ABSTRACT
In December 2016, a low-pathogenic avian influenza (LPAI) A(H7N2) virus was identified to be the causative source of an outbreak in a cat shelter in New York City, which subsequently spread to multiple shelters in the states of New York and Pennsylvania. One person with occupational exposure to infected cats became infected with the virus, representing the first LPAI H7N2 virus infection in a human in North America since 2003. Considering the close contact that frequently occurs between companion animals and humans, it was critical to assess the relative risk of this novel virus to public health. The virus isolated from the human case, A/New York/108/2016 (NY/108), caused mild and transient illness in ferrets and mice but did not transmit to naive cohoused ferrets following traditional or aerosol-based inoculation methods. The environmental persistence of NY/108 virus was generally comparable to that of other LPAI H7N2 viruses. However, NY/108 virus replicated in human bronchial epithelial cells with an increased efficiency compared with that of previously isolated H7N2 viruses. Furthermore, the novel H7N2 virus was found to utilize a relatively lower pH for hemagglutinin activation, similar to human influenza viruses. Our data suggest that the LPAI H7N2 virus requires further adaptation before representing a substantial threat to public health. However, the reemergence of an LPAI H7N2 virus in the northeastern United States underscores the need for continuous surveillance of emerging zoonotic influenza viruses inclusive of mammalian species, such as domestic felines, that are not commonly considered intermediate hosts for avian influenza viruses.
IMPORTANCE Avian influenza viruses are capable of crossing the species barrier to infect mammals, an event of public health concern due to the potential acquisition of a pandemic phenotype. In December 2016, an H7N2 virus caused an outbreak in cats in multiple animal shelters in New York State. This was the first detection of this virus in the northeastern United States in over a decade and the first documented infection of a felid with an H7N2 virus. A veterinarian became infected following occupational exposure to H7N2 virus-infected cats, necessitating the evaluation of this virus for its capacity to cause disease in mammals. While the H7N2 virus was associated with mild illness in mice and ferrets and did not spread well between ferrets, it nonetheless possessed several markers of virulence for mammals. These data highlight the promiscuity of influenza viruses and the need for diligent surveillance across multiple species to quickly identify an emerging strain with pandemic potential.
KEYWORDS: H7N2, cats, ferret, influenza, low-pathogenic avian influenza virus, pathogenesis, transmission
INTRODUCTION
Low-pathogenic avian influenza (LPAI) A(H7N2) viruses circulated for several years in the northeastern United States, until their elimination from live bird markets in this region in 2006 (1, 2). This virus subtype was associated with several outbreaks, notably an outbreak in turkeys in Virginia in 2002 that resulted in one case of human seroconversion (3). H7N2 virus was also isolated from a person in New York State in 2003 (4). These represent the only previously documented human cases of LPAI H7N2 virus infection in North America; additional cases have been reported in Europe (5). Since 2006, H7N3, H7N8, and H7N9 viruses have been associated with avian outbreaks in North America, and two cases of human conjunctivitis associated with a highly pathogenic avian influenza (HPAI) H7N3 virus were reported in Jalisco, Mexico, in 2012 (6, 7).
In December 2016, an outbreak of respiratory illness in shelter cats in New York City was identified to be caused by an LPAI H7N2 virus (8). This was the first detection of this subtype in North America in over a decade. One person with occupational exposure to infected cats contracted the virus, displaying a mild respiratory illness prior to recovery (9). The genome of the causative virus was found to share close sequence similarity with the genomes of LPAI H7N2 viruses detected in poultry a decade earlier (A. Marinova-Petkova, Y. Jang, B. Lynch, N. Zanders, M. Rodriguez, J. Jones, S. Thor, E. Hodges, J. de la Cruz, J. Belser, H. Yang, P. Carney, B. Shu, L. Berman, J. Barnes, F. Havers, S. C. Trock, A. Fry, L. Gubareva, J. S. Bresee, J. Stevens, M. K. Torchetti, K. Toohey-Kurth, K. St. George, D. E. Wentworth, S. Lindstrom, and C. T. Davis, submitted for publication); it is currently unknown how the virus reemerged in the shelter cat population.
Infection of domestic cats with influenza virus in North America has been reported previously (10–12), and it has been shown that cats infected with influenza virus can transmit virus to naive cats either in a direct-contact (DC) setting or via respiratory droplets (RDs) (13). As such, cats are one of several mammalian species which can serve as intermediate hosts for influenza viruses, underscoring the need to better understand influenza virus infection in this species and the potential role that cats may play in the emergence of reassortant or mutant viruses with pandemic potential. While many human and avian influenza viruses have been detected previously in domestic cats (14), the 2016 LPAI H7N2 outbreak in shelter cats represents the first known outbreak of an H7 subtype virus in this species. Considering the high risk of interspecies exposure and possible transmission between companion animals and humans, there is a need to examine the capacity of this novel H7N2 virus to cause infection and disease in mammals. Here, we examined the pathogenicity and transmissibility of the first human isolate from this outbreak, A/New York/108/2016 (NY/108). We found that this virus exhibited mild virulence in mouse and ferret models but displayed an increased replicative ability in human respiratory cells compared with that of previously studied LPAI H7N2 viruses.
RESULTS
Pathogenicity and transmissibility of NY/108 H7N2 influenza virus following intranasal inoculation of ferrets and mice.
On 14 December 2016, an outbreak of avian lineage H7N2 virus was reported among cats housed in an animal shelter in New York City, resulting in one human infection (8, 9). The genomes of both NY/108 virus and a closely related feline isolate (A/feline/New York/16-040082-1/2016 [feline/NY]) share nearly 100% amino acid identity (Marinova-Petkova et al., submitted) and possess several genetic features common among North American LPAI viruses, including the presence of 271Thr, 590Gly/591Gln, 627Glu, and 701Asp amino acids in PB2, the absence of multiple polybasic amino acids at the hemagglutinin (HA) cleavage site, and the presence of stalk deletions in the neuraminidase (NA) (Marinova-Petkova et al., submitted). Prior studies have identified that these related North American LPAI H7N2 viruses can replicate efficiently throughout the respiratory tract of ferrets and are capable of high-titer replication in the murine lung and nose without prior adaptation (15, 16). However, it was unknown if the recently isolated H7N2 virus shared these properties.
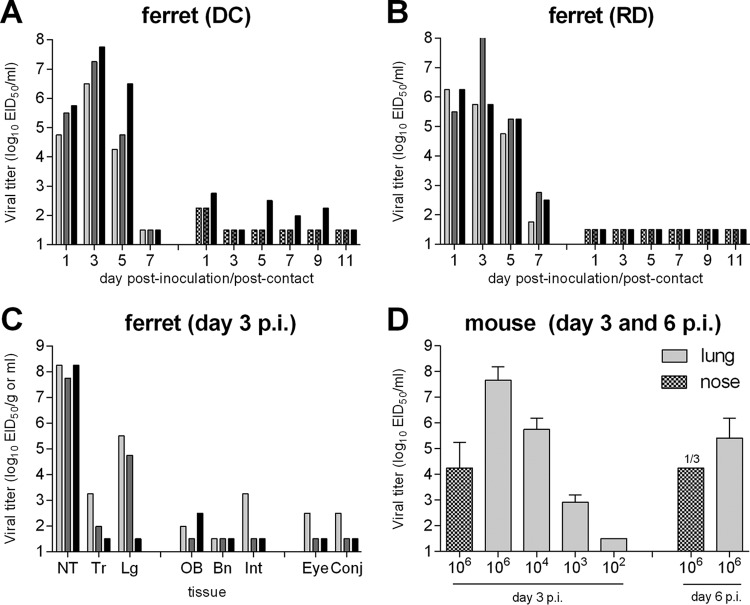
To assess the virulence of this novel virus for mammals, six ferrets were inoculated intranasally (i.n.) with 106 50% egg infectious doses (EID50) of NY/108 virus and observed daily for clinical signs and symptoms of infection. All inoculated ferrets became productively infected, with NY/108 virus replicating to high titers in ferret nasal wash (NW) samples and reaching peak mean maximum titers of 107 EID50/ml (Table 1; Fig. 1A and B, left sets of bars). The ferrets exhibited mild and transient weight loss during the acute phase of infection (<5% mean maximum weight loss) with a mild and unsustained increase in body temperature (<1°C) (Table 1). No respiratory signs, such as nasal discharge and sneezing, were observed. In agreement with the high nasal wash titers, robust virus replication in the nasal turbinates was observed on day 3 postinoculation (p.i.) (mean titer > 108 EID50/ml) in three additional ferrets that had also been i.n. inoculated with 106 EID50 of NY/108 virus (Fig. 1C). Virus was further detected in the lung and trachea of 2/3 ferrets, though the titers were >100-fold higher in the lung than the trachea among ferrets with detectable virus in these tissues. Typical of other LPAI viruses (15, 17), low titers of NY/108 virus were detected in the olfactory bulb of 2/3 ferrets (mean titer < 103 EID50/g) but not in anterior or posterior brain tissues. Infectious virus was not detected in other systemic tissues (liver, spleen, kidneys) or blood, with the exception of intestinal tissue in one ferret, further supporting the low-virulence phenotype of this virus.
TABLE 1.
NY/108 virus virulence in ferrets
| Routea | Doseb (EID50) | No. of infected ferrets/total no.c | % wt lossd | Rise in tempe (°C) | Peak titer in NWf | No. of ferrets/total no.g: |
No. of ferrets that seroconverted/total no.h | |
|---|---|---|---|---|---|---|---|---|
| CW | RS | |||||||
| i.n. | 106 | 6/6 | 4.9 | 0.6 | 7.0 ± 0.8 (1–3) | 1/6 | 0/6 | 6/6 (160–640) |
| AR | 105.7 | 3/3 | 7.3 | 1.2 | 6.2 ± 0.6 (3–5) | 0/3 | 2/3 | 3/3 (320–640) |
| AR | 103.5 | 1/3 | 7.0 | 0.8 | 5.8 (5) | 0/3 | 0/3 | 1/3 (320) |
| OA | 102.9–103.6 | 2/3 | 8.0 | 0.9 | 6.0 ± 0.4 (7) | 1/3 | 1/3 | 2/3 (160–320) |
i.n., 1 ml liquid intranasal administration; AR, aerosol inhalation; OA, ocular aerosol exposure.
The specific aerosol dosing information is presented in the table in Fig. 2.
Number of ferrets with positive virus detection in nasal wash and seroconversion against homologous virus by the end of experiment/total number of ferrets tested.
Mean maximum weight loss observed among all infected ferrets through day 10 p.i.
Mean maximum rise in body temperature among all infected ferrets through day 10 p.i.
Mean maximum viral titer detected in nasal wash (NW) samples ± standard deviation. The day(s) of peak virus detection is specified in parentheses.
Number of ferrets with positive virus detection in conjunctival wash (CW) or rectal swab (RS) samples through day 5 p.i./total number of ferrets tested. The limits of virus detection were 100.8 EID50 and 101.5 EID50 for CW and RS samples, respectively.
Number of ferrets that seroconverted to homologous virus/total number of ferrets tested. The range of the hemagglutination inhibition titers is specified in parentheses.
FIG 1.
Pathogenicity and transmissibility of NY/108 virus following intranasal inoculation in ferrets and mice. (A and B) Six ferrets were inoculated i.n. with 106 EID50 of virus, and nasal wash specimens were collected from each ferret on alternate days postinoculation (p.i.) to assess viral replication (left sets of bars). A naive ferret was placed in the same cage at 24 h p.i. to assess transmission via DC (A) or in an adjacent cage with perforated sidewalls to assess transmission via RD (B), and nasal washes were collected on alternate days postcontact (p.c.) to assess virus transmission in the presence of direct contact or via respiratory droplets (right sets of bars). (C) Tissues were collected at day 3 p.i. from three ferrets that had been inoculated i.n. with 106 EID50 of virus for viral titration. Bars represent individual ferrets. NT, nasal turbinates; Tr, tracheal tissue; Lg, lung tissue; OB, olfactory bulb tissue; Bn, brain tissue (pooled anterior and posterior); Int, intestinal tissue (pooled duodenum, jejunoileal loop, and descending colon); Eye, pooled left and right eye tissue; Conj, conjunctival tissue (pooled left and right). The titers in all tissues are expressed as the mean titer per gram of tissue, with the exception of nasal turbinates and eye and conjunctival tissues, for which the titers are expressed as the mean titer per milliliter of tissue homogenate. (D) Groups of 3 mice each were inoculated i.n. with virus (serial dilution range, 106 to 102 EID50 of virus) and euthanized at day 3 or 6 p.i. for collection of nose or lung tissue for virus titration. Titers are presented as the mean titer per milliliter of tissue plus the standard deviation and are inclusive for 3 mice unless otherwise specified. The limit of virus detection was 101.5 EID50.
To assess the pathogenicity of the LPAI H7N2 virus in a second mammalian species, mice were inoculated i.n. with 106 EID50 NY/108 virus either for collection of systemic tissues p.i. or observation of morbidity and mortality. Mice inoculated with NY/108 virus exhibited mild, transient weight loss (5.8% mean maximum weight loss at day 2 p.i. [data not shown]) with efficient virus replication in nose and lung tissues day 3 p.i. (mean titers, >104 and >107.5 EID50/ml, respectively) (Fig. 1D), but the virus was not detected in the brain (data not shown). The 50% mouse infectious dose (MID50) for this virus (102.5 EID50) was comparable to that for other LPAI H7N2 viruses in this species (15).
Select North American LPAI H7 influenza viruses have demonstrated the capacity to transmit between ferrets (7, 18). To determine if NY/108 virus shared this property, we placed a naive ferret either in the same cage as an inoculated ferret (direct contact [DC] ferrets) or in a cage with modified sidewalls adjacent to the cage with an inoculated ferret (respiratory droplet [RD] ferrets) 1 day after inoculation. Contact ferrets (either DC or RD ferrets) did not shed high titers of virus in nasal wash specimens and did not seroconvert to homologous virus at the end of the experiment (Fig. 1A and B). However, the detection of low-titer virus in nasal wash samples of 3/3 DC ferrets (<103 EID50/ml) suggests the low-level spread of virus between cohoused ferrets in the absence of productive infection of the contact animal; this phenomenon has been reported previously for an North American H7 virus (18). Taken together, we found that NY/108 virus replicates robustly in two mammalian species following high-titer inoculation but does not transmit efficiently between ferrets.
Infectivity and transmissibility of NY/108 H7N2 influenza virus following aerosol inoculation of ferrets.
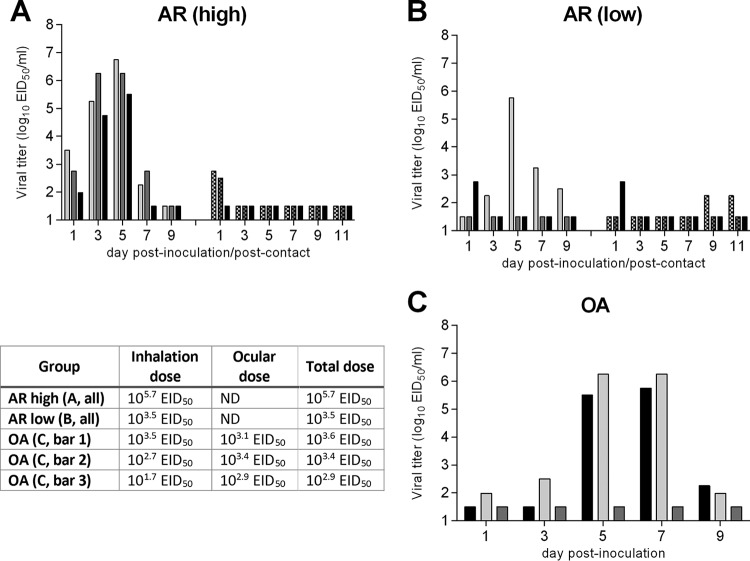
Intranasal inoculation of ferrets with a liquid inoculum does not reflect the capacity for natural virus infection by contact or the airborne route; the increasing use of aerobiology in recent years has enabled the modeling of more complex modes of virus transmission which are relevant to our understanding of the true capacity of an influenza virus to cause disease in mammals. Prior studies from our laboratory and others have found that ferrets are susceptible to infection with low doses of aerosolized influenza virus, often displaying symptoms more comparable to those occurring during natural human infection (19, 20). To determine if aerosol administration of NY/108 virus would alter its virulence in ferrets compared to that after i.n. administration, ferrets were exposed to a high dose (105.7 EID50) or a low dose (103.5 EID50) of virus by the aerosol inhalation (AR) route (Fig. 2A and B). All three ferrets that received a challenge with a high dose by the AR route became infected, but only 1/3 of the ferrets that were exposed to the lower dose shed high-titer infectious virus in nasal wash specimens (Table 1). Among infected AR-inoculated ferrets, transient weight loss and increases in body temperature were detected at levels modestly higher than those following i.n. administration. Peak viral titers in nasal wash specimens from AR-inoculated ferrets were delayed 1 to 3 days compared with the time of peak titers after i.n. inoculation and were approximately 10-fold lower than those after i.n. inoculation. Similar to the findings after i.n. inoculation, naive ferrets placed in the same cage with ferrets inoculated via the AR route did not become productively infected with virus, even though low levels (<103 EID50/ml) of infectious virus were sporadically detected in nasal washes collected from them (Fig. 2A and B, right sets of bars). A lack of seroconversion against homologous virus confirmed the absence of productive infection among all contact ferrets.
FIG 2.
Infectivity and transmissibility of NY/108 virus following aerosol inoculation in ferrets. Ferrets were inoculated by the aerosol inhalation (AR) route with a high (A) or low (B) dose of virus, and nasal washes were collected from each ferret on alternate days p.i. to assess viral replication (left sets of bars). At 24 h p.i., a naive ferret was placed in the same cage as each inoculated ferret, and nasal washes were collected on alternate days p.c. to assess virus transmission (right sets of bars). (C) Ferrets were inoculated by the ocular aerosol (OA) route, and nasal washes were collected from each ferret on alternate days p.i. to assess viral replication. Bars represent individual ferrets. The limit of virus detection was 101.5 EID50. Inoculum doses are listed in the table. ND, the ferret ocular surface was exposed to aerosolized virus during inhalation exposure, but ocular doses were not specifically determined.
In addition to the inhalation of virus-containing aerosols, our laboratory has shown previously that ferrets may become productively infected with influenza virus following an ocular-only aerosol exposure or concurrent ocular-aerosol inhalation exposure (21, 22). This work has revealed that very low levels of exposure of the ocular surface to aerosols can result in a productive respiratory infection in mammals, with heightened replication occurring when mammals are infected with aerosolized virus via both the ocular and respiratory routes. To further assess the infectivity of NY/108 virus, the ocular surface of ferrets was exposed to aerosolized virus and the ferrets concurrently inhaled increasing doses of aerosolized virus during the period of exposure (Fig. 2C). Similar to the findings for the AR-inoculated ferrets, ferrets receiving a combined dose of aerosolized virus of 103.4 or 103.6 EID50 became productively infected with NY/108 virus, achieving peak mean titers in nasal wash specimens comparable to those in AR-inoculated ferrets, whereas a ferret receiving the lowest combined dose (102.9 EID50) did not shed infectious virus in nasal wash specimens and did not seroconvert (Table 1). Collectively, this finding shows that NY/108 virus requires a higher AR infectious dose in ferrets (>103 EID50) compared with the dose of some previously examined highly transmissible seasonal influenza viruses, which can possess 50% ferret infectious doses (FID50) of ≤101.5 EID50 or ≤10 PFU following i.n. or AR inoculation, respectively (19, 23).
Environmental persistence of NY/108 virus.
Infectious influenza virus has been shown to persist in the environment for multiple days postdeposition (24), illustrating the contribution that environmental contamination may play in the spread of the virus. As in humans, sneezing and coughing by cats represent opportunities for the expulsion of respiratory secretions, which may contain virus (25, 26). To measure if NY/108 virus could maintain infectivity in ex vivo secretions, representing a potential source for the spread of the LPAI H7N2 virus in cat shelters, we assessed the ability of infectious NY/108 virus to survive in droplets over time in an environmental setting recommended for the housing of cats (20°C, 50% relative humidity) and compared it with the ability of related H7N2 viruses to survive under such conditions (27). Both NY/108 virus and a 2003 LPAI H7N2 human isolate, A/New York/107/2003 (NY/107), maintained infectivity under these conditions in this simulated environment for an average of 3 and 1.6 h, respectively (Table 2). A closely related LPAI H7N2 avian virus, A/Turkey/Virginia/4529/2002 (Tky/VA), was similarly detected for ≥2 h under these conditions. These data suggest that infectious NY/108 virus could be recovered from evaporated droplets several hours postdeposition on a hard surface, identifying one potential route of virus spread in a shelter housing environment.
TABLE 2.
Persistence of infectious LPAI H7N2 virus under cat shelter environmental conditions
| Virusa | Titerb (EID50/ml) | Virus detection at the following times postincubationc (h): |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 | 3.5 | 4 | ||
| NY/108 | 1.34 × 104 | + | + | + | + | − | − | − | − | − |
| 3.11 × 104 | + | + | + | + | + | + | + | − | − | |
| 3.11 × 104 | + | + | + | + | + | + | + | + | + | |
| NY/107 | 2.24 × 103 | + | + | − | − | − | − | − | − | − |
| 2.65 × 103 | + | + | + | + | + | + | + | − | − | |
| 1.37 × 104 | + | + | + | + | − | − | − | − | − | |
| Tky/VA | 2.65 × 104 | + | + | + | + | + | − | − | − | − |
| 6.22 × 105 | + | + | + | + | + | + | + | + | + | |
| 1.37 × 104 | + | + | + | + | + | + | + | + | + | |
For each virus, each row represents an independent experiment.
Baseline titer at time zero postincubation at 20°C and 50% relative humidity.
Virus (5 μl) was deposited in individual wells of a 96-well plate and allowed to dry. At the indicated times, 200 μl of PBS was added to each well and the contents of the wells were pipetted for ∼10 s to rehydrate and resuspend any viral material present and frozen at −80°C until titration in eggs. +, infectious virus was detected in >50% of the wells (limit of detection, 6.3 EID50/ml); −, no infections virus was detected in >50% of the wells.
Tropism of NY/108 H7N2 influenza virus in human and ferret respiratory tract cells.
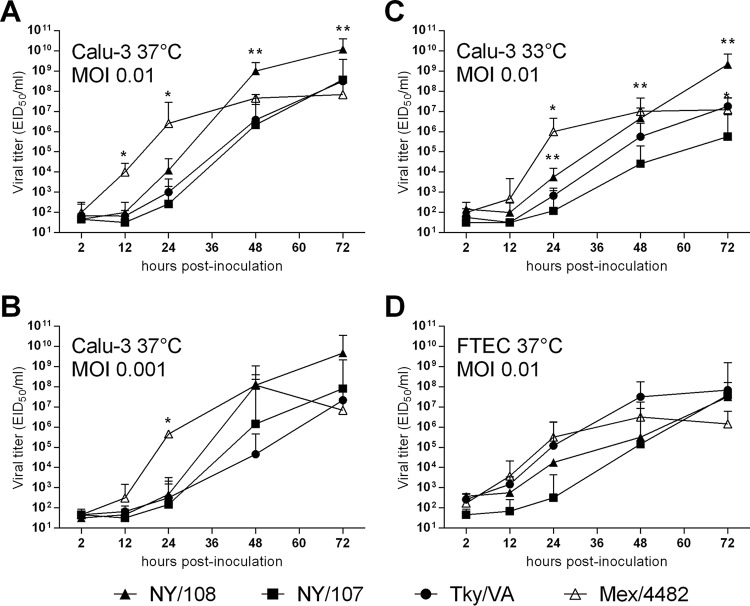
High-titer virus replication in epithelial cells of the respiratory tract is a common feature among pandemic influenza viruses and avian influenza viruses with pandemic potential (28, 29). Previous work from our laboratory identified that an LPAI H7N2 virus from 2003 associated with human infection (NY/107) exhibited a reduced replication capacity in human bronchial epithelial cells compared with that of other North American-lineage H7 viruses (30). To determine the relative replication efficiency of NY/108 virus, we infected cells of the bronchial epithelial cell line Calu-3 with the H7N2 viruses described above, in addition to the 2009 H1N1 pandemic virus A/Mexico/4482/2009 (Mex/4482). Interestingly, NY/108 virus replicated to significantly higher titers than NY/107 virus in Calu-3 cells at 48 and 72 h p.i. (P < 0.05) (Fig. 3A). Comparable results were observed when Calu-3 cells were infected with a lower dose (multiplicity of infection [MOI] = 0.001), though the differences between NY/108 and NY/107 viruses were not statistically significant (Fig. 3B). When cultured at a temperature that more closely resembles that in the upper airway in humans (33°C), NY/108 virus replicated to a significantly higher titer than NY/107 virus at 24 through 72 h p.i. (P < 0.05) (Fig. 3C). Strikingly, at an MOI of 0.01, NY/108 virus replicated to a significantly higher titer in Calu-3 cells than all other viruses tested at 72 h p.i. at either 37°C or 33°C (P < 0.05). These data indicate that NY/108 virus has an enhanced ability to replicate in a human respiratory cell line compared with that of other LPAI H7N2 viruses associated with human infection.
FIG 3.
Kinetics of H7N2 and pandemic 2009 H1N1 virus replication in human and ferret respiratory tract cells. Human bronchial epithelial (Calu-3) cells (A to C) and ferret differentiated primary tracheal epithelial cells (FTECs) (D) were grown on transwell inserts and infected apically in triplicate at a multiplicity of infection (MOI) of approximately 0.01 (103.7 EID50/well) or 0.001, as specified, with A/New York/108/16, A/New York/107/03, A/Tky/VA/4529/02 (LPAI H7N2), or A/Mexico/4482/09 (pandemic H1N1) virus. (D) NY/108 virus replicated in only 1/3 cultures, so only the results for this replicate are shown. Cells were incubated at 37°C or 33°C, as specified. Culture supernatants were collected at the indicated times p.i. for titration in eggs. The limit of virus detection was 101.5 EID50. Samples with no virus detection were assigned a value of 101.5. *, P < 0.05 between Mex/4482 virus and each of the H7N2 viruses; **, P < 0.05 between NY/108 and NY/107 viruses.
The concurrent use of human and ferret cell culture systems here provided a unique opportunity to bridge and contextualize our results from both species. Results from the ferret model showed that NY/108 virus replicated efficiently in nasal tissue, as demonstrated by the high viral titers detected in nasal washes and nasal turbinate tissue early after infection (Table 1 and Fig. 1C), with its replicative capacity in ferret lung tissue being comparable to that of other LPAI H7N2 viruses (15). However, the mean titers of NY/108 virus in tracheal tissue from infected ferrets at day 3 p.i. were >100,000-fold lower than the mean titers in the nasal turbinates. To better assess the replicative ability of NY/108 virus in the trachea, we infected cultures of ferret differentiated primary tracheal epithelial cells (FTECs) isolated from individual ferrets (31) with the panel of viruses described above. We found that NY/108 virus demonstrated productive replication in cultures of FTECs from only 1/3 ferrets, supporting in vivo data suggesting that this virus does not preferentially replicate in this tissue (Fig. 3D). While a peak viral titer of >107 EID50/ml was detected in the one infected culture by 72 h, indicating that NY/108 virus is capable of replicating to a high titer in tracheal tissue, the lack of infection in cultures of cells derived from 2/3 individual ferrets suggests that this property is not uniformly achieved, as was observed for the other H7N2 and H1N1 viruses tested in these cultures. In summary, we found that NY/108 virus replicates efficiently in human respiratory tract epithelial cells but does not demonstrate a tropism for ferret tracheal cells.
NY/108 H7N2 virus exhibited a lower pH threshold for fusion than most avian influenza H7 viruses.
It has recently been shown that adaptation of avian influenza viruses to mammals is often associated with a decrease in the HA activation threshold and that an acid-stable HA is necessary for the generation of an airborne transmissible H5 influenza virus (32–35). Like most avian influenza viruses, avian-origin H7 viruses fuse at relatively high pH values compared to those for human seasonal influenza viruses (36). We used a syncytium formation assay to measure the HA activation pH of NY/108 virus and related viruses in order to investigate whether the newly emerged NY/108 virus had acquired the trait in the HA protein to better adapt to mammals. As shown in Fig. 4, NY/108 virus was able to induce syncytia at pH values of ≤5.4, comparable to recent 2009 pandemic A(H1N1) viruses (35). In contrast, other H7 viruses, including the avian LPAI H7N2 virus Tky/VA and the human LPAI H7N9 virus A/Anhui/1/2013 (Anhui/1), had a higher pH threshold (pH ≤ 5.8) for fusion. Our results indicate that NY/108 virus possesses a relatively lower pH for HA activation than most avian influenza H7 viruses, which may be advantageous for viral replication in mammalian hosts.
FIG 4.
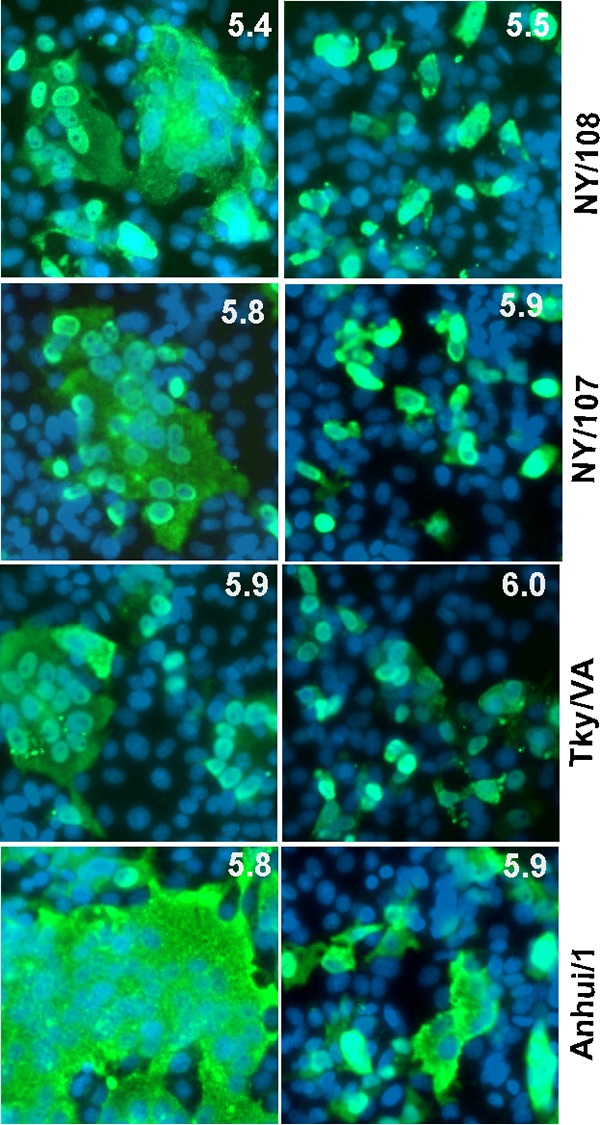
Influenza virus fusion threshold measured by syncytium formation assay. Vero cells were infected with H7 influenza viruses for 16 h before treatment with 5 μg/ml of TPCK-trypsin for 15 min, and then the cells were incubated with fusion buffer with pH values ranging from 4.8 to 7.4 at 37°C for 5 min, followed by incubation with cell culture medium containing 10% FBS for three additional hours. NP-positive cells were identified by immunofluorescence microscopy with anti-NP antibody after fixation. The syncytia among NP-positive cells at the highest pH are shown on the left, and the syncytia among NP-positive cells upon fusion induction at a pH 0.1 unit higher are shown on the right.
DISCUSSION
Influenza virus infection of felids is rare but documented. Isolation of H5N1 and H5N6 viruses from cats, leopards, lions, and tigers and subsequent experimental inoculation of these and related species illustrate the capacity for avian influenza viruses to cause severe disease and death in domesticated, wild, or captive felids (37–41). However, while serological studies suggest that avian influenza viruses do not appear to be widely prevalent in cats (42, 43), they have detected a relatively elevated seroprevalence of seasonal H1N1, H3N2, and, especially, 2009 pandemic H1N1 viruses in domestic felids (44–46). Confirmed influenza virus infection of domestic cats has occasionally resulted in fatal infection in these animals, most frequently documented with 2009 H1N1 viruses, and it is believed that human-to-cat virus transmission is responsible in at least some of these cases (10–12, 47).
Similar to H5 subtype viruses, H7 viruses are of special public health concern because of the ability of the H7 virus HA to acquire polybasic amino acids at the HA cleavage site, which can result in a highly pathogenic phenotype (48). As such, when H7 viruses cross the species barrier to mammals, it is critical to assess their genetic features, pathogenicity, transmissibility, and tropism, to best assess the relative risk posed to human health. This study found that the LPAI NY/108 H7N2 virus from 2016 exhibits low virulence in two mammalian models and does not readily transmit between cohoused ferrets. However, NY/108 virus possesses several features (including a high replication efficiency in human respiratory cells and a relatively low threshold of pH fusion) that suggest a potential for mammalian host adaptation not found in most avian LPAI H7N2 viruses. Further study is needed to identify the source of the LPAI H7N2 virus causing this 2016 outbreak in shelter cats, especially considering the decade-long absence of this virus subtype from surveillance activities in this geographic region.
The respiratory tract of domestic cats expresses both α2-3- and α2-6-linked sialic acids (SA) (49, 50), suggesting that felines are capable of serving as an intermediate host for avian influenza viruses prior to human exposure. Similar to previously studied North American LPAI H7N2 viruses, NY/108 virus possesses a deletion of the 220 loop in HA (in which the 226Gln and 228Gly receptor binding amino acids, known to interact with avian α2-3-linked sialosides, were lost) and binds to both α2-3- and α2-6-linked SA (18, 51–53; Marinova-Petkova et al., submitted). Additionally, our finding of a reduced replicative ability of NY/108 virus in ferret tracheal cells but not human bronchial epithelial cells (Fig. 3) is in agreement with the findings of a prior study showing enhanced H5N1 virus attachment to feline type II pneumocytes in the lower respiratory tract but not to tracheal tissue (54). Thus, the reduced infectivity of NY/108 virus but not NY/107 virus in cultures of FTECs (which possess a greater density of α2-6-linked than α2-3-linked SA [31]) and the reduced transmissibility of NY/108 virus compared with that of NY/107 virus among ferrets (18) suggest that differences between the HAs of these two LPAI H7N2 viruses may be present. One potential change may be an Ala135Ser substitution in NY/108 virus HA that predicts a 4th N-glycosylation site at position 133, which is not present in NY/107 virus, Tky/VA virus, or the great majority of sequenced North American avian viruses. Although the acquisition of N-linked glycosylation may not interfere with the binding affinity of NY/107 virus to glycan receptors (52), further investigation is warranted to better understand differences in SA binding which may be present between NY/108 and NY/107 viruses.
Interestingly, we found that NY/108 virus was able to fuse at a pH of ≤5.4, 0.4 pH unit lower than that required for the fusion of the phylogenetically related NY/107 and Tky/VA viruses. Both a switch in receptor binding specificity to α2-6-linked SA and a lowered HA activation pH have been found to be essential for the heightened replicative ability in upper respiratory tract tissues and the aerosol transmissibility of H5N1 mutant viruses in ferrets (34, 55, 56). A few substitutions at the HA trimer interface and stem region have been identified to be responsible for the changes in the HA fusion threshold (34, 55, 56), though further study is required to identify the precise substitution(s) in the 2016 H7N2 virus compared with the sequence of other LPAI H7N2 viruses that may be present and might contribute to the reduced HA fusion threshold for NY/108 virus observed here. In addition to differences in activation pH, the presence of 702Arg in PB2 in both human and feline H7N2 isolates, in contrast to the 702Lys present in NY/107 virus, represents an additional marker for mammalian host adaptation (57) and was found in only a subset of H7N2 avian viruses isolated from 1999 to 2002. Collectively, the combination of genetic and phenotypic changes from previous H7N2 viruses observed in NY/108 virus warrants further investigation regarding the role that these new changes may play in the receptor binding and transmission of NY/108 virus to mammals.
An outbreak of H7 subtype influenza virus infection has not been previously reported in cats. Experimental inoculation of cats with HPAI H7N7 and H5N1 viruses has shown that felids can support infection with avian influenza viruses (58, 59). Furthermore, ferrets have been shown to support the replication of influenza viruses isolated from felids (60). Ferrets represent a well-suited but not exact mammalian match to humans for the study of avian influenza viruses and the potential risk that avian influenza viruses pose to human health (37). The low virulence of NY/108 virus observed in both ferrets and mice is in accord with the mild respiratory illness reported in the infected human case and the mild respiratory illness reported among influenza virus-positive shelter cats (9). The general lack of the extrapulmonary spread of NY/108 virus in both mice and ferrets is in agreement with the clinical findings for other LPAI H7N2 viruses in these species and in cats (15, 58). Sanger sequencing of nasal wash, nasal turbinate, and tracheal and lung tissue specimens from NY/108 virus-infected ferrets did not show evidence of genetic variability in the HA, suggesting that mutations did not arise in vivo during the acute phase of infection (data not shown).
We did not detect efficient transmission of NY/108 virus between ferrets, regardless of the inoculation route employed, the inoculation dose administered, or the transmission model used (Fig. 1 and 2). Despite levels of environmental persistence comparable to those of LPAI H7N2 NY/107 virus, which has shown the capacity for transmission in a ferret direct-contact model (Fig. 4) (18), the sporadic and low-level detection of virus in NW samples from ferrets placed in direct contact with ferrets shedding virus to the environment suggests that ferrets in contact with infected ferrets were exposed to infectious virus but that the dose of NY/108 virus required for infection may be higher than what is typical for highly transmissible viruses in ferrets. The FID50 of human and avian influenza viruses can be <10 infectious particles by intranasal or aerosol inoculation (19, 23); ocular-only aerosol exposure of ferrets to <10 infectious particles of human and avian influenza viruses can result in robust infection (21). As such, our finding that ferrets exposed by the AR and concurrent ocular-aerosol inhalation routes to doses of 102.9 to 103.5 EID50 of NY/108 virus did not become uniformly infected supports the suggestion that this virus has a higher threshold of infectivity and offers a possible explanation for why transmissibility in the ferret model was not observed, even though LPAI H7N2 viruses display a high capacity for transmission between shelter cats. Previous studies with reassortant viruses in the ferret model have shown that ferret infectivity is a multifactorial trait, with the HA, NA, M, and NS genes contributing to this property (23). Further investigation regarding the FID50 differences between H7 subtype viruses, especially H7N2 subtype viruses that exhibit differential transmissibility in the ferret model, is warranted. Interestingly, the lack of detection of nasal discharge or sneezing among NY/108 virus-infected ferrets suggests that the elicitation of host responses in upper respiratory tract cells may also differ between this virus and other closely related strains (61). It is also possible that LPAI H7N2 virus spread in the shelter cat population was facilitated by nonrespiratory transmission routes, as one study found that experimentally H5N1 virus-infected cats shed virus via the digestive tract (62). While rectal swab and intestinal tissue specimens from H7N2 virus-infected ferrets were only sporadically positive for virus detection, this nonetheless indicates a potential role that contaminated bedding or litter may play in virus transmission among felines. Furthermore, while cats and ferrets have been identified to be suitable models for H5N1 viral pneumonia because of the viral attachment patterns (54), the attachment patterns of viruses transmissible between mammals in upper respiratory tract tissues, particularly the trachea and submucosal glands, differ between these two species (63). Species-specific differences between mammalian species regarding LPAI H7N2 virus attachment, the composition and production of mucus from upper respiratory tract tissues, and the SA distribution in mucus may thus contribute to the reduced infectivity of NY/108 virus observed in the ferret model.
H7 subtype influenza viruses typically display an ocular tropism in humans, yet H7N9 viruses isolated from 2013 to the present have been associated with respiratory but not ocular disease (64, 65). While a paucity of available specimens has limited the study of ocular infection with H7N2 viruses in the laboratory, there is evidence to suggest that, like H7N9 viruses, H7N2 viruses are more frequently associated with respiratory disease in humans and do not possess the ocular tropism evident among H7N3 and H7N7 viruses (66). The infrequent detection of NY/108 virus in ferret conjunctival wash, eye tissue, and conjunctival tissue specimens during the acute phase of infection is likely reflective of high viral titers in the nasopharyngeal space, and the virus detected may represent virus transferred via the lacrimal duct and not virus that productively replicated in ocular tissue. Nonetheless, the ocular surface represents a vulnerable secondary mucosal site for respiratory virus exposure (67), and personal protective equipment, including eye protection, should be employed in the event of laboratory or occupational exposure to H7N2 virus (68, 69).
It is clear that the interspecies transmission of influenza viruses represents an ongoing public health threat. With regard to companion animals, dogs have received increased attention due to the establishment and circulation of multiple canine influenza virus subtypes on several continents (70). The isolation of H3N2 canine influenza viruses from cats (71, 72) and identification of the capacity for cat-to-cat transmission of this virus subtype (73, 74) underscore that cats are a poorly studied yet relevant mammalian species when considering the role that companion animals may play in zoonotic human infection with influenza viruses. There remains a need for risk assessments of novel influenza viruses associated with human infection, regardless of the zoonotic source. Employment of multiple inoculation modes and various inoculation doses when performing these risk assessments permits a greater understanding and contextualization of the data collected, which will ultimately contribute to an enhanced applicability of the results generated in mammalian models to human health.
MATERIALS AND METHODS
Viruses.
Influenza A viruses (A/New York/108/2016 [GISAID accession numbers EPI944622 to EPI944629], A/New York/107/2003 [GenBank accession numbers EU587368 to EU587374 and EU783920], A/Turkey/Virginia/4529/2002 [GISAID accession numbers EPI993028 to EPI993035], and A/Mexico/4482/2009 [GISAID accession number EPI335032 and GenBank accession numbers GQ149675 to GQ149678, GQ379820, GQ162192, and GQ379818]) were propagated in the allantoic cavity of 10- to 11-day-old embryonated chicken eggs (Hyline) at 33.5 to 35°C for 40 to 48 h (H7 viruses) or were propagated in Madin-Darby canine kidney cells (H1 viruses) as described previously (64, 75). Pooled allantoic fluid or cell culture supernatant was clarified by centrifugation, and aliquots were stored at −80°C until use. The titers in the stocks, given as the 50% egg infectious doses (EID50), were determined using standard methods (76). All experiments were conducted under biosafety level 2 or 3 containment conditions including enhancements required by the U.S. Department of Agriculture.
Ethics statement.
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Centers for Disease Control and Prevention and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility.
Mouse experiments.
Female BALB/c mice (Jackson Laboratories), 6 weeks of age, were anesthetized by the intraperitoneal (i.p.) route with 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich) and inoculated with serial dilutions (106 to 102 EID50) of H7N2 virus by the intranasal (i.n.) route in a 50-μl volume. Five mice inoculated with 106 EID50 of virus were monitored for morbidity and mortality for 14 days postinoculation (p.i.). Replication and systemic spread of virus were determined by harvesting tissues of the nose, lung, and brain of mice (n = 3) on days 3 and 6 p.i., followed by tissue homogenization and sample titration in eggs (limit of detection, 101.5 EID50/ml); the 50% mouse infectious dose (MID50) was calculated by the method of Reed and Muench (76) as previously described (15).
Ferret experiments.
Male Fitch ferrets (Triple F Farms), 9 months of age and serologically negative by a standard hemagglutination assay for currently circulating influenza viruses, were used in this study. Ferrets were housed in a Duo-Flo Bioclean mobile environmental enclosure (Lab Products) for the duration of each experiment. For traditional i.n. inoculation experiments, ferrets were inoculated with 106 EID50 of H7N2 virus diluted in phosphate-buffered saline (PBS) to a 1-ml total volume (15). For aerosol inhalation inoculation experiments, ferrets were exposed to a high dose (105.7 EID50) or a low dose (103.5 EID50) of H7N2 virus as described previously (19). For concurrent ocular-aerosol inhalation inoculation, ferrets were exposed to a range of doses (102.9 to 103.6 EID50) of H7N2 virus as described previously (21, 22). All virus-inoculated ferrets were observed daily for clinical signs and symptoms of infection, with nasal wash (NW), conjunctival wash (CW), and rectal swab (RS) specimens being collected on alternate days p.i. to measure virus shedding as previously described (77). Three additional ferrets inoculated by the i.n. route were euthanized on day 3 p.i. for assessment of virus replication and systemic spread, as previously described (75).
Virus transmissibility was assessed by placing a naive ferret in the same cage as an inoculated ferret (to assess transmission in the presence of direct contact [DC]) or in an adjacent cage with modified sidewalls to allow air exchange in the absence of direct or indirect contact between animals (to assess transmission by respiratory droplets [RD]) as previously described (19). Serum was collected on days 14 to 18 p.i./postcontact (p.c.) to measure seroconversion against homologous virus by hemagglutinin inhibition assay (19).
Sequence analysis.
Ferret specimens collected during transmission and necropsy experiments were selected for sequencing analysis. Viral RNA was extracted using a QIAamp viral RNA minikit (Qiagen Corporation, USA), and reverse transcription-PCR was performed using an AccessQuick system (Promega, USA) along with influenza virus-specific oligonucleotides to amplify HA1 and PB2 (nucleotides 882 to 2285). Amplified PCR products were gel purified (MinElute gel extraction kit; Qiagen, USA) and sequenced using a BigDye Terminator (v3.1) cycle sequencing kit and an ABI 3130 XL analyzer (Applied Biosystems, USA). Sequences were assembled in SeqMan Pro software (DNAStar, Madison, WI), using the original sequences for comparison.
Influenza virus genome sequences were downloaded from the Influenza Research Database (http://www.fludb.org/), GenBank-NCBI (https://www.ncbi.nlm.nih.gov/GenBank/), or the Influenza Division Sequence Database for the viruses indicated above or for A/feline/New York/16-040082-1/2016 (GenBank accession numbers KY888121 to KY888128). Alignment of full-length coding sequences was performed using BioEdit (v7.1.3.0) (78) and MUSCLE (79) software. Analyses of the identities and similarities of influenza virus protein sequences were performed using the LALIGN (http://www.ch.embnet.org/software/LALIGN_form.html) and BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) programs. N-glycosylation site prediction was conducted on the NetGlyc (v1.0) server (http://www.cbs.dtu.dk/services/NetNGlyc/).
Cell culture and viral replication.
Cells of the human bronchial epithelial cell line Calu-3 (ATCC) were grown on 12-well membrane inserts and cultured as previously described (28). Ferret differentiated primary tracheal epithelial cells (FTECs) were isolated and cultured as previously described (31). Calu-3 cells were cultured under submerged conditions, and ferret primary cells were cultured under air-liquid interface (ALI) conditions. To measure replication kinetics, 103.7 EID50/well of each virus was added apically in serum-free medium (multiplicity of infection [MOI], approximately 0.01) and the mixture was incubated for 1 h before washing and culturing under submerged (Calu-3 cells) or ALI (ferret primary cells) conditions for the duration of the experiment at 37°C (all cell types) and 33°C (Calu-3 cells only). Additionally, 102.7 EID50/well was added to Calu-3 cells at 37°C for an MOI of approximately 0.001. Aliquots of culture supernatants collected at the times p.i. indicated above were immediately frozen at −80°C until titration in eggs. The statistical significance of viral replication kinetics was assessed using two-way analysis of variance with a Tukey posttest.
Environmental persistence assay.
Diluted influenza viruses (103.3 to 105.7 EID50) were added to 96-well plates (5 μl/well) without a cover, immediately placed in a MicroClimate MCBHS-1.2 environmental chamber in which the conditions were set at 20°C and 50% relative humidity (Cincinnati Sub-Zero), and allowed to dry. At the times postaddition indicated above and in Table 2, 200 μl of PBS was added to each well and the contents of the wells were pipetted for ∼10 s for rehydration and resuspension of any viral material present and immediately frozen at −80°C until titration in eggs. Assays were performed three times in triplicate for each virus per assay.
H7 virus HA fusion pH measured by virus-induced syncytium formation assay.
Following previously established methods (80), Vero cells (ATCC) were infected with various strains of influenza viruses at an MOI of 1 to 20 to achieve at least 50% infectivity (on the basis of nucleoprotein staining). At 16 h p.i., infected cells were treated with 5 μg/ml of N-p-tosyl-l-phenylalanine chloromethyl ketone (TPCK)-trypsin for 15 min at 37°C and, to induce fusion, were then incubated for 5 min at 37°C with warm fusion buffer (20 mM HEPES, 2 mM CaCl2, 150 mM NaCl, 20 mM citric acid monohydrate/sodium citrate tribasic dehydrate) at pH values ranging from 4.8 to 7.4, with the pH being increased in increments of 0.1 or 0.2 pH unit. The cells were then incubated with Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum (FBS) for 3 h before fixation with 4% paraformaldehyde and stained for anti-NP antibody (clone A1, A3 blend; EMD Millipore) for immunofluorescence microscopy. The fusion pH was defined as the highest pH value at which syncytia were observed.
ACKNOWLEDGMENTS
We thank Jaber Hossain, Adam Johnson, and Angie Foust for access to embryonated eggs and Catherine Smith for assistance with database accession numbers.
H.M.C. was supported by the Oak Ridge Institute for Science and Education.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry.
REFERENCES
- 1.Senne DA. 2007. Avian influenza in North and South America, 2002–2005. Avian Dis 51:167–173. doi: 10.1637/7621-042606R1.1. [DOI] [PubMed] [Google Scholar]
- 2.Senne D. 2007. National Veterinary Services Laboratory avian influenza and Newcastle disease activities FY 2007. National Veterinary Services Laboratory, Ames, IA. [Google Scholar]
- 3.CDC. 2004. Update: influenza activity—United States, 2003–04 season. MMWR Morb Mortal Wkly Rep 53:284–287. [PubMed] [Google Scholar]
- 4.Ostrowsky B, Huang A, Terry W, Anton D, Brunagel B, Traynor L, Abid S, Johnson G, Kacica M, Katz J, Edwards L, Lindstrom S, Klimov A, Uyeki TM. 2012. Low pathogenic avian influenza A (H7N2) virus infection in immunocompromised adult, New York, USA, 2003. Emerg Infect Dis 18:1128–1131. doi: 10.3201/eid1807.111913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. 2007. Avian influenza A/(H7N2) outbreak in the United Kingdom. Euro Surveill 12(22):pii=3206 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3206. [PubMed] [Google Scholar]
- 6.Lopez-Martinez I, Balish A, Barrera-Badillo G, Jones J, Nunez-Garcia TE, Jang Y, Aparicio-Antonio R, Azziz-Baumgartner E, Belser JA, Ramirez-Gonzalez JE, Pedersen JC, Ortiz-Alcantara J, Gonzalez-Duran E, Shu B, Emery SL, Poh MK, Reyes-Teran G, Vazquez-Perez JA, Avila-Rios S, Uyeki T, Lindstrom S, Villanueva J, Tokars J, Ruiz-Matus C, Gonzalez-Roldan JF, Schmitt B, Klimov A, Cox N, Kuri-Morales P, Davis CT, Diaz-Quinonez JA. 2013. Highly pathogenic avian influenza A(H7N3) virus in poultry workers, Mexico, 2012. Emerg Infect Dis 19:1531–1534. doi: 10.3201/eid1909.130087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Belser JA, Pulit-Penaloza JA, Zeng H, Lewis A, Shieh WJ, Tumpey TM, Maines TR. 2016. Pathogenesis and transmission assessments of two H7N8 influenza A viruses recently isolated from turkey farms in Indiana using mouse and ferret models. J Virol 90:10936–10944. doi: 10.1128/JVI.01646-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanton L, Mustaquim D, Alabi N, Kniss K, Kramer N, Budd A, Garg S, Cummings CN, Fry AM, Bresee J, Sessions W, Garten R, Xu X, Elal AI, Gubareva L, Barnes J, Wentworth DE, Burns E, Katz J, Jernigan D, Brammer L. 2017. Update: influenza activity—United States, October 2, 2017-February 4, 2017. MMWR Morb Mortal Wkly Rep 66:159–166. doi: 10.15585/mmwr.mm6606a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. 2016. Avian influenza A (H7N2) in cats in animal shelters in NY; one human infection. CDC, Atlanta, GA: https://www.cdc.gov/flu/spotlights/avian-influenza-cats.htm. Accessed 21 February 2017. [Google Scholar]
- 10.Campagnolo ER, Rankin JT, Daverio SA, Hunt EA, Lute JR, Tewari D, Acland HM, Ostrowski SR, Moll ME, Urdaneta VV, Ostroff SM. 2011. Fatal pandemic (H1N1) 2009 influenza A virus infection in a Pennsylvania domestic cat. Zoonoses Public Health 58:500–507. doi: 10.1111/j.1863-2378.2011.01390.x. [DOI] [PubMed] [Google Scholar]
- 11.Sponseller BA, Strait E, Jergens A, Trujillo J, Harmon K, Koster L, Jenkins-Moore M, Killian M, Swenson S, Bender H, Waller K, Miles K, Pearce T, Yoon KJ, Nara P. 2010. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerg Infect Dis 16:534–537. doi: 10.3201/eid1603.091737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight CG, Davies JL, Joseph T, Ondrich S, Rosa BV. 2016. Pandemic H1N1 influenza virus infection in a Canadian cat. Can Vet J 57:497–500. [PMC free article] [PubMed] [Google Scholar]
- 13.Paniker CK, Nair CM. 1970. Infection with A2 Hong Kong influenza virus in domestic cats. Bull World Health Organ 43:859–862. [PMC free article] [PubMed] [Google Scholar]
- 14.Horimoto T, Gen F, Murakami S, Iwatsuki-Horimoto K, Kato K, Hisasue M, Sakaguchi M, Nidom CA, Kawaoka Y. 2015. Cats as a potential source of emerging influenza virus infections. Virol Sin 30:221–223. doi: 10.1007/s12250-015-3580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belser JA, Lu X, Maines TR, Smith C, Li Y, Donis RO, Katz JM, Tumpey TM. 2007. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J Virol 81:11139–11147. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. 2007. Evaluation of replication and pathogenicity of avian influenza A H7 subtype viruses in a mouse model. J Virol 81:10558–10566. doi: 10.1128/JVI.00970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. 2013. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature 501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. 2008. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci U S A 105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustin KM, Belser JA, Wadford DA, Pearce MB, Katz JM, Tumpey TM, Maines TR. 2011. Influenza virus aerosol exposure and analytical system for ferrets. Proc Natl Acad Sci U S A 108:8432–8437. doi: 10.1073/pnas.1100768108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varble A, Albrecht RA, Backes S, Crumiller M, Bouvier NM, Sachs D, Garcia-Sastre A, ten Oever BR. 2014. Influenza A virus transmission bottlenecks are defined by infection route and recipient host. Cell Host Microbe 16:691–700. doi: 10.1016/j.chom.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belser JA, Gustin KM, Katz JM, Maines TR, Tumpey TM. 2014. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J Virol 88:9647–9654. doi: 10.1128/JVI.01067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belser JA, Maines TR, Creager HM, Katz JM, Tumpey TM. 2015. Oseltamivir inhibits influenza virus replication and transmission following ocular-only aerosol inoculation of ferrets. Virology 484:305–312. doi: 10.1016/j.virol.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, Ortin J, Falcon A, Nguyen TH, Mai LQ, Sedyaningsih ER, Harun S, Tumpey TM, Donis RO, Cox NJ, Subbarao K, Katz JM. 2006. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A 103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas Y, Vogel G, Wunderli W, Suter P, Witschi M, Koch D, Tapparel C, Kaiser L. 2008. Survival of influenza virus on banknotes. Appl Environ Microbiol 74:3002–3007. doi: 10.1128/AEM.00076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoh I, Shiba K, Kobayashi N, Nakajima Y, Konno A. 1998. Upper airway motor outputs during sneezing and coughing in decerebrate cats. Neurosci Res 32:131–135. doi: 10.1016/S0168-0102(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 26.Fiegel J, Clarke R, Edwards DA. 2006. Airborne infectious disease and the suppression of pulmonary bioaerosols. Drug Discov Today 11:51–57. doi: 10.1016/S1359-6446(05)03687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Research Council. 2011. Environment, housing, and management (terrestrial housing). In Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 28.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. 2007. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J Virol 81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan MC, Chan RW, Yu WC, Ho CC, Yuen KM, Fong JH, Tang LL, Lai WW, Lo AC, Chui WH, Sihoe AD, Kwong DL, Wong DS, Tsao GS, Poon LL, Guan Y, Nicholls JM, Peiris JS. 2010. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol 176:1828–1840. doi: 10.2353/ajpath.2010.091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belser JA, Zeng H, Katz JM, Tumpey TM. 2011. Infection with highly pathogenic H7 influenza viruses results in an attenuated proinflammatory cytokine and chemokine response early after infection. J Infect Dis 203:40–48. doi: 10.1093/infdis/jiq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng H, Goldsmith CS, Maines TR, Belser JA, Gustin KM, Pekosz A, Zaki SR, Katz JM, Tumpey TM. 2013. Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J Virol 87:2597–2607. doi: 10.1128/JVI.02885-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilyushina NA, Govorkova EA, Russell CJ, Hoffmann E, Webster RG. 2007. Contribution of H7 haemagglutinin to amantadine resistance and infectivity of influenza virus. J Gen Virol 88:1266–1274. doi: 10.1099/vir.0.82256-0. [DOI] [PubMed] [Google Scholar]
- 33.Zaraket H, Bridges OA, Russell CJ. 2013. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus replication and pathogenesis in mice. J Virol 87:4826–4834. doi: 10.1128/JVI.03110-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, Shinya K, Zhong G, Hanson A, Katsura H, Watanabe S, Li C, Kawakami E, Yamada S, Kiso M, Suzuki Y, Maher EA, Neumann G, Kawaoka Y. 2012. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486:420–428. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russier M, Yang G, Rehg JE, Wong SS, Mostafa HH, Fabrizio TP, Barman S, Krauss S, Webster RG, Webby RJ, Russell CJ. 2016. Molecular requirements for a pandemic influenza virus: an acid-stable hemagglutinin protein. Proc Natl Acad Sci U S A 113:1636–1641. doi: 10.1073/pnas.1524384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galloway SE, Reed ML, Russell CJ, Steinhauer DA. 2013. Influenza HA subtypes demonstrate divergent phenotypes for cleavage activation and pH of fusion: implications for host range and adaptation. PLoS Pathog 9:e1003151. doi: 10.1371/journal.ppat.1003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belser JA, Tumpey TM. 2013. H5N1 pathogenesis studies in mammalian models. Virus Res 178:168–185. doi: 10.1016/j.virusres.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Q, Wang H, Zhao L, Ma L, Wang R, Lei Y, Li Y, Yang G, Chen J, Chen G, Li L, Jin T, Li J, Liu X, Xu X, Wong G, Liu L, Liu Y, Shi W, Bi Y, Gao GF. 2016. First documented case of avian influenza (H5N1) virus infection in a lion. Emerg Microbes Infect 5:e125. doi: 10.1038/emi.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Z, Gao X, Wang T, Li Y, Li Y, Xu Y, Chu D, Sun H, Wu C, Li S, Wang H, Li Y, Xia Z, Lin W, Qian J, Chen H, Xia X, Gao Y. 2015. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Sci Rep 5:10704. doi: 10.1038/srep10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He S, Shi J, Qi X, Huang G, Chen H, Lu C. 2015. Lethal infection by a novel reassortant H5N1 avian influenza A virus in a zoo-housed tiger. Microbes Infect 17:54–61. doi: 10.1016/j.micinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Gordy JT, Jones CA, Rue J, Crawford PC, Levy JK, Stallknecht DE, Tripp RA, Tompkins SM. 2012. Surveillance of feral cats for influenza A virus in north central Florida. Influenza Other Respir Viruses 6:341–347. doi: 10.1111/j.1750-2659.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun L, Zhou P, He S, Luo Y, Jia K, Fu C, Sun Y, He H, Tu L, Ning Z, Yuan Z, Wang H, Li S, Yuan L. 2015. Sparse serological evidence of H5N1 avian influenza virus infections in domestic cats, northeastern China. Microb Pathog 82:27–30. doi: 10.1016/j.micpath.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Zhao FR, Zhou DH, Zhang YG, Shao JJ, Lin T, Li YF, Wei P, Chang HY. 2015. Detection prevalence of H5N1 avian influenza virus among stray cats in eastern China. J Med Virol 87:1436–1440. doi: 10.1002/jmv.24216. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Shen Y, Du L, Wang R, Jiang B, Sun H, Pu J, Lin D, Wang M, Liu J, Sun Y. 2015. Serological survey of canine H3N2, pandemic H1N1/09, and human seasonal H3N2 influenza viruses in cats in northern China, 2010-2014. Virol J 12:50. doi: 10.1186/s12985-015-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCullers JA, Van De Velde LA, Schultz RD, Mitchell CG, Halford CR, Boyd KL, Schultz-Cherry S. 2011. Seroprevalence of seasonal and pandemic influenza A viruses in domestic cats. Arch Virol 156:117–120. doi: 10.1007/s00705-010-0809-7. [DOI] [PubMed] [Google Scholar]
- 46.Ali A, Daniels JB, Zhang Y, Rodriguez-Palacios A, Hayes-Ozello K, Mathes L, Lee CW. 2011. Pandemic and seasonal human influenza virus infections in domestic cats: prevalence, association with respiratory disease, and seasonality patterns. J Clin Microbiol 49:4101–4105. doi: 10.1128/JCM.05415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohr CV, DeBess EE, Baker RJ, Hiett SL, Hoffman KA, Murdoch VJ, Fischer KA, Mulrooney DM, Selman RL, Hammill-Black WM. 2010. Pathology and viral antigen distribution of lethal pneumonia in domestic cats due to pandemic (H1N1) 2009 influenza A virus. Vet Pathol 47:378–386. doi: 10.1177/0300985810368393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CW, Lee YJ, Senne DA, Suarez DL. 2006. Pathogenic potential of North American H7N2 avian influenza virus: a mutagenesis study using reverse genetics. Virology 353:388–395. doi: 10.1016/j.virol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Wang H, Wu X, Cheng Y, An Y, Ning Z. 2013. Tissue distribution of human and avian type sialic acid influenza virus receptors in domestic cat. Acta Vet Hung 61:537–546. doi: 10.1556/AVet.2013.030. [DOI] [PubMed] [Google Scholar]
- 50.Thongratsakul S, Suzuki Y, Hiramatsu H, Sakpuaram T, Sirinarumitr T, Poolkhet C, Moonjit P, Yodsheewan R, Songserm T. 2010. Avian and human influenza A virus receptors in trachea and lung of animals. Asian Pac J Allergy Immunol 28:294–301. [PubMed] [Google Scholar]
- 51.Suarez DL, Garcia M, Latimer J, Senne D, Perdue M. 1999. Phylogenetic analysis of H7 avian influenza viruses isolated from the live bird markets of the Northeast United States. J Virol 73:3567–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan K, Raman R, Jayaraman A, Viswanathan K, Sasisekharan R. 2013. Quantitative description of glycan-receptor binding of influenza A virus H7 hemagglutinin. PLoS One 8:e49597. doi: 10.1371/journal.pone.0049597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, Chen LM, Carney PJ, Donis RO, Stevens J. 2010. Structures of receptor complexes of a North American H7N2 influenza hemagglutinin with a loop deletion in the receptor binding site. PLoS Pathog 6:e1001081. doi: 10.1371/journal.ppat.1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2006. H5N1 virus attachment to lower respiratory tract. Science 312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 55.Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, Rimmelzwaan GF, Osterhaus AD, Fouchier RA. 2012. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336:1534–1541. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shelton H, Roberts KL, Molesti E, Temperton N, Barclay WS. 2013. Mutations in haemagglutinin that affect receptor binding and pH stability increase replication of a PR8 influenza virus with H5 HA in the upper respiratory tract of ferrets and may contribute to transmissibility. J Gen Virol 94:1220–1229. doi: 10.1099/vir.0.050526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taft AS, Ozawa M, Fitch A, Depasse JV, Halfmann PJ, Hill-Batorski L, Hatta M, Friedrich TC, Lopes TJ, Maher EA, Ghedin E, Macken CA, Neumann G, Kawaoka Y. 2015. Identification of mammalian-adapting mutations in the polymerase complex of an avian H5N1 influenza virus. Nat Commun 6:7491. doi: 10.1038/ncomms8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Riel D, Rimmelzwaan GF, van Amerongen G, Osterhaus AD, Kuiken T. 2010. Highly pathogenic avian influenza virus H7N7 isolated from a fatal human case causes respiratory disease in cats but does not spread systemically. Am J Pathol 177:2185–2190. doi: 10.2353/ajpath.2010.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiry E, Zicola A, Addie D, Egberink H, Hartmann K, Lutz H, Poulet H, Horzinek MC. 2007. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet Microbiol 122:25–31. doi: 10.1016/j.vetmic.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Crossley B, Hietala S, Hunt T, Benjamin G, Martinez M, Darnell D, Rubrum A, Webby R. 2012. Pandemic (H1N1) 2009 in captive cheetah. Emerg Infect Dis 18:315–317. doi: 10.3201/eid1802.111245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maines TR, Belser JA, Gustin KM, van Hoeven N, Zeng H, Svitek N, von Messling V, Katz JM, Tumpey TM. 2012. Local innate immune responses and influenza virus transmission and virulence in ferrets. J Infect Dis 205:474–485. doi: 10.1093/infdis/jir768. [DOI] [PubMed] [Google Scholar]
- 62.Rimmelzwaan GF, van Riel D, Baars M, Bestebroer TM, van Amerongen G, Fouchier RA, Osterhaus AD, Kuiken T. 2006. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am J Pathol 168:176–183. doi: 10.2353/ajpath.2006.050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am J Pathol 171:1215–1223. doi: 10.2353/ajpath.2007.070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. 2009. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science 325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ. 2013. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med 368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- 66.Belser JA, Sun X, Creager HM, Johnson A, Ridenour C, Chen LM, Tumpey TM, Maines TR. 2017. Role of H7 hemagglutinin in murine infectivity of influenza viruses following ocular inoculation. Virology 502:13–19. doi: 10.1016/j.virol.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belser JA, Rota PA, Tumpey TM. 2013. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev 77:144–156. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.CDC. 2006. Interim guidance for protection of persons involved in U.S. avian influenza outbreak disease control and eradication activities. CDC, U.S. Department of Health and Human Services, Atlanta, GA: http://www.cdc.gov/flu/avian/professional/protect-guid.htm. [Google Scholar]
- 69.CDC. 2010. Guidelines and recommendations: prevention strategies for seasonal influenza in healthcare settings. CDC, Atlanta, GA: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm. [Google Scholar]
- 70.Gibbs EP, Anderson TC. 2010. Equine and canine influenza: a review of current events. Anim Health Res Rev 11:43–51. doi: 10.1017/S1466252310000046. [DOI] [PubMed] [Google Scholar]
- 71.Song DS, An DJ, Moon HJ, Yeom MJ, Jeong HY, Jeong WS, Park SJ, Kim HK, Han SY, Oh JS, Park BK, Kim JK, Poo H, Webster RG, Jung K, Kang BK. 2011. Interspecies transmission of the canine influenza H3N2 virus to domestic cats in South Korea, 2010. J Gen Virol 92:2350–2355. doi: 10.1099/vir.0.033522-0. [DOI] [PubMed] [Google Scholar]
- 72.Jeoung HY, Lim SI, Shin BH, Lim JA, Song JY, Song DS, Kang BK, Moon HJ, An DJ. 2013. A novel canine influenza H3N2 virus isolated from cats in an animal shelter. Vet Microbiol 165:281–286. doi: 10.1016/j.vetmic.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 73.Kim H, Song D, Moon H, Yeom M, Park S, Hong M, Na W, Webby RJ, Webster RG, Park B, Kim JK, Kang B. 2013. Inter- and intraspecies transmission of canine influenza virus (H3N2) in dogs, cats, and ferrets. Influenza Other Respir Viruses 7:265–270. doi: 10.1111/j.1750-2659.2012.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei N, Yuan ZG, Huang SF, Zhang DW, Zhang AG, Huang BH, Zhang GH, Li SJ. 2012. Transmission of avian-origin canine influenza viruses A (H3N2) in cats. Vet Microbiol 160:481–483. doi: 10.1016/j.vetmic.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 75.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. 2005. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol 79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reed LJ, Muench HA. 1938. A simple method of estimating fifty per cent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 77.Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. 2012. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog 8:e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98. [Google Scholar]
- 79.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reed ML, Bridges OA, Seiler P, Kim JK, Yen HL, Salomon R, Govorkova EA, Webster RG, Russell CJ. 2010. The pH of activation of the hemagglutinin protein regulates H5N1 influenza virus pathogenicity and transmissibility in ducks. J Virol 84:1527–1535. doi: 10.1128/JVI.02069-09. [DOI] [PMC free article] [PubMed] [Google Scholar]