were incubated 24 h at 37°C with the different detergent extracts. Supernatants were collected, and IL-23 concentration was determined by ELISA. Results are shown as the means and standard errors of the means from three independent experiments. PBS, negative control; LPS, positive control; Mh, M. hominis PG21; Mb, M. hominis PG21 membranes; DPTX, Triton X-114 (TX-114) detergent phase (extracted proteins); APTX, TX-114 aqueous phase (nonextracted proteins); SFSar, Sarkosyl-soluble fraction (extracted proteins); IFSar, Sarkosyl-insoluble fraction (nonextracted proteins); IFSar-DPTX, Sarkosyl-insoluble fraction extracted by TX-114 (detergent phase); IFSar-APTX, Sarkosyl-insoluble fraction extracted by TX-114 (aqueous phase); NS, statistically nonsignificant according to the Newman-Keuls test.
A second round of extraction was undertaken by TX-114 partitioning applied to the IFSar. Upon electrophoretic separation, the IFSar-DPTX sequential extract (i.e., Sarkosyl-insoluble fraction extracted by TX-114) showed fewer bands than a simple TX-114 extract (DPTX) (see Fig. S3 in the supplemental material). These bands were also of higher intensity, suggesting an enrichment in specific proteins. To check whether the bioactive lipoproteins were recovered at the end of the sequential extraction procedure, IL-23 released by hDCs incubated for 24 h with IFSar-DPTX was measured (Fig. 1). The IFSar-DPTX sequential extract triggered a substantial release of IL-23, and the levels of IL-23 production were similar to those measured after incubation with M. hominis cells, with M. hominis membranes, or with the simple TX-114 extract (DPTX).
Finally, the use of Sarkosyl as the initial detergent allowed the removal of many proteins without eliminating bioactive lipoproteins, validating the use of the sequential extraction procedure using Sarkosyl followed by TX-114 in further experiments.
Identification of the lipoproteins in the bioactive IFSar-DPTX sequential extract.
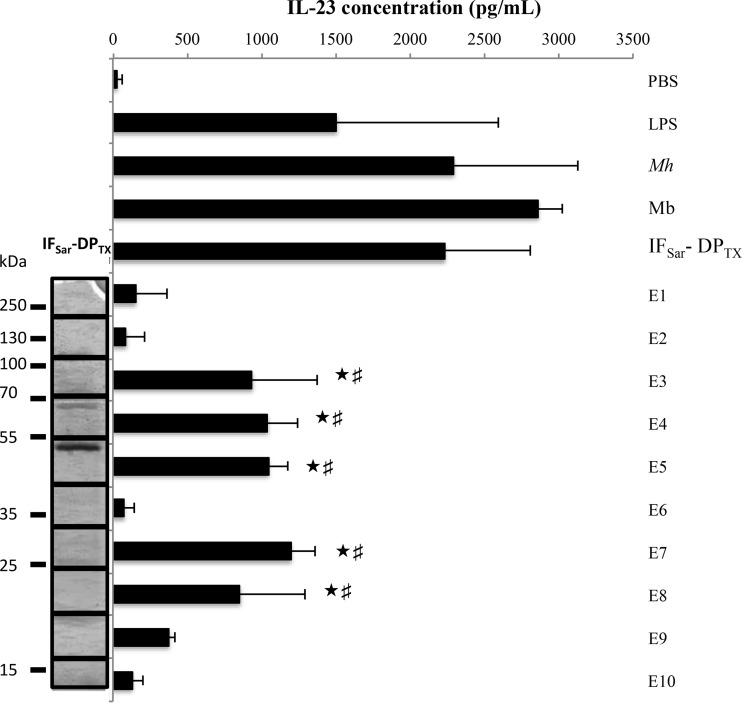
The IFSar-DPTX sequential extract was further fractionated based on the protein molecular mass (MM). After electrophoretic separation, the gel slice was cut into 10 equal bands, from which proteins were extracted using OG in order to obtain 10 eluates, E1 (higher MM) to E10 (lower MM). The dendritic cell-activating potency of the eluates was evaluated by measuring the IL-23 release by hDCs incubated in the presence of each of the E1 to E10 fractions for 24 h (Fig. 2).
FIG 2.
Release of IL-23 by hDCs after coincubation with the 10 eluates from the gel-separated sequential extract. Results are shown as the means and standard errors of the means from three independent experiments. PBS, negative control; LPS, positive control; Mh, M. hominis PG21; Mb, M. hominis PG21 membranes; IFSar-DPTX, Sarkosyl-insoluble fraction treated by TX-114 (detergent phase). ★, significantly different (P < 0.05) from PBS according to the Newman-Keuls post hoc test; #, significantly different (P < 0.05) from IL-23 concentration released after coincubation with E2, E6, and E9 eluates according to the Newman-Keuls test.
Five eluates (E3 to E5, E7, and E8) triggered the release of a significant amount of IL-23 by hDCs. The bioactive proteins present in eluates E3, E4, and E5 had a high apparent molecular mass (AMM), ranging from 40 to 100 kDa. The proteins in eluates E7 and E8 had a low AMM, from 20 to 35 kDa. No significant IL-23 release was observed for the E2 and E6 eluates. The bioactive compounds able to induce IL-23 release could correspond to two or more unrelated proteins or to different fragments of the same protein.
The lipoproteins from the bioactive eluates E3, E4, E5, E7, and E8 were identified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Table 1). The E2 and E6 eluates that did not induce IL-23 production were also analyzed by LC-MS/MS as reference. Lipoproteins were considered nonfragmented when the AMM was close to that calculated on the basis of their predicted molecular mass. A total of 26 of the 48 M. hominis predicted lipoproteins (19, 20) were detected, i.e., 54.2% of the lipoproteins predicted in silico (Table 1). Two lipoproteins, MHO_1640 and MHO_3620, which were solely present in the E2 and E6 eluates, which did not induce IL-23 release from hDCs, were not considered to be bioactive. Vaa (MHO_3470) could not be considered inactive, although it was identified in the E2 and E6 eluates. Indeed, Vaa-derived peptides were recovered mainly in the bioactive E5 eluate, and either the entire Vaa or a Vaa-derived fragment with an AMM of >40 kDa could be responsible for the observed bioactivity in this eluate. Nonfragmented OppA (MHO_1510), Lmp1 (MHO_0530), and P120 (MHO_3660) proteins were also identified in the inactive E2 eluate. Consequently, the nonfragmented forms of these proteins could be considered inactive. However, for all three proteins, the fragmented forms detected in the E3 and E4 eluates could represent bioactive polypeptides.
TABLE 1.
Lipoproteins identified by LC-MS/MS in eluates E2 to E8a
| Gene locus | Product | No. of peptides identified in eluate: |
Calculated MM (kDa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E2 (100–200 kDa) | E3 (70–100 kDa) | E4 (55–70 kDa) | E5 (40–55 kDa) | E6 (30–40 kDa) | E7 (25–30 kDa) | E8 (15–25 kDa) | |||
| MHO_0280 | Hypothetical protein, predicted lipoprotein | 47 | 28 | 8 | 6 | 80.5 | |||
| MHO_0290 | Pseudogene of hypothetical protein (N-terminal part), predicted lipoprotein | 55 | 21 | 11 | 2 | 67.7 | |||
| MHO_0530 | Lmp1 protein | 13 | 5 | 2 | 170.7 | ||||
| MHO_0540 | Lmp-related protein | 3 | 28 | 70.0 | |||||
| MHO_0720 | Conserved hypothetical protein, predicted lipoprotein | 53 | 20 | 75.2 | |||||
| MHO_0730 | Hypothetical protein, putative nuclease, predicted lipoprotein | 2 | 34.3 | ||||||
| MHO_0780 | Conserved hypothetical protein, predicted lipoprotein | 24 | 10 | 78.2 | |||||
| MHO_0790 | Conserved hypothetical protein, predicted lipoprotein | 5 | 61 | 27 | 13 | 2 | 2 | 78.2 | |
| MHO_1510 | P100 = OppA, oligopeptide ABC transporter substrate-binding protein | 44 | 36 | 24 | 7 | 105.8 | |||
| MHO_1640b | Lmp3 protein | 4 | 178.0 | ||||||
| MHO_1730 | Hypothetical protein, predicted lipoprotein | 11 | 27.1 | ||||||
| MHO_2080 | Conserved hypothetical protein, predicted lipoprotein | 8 | 64.3 | ||||||
| MHO_2340 | Hypothetical protein, putative adhesin, predicted lipoprotein | 18 | 64.6 | ||||||
| MHO_2440 | Hypothetical protein, predicted lipoprotein | 4 | 10 | 24.3 | |||||
| MHO_2620 | Hypothetical protein, predicted lipoprotein | 18 | 2 | 50.5 | |||||
| MHO_3070 | Lmp related protein | 24 | 44.2 | ||||||
| MHO_3100 | P75 related protein, predicted lipoprotein | 39 | 62.3 | ||||||
| MHO_3200 | Conserved hypothetical protein, putative peptidase, predicted lipoprotein | 5 | 85 | 47 | 14 | 11 | 4 | 10 | 89.6 |
| MHO_3470 | P50 = Vaa, surface lipoprotein adhesin | 17 | 17 | 32 | 76 | 18 | 9 | 13 | 51.1 |
| MHO_3490 | P60 | 30 | 64.2 | ||||||
| MHO_3620b | P37-like | 4 | 41.8 | ||||||
| MHO_3660 | P120 | 25 | 6 | 2 | 119.7 | ||||
| MHO_3720 | P75 protein precursor | 4 | 25 | 65 | 4 | 2 | 4 | 5 | 72.4 |
| MHO_3730 | Lmp-related protein | 27 | 2 | 77.8 | |||||
| MHO_4400 | Hypothetical protein, predicted lipoprotein | 3 | 29.7 | ||||||
| MHO_4720 | Hypothetical protein, predicted lipoprotein | 2 | 9 | 7 | 28.3 | ||||
Underlined numbers correspond to the numbers of distinct peptides detected for a nonfragmented lipoprotein. The calculated molecular mass (MM) ignores the signal peptide. E2 and E6 eluates, which did not induce IL-23 production, were analyzed for reference.
MHO_1640 and MHO_3620 were not considered bioactive because they were present solely in the E2 and E6 eluates, which did not induce IL-23 release from hDCs.
MHO_4720 was the only unfragmented lipoprotein identified in the E8 eluate. Although we could not exclude the bioactivity of fragments of the other lipoproteins detected in the E8 eluate (MHO_3200, MHO_3470, MHO_3720), MHO_4720 could by itself be responsible for the bioactivity of E7 and E8 eluates.
Overall, 24 M. hominis lipoproteins (entire or degraded forms) were able to induce the secretion of IL-23 by hDCs, 5 with an AMM between 20 and 35 kDa and 19 with an AMM between 40 and 100 kDa (Table 1). In further experiments, more attention will be focused on MHO_4720, which may be the only bioactive protein in the E7 and E8 eluates.
Role of the polypeptidic portion of the lipoproteins in IL-23 production by hDCs.
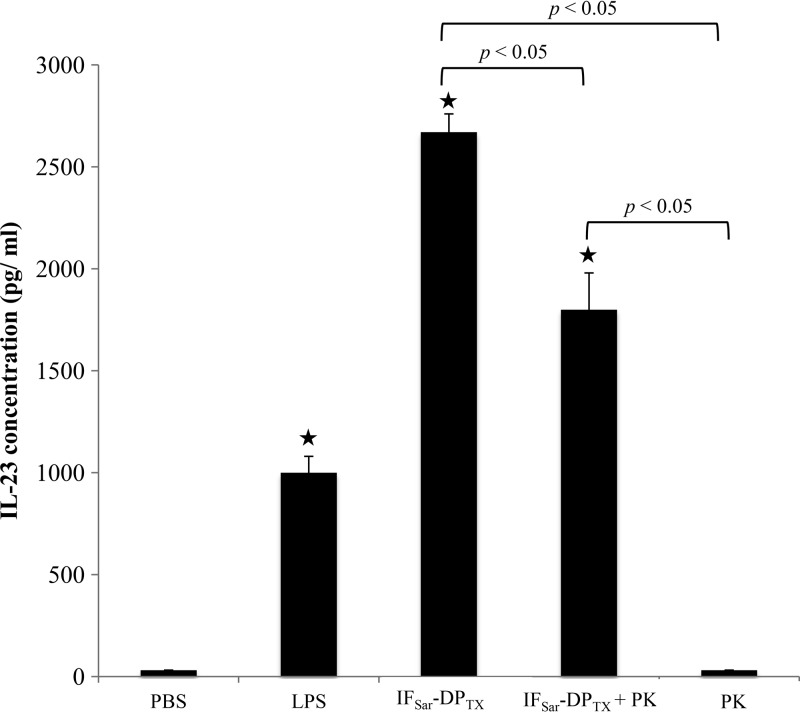
To assess whether M. hominis lipoprotein polypeptide fragments were responsible for the hDC stimulation, the IFSar-DPTX sequential extract was incubated with proteinase K (PK) for 1 h (Fig. 3), and IL-23 release was measured after incubating hDCs in the presence of the PK-digested extract. The bioactivity of the PK-treated IFSar-DPTX extract was significantly decreased but not abolished. There was no difference between results after 1 h, 4 h, 8 h, and 24 h of proteinase K digestion (data not shown), suggesting that the digestion was complete after 1 h. These data indicate that the protein portion of the lipoproteins only partly contributes to the stimulation of the hDCs and that some proteinase K-resistant compounds, possibly the acylated part of lipoproteins, are major determinants for full expression of this bioactivity.
FIG 3.
Effect of proteinase K on activity of M. hominis PG21 IFSar-DPTX sequential TX-114 extract. Three independent experiments were performed. ★, significantly different (P < 0.05) from PBS according to the Newman-Keuls test. PBS, negative control; LPS, positive control; IFSar-DPTX, Sarkosyl-insoluble fraction extracted by TX-114 (detergent phase); PK, proteinase K.
Stimulation of hDCs by a dendritic cell-stimulating lipopeptide DSL-1 derived from MHO_4720.
Because MHO_4720, in contrast to MHO_1730 and MHO_2440, was present in both bioactive eluates E7 and E8, it appeared as an M. hominis surface-exposed lipoprotein that could induce IL-23 secretion by hDCs. We aimed at confirming its dendritic cell-activating potency and therefore designed a lipopeptide with a minimal peptidic sequence derived from MHO_4720 that could stimulate IL-23 production by hDCs. We defined the peptide length of the lipopeptide, the amino acid composition, and the acyl chain composition based on data acquired from the analysis of Braun's lipoprotein, the first bacterial lipoprotein characterized in Escherichia coli (21). Data and sequence alignments from other bacterial lipoproteins and from bioactive lipopeptides derived from mycoplasmas such as MALP-2 from M. fermentans, FSL-1 from M. salivarium, and MPPL-1 from M. pneumoniae, all known to stimulate immune human cells, were also used (Table 2) (11–13). We eventually synthesized the dendritic stimulating lipopeptide 1 (DSL-1), derived from MHO_4720, which was S-(2,3-bispalmitoyloxypropyl)-cysteine-GGEKFN.
TABLE 2.
Alignment of N-terminal parts of the 24 potential bioactive lipoproteins of M. hominis PG21 and of mycoplasmal bioactive lipopeptidesa
| Gene locus | Species | N-terminal amino acid sequence of protein | MM (kDa) | pI | GRAVY score | Acylation | Reference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MHO_0280 | M. hominis | C | N | K | T | T | N | H | E | T | N | 1,161.2 | 6.74 | −2.070 | This study | |||||
| MHO_0290 | M. hominis | C | K | D | P | N | K | P | E | V | K | 1,157.3 | 8.18 | −1.870 | This study | |||||
| MHO_530 | M. hominis | C | G | G | L | A | I | A | T | T | A | 877.0 | 5.52 | 1.400 | This study | |||||
| MHO_0540 | M. hominis | C | R | F | C | K | K | P | N | K | Q | 1,251.5 | 9.85 | −1.700 | This study | |||||
| MHO_0720 | M. hominis | C | G | H | T | G | T | G | Y | G | F | 999.0 | 6.73 | −0.220 | This study | |||||
| MHO_0730 | M. hominis | C | K | T | V | N | K | E | K | I | N | 1,176.4 | 9.20 | −1.170 | This study | |||||
| MHO_0780 | M. hominis | C | S | T | T | E | N | S | K | Y | G | 1,089.1 | 5.99 | −1.310 | This study | |||||
| MHO_0790 | M. hominis | C | S | T | T | E | N | S | K | Y | G | 1,089.1 | 5.99 | −1.310 | This study | |||||
| MHO_1510 | M. hominis | C | K | I | D | P | A | Y | E | K | G | 1,123.3 | 6.06 | −0.930 | This study | |||||
| MHO_1730 | M. hominis | C | N | D | P | K | N | K | K | N | P | 1,157.3 | 9.20 | −2.640 | This study | |||||
| MHO_2080 | M. hominis | C | A | I | L | P | V | S | C | S | L | 1,005.2 | 5.51 | 1.990 | This study | |||||
| MHO_2340 | M. hominis | C | I | K | T | K | K | P | E | V | K | 1,173.4 | 9.63 | −1.020 | This study | |||||
| MHO_2440 | M. hominis | C | K | T | T | N | D | S | Q | N | S | 1,097.1 | 5.83 | −1.840 | This study | |||||
| MHO_2620 | M. hominis | C | V | K | T | K | K | P | E | G | E | 1,118.3 | 8.18 | −1.470 | This study | |||||
| MHO_3070 | M. hominis | C | N | N | K | N | S | K | L | T | K | 119.3 | 9.79 | −1.740 | This study | |||||
| MHO_3100 | M. hominis | C | S | N | K | Q | D | K | K | E | D | 1,194.2 | 6.11 | −2.750 | This study | |||||
| MHO_3200 | M. hominis | C | K | N | T | N | K | K | T | N | N | 1,164.3 | 9.79 | −2.460 | This study | |||||
| MHO_3470 | M. hominis | C | N | D | D | K | L | A | E | K | N | 1,149.2 | 4.56 | −1.720 | This study | |||||
| MHO_3490 | M. hominis | C | K | K | E | K | E | D | S | Q | Q | 1,222.3 | 6.17 | −2.750 | This study | |||||
| MHO_3660 | M. hominis | C | T | H | T | N | N | D | E | L | N | 1,160.1 | 4.35 | −1.580 | This study | |||||
| MHO_3720 | M. hominis | C | K | N | E | K | S | N | A | E | Y | 1,185.2 | 6.13 | −1.960 | This study | |||||
| MHO_3730 | M. hominis | C | T | V | T | V | K | V | K | E | K | 1,134.4 | 9.20 | −0.150 | This study | |||||
| MHO_4400 | M. hominis | C | N | K | T | A | T | I | T | L | N | 1,078.2 | 8.22 | −0.040 | This study | |||||
| MHO_4720 | M. hominis | C | G | G | E | K | F | N | A | F | A | 1,043.1 | 5.99 | 0.000 | This study | |||||
| MALP-2 | M. fermentans | C | G | N | N | D | E | S | N | I | S | F | K | E | K | 2,134.1 | 4.68 | −1.500 | Pam2 | 36 |
| FSL-1 | M. salivarium | C | G | D | P | K | H | P | K | S | F | 1,666.1 | 8.21 | −1.360 | Pam2 | 12 | ||||
| MPPL-1 | M. pneumoniae | C | T | G | I | Q | A | D | L | R | N | L | I | K | 1,341.5 | 8.41 | −0.133 | Pam2 | 13 | |
| P2C-RGDS | Mycobacterium tuberculosis | C | R | G | D | S | 1,087.5 | 5.83 | −1.34 | Pam2 | 25 | |||||||||
| PGTP2-RL | Porphyromonas gingivalis | C | N | S | Q | A | K | 649.7 | 8.22 | −1.233 | Pam3 | 37 | ||||||||
| Murein LPP | E. coli | C | S | S | N | K | I | D | E | L | S | D | D | 1,325.3 | 3.84 | −1.083 | Pam3 | 21 | ||
| MDPL | M. synoviae | C | G | D | Q | T | P | A | P | E | P | T | P | G | N | 1,383 | 3.67 | −1.307 | Pam2 | 38 |
| DSL-1 | M. hominis | C | G | G | E | K | F | N | 753.8 | 5.99 | −0.914 | Pam2 | This study | |||||||
Molecular mass (MM), isoelectric point (pI), and GRAVY score were determined using ProtParam software (http://web.expasy.org/cgi-bin/protparam/protparam). Hydrophobic amino acids are in boldface. Pam, palmitic acid; Pam2, diacylated lipoproteins with two palmitic acids; Pam3, triacylated lipoproteins with three palmitic acids.
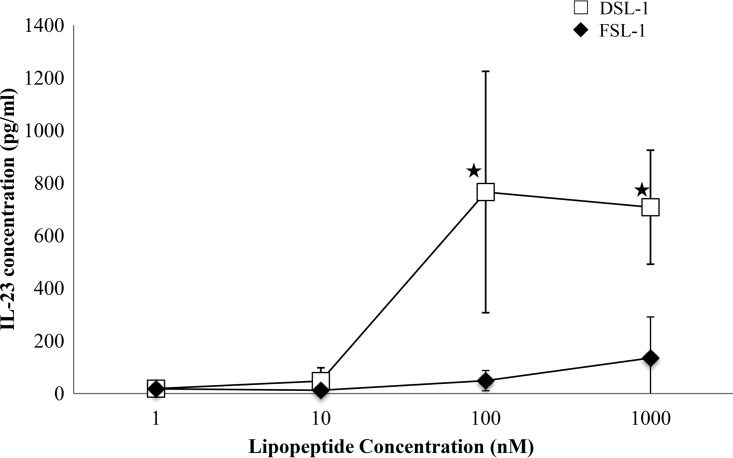
A 10-fold serial dilution of DSL-1 (1 to 1,000 nM) was tested for IL-23 release after coincubation with hDCs. DSL-1 stimulated the production of IL-23 by hDCs at a concentration higher than 10 nM (Fig. 4). The IL-23 production increased up to a DSL-1 concentration of 100 nM and slightly decreased over this concentration. FSL-1 did not induce a significant production of IL-23.
FIG 4.
Release of IL-23 by hDCs after coincubation with DSL-1 and FSL-1. Results are shown as the means and standard errors of the means from four independent experiments. ★, significantly different (P < 0.05) from IL-23 concentration after coincubation with 1 nM or 10 nM DSL-1 according to the Newman-Keuls test.
M. hominis PG21 induces caspase-dependent IL-1β secretion.
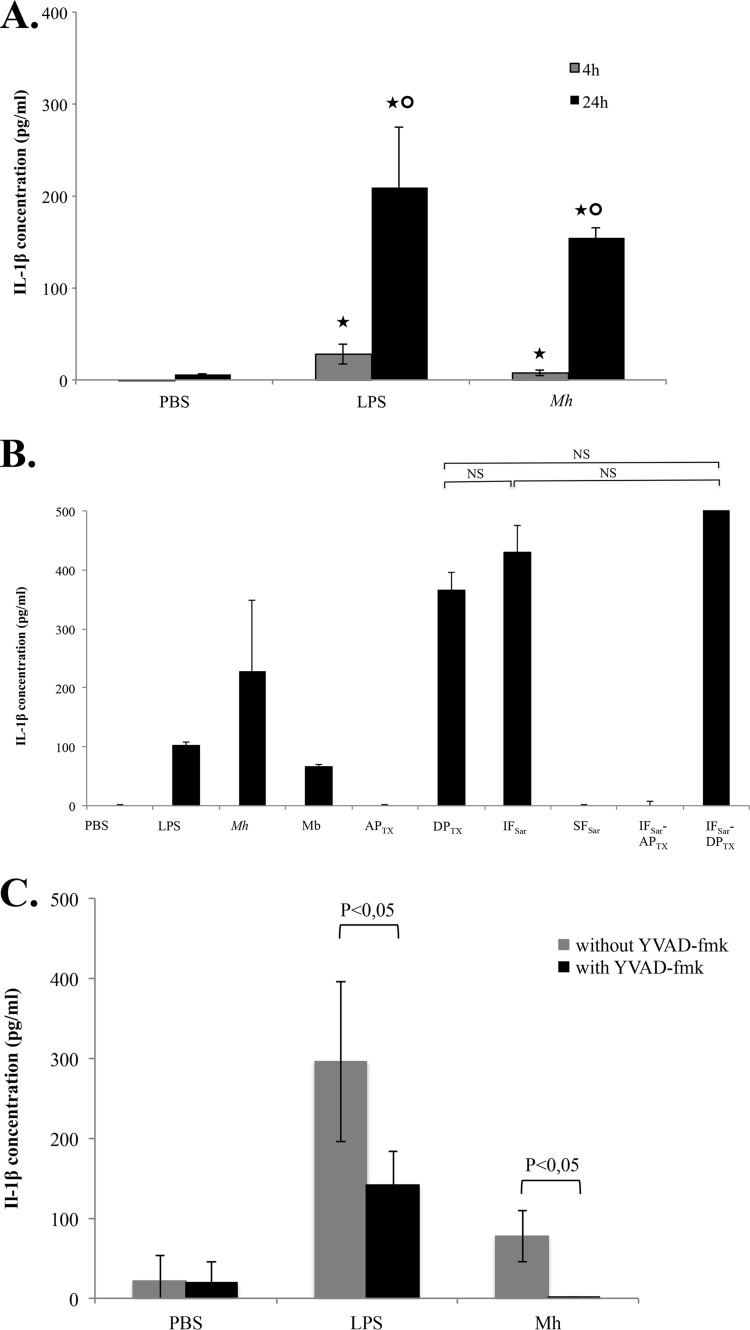
We previously partially described the hDC immune response upon exposure to M. hominis PG21 (8). To obtain a deeper understanding of the inflammation response of hDCs, we performed a PCR array targeting the inflammation-related genes of hDCs after 24 h of coincubation with M. hominis. A significantly strong upregulation of the IL-1β gene was observed, suggesting the activation of the inflammasome (data not shown). We confirmed the strong IL-1β release after 24 h of coincubation of M. hominis with hDCs by ELISA (Fig. 5A). A weaker IL-1β release was observed after 4 h of coincubation. IL-1β release was also observed after exposure of hDCs to M. hominis fractions enriched in lipoproteins, especially the sequential extract IFSar-DPTX (Fig. 5B). To determine whether caspases were involved in this IL-1β release, a caspase-1- and caspase-5-specific inhibitor, Ac-YVAD-fmk, was added to the hDCs before a 24-h coincubation with M. hominis (Fig. 5C). IL-1β release was abolished when the cells were exposed beforehand to Ac-YVAD-fmk, suggesting that caspase-1 and/or caspase-5 was involved in IL-1β production by hDCs after coincubation with M. hominis.
FIG 5.
IL-1β release by hDCs. (A) Results after incubation with M. hominis PG21 for 4 h and 24 h. ★, significantly different (P < 0.05) from IL-1β concentration released after coincubation with PBS at the same time point; ○, significantly different (P < 0.05) from IL-1β concentration released after coincubation for 4 h according to the Newman-Keuls test. (B) Results after incubation with M. hominis PG21 and its detergent fractions. (C) Results after coincubation with M. hominis PG21 with and without the caspase inhibitor YVAD-fmk. Mh, M. hominis PG21. Results are shown as the means and standard errors of the means from three independent experiments. PBS, negative control; LPS, positive control; Mh, M. hominis PG21; Mb, M. hominis PG21 membranes; APTX, TX-114 aqueous phase (nonextracted proteins); DPTX, TX-114 detergent phase (extracted proteins); IFSar, Sarkosyl-insoluble fraction (nonextracted proteins); SFSar, Sarkosyl-soluble fraction (extracted proteins); IFSar-DPTX, Sarkosyl-insoluble fraction extracted by TX-114 (detergent phase); IFSar-APTX, Sarkosyl-insoluble fraction extracted by TX-114 (aqueous phase); NS, statistically nonsignificant according to the Newman-Keuls test.
To assess which caspase and what other inflammasome components were activated in hDCs, we performed a PCR array targeting inflammasome genes after 4 h and 24 h of coincubation with M. hominis. The expression of 85 hDC genes involved in inflammasome activation was quantified in hDCs alone and in hDCs coincubated with M. hominis PG21 (Table 3). As expected, a strong upregulation of the IL-1β gene and of the IL-12β genes (IL-23 subunit gene) was observed (fold changes, 285 and 2,660, respectively, at 24 h). There was no change in the IL-18-encoding gene expression. The IL-6 inflammatory cytokine and NF-κB genes were also upregulated at 24 h. Among the caspase-coding genes tested, only the caspase-5 gene was upregulated at 4 h and 24 h, whereas there was no variation of the caspase-1 and caspase-8 gene expression. In the NOD-like receptor (NLR) family, the expression levels of NLRX1-, NOD1-, and NOD2-coding genes were downregulated at 4 h, and the expression level of NLRP3 was downregulated at 24 h (fold change, −21.8). The gene encoding the adaptor protein PYCARD was also downregulated at 24 h (fold change, −23.7). The expression level of the NLRP7-coding gene was assessed by reverse transcription-quantitative PCR (qRT-PCR) but did not show any change upon exposure of hDCs to M. hominis. No upregulation of NLR was observed at 4 h or at 24 h of coincubation. Taken together, these data suggest that M. hominis PG21 may activate the inflammasome and induce IL-1β release in a caspase-5-dependent manner.
TABLE 3.
Differential expression of the hDC inflammasome genes after 4 h and 24 h of coincubation with M. hominis PG21a
| Protein name |
t = 4 h |
t = 24 h |
||
|---|---|---|---|---|
| Fold change | P value | Fold change | P value | |
| Absent in melanoma 2 | 0.7 | 0.269 | 4.3 | 0.057 |
| B-cell CLL/lymphoma 2 | 4.4 | 0.036 | 20.7 | 0.034 |
| BCL2-like 1 | 1.4 | 0.100 | 3.4 | 0.059 |
| Baculoviral IAP repeat containing 2 | 1.2 | 0.577 | 3.5 | 0.039 |
| Baculoviral IAP repeat containing 3 | 2.1 | 0.708 | 34.7 | 0.029 |
| Caspase recruitment domain family, member 18 | Undetected | Undetected | Undetected | Undetected |
| Caspase recruitment domain family, member 6 | −1.7 | 0.309 | −1.7 | 0.155 |
| Caspase 1, apoptosis-related cysteine peptidase (interleukin 1β convertase) | 1.1 | 0.677 | 1.1 | 0.767 |
| Caspase 5, apoptosis-related cysteine peptidase | 3.1 | 0.019 | 5.8 | 0.030 |
| Caspase 8, apoptosis-related cysteine peptidase | −1.8 | 0.492 | −1.2 | 0.412 |
| Chemokine (C-C motif) ligand 2 | 1.1 | 0.975 | 80.9 | 0.010 |
| Chemokine (C-C motif) ligand 5 | 30.3 | 0.042 | 35.1 | 0.221 |
| Chemokine (C-C motif) ligand 7 | −1.1 | 0.720 | Undetected | Undetected |
| CD40 ligand | −4.3 | 0.401 | −2.1 | 0.205 |
| CASP8 and FADD-like apoptosis regulator | 1.7 | 0.604 | 5.9 | 0.024 |
| Conserved helix-loop-helix ubiquitous kinase | −1.1 | 0.332 | 1.6 | 0.467 |
| Class II major histocompatibility complex, transactivator | −1.1 | 0.938 | −4.3 | 0.024 |
| Cathepsin B | 1.0 | 0.999 | −2.8 | 0.011 |
| Chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity alpha) | 2.0 | 0.038 | 934.5 | 0.031 |
| Chemokine (C-X-C motif) ligand 2 | 1.0 | 0.540 | 265.2 | 0.054 |
| Fas (TNFRSF6)-associated via death domain | −2.0 | 0.254 | 1.0 | 0.978 |
| Heat shock protein 90kDa alpha (cytosolic), class A member 1 | −1.2 | 0.317 | −1.3 | 0.296 |
| Heat shock protein 90kDa alpha (cytosolic), class B member 1 | 1.0 | 0.905 | −1.9 | 0.150 |
| Heat shock protein 90kDa beta (Grp94), member 1 | −1.2 | 0.250 | −1.1 | 0.848 |
| Interferon beta 1 fibroblast | −2.4 | 0.570 | Undetected | Undetected |
| Interferon gamma | Undetected | Undetected | Undetected | Undetected |
| Inhibitor of kappa light polypeptide gene enhancer in B cells. kinase beta | 1.1 | 0.711 | 1.2 | 0.585 |
| Inhibitor of kappa light polypeptide gene enhancer in B cells. kinase gamma | −1.2 | 0.659 | 1.2 | 0.500 |
| Interleukin 12A (natural killer cell stimulatory factor 1, cytotoxic lymphocyte maturation factor 1, p35) | Undetected | Undetected | Undetected | Undetected |
| Interleukin 12B (natural killer cell stimulatory factor 2, cytotoxic lymphocyte maturation factor 2, p40) | 43.4 | 0.010 | 2,660.2 | 0.018 |
| Interleukin 18 (interferon-gamma-inducing factor) | −1.5 | 0.319 | 1.7 | 0.435 |
| Interleukin 1β | 8.0 | 0.040 | 285.5 | 0.035 |
| Interleukin 33 | Undetected | Undetected | Undetected | Undetected |
| Interleukin 6 (interferon beta 2) | −1.1 | 0.973 | 4,784.0 | 0.016 |
| Interleukin-1 receptor-associated kinase 1 | 1.4 | 0.482 | −1.3 | 0.385 |
| Interferon regulatory factor 1 | 1.8 | 0.184 | 8.8 | 0.040 |
| Interferon regulatory factor 2 | 1.2 | 0.811 | 2.7 | 0.046 |
| Mitogen-activated protein kinase kinase kinase 7 | −1.3 | 0.185 | −1.1 | 0.455 |
| Mitogen-activated protein kinase 1 | −1.2 | 0.729 | −1.2 | 0.299 |
| Mitogen-activated protein kinase 11 | 1.4 | 0.138 | 3.5 | 0.047 |
| Mitogen-activated protein kinase 12 | 2.0 | 0.105 | −1.9 | 0.202 |
| Mitogen-activated protein kinase 13 | 1.0 | 0.999 | 2.6 | 0.047 |
| Mitogen-activated protein kinase 3 | −1.5 | 0.623 | −3.8 | 0.035 |
| Mitogen-activated protein kinase 8 | 1.0 | 0.792 | 3.3 | 0.021 |
| Mitogen-activated protein kinase 9 | −1.2 | 0.523 | −2.9 | 0.038 |
| Mediterranean fever | 1.9 | 0.055 | −2.2 | 0.111 |
| Myeloid differentiation primary response gene (gene 88 product) | −1.4 | 0.630 | 1.3 | 0.112 |
| NLR family, apoptosis inhibitory protein | −2.7 | 0.478 | −12.2 | 0.026 |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells 1 | 2.3 | 0.537 | 11.5 | 0.010 |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor. alpha | 2.2 | 0.473 | 6.5 | 0.018 |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor. beta | 1.8 | 0.139 | 1.7 | 0.073 |
| NLR family, CARD domain containing 4 | −1.1 | 0.782 | −2.3 | 0.069 |
| NLR family, CARD domain containing 5 | 1.0 | 0.822 | 1.9 | 0.166 |
| NLR family, pyrin domain containing 1 | 1.5 | 0.352 | 1.3 | 0.590 |
| NLR family, pyrin domain containing 12 | 1.9 | 0.284 | Undetected | Undetected |
| NLR family, pyrin domain containing 3 | −1.1 | 0.952 | −21.8 | 0.030 |
| NLR family, pyrin domain containing 4 | Undetected | Undetected | Undetected | Undetected |
| NLR family, pyrin domain containing 5 | Undetected | Undetected | Undetected | Undetected |
| NLR family, pyrin domain containing 6 | Undetected | Undetected | Undetected | Undetected |
| NLR family, pyrin domain containing 7b | 0.9 | 0.788 | 1.9 | 0.329 |
| NLR family, pyrin domain containing 9 | −1.3 | 0.719 | 1.3 | 0.414 |
| NLR family member X1 | −5.4 | 0.034 | −2.1 | 0.280 |
| Nucleotide-binding oligomerization domain containing 1 | −8.2 | 0.004 | −1.3 | 0.276 |
| Nucleotide-binding oligomerization domain containing 2 | −6.0 | 0.030 | −1.2 | 0.668 |
| Purinergic receptor P2X, ligand-gated ion channel, 7 | 18.6 | 0.070 | 7.2 | 0.064 |
| Pannexin 1 | −1.3 | 0.267 | 2.9 | 0.017 |
| Phosphoprotein enriched in astrocytes 15 | 1.1 | 0.665 | 2.0 | 0.233 |
| Proline-serine-threonine phosphatase interacting protein 1 | −1.9 | 0.352 | −13 | 0.029 |
| Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) | 4.7 | 0.033 | 152.6 | 0.032 |
| PYD and CARD domain containing | −2.3 | 0.204 | −23.7 | 0.045 |
| PYD (pyrin domain) containing 1 | 1.5 | 0.507 | 3.2 | 0.920 |
| Renal tumor antigen | −1.3 | 0.038 | −1.3 | 0.314 |
| V-rel reticuloendotheliosis viral oncogene homolog A (avian) | 0.5 | 0.031 | 2.2 | 0.045 |
| Receptor-interacting serine-threonine kinase 2 | 2.1 | 0.579 | 9.5 | 0.023 |
| SGT1, suppressor of G2 allele of SKP1 (Saccharomyces cerevisiae) | −1.1 | 0.652 | 1.1 | 0.157 |
| TGF-beta activated kinase 1/MAP3K7 binding protein 1 | −2.6 | 0.490 | −1.6 | 0.038 |
| TGF-beta activated kinase 1/MAP3K7 binding protein 2 | −1.2 | 0.756 | 2.6 | 0.027 |
| Toll-interleukin 1 receptor (TIR) domain containing adaptor protein | −1.6 | 0.437 | −1.9 | 0.264 |
| Tumor necrosis factor | 2.4 | 0.489 | 7.6 | 0.110 |
| Tumor necrosis factor (ligand) superfamily, member 11 | Undetected | Undetected | Undetected | Undetected |
| Tumor necrosis factor (ligand) superfamily, member 14 | 4.0 | 0.054 | 1.4 | 0.350 |
| Tumor necrosis factor (ligand) superfamily, member 4 | 1.0 | 0.861 | 38.7 | 0.006 |
| TNF receptor-associated factor 6 | −1.2 | 0.140 | 1.6 | 0.121 |
| Thioredoxin interacting protein | −1.5 | 0.062 | −3.3 | 0.264 |
| X-linked inhibitor of apoptosis | 1.0 | 0.979 | 2.4 | 0.041 |
The lipoproteins for which significant fold changes (P value < 0.05) were observed are in boldface. Three independent biological replicates were performed for each time point.
The expression level of the NLRP7-coding gene, not present in the PCR Human Inflammasome (Qiagen) array, was determined by qRT-PCR.
In addition, the prostaglandin-endoperoxide synthase 2 (COX2)-encoding gene, ptgs2, was upregulated at 4 h (fold change, 4.7) and highly upregulated at 24 h (fold change, 152.6). Chemoattractant-coding genes were also regulated, with an increased expression of the CCL5-encoding gene at 4 h and an increased expression of CCL2- and CXCL1-encoding genes at 24 h.
DISCUSSION
Identification of IL-23 release-inducing lipoproteins in a sequential Sarkosyl/TX-114 extract of M. hominis PG21.
In the present study, 24 lipoproteins from the bioactive eluates of the IFSar-DPTX sequential extract were identified and divided into two groups: lipoproteins with apparent molecular masses ranging from 20 to 35 kDa and from 40 to 100 kDa. These data suggest that IL-23 production by hDCs results either from the action of several lipoproteins or from the action of different forms of the same lipoprotein (whole or fragmented). As a comparison, three fractions (from 22 to 25 kDa, from 25 to 32 kDa, and from 36 to 43 kDa) from Mycoplasma arthritidis, a mycoplasma phylogenetically close to M. hominis, were able to induce TNF-α secretion by murine monocytes (2). Peltier et al. showed that the stimulation of TNF-α production by THP-1 cells after incubation with M. hominis PG21 fractions was due to proteins that had molecular masses from 10 to 50 kDa (3). Hasebe et al. isolated and purified a lipoprotein of 40 kDa from M. hominis membranes that stimulated TNF-α production by THP-1 cells (14). This study reports for the first time that a group of high-molecular-mass lipoproteins from M. hominis could be involved in the immune response of hDCs.
Among the 24 potential bioactive lipoproteins, we focused on MHO_4270 because this lipoprotein could alone be responsible for the bioactivity of the E7 and E8 eluates. Many orthologs of this hypothetical lipoprotein were found in other mycoplasmal species, such as Mycoplasma spumans, M. canadense, M. alkalescens, and M. arginini, involved in animal infections. These hypothetical proteins and lipoproteins are all of unknown function. However, a leucine-rich repeat (LRR) domain is conserved among all these lipoproteins. The LRR domain is a widespread sequence of 20 to 30 amino acids with a characteristic repetitive sequence pattern rich in leucines. The LRR corresponds to a short β-strand and α-helix region, which is involved in protein-protein interactions (22). Indeed, it was reported that mycoplasma diacylated lipoproteins and FSL-1, an M. salivarium-derived diacylated lipopeptide, were recognized via their LRR domain by the LRR domain of the Toll-like receptor 2 (TLR2) (23). As TLR2 was previously reported to interact with M. hominis (8), we can speculate that MHO_4720 could interact via its LRR domain with TLR2 of the hDCs.
Lipopeptide DSL-1, corresponding to the N-terminal part of MHO_4720, stimulates IL-23 release by hDCs.
We noted that the protein moiety was important to stimulate hDCs because proteinase K digestion significantly reduced the bioactivity of the sequential extract IFSar-DPTX. Peltier et al. also showed that the activity of the stimulating factor of THP-1 cells present in a TX-114 extract from M. hominis was significantly decreased by proteinase K digestion (3). To determine if a minimal lipoprotein structure could stimulate the hDCs, we designed a synthetic lipopeptide. As suggested for the generation of the bioactive lipopeptide MPPL-1 from M. pneumoniae (13), we first attempted to find a consensus sequence by the alignment of the N-terminal sequence of the 24 potential bioactive lipoproteins identified in the present study. However, no consensus sequence could be identified. Synthetic lipopeptides derived from Braun's lipoprotein were shown to exhibit stimulatory activity comparable to that of native lipoproteins (24). It was also shown that a TLR2 agonist, which is the hDC TLR that recognizes M. hominis (8), required a hydrophilic N-terminal part (25). The DSL-1 peptide chosen had seven N-terminal amino acid residues (CGGEKFN), which conferred a negative grand average of hydropathy (GRAVY) score close to that of the bioactive mycoplasmal lipopeptides FSL-1 and MALP-2 (11, 12) (Table 2). In addition, the presence of only two acyl chains bound to the cysteine residue would be adequate for the bioactivity because many mycoplasmal bioactive lipopeptides such as FSL-1, MALP-2, and MPPL-1 are diacylated (11–13).
DSL-1 induced IL-23 production in two stages, an increasing stage followed by a steady-state stage. Okusawa et al. also observed two phases in the production of IL-6, IL-8, and MCP-1 by human gingival fibroblasts stimulated by FSL-1 and in the production of TNF-α by THP-1 cells induced by FSL-1 and MALP-2 (26). In addition, FSL-1 failed to induce significant IL-23 production, highlighting that the immune hDC response was specific to the DSL-1 lipopeptide.
M. hominis PG21 activates the hDC inflammasome.
Results of the PCR array targeting inflammasome genes were validated by the strong upregulation of the IL-23- and IL-1β-coding genes, which confirmed the IL-23 and IL-1β release observed in coincubation supernatants. The increase of the transcription of the IL-6 gene was also confirmed by IL-6 ELISA detection in the supernatants after coincubation (data not shown). IL-1β release after coincubation of M. hominis with hDCs may result from caspase-5-dependent inflammasome activation. However, the role of caspase-1 in the IL-1β release observed after coincubation of hDCs with M. hominis cannot be ruled out. Caspase-1 could be constitutively expressed, as it was shown to be the case in the monocytic cell line THP-1 (27). In addition, caspase-1 and caspase-5 were both reported to be recruited by the NLRP1/PYCARD complex and were reported to be capable of forming heterocomplexes in the THP1 cell line stimulated by LPS or phorbol myristate acetate (PMA) (28).
In the present study, the inflammasome sensor remained unidentified at the gene expression level among the 13 NLR genes tested. The NLR protein family comprises 22 members in humans; therefore, we can speculate that either the sensor is part of the 9 untested NLRs or the molecule does not trigger a priming step of gene expression; rather, it may directly activate NLR(s) at the protein level to assemble the inflammasome. The NLRP7-encoding gene was not regulated in our model of coincubation, although (i) NLRP7 had been specifically identified as an intracellular sensor of mycoplasmal acylated lipoproteins and (ii) NLRP7 was reported to activate the inflammasome of the THP-1 cell line after exposure to A. laidlawii, to lipopeptides derived from mycoplasmas such as FSL-1 and MALP-2, and to the synthetic lipopeptides Pam2CSK4 and Pam3CSK4 (16). Notably, we observed a significant downregulation of the NLRP3-encoding gene and of the adaptor PYCARD-coding gene at 24 h. The ability of LPS to activate NLRP3 was described to be weak and transient, with a low capacity of NLRP3 activation after 24 h (29). Chronic LPS stimulation was also reported to trigger IL-10-dependent regulatory mechanisms. Similarly, we previously showed that M. hominis PG21 induces IL-10 release after coincubation with the hDCs (8). In the present study, an early time point (4 h) and a late time point (24 h) were chosen to assess major differences in the transcriptional levels of individual genes. At 4 h, we might have missed the upregulation of the NLRP3 gene. In addition, even though we did not observe a modulation of NLRP3 gene expression at 4 h, a small amount of constitutively expressed NLRP3 protein might be sufficient to activate and trigger inflammasome assembly. In addition, for several other mycoplasma species, the NLRP3 protein is the sensor of the inflammasome. Indeed, M. pneumoniae community-acquired respiratory distress syndrome (CARDS) toxin, A. laidlawii, M. hyorhinis, and M. salivarium were reported to activate the NLRP3 inflammasome of mouse primary bone marrow-derived macrophages (30), of human THP-1 cells (16, 17), and of murine dendritic cells (18), respectively. For all these reasons, NLRP3 may be the inflammasome receptor involved in the caspase-dependent IL-1β release.
We observed an upregulation of the prostaglandin-endoperoxide synthase 2 (COX-2)-encoding gene at 24 h. The COX-2-inducible enzyme is responsible for the synthesis of prostaglandin E2 (PGE2) from arachidonic acid derived from membrane phospholipids. PGE2 is a potent lipid mediator involved in maintaining homeostasis but also in promoting acute inflammation or immune suppression in chronic inflammation. The overexpression of COX-2 in human placental trophoblast cells was previously described to be induced by MALP-2, a mycoplasmal lipopeptide (31). Moreover, in the present study, the overexpression of COX-2 may be related to the downregulation of NLRP3. In accordance with this hypothesis, an inverse relation between the expression of COX-2 and NLRP3 was previously reported in human primary monocyte-derived macrophages (32).
Conclusion.
We studied the interaction of M. hominis PG21 with hDCs by analyzing both sides of the interaction, the mycoplasmal lipoproteins that induce IL-23 production and the cell innate immune response. We defined a list of 24 lipoproteins that could induce the secretion of IL-23 by hDCs. Among them, lipoprotein MHO_4720 was identified as a potential stimulating factor of hDCs. Inflammasome activation of hDCs with caspase-5-dependent production of IL-1β was also observed. After 24 h of coincubation, a downregulation of NLRP3- and of the adaptor PYCARD-encoding genes associated with an upregulation of the COX2-encoding gene suggests a regulation of the inflammasome and a potential involvement of NLRP3 as an inflammasome sensor. Further studies are necessary to specify the role of NLRP3 in hDC inflammasome activation after incubation with M. hominis PG21.
MATERIALS AND METHODS
Growth of bacteria and membrane preparations.
M. hominis strain PG21 (ATCC 23114) was cultivated, and membranes were isolated as described previously (8) with slight modifications. Briefly M. hominis was harvested by centrifugation (20,000 × g, 30 min, 4°C) and washed three times in phosphate-buffered saline (PBS). The cells were lysed by three 1-min bursts of sonication on ice at the maximum setting for the micro tip (Vibra-Cell sonicator; Branson). Membranes were recovered by centrifugation for 1 h at 30,000 × g (4°C). The pellets were dispersed in PBS and washed in the same buffer four times. The final pellets were resuspended in PBS.
Membrane protein extraction.
M. hominis PG21 membranes were separated into detergent-soluble and detergent-insoluble fractions using five detergents: 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 50 nM (Thermo Fisher Scientific, San Jose, CA); octyl-glucopyranoside (OG), 75 nM (Sigma-Aldrich, St. Louis, MO); sodium deoxycholate (DOC), 50 nM (Sigma-Aldrich); Triton X-100 (TX-100), 1% (Sigma-Aldrich); N-lauroylsarcosine sodium salt (Sarkosyl), 100 nM (Sigma-Aldrich); and 1% TX-114 (Sigma-Aldrich). One volume of membrane suspension (1 mg of protein/ml) was mixed with 4 volumes of detergent in 0.1 M sodium phosphate buffer (pH 7.4) and gently rocked for 1 h at 4°C. The mixtures were centrifuged at 285,000 × g for 15 min at 4°C (Beckman TL-100 ultracentrifuge, TLA 100.1 rotor) to separate the solubilized proteins from the insoluble material. For the sequential extraction procedure using two detergents, the Sarkosyl-soluble fraction (SFSar) and the Sarkosyl-insoluble fraction (IFSar) were subjected to TX-114 1% extraction. A volume of 900 μl of ice-cold acetone was added to 100 μl of sample (final detergent extract) to precipitate the proteins. The proteins were further washed using ice-cold acetone. The protein concentration was determined using the DC protein assay kit (Bio-Rad, Hercules, CA).
Preparative SDS-PAGE experiments.
Each detergent-soluble and detergent-insoluble fraction obtained from a quantity of 200 μg of total membrane protein was heated for 3 min at 100°C and then separated on a 12.5% acrylamide gel by SDS-PAGE. The proteins were visualized using the ProteoSilver silver stain kit (Sigma-Aldrich).
To identify the lipoproteins present in the IFSar-DPTX sequential extract (i.e., the TX-114 detergent-enriched phase obtained by extraction of the Sarkosyl-insoluble fraction), the extract was separated by 12.5% SDS-PAGE. The gel was cut into 10 equal bands, and each band was eluted with 75 nM OG. The proteins were precipitated using ice-cold acetone (9 volumes of acetone for 1 volume of sample). This precipitation step allowed the removal of OG. The protein pellet was further washed using ice-cold acetone before being dissolved in PBS. Each eluate was incubated with hDCs for 24 h as described below.
Proteins from the eluates of interest were identified as previously described by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (19).
Proteinase K digestion of the IFSar-DPTX sequential extract.
The IFSar-DPTX sequential extract was incubated with 0.4 U proteinase K coupled to EupergitC (Sigma-Aldrich), which is a carrier consisting of macroporous beads for immobilizing enzymes, and gently rocked for 1 h at 37°C. The insoluble enzyme was subsequently removed by centrifugation at 800 × g for 5 min. The digested extracts were tested for IL-23 release after coincubation with hDCs.
Design and activity of DSL-1.
In silico analysis of the N-terminal part of the hypothetical bioactive lipoproteins from eluates E3, E4, E5, E7, and E8 was performed using Molligen 3.0 software (http://www.cbib.u-bordeaux2.fr/outils/molligen/) (33). The search for a consensus peptide sequence was carried out using ClustalW2 software (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The hydropathy (GRAVY scores) of the peptidic fragments was determined using ProtParam software (http://web.expasy.org/cgi-bin/protparam/protparam). FSL-1, S-(2,3-bispalmitoyloxypropyl)-CGDPKHSPKSF, synthesized on the basis of a 44-kDa lipoprotein of M. salivarium (12), and S-(2,3-bispalmitoyloxypropyl)-CGGEKFN (DSL-1) were synthesized by EMC microcollections GmbH (Tübingen, Germany). The DSL-1 purity was confirmed by analytical high-performance liquid chromatography (HPLC) with a reverse-phase (RP) C18 column, and the mass of the synthetic product was checked by electrospray ionization mass spectrometry (ESI-MS) (see Fig. S1 in the supplemental material). A 1-mg/ml stock solution was prepared in PBS.
Human monocyte-derived DC generation and M. hominis stimulation.
Human monocyte-derived DC generation was performed as described elsewhere (8). Briefly, human monocytes were selected from human peripheral blood mononuclear cells (PBMC) using CD14 microbeads (Miltenyi Biotec, San Diego, CA). Plastic-adherent monocytes were cultured for 5 days with 25 ng/ml of human granulocyte-macrophage colony-stimulating factor (hGM-CSF) and 10 ng/ml of human interleukin-4. The purity and viability of DC preparation were >95%. For IL-23 and IL-1β release analysis, hDCs were transferred into 96-well plates at 5 × 105 cells per well along with the different extracts at 20 μg/ml, except for the sequential extractions, for which the protein concentrations were lower and could not be adjusted. An M. hominis suspension at 108 color changing units (CCU)/ml for a multiplicity of infection of 50, LPS from Escherichia coli serotype O26:B6 (Sigma-Aldrich) at 10 ng/ml, and mycoplasma membranes at 20 μg/ml were used. The mixtures were incubated at 37°C for 24 h. The cells were pelleted by centrifugation at 1,300 × g for 10 min at room temperature. The supernatants were collected for the determination of IL-23 and IL-1β using the human ELISA Ready-Set-Go system (eBioscience, San Diego, CA). The cell viability was assessed using trypan blue (Sigma-Aldrich). When appropriate, the caspase-1 and -5 inhibitor, Ac-YVAD-fmk (40 μM; Miltenyi Biotec), was added to the cells 30 min before coincubation of the hDCs with M. hominis. At least three independent biological replicates were performed for each quantitative analysis.
RNA extraction, reverse transcription, and PCR array.
To evaluate the differential expression of hDC genes upon contact with M. hominis, hDCs were coincubated with M. hominis as described above at 37°C for 4 and 24 h. After coincubation, the cells were pelleted, total RNA was extracted from the pellet, and the RNA purity and integrity were assessed as previously described (19).
The differential expression of hDC genes was determined using the Human Inflammatory Response & Autoimmunity and the Human Inflammasomes PCR arrays (Qiagen, Hilden, Germany). The amplification settings, cycle threshold (CT) determination, and expression ratios were determined as previously described (19). The limit of detection and CT cutoff were 35 for each gene, as recommended by the manufacturer. The expression level of the NLRP7-encoding gene, not present in the PCR array, was determined by qRT-PCR with previously reported primers (34) and the same PCR conditions as for the PCR arrays.
Statistics.
The P values were calculated based on analysis of variance (ANOVA) and the Newman-Keuls test as the post hoc test in ELISAs. For the expression ratios of hDC genes, the P values were calculated based on Student's t test of the ΔCT values between hDCs incubated with M. hominis PG21 and hDCs incubated without M. hominis, independently for each gene (35). A P value of <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by internal funding.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00213-17.
REFERENCES
- 1.Browning GF, Marenda MS, Noormohammadi AH, Markham PF. 2011. The central role of lipoproteins in the pathogenesis of mycoplasmoses. Vet Microbiol 153:44–50. doi: 10.1016/j.vetmic.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Cole BC, Mu HH, Pennock ND, Hasebe A, Chan FV, Washburn LR, Peltier MR. 2005. Isolation and partial purification of macrophage- and dendritic cell-activating components from Mycoplasma arthritidis: association with organism virulence and involvement with Toll-like receptor 2. Infect Immun 73:6039–6047. doi: 10.1128/IAI.73.9.6039-6047.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltier MR, Freeman AJ, Mu HH, Cole BC. 2005. Characterization and partial purification of a macrophage-stimulating factor from Mycoplasma hominis. Am J Reprod Immunol 54:342–351. doi: 10.1111/j.1600-0897.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, Kida Y, Kuwano K. 2007. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology 121:473–483. doi: 10.1111/j.1365-2567.2007.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimizu T, Kida Y, Kuwano K. 2008. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-kappaB through TLR1, TLR2 and TLR6. Microbiology 154:1318–1325. doi: 10.1099/mic.0.2007/016212-0. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Kida Y, Kuwano K. 2008. A triacylated lipoprotein from Mycoplasma genitalium activates NF-kappaB through Toll-like receptor 1 (TLR1) and TLR2. Infect Immun 76:3672–3678. doi: 10.1128/IAI.00257-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, You X, Zeng Y, Yu M, Zuo L, Wu Y. 2009. Mycoplasma genitalium-derived lipid-associated membrane proteins activate NF-kappaB through toll-like receptors 1, 2, and 6 and CD14 in a MyD88-dependent pathway. Clin Vaccine Immunol 16:1750–1757. doi: 10.1128/CVI.00281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truchetet ME, Béven L, Renaudin H, Douchet I, Férandon C, Charron A, Blanco P, Schaeverbeke T, Contin-Bordes C, Bébéar C. 2011. Potential role of Mycoplasma hominis in interleukin (IL)-17-producing CD4+ T-cell generation via induction of IL-23 secretion by human dendritic cells. J Infect Dis 204:1796–1805. doi: 10.1093/infdis/jir630. [DOI] [PubMed] [Google Scholar]
- 9.Calcutt MJ, Kim MF, Karpas AB, Muhlradt PF, Wise KS. 1999. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect Immun 67:760–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YC, Lin IH, Chung WJ, Hu WS, Ng WV, Lu CY, Huang TY, Shu HW, Hsiao KJ, Tsai SF, Chang CH, Lin CH. 2012. Proteomics characterization of cytoplasmic and lipid-associated membrane proteins of human pathogen Mycoplasma fermentans M64. PLoS One 7:e35304. doi: 10.1371/journal.pone.0035304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlradt PF, Kiess M, Meyer H, Sussmuth R, Jung G. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med 185:1951–1958. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibata K, Hasebe A, Into T, Yamada M, Watanabe T. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J Immunol 165:6538–6544. doi: 10.4049/jimmunol.165.11.6538. [DOI] [PubMed] [Google Scholar]
- 13.Into T, Dohkan J, Inomata M, Nakashima M, Shibata K, Matsushita K. 2007. Synthesis and characterization of a dipalmitoylated lipopeptide derived from paralogous lipoproteins of Mycoplasma pneumoniae. Infect Immun 75:2253–2259. doi: 10.1128/IAI.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasebe A, Mu HH, Cole BC. 2014. A potential pathogenic factor from Mycoplasma hominis is a TLR2-dependent, macrophage-activating, P50-related adhesin. Am J Reprod Immunol 72:285–295. doi: 10.1111/aji.12279. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Kida Y, Kuwano K. 2011. Cytoadherence-dependent induction of inflammatory responses by Mycoplasma pneumoniae. Immunology 133:51–61. doi: 10.1111/j.1365-2567.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. 2012. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Li H, Chen W, Yao X, Xing Y, Wang X, Zhong J, Meng G. 2013. Mycoplasma hyorhinis activates the NLRP3 inflammasome and promotes migration and invasion of gastric cancer cells. PLoS One 8:e77955. doi: 10.1371/journal.pone.0077955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama M, Saeki A, Hasebe A, Kamesaki R, Yoshida Y, Kitagawa Y, Suzuki T, Shibata K. 2016. Activation of inflammasomes in dendritic cells and macrophages by Mycoplasma salivarium. Mol Oral Microbiol 31:259–269. doi: 10.1111/omi.12117. [DOI] [PubMed] [Google Scholar]
- 19.Goret J, Le Roy C, Touati A, Mesureur J, Renaudin H, Claverol S, Bébéar C, Beven L, Pereyre S. 2016. Surface lipoproteome of Mycoplasma hominis PG21 and differential expression after contact with human dendritic cells. Future Microbiol 11:179–194. doi: 10.2217/fmb.15.130. [DOI] [PubMed] [Google Scholar]
- 20.Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, Barré A, Avenaud P, Jacob D, Couloux A, Barbe V, de Daruvar A, Blanchard A, Bébéar C. 2009. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet 5:e1000677. doi: 10.1371/journal.pgen.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun V, Rehn K. 1969. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem 10:426–438. [DOI] [PubMed] [Google Scholar]
- 22.Bella J, Hindle KL, McEwan PA, Lovell SC. 2008. The leucine-rich repeat structure. Cell Mol Life Sci 65:2307–2333. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita M, Into T, Yasuda M, Okusawa T, Hamahira S, Kuroki Y, Eto A, Nisizawa T, Morita M, Shibata K. 2003. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J Immunol 171:3675–3683. doi: 10.4049/jimmunol.171.7.3675. [DOI] [PubMed] [Google Scholar]
- 24.Bessler WG, Cox M, Lex A, Suhr B, Wiesmuller KH, Jung G. 1985. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol 135:1900–1905. [PubMed] [Google Scholar]
- 25.Akazawa T, Inoue N, Shime H, Kodama K, Matsumoto M, Seya T. 2010. Adjuvant engineering for cancer immunotherapy: development of a synthetic TLR2 ligand with increased cell adhesion. Cancer Sci 101:1596–1603. doi: 10.1111/j.1349-7006.2010.01583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, Hara Y, Hasebe A, Golenbock DT, Morita M, Kuroki Y, Ogawa T, Shibata K. 2004. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by Toll-like receptors 2 and 6. Infect Immun 72:1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin XY, Choi MS, Porter AG. 2000. Expression analysis of the human caspase-1 subfamily reveals specific regulation of the CASP5 gene by lipopolysaccharide and interferon-gamma. J Biol Chem 275:39920–39926. doi: 10.1074/jbc.M007255200. [DOI] [PubMed] [Google Scholar]
- 28.Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 29.Gurung P, Li B, Subbarao Malireddi RK, Lamkanfi M, Geiger TL, Kanneganti TD. 2015. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Sci Rep 5:14488. doi: 10.1038/srep14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bose S, Segovia JA, Somarajan SR, Chang TH, Kannan TR, Baseman JB. 2014. ADP-ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS toxin regulates inflammasome activity. mBio 5(6):302186–14. doi: 10.1128/mBio.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, Terakawa N. 2006. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol 72:46–59. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Sokolowska M, Chen LY, Liu Y, Martinez-Anton A, Qi HY, Logun C, Alsaaty S, Park YH, Kastner DL, Chae JJ, Shelhamer JH. 2015. Prostaglandin E2 inhibits NLRP3 inflammasome activation through EP4 receptor and intracellular cyclic AMP in human macrophages. J Immunol 194:5472–5487. doi: 10.4049/jimmunol.1401343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barré A, de Daruvar A, Blanchard A. 2004. MolliGen, a database dedicated to the comparative genomics of Mollicutes. Nucleic Acids Res 32(Database issue):D307–D310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang P, Dixon M, Zucchelli M, Hambiliki F, Levkov L, Hovatta O, Kere J. 2008. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS One 3:e2755. doi: 10.1371/journal.pone.0002755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellemans J, Vandesompele J. 2011. qPCR data analysis—unlocking the secret to successful results, p 1–13. In Kennedy S, Oswald N (ed), PCR troubleshooting and optimization: the essential guide. Caister Academic Press, Poole, UK. [Google Scholar]
- 36.Shimizu T, Kida Y, Kuwano K. 2004. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology 113:121–129. doi: 10.1111/j.1365-2567.2004.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asai Y, Makimura Y, Ogawa T. 2007. Toll-like receptor 2-mediated dendritic cell activation by a Porphyromonas gingivalis synthetic lipopeptide. J Med Microbiol 56:459–465. doi: 10.1099/jmm.0.46991-0. [DOI] [PubMed] [Google Scholar]
- 38.Oven I, Resman Rus K, Dusanic D, Bencina D, Keeler CL Jr, Narat M. 2013. Diacylated lipopeptide from Mycoplasma synoviae mediates TLR15 induced innate immune responses. Vet Res 44:99. doi: 10.1186/1297-9716-44-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.