ABSTRACT
Autophagy is closely associated with the regulation of hepatitis B virus (HBV) replication. HBV X protein (HBx), a multifunctional regulator in HBV-associated biological processes, has been demonstrated to be crucial for autophagy induction by HBV. However, the molecular mechanisms of autophagy induction by HBx, especially the signaling pathways involved, remain elusive. In the present investigation, we demonstrated that HBx induced autophagosome formation independently of the class I phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway. In contrast, the class III PI3K(VPS34)/beclin-1 pathway was revealed to be critical for HBx-induced autophagosome formation. Further study showed that HBx did not affect the level of VPS34 and beclin-1 expression but inhibited beclin-1/Bcl-2 association, and c-Jun NH2-terminal kinase (JNK) signaling was found to be important for this process. Moreover, it was found that HBx treatment led to the generation of reactive oxygen species (ROS), and inhibition of ROS activity abrogated both JNK activation and autophagosome formation. Of importance, ROS-JNK signaling was also revealed to play an important role in HBV-induced autophagosome formation and subsequent HBV replication. These data may provide deeper insight into the mechanisms of autophagy induction by HBx and help in the design of new therapeutic strategies against HBV infection.
IMPORTANCE HBx plays a key role in diverse HBV-associated biological processes, including autophagy induction. However, the molecular mechanisms of autophagy induction by HBx, especially the signaling pathways involved, remain elusive. In the present investigation, we found that HBx induced autophagy independently of the class I PI3K/AKT/mTOR signaling pathway, while the class III PI3K(VPS34)/beclin-1 pathway was revealed to be crucial for this process. Further data showed that ROS-JNK activation by HBx resulted in the release of beclin-1 from its association with Bcl-2 to form a complex with VPS34, thus enhancing autophagosome formation. Of importance, ROS-JNK signaling was also demonstrated to be critical for HBV replication via regulation of autophagy induction. These data help to elucidate the molecular mechanisms of autophagy induction by HBx/HBV and might be useful for designing novel therapeutic approaches to HBV infection.
KEYWORDS: HBV X protein, autophagy, signaling pathway, viral replication
INTRODUCTION
Hepatitis B virus (HBV) is an important human pathogen that can cause a wide spectrum of liver diseases, including hepatitis, liver cirrhosis, and hepatocellular carcinoma (1, 2). The HBV genome is about 3.2 kb in length, carrying four genes named the C, S, X, and P genes. The X protein encoded by the X gene has the ability to activate the transcription of HBV regulatory elements, as well as a variety of cellular and viral genes (3–5). Currently, anti-HBV drugs including interferon alpha and nucleoside analogs are either fraught with adverse effects or only effective in short-term therapy. Biological elucidation of the intricate interactions between HBx and host factors may facilitate the development of new therapeutic strategies against HBV infection (6–10). Autophagy is emerging as an important host factor in the regulation of infections with diverse pathogens (11, 12), and autophagy modulation has been highlighted as a potential therapeutic strategy against a variety of infectious diseases in recent years (13, 14).
Autophagy is a self-degradative process that is important for maintaining cellular homeostasis (15, 16). The process of autophagy is regulated by diverse signaling pathways, such as class I and III phosphatidylinositol 3-kinase (PI3K) pathways and mitogen-activated protein kinase (MAPK) pathways (16). The class I PI3K/AKT/mTOR pathway plays an important role in the phosphorylation and suppression of ULK1 (16), while the class III PI3K(VPS34)/beclin-1 pathway is essential for initiation of autophagy (17), in which Bcl-2 has been reported to play a negative role via binding to beclin-1 (18, 19). The MAPKs, including extracellular signal-regulated kinase (ERK), p38, and c-Jun NH2-terminal kinase (JNK), have also been reported to be implicated in autophagy regulation in recent investigations (20–22). Accumulating evidence indicates that autophagy is involved in various pathological processes, including cancer and metabolic and neurodegenerative disorders (23, 24). Recent studies have shown that autophagy is an important host factor in the regulation of the replication of diverse viruses, such as dengue virus, poliovirus, influenza virus A, and coxsackievirus B3 virus, as well as HBV (25–32). Evidence indicates that HBV can activate autophagosome formation, which is required for its own replication (28–32). Furthermore, HBx appears to play an important role in HBV-induced autophagosome formation (28, 33–36). HBx may contribute to the induction of autophagy in hepatocytes by enhancing the activity of VPS34 (28) or by upregulating beclin-1 expression (33, 34). It has also been reported that HBx can suppress autophagic degradation by impairing lysosomal maturation (35). However, the signaling pathways involved in HBx-induced autophagosome formation and its cellular consequences remain unclear.
In this study, we found that HBx induced autophagosome formation independently of the class I PI3K/AKT/mTOR pathway; however, the class III PI3K (VPS34)/beclin-1 complex was demonstrated to be crucial for HBx-induced autophagy. HBx did not significantly affect the level of VPS34 and beclin-1 expression but inhibited beclin-1/Bcl-2 association significantly. Further study showed that JNK activation is critical for HBx-induced autophagy through regulation of the beclin-1/Bcl-2 interaction and that reactive oxygen species (ROS) signaling is crucial for HBx-induced JNK activation and autophagosome formation. Finally, it was found that ROS-JNK signaling plays an important role in HBV-induced autophagosome formation and the ensuing viral replication.
RESULTS
HBx induces autophagosome formation independently of class I PI3K/AKT/mTOR signaling.
To investigate the effect of HBx on autophagy induction, we transfected HBx-expressing plasmid pHBx into HepG2 hepatoma cells. At 48 h posttransfection, the accumulation of LC3-II, the hallmark of autophagy formation, was examined by Western blot analysis. It was found that HBx overexpression markedly upregulated LC3-II levels (Fig. 1A). We also determined the effect of HBx treatment on the p62 protein level, a widely used indicator of autophagic flux (11, 15). As shown in Fig. 1A, HBx treatment did not have a significant effect on p62 expression, indicating that HBx induced incomplete autophagy. It is well established that the mTOR pathway plays an important role in autophagy induction by negatively regulating the phosphorylation of some important autophagy-related (ATG) proteins (15, 16). We thus investigated whether HBx induces autophagy formation via mTOR inhibition. Surprisingly, our results showed that HBx activated, rather than suppressed, the mTOR signaling pathway, as evidenced by the increased level of phosphorylated mTOR and its substrates p70S6K and 4EBP1 (Fig. 1B). As the activation of mTOR is often under the control of class I PI3K-AKT signaling (37, 38), we further investigated the effect of HBx on AKT phosphorylation. As expected, HBx enhanced AKT phosphorylation significantly (Fig. 1C). Furthermore, we treated HBx-transfected cells with rapamycin or an AKT inhibitor (AKTi). It was found that treatment with rapamycin inhibited the phosphorylation of p70s6K and that AKTi treatment suppressed the phosphorylation of AKT and p70S6k efficiently in HBx-transfected cells; however, neither of them had a significant effect on LC3-II accumulation triggered by HBx transfection (Fig. 1D). Together, these data indicate that HBx induces autophagosome formation independently of the class I PI3K/AKT/mTOR signaling pathway.
FIG 1.
HBx induces autophagosome formation independently of the PI3K/AKT/mTOR signaling pathway. (A) HepG2 cells were transfected with HBx-expressing plasmid pHBx or the control empty vector for 48 h, and LC3 and p62 expression levels were examined by Western blot analysis. GAPDH was used as a loading control. (Left panel) The immunoblots from three independent experiments were scanned and subjected to densitometric analysis. Levels of LC3-II and p62 relative to the level of GAPDH were normalized to the level of empty-vector-transfected cells, which was set at 1.0 (n = 5). *, P < 0.05. (B) HepG2 cells were treated as described for panel A. Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-mTOR, p-p70s6k, and p-4EBP1 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (C) HepG2 cells were treated as described for panel A. Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-AKT relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (D) pHBx-transfected HepG2 cells were treated with rapamycin (20 nM) or AKTi (1 μM) for 16 h. Cells were then collected and subjected to Western blot analysis with the antibodies indicated. (Left panel) Levels of LC3-II, p-p70s6k, and p-AKT relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 3). *, P < 0.05.
The class III PI3K (VPS34)/beclin-1 complex plays a pivotal role in HBx-induced autophagy.
Class III PI3K, also named VPS34, plays an important role in autophagy induction by interacting with beclin-1 (17, 18). As the data described above showed that the class I PI3K/AKT/mTOR pathway might not be involved in HBx-induced autophagy, we further investigated the role of the VPS34/beclin-1 complex in HBx-induced autophagy. It was found that inhibition of class III PI3K activity by 3-methyladenine (3-MA) significantly suppressed HBx-induced autophagosome formation (Fig. 2A). Further, we downregulated the level of VPS34 or beclin-1 expression by the siRNA technique and then tested the effect of HBx on autophagosome formation. The results showed that downregulation of VPS34 or beclin-1 significantly inhibited HBx-induced autophagy (Fig. 2B and C). Taken together, these data indicated that HBx induced autophagosome formation via a VPS34/beclin-1 complex-dependent pathway.
FIG 2.
The beclin-1/VPS34 complex is critical for HBx-induced autophagosome formation. (A) pHBx-transfected HepG2 cells were treated with 3-MA (5 mM) for 16 h or left untreated. Cells were then collected and subjected to Western blot analysis with the antibodies indicated. (Lower panel) Levels of LC3II relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 5). *, P < 0.05. (B) HepG2 cells were transfected with control, beclin-1, or VPS34 siRNA. At 72 h posttransfection, Western blot analysis was performed with antibodies against beclin-1, VPS34, or GAPDH. (C) HepG2 cells were transfected with control, beclin-1, or VPS34 siRNA. At 48 h after siRNA transfection, cells were transfected with pHBx for 48 h. Western blot analysis was performed with the antibodies indicated. (Lower panel) Levels of LC3II relative to the level of GAPDH were examined by densitometric analysis, and the value from control empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05.
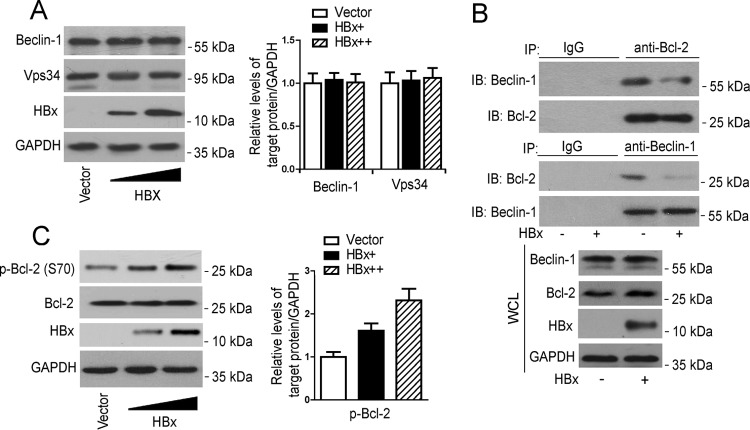
HBx does not have a significant effect on the expression of VPS34 or beclin-1 but enhances the disassociation of beclin-1 from Bcl-2.
Given the importance of the VPS34/beclin-1 complex in HBx-induced autophagosome formation, we examined the effect of HBx on the expression of VPS34 and beclin-1. The results showed that HBx overexpression did not have a significant effect on the level of VPS34 and beclin-1 expression in HepG2 cells (Fig. 3A), suggesting that HBx-induced autophagosome formation via upregulation of the level of VPS34 or beclin-1 expression was unlikely. It has been reported that Bcl-2 functions as a negative regulator of VPS34/beclin-1 complex-dependent autophagy through binding to beclin-1 (18, 19). We thus investigated the effect of HBx on the interaction between beclin-1 and Bcl-2. Data from an immunoprecipitation assay showed that HBx overexpression significantly attenuated the interaction between beclin-1 and Bcl-2 whether the precipitating antibody was an anti-Bcl-2 or an anti-beclin-1 antibody (Fig. 3B). As the phosphorylated modification of Bcl-2 has been suggested to be crucial for its interaction with beclin-1 (39, 40), we further examined the effect of HBx on the phosphorylation of Bcl-2. As shown in Fig. 3C, HBx treatment could dose dependently upregulate the phosphorylation of Bcl-2.
FIG 3.
HBx suppresses the association of beclin-1 with Bcl-2. (A) HepG2 cells were transfected with increasing doses of pHBx. At 48 h posttransfection, Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of beclin-1 and VPS34 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (B) HepG2 cells were transfected with pHBx or the empty vector. At 48 h posttransfection, cell lysates were subjected to immunoprecipitation with anti-Bcl-2 or anti-beclin-1 antibody, followed by immunoblotting (IB) with anti-beclin-1 or anti-Bcl-2 antibody. (C) HepG2 cells were treated as described for panel A, Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-Bcl2 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. WCL, whole-cell lysate.
The JNK signaling pathway is required for HBx-induced autophagosome formation.
Besides the class I PI3K/AKT/mTOR and class III PI3K/beclin-1 signaling pathways, MAPKs (ERK, p38, and JNK) may also play an important role in autophagy induction (21), while HBx, as a multifunctional protein, is well known for its role in the regulation of diverse signaling pathways (41, 42). We therefore further examined the contribution of MAPKs to HBx-induced autophagosome formation. Consistent with previous investigations (43–45), HBx transfection led to the activation of ERK, p38, and JNK signaling in HepG2 cells (Fig. 4A), which could be significantly suppressed by the ERK-specific inhibitor U0126, the p38-specific inhibitor SB202190, and the JNK-specific inhibitor SP600125, respectively (Fig. 4B). However, of the three MAPK inhibitors, only SP600125 could effectively abrogate HBx-induced LC3-II accumulation (Fig. 4C). To further explore the importance of JNK in the modulation of autophagosome formation induced by HBx, we downregulated JNK expression in HepG2 cells by the small interfering RNA (siRNA) technique and then tested the effect of HBx on autophagosome formation. Consistent with the data obtained with the pharmacological inhibitor, siRNA-mediated JNK downregulation significantly decreased HBx-induced autophagosome formation (Fig. 4D and E). Collectively, these data indicate that HBx-induced autophagy involves JNK activity.
FIG 4.
The JNK signaling pathway is crucial for HBx-induced autophagosome formation. (A) HepG2 cells were transfected with increasing doses of pHBx. At 24 h posttransfection, Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-ERK, p-p38, and p-JNK relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). (B) HepG2 cells were pretreated with the ERK inhibitor U0126 (10 μM), the p38 inhibitor SB202190 (10 μM), or the JNK inhibitor SP600125 (10 μM) for 30 min and then transfected with pHBx for 48 h. Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-ERK, p-p38, and p-JNK relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 5). *, P < 0.05. (C) Cells were treated as described for panel B. Western blot analysis was performed with the antibodies indicated. (Lower panel) Levels of LC3-II relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (D) HepG2 cells were transfected with control or JNK siRNA. At 72 h posttransfection, Western blot analysis was performed to determine the level of JNK expression. GAPDH was used as a loading control. (E) HepG2 cells were transfected with control (Contr) or JNK siRNA. At 48 h after siRNA transfection, cells were transfected with pHBx for an additional 48 h and Western blot analysis was then performed with the antibodies indicated. (Lower panel) Levels of LC3II relative to the level of GAPDH were examined by densitometric analysis, and the value from control empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05.
JNK signaling contributes to HBx-mediated Bcl-2 phosphorylation and disassociation of beclin-1 from Bcl-2.
The data described above showed that HBx overexpression significantly led to the disassociation of beclin-1 from Bcl-2, which might involve the phosphorylation of Bcl-2. We thus further investigated the roles of JNK signaling in Bcl-2 phosphorylation and the beclin-1/Bcl-2 interaction. Our data showed that treatment with the JNK inhibitor SP600125 indeed decreased HBx-mediated Bcl-2 phosphorylation (Fig. 5A) and attenuated the HBx-mediated disassociation of beclin-1 from Bcl-2 (Fig. 5B). The data from an RNA interference assay also revealed that downregulation of JNK expression significantly suppressed HBx-mediated Bcl-2 phosphorylation (Fig. 5C) and the beclin-1/Bcl-2 interaction (Fig. 5D), further suggesting that JNK signaling contributes to HBx-mediated Bcl-2 phosphorylation and the disassociation of beclin-1 from Bcl-2.
FIG 5.
JNK signaling is important for HBx-mediated Bcl-2 phosphorylation and disassociation of beclin-1 from Bcl-2. (A) HepG2 cells were pretreated with the JNK inhibitor SP600125 (10 μM) for 30 min and then transfected with pHBx. At 48 h posttransfection, Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-Bcl-2 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 5). *, P < 0.05. (B) HepG2 cells were treated as described for panel A, and cell lysates were subjected to immunoprecipitation (IP) with anti-Bcl-2 or beclin-1 antibody, followed by Western blot (immunoblot [IB]) analysis with anti-beclin-1 or anti-Bcl-2 antibody. (C) HepG2 cells were transfected with control or JNK siRNA. At 48 h after siRNA transfection, cells were transfected with pHBx for 48 h and Western blot analysis was then performed with the antibodies indicated. (Left panel) Levels of p-Bcl-2 relative to the level of GAPDH were examined by densitometric analysis, and the value from control empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (D) HepG2 cells were treated as described for panel C, and cell lysates were subjected to immunoprecipitation with anti-Bcl-2 or anti-beclin-1 antibody, followed by Western blot analysis with anti-beclin-1 or anti-Bcl-2 antibody. WCL, whole-cell lysate.
ROS is crucial for HBx-induced JNK activation and autophagosome formation.
Recent work has revealed that ROS may play an important role in JNK-dependent autophagy regulation (46–49), while HBx treatment has been reported to cause ROS generation in hepatoma cells (50, 51). We thus investigated the role of ROS in HBx-induced JNK activation and the ensuing autophagosome formation. As shown in Fig. 6A, HBx transfection led to upregulation of ROS in HepG2 cells, which could be significantly suppressed by the ROS scavenger N-acetylcysteine (NAC). Of importance, it was revealed that inhibition of ROS activity by NAC significantly downregulated JNK phosphorylation in HBx-transfected cells (Fig. 6B), indicating that JNK activation by HBx was dependent on ROS generation. Further study showed that NAC treatment, as expected, abrogated HBx-mediated disruption of the beclin-1/Bcl-2 complex (Fig. 6C) and the subsequent autophagosome formation induced by HBx (Fig. 6D).
FIG 6.
ROS is crucial for HBx-induced JNK activation and autophagosome formation. (A) HepG2 cells were transfected with pHBx or the control vector. At 48 h posttransfection, cells were treated with the ROS inhibitor NAC (10 mM) for 24 h or left untreated and ROS activity was determined by FACS analysis. (B) HepG2 cells were treated as described for panel A, and Western blot (immunoblot [IB]) analysis was performed with the antibodies indicated. (Left panel) Levels of p-JNK and p-Bcl-2 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (C) HepG2 cells were treated as described for panel A, and cells were subjected to immunoprecipitation (IP) with anti-Bcl-2 or anti-beclin-1 antibody, followed by Western blot analysis with anti-beclin-1 or anti-Bcl-2 antibody. (D) HepG2 cells were treated as described for panel A, and Western blot analysis was performed with the antibodies indicated. (Lower panel) Levels of LC3II relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 5). *, P < 0.05. WCL, whole-cell lysate.
ROS-JNK signaling is important for HBV-induced autophagosome formation and its ensuing replication.
Several investigations have demonstrated that HBV induces incomplete autophagy, which is critical for its own replication (28–32). As the data described above revealed that HBx induces autophagosome formation via the ROS-JNK signaling pathway, we further investigated the role of this signaling pathway in HBV-induced autophagosome formation and HBV replication. It was found that transfection with HBV replication-competent plasmid pHBV1.3, but not with pHBV1.3 with HBx-minus (an HBV genome mutant incompetent to express HBx protein), significantly upregulated the ROS level, and inhibition of ROS generation by NAC significantly decreased the phosphorylation of JNK and Bcl-2 induced by HBV (Fig. 7A and B), further supporting the crucial role of HBx in the activation of the ROS-JNK signaling pathway. Of note, it was found that inhibition of ROS-JNK signaling significantly attenuated LC3-II accumulation but had little effect on the p62 level (Fig. 7C), indicating that ROS-JNK signaling contributed to HBV-induced incomplete autophagy. Furthermore, we evaluated the role of ROS-JNK signaling in HBV gene expression and replication. Quantitative PCR results showed that inhibition of the ROS-JNK pathway downregulated the HBV DNA level in a dose-dependent manner (Fig. 7D) but had no significant effect on the HBV RNA level (Fig. 7E). Further Southern blot analysis confirmed the role of this signaling pathway in the regulation of HBV replication (Fig. 7F). We also investigated the role of ROS-JNK signaling in HBV-induced autophagy and HBV replication in an infection system. As shown in Fig. 7G, HBV infection induced the accumulation of LC3-II but had no significant effect on the p62 protein level in primary human hepatocytes (PHHs), indicating that HBV infection might also induce incomplete autophagy. Inhibition of ROS-JNK signaling significantly decreased the LC3-II level (Fig. 7G) and downregulated the HBV DNA level in HBV-infected PHHs (Fig. 7H), suggesting that this pathway plays a role in natural HBV infection.
FIG 7.
Inhibition of ROS-JNK signaling decreases HBV-induced autophagosome formation and its following replication. (A) HepG2 cells were transfected with pHBV1.3, pHBV1.3 with HBx-minus, or the control vector. At 48 h posttransfection, cells were treated with NAC (10 mM) for 24 h or left untreated and ROS activity was determined by FACS analysis. (B) HepG2 cells were transfected with pHBV1.3, pHBV1.3 with HBx-minus, or the control vector as in panel A. Cells were then treated with NAC (10 mM) or SP600125 (10 μM) or left untreated for 24 h, and Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of p-JNK and p-Bcl-2 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (C) HepG2 cells were treated as described for panel B, and Western blot analysis was performed with the antibodies indicated. (Left panel) Levels of LC3-II and p62 relative to the level of GAPDH were examined by densitometric analysis, and the value from empty-vector-transfected cells was set at 1.0 (n = 4). *, P < 0.05. (D) HepG2 cells were transfected with pHBV1.3 or the control vector. At 48 h posttransfection, transfected cells were further treated with increasing doses of NAC or SP600125 for another 24 h or left untreated. Quantitative PCR was then performed to determine the HBV DNA level (n = 5). N.D., not detected. (E) HepG2 cells were treated as described for panel D. qRT-PCR analysis for HBV RNAs and preC-pgRNA was performed in the presence or absence of reverse transcriptase (RT) (n = 4). N.D., not detected. (F) pHBV1.3-transfected HepG2 cells were treated with NAC or SP600125 for 24 h, and HBV-DNA was extracted and subjected to Southern blot analysis. (G) PHHs were infected with HBV for 16 h or not infected. At day 7 posttransfection, cells were treated with NAC (10 mM) or SP600125 (10 μM) for another 3 days. Cells were then subjected to Western blot analysis with the antibodies indicated. (Left panel) Levels of LC3-II, p62, and p-JNK relative to the level of GAPDH were examined by densitometric analysis, and the value from cells without HBV infection was set at 1.0 (n = 4). *, P < 0.05. (H) PHHs were treated as described for panel G, and quantitative PCR was then performed to determine the HBV DNA level (n = 4). N.D., not detected. *, P < 0.05.
DISCUSSION
HBV infection remains a global health problem; however, there is still no effective measure for the cure of HBV infection. Manipulation of host autophagy has been proposed as a promising therapeutic strategy against diverse viruses, including HBV (13, 14, 31). It is now evident that HBx plays a crucial role in HBV-induced autophagy (28, 33–36); however, the underlying mechanisms, especially the signaling pathways involved, remain elusive.
The mTOR signaling pathway is often regarded as a brake for autophagy induction (15, 16). To our surprise, it was found that although HBx efficiently induced autophagosome formation, it activated, rather than suppressed, mTOR activation. We also examined the activation of class I PI3K/AKT signaling, which acts upstream of mTOR, and found that HBx indeed enhanced AKT activation. Of note, treatment with an mTOR inhibitor (RAPA) or a specific AKTi did not have a significant effect on the increase in LC3-II triggered by HBx, further suggesting that HBx induces autophagosome formation independently of the class I PI3K/AKT/mTOR signaling pathway. Wang et al. (36) showed that HBx activates autophagosome formation; their data also showed that HBx activates, rather than suppresses, the PI3K-Akt-mTOR pathway, which is similar to our investigation. However, we cannot agree with their conclusion that HBx activates autophagosome formation through the PI3K-Akt-mTOR pathway. Some agents, including viruses, can indeed activate autophagosome formation by regulating mTOR signaling; however, they suppress, rather than activate, the mTOR signaling pathway. For example, it has been reported that human papillomavirus 16, chikungunya virus, or avian influenza A virus induces autophagy by downregulating mTOR activation (52–54). Besides Wang et al. and us, several research groups have also reported that HBx can activate the class I PI3K/AKT/mTOR signaling pathway, which may help HBV-infected cells to survive (55, 56).
The class III PI3K(VPS34)/beclin-1 complex is well known for its role in autophagy induction (16, 17), although there are also reports of class III PI3K-independent autophagosome formation (57, 58). By downregulating the level of VPS34 or beclin-1 expression by the siRNA technique, we demonstrated that the VPS34/beclin-1 complex is required for HBx-induced autophagosome formation. It has been reported that HBx treatment may increase the level of beclin-1 expression, sensitizing cells to starvation-induced autophagy (33); however, our data show that HBx appears not to affect the level of beclin-1 expression significantly. Consistent with our results, Sir et al. (28) have also reported that neither HBx nor HBV has a significant effect on beclin-1 induction. Beclin-1 contains a Bcl-2-binding domain, and Bcl-2 has been reported to negatively regulate autophagosome formation through binding to beclin-1 (17, 18). We therefore investigated the effect of HBx on the interaction between Bcl-2 and beclin-1. It was found that HBx transfection indeed inhibited the association of beclin-1 with Bcl-2. Further, we found that HBx expression led to the phosphorylation of Bcl-2, which might contribute to its disassociation from beclin-1.
Besides the class I and III PI3K signaling pathways, MAPK (ERK1/2, p38, and JNK) pathways have also been reported to play important roles in autophagy regulation (20, 21, 39). As a multifunctional protein, HBx is well known for its role in the activation of diverse signaling pathways, and MAPK signaling is especially involved in diverse HBx-mediated biological processes (59). We found that HBx significantly activates MAPK signaling in hepatoma cells, consistent with previous investigations (44, 45, 60). However, JNK, but not ERK1/2 and p38, was revealed to be involved in HBx-induced autophagosome formation. Further data from either pharmacological inhibition or RNA interference studies revealed that inhibition of JNK activity could lead to the inhibition of HBx-induced Bcl-2 phosphorylation and the disassociation of beclin-1 from Bcl-2, thereby enhancing autophagosome formation. Zhang et al. (34) reported that HBx induces autophagy and also activates JNK signaling, which is consistent with our data. However, their data showed that downregulation of JNK expression by the siRNA technique in Chang cells did not affect HBx-induced autophagosome formation significantly. The reason for this discrepancy is unclear, but it may be due to the different cell lines or experimental conditions used. Recent evidence highlights the role of ROS in JNK-activated autophagosome formation (46, 47), and ROS has also been reported to be closely associated with HBx- and HBV-mediated biological processes (50, 51, 61). We therefore further investigated the role of ROS in HBx-activated JNK signaling and autophagosome formation. It was found that inhibition of ROS activity indeed decreased HBx-triggered JNK activation and autophagosome formation. A variety of reports have indicated that ROS activation often leads to inhibition of the AKT/mTOR axis (62, 63); however, our data show that HBx overexpression in HepG2 cells not only leads to the generation of ROS but also activates class I PI3K/AKT/mTOR signaling. This scenario might be due to the inhibitory effect of HBx on PTEN, a negative regulator of AKT activation, as reported by Ha and Yu (50).
Evidence indicates that HBx plays a crucial role in HBV-induced autophagosome formation, which is required for HBV replication (28–32). As our data revealed that HBx induces autophagy via the ROS-JNK signaling pathway, we further examined the role of this signaling pathway in HBV-induced autophagy and its replication. It was found that the activation of ROS-JNK signaling is important for HBV-induced incomplete autophagy, while HBx was revealed to play a major role in these processes. Furthermore, inhibition of ROS-JNK signaling, as expected, significantly suppressed HBV replication but did not have a significant effect on HBV RNA levels, which is consistent with the notion that HBV-induced autophagosome formation is required for its replication but not for its transcription (28–30). We also determined the effect of ROS-JNK signaling on HBV-induced autophagy and HBV replication in an HBV infection system. It was found that inhibition of ROS-JNK signaling significantly inhibits HBV-induced autophagosome formation and suppresses the subsequent HBV replication in HBV-infected PHHs, indicating a possible role for the ROS-JNK-autophagy pathway in HBV infection under physiological conditions.
In summary, we found that HBx induces autophagosome formation independently of the class I PI3K/AKT/mTOR signaling pathway, while the class III PI3K(VPS34)/beclin-1 complex was found to be required for this process, which might involve disruption of the beclin-1/Bcl-2 interaction. Further data showed that the ROS-JNK signaling pathway plays an important role in HBx-induced autophagosome formation through regulation of the beclin-1/Bcl-2 association. Of importance, ROS-JNK signaling was also demonstrated to be critical for HBV replication via regulation of autophagy induction. These data help to decipher the molecular mechanisms of autophagy induction by HBx/HBV, and ROS-JNK-mediated autophagic process might be a potential therapeutic target for HBV infection.
MATERIALS AND METHODS
Antibodies and reagents.
The antibodies against p-mTOR (catalog no. 2971), mTOR (catalog no. 2972), p-p70S6K (catalog no. 9234), p-4EBP1 (catalog no. 2855), p-Akt (catalog no. 9271), Akt (catalog no. 9272), beclin-1 (catalog no. 3738), VPS34 (catalog no. 3811), beclin-1 (catalog no. 3495 and 4122), p-JNK (catalog no. 9251), JNK (catalog no. 9252), p-ERK1/2 (catalog no. 9101), p-p38 (catalog no. 9211), p-Bcl-2 (catalog no. 2827), Bcl-2 (catalog no. 15071 and 4223), p62 (catalog no. 3912), horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (catalog no. 7074), and HRP-conjugated anti-mouse IgG (catalog no. 7076) were from Cell Signaling Technology (Beverly, MA, USA). The antibody against LC3 (catalog no. L7543) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The antibody against HBx (catalog no. ab2741) was obtained from Abcam (Cambridge, MA, USA). The antibody against HBc (catalog no. B0586) was from Dako (Glostrup, Denmark). Chemicals, including the ERK inhibitor U0126 (catalog no. U120), the p38 inhibitor SB202190 (catalog no. S7067), the JNK inhibitor SP600125 (catalog no. S5567), the class III PI3K inhibitor 3-MA (catalog no. M9281), and the ROS inhibitor NAC (catalog no. A7250), were obtained from Sigma-Aldrich. The AKTi 1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate (catalog no. 124005) was obtained from Calbiochem (San Diego, CA, USA).
Cell culture.
The human hepatoma cell line HepG2 was cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum at 37°C. PHHs were purchased from BioreclamationIVT (Baltimore, MD, USA). PHHs were thawed in prewarmed InVitroGro CP (plating) medium supplemented with 10% fetal calf serum and plated on collagen-coated tissue culture plates at approximately 0.7 × 106/ml. Cells were allowed to attach for approximately 8 h, and the plating medium was then replaced with InVitroGro HI (incubation) medium.
Plasmid or siRNA transfection and HBV infection.
HBV replication-competent plasmid pHBV1.3 and plasmid pHBV1.3 with HBx-minus were described previously (64, 65). To construct HBx expression plasmid pHBx, the DNA sequence encoding HBx was amplified from pHBV1.3 and cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA). Transfection of pHBx, pHBV1.3, or pHBV1.3 with HBx-minus into HepG2 cells was performed with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions. For siRNA transfection into HepG2 cells, cells were trypsinized and mixed with 100 nM siRNA against beclin-1, VPS34, or JNK (GenePharma, Shanghai, China) and then subjected to electrotransfection by Amaxa Nucleofector technology (Amaxa GmbH, Cologne, Germany). The optimal sense sequences of the VPS34, beclin-1, and JNK-1 siRNAs were 5′-CGCGAAAGUGGAAAUCGUA-3′, 5′-CAGUUUGGCACAAUCAAUA-3′, and 5′-UCACAGUCCUGAAACGAUA-3′, respectively. HBV virions were generated from stable HBV-expressing human hepatoma HepAD38 cells. To infect PHHs, cells were incubated with an HBV inoculum for 16 h at a multiplicity of infection of 100 virus genome equivalents per cell in the presence of 4% PEG 8000 as described previously (66). The infection experiments were repeated at least three times on different days.
Western blot analysis.
Equal amounts of cell lysates were resolved by SDS-polyacrylamide gel electrophoresis, and the proteins were transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 10% nonfat milk in Tris-buffered saline containing 0.1% (vol/vol) Tween 20 for 1 h and then incubated with 10% nonfat milk containing the primary antibodies indicated for 2 h at room temperature. After being washed, the membrane was incubated with 10% nonfat milk containing HRP-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Immunoprecipitation.
Immunoprecipitation was performed as described previously (67, 68). Briefly, cells were lysed in RIPA buffer (50 mM Tris at pH 8.0, 280 mM NaCl, 0.5% NP-40, 2 mM EDTA, 10% glycerol, 1 mM dithiothreitol) supplemented with a protease inhibitor cocktail (Roche). After being precleared with protein A/G agarose beads for 2 h at 4°C, lysates were incubated with an anti-Bcl-2 or anti-beclin-1 antibody at 4°C overnight; this was followed by incubation with protein A/G agarose beads for 2 h. The beads were washed five times with lysis buffer. Immunoprecipitated proteins were subjected to Western blot analysis with an anti-beclin-1 or anti-Bcl-2 antibody.
Measurement of intracellular ROS.
The 2,7-dichlorofluorescein diacetate (DCF-DA) method was used to measure the intracellular ROS level in HepG2 cells. Cells were transfected with pHBx or pHBV1.3 in the presence or absence of 10 mM NAC. After medium was changed, 100 mM DCF-DA was added to each well and incubated for an additional 30 min at 37°C in the dark. The treated cells were then detached with trypsin, and cellular mean fluorescence intensity was quantified by fluorescence-activated cell sorter (FACS) analysis.
Intracellular core particle-associated HBV-DNA extraction and analysis.
The method used to extract and analyze intracellular core particle-associated HBV DNA was adapted from previous investigations (32, 69, 70). Briefly, the pHBV1.3-transfected HepG2 cells from a 6-cm dish were rinsed twice with phosphate-buffered saline and lysed with 700 μl of lysis buffer (10 mM HEPES at pH 7.5, 100 mM NaCl, 1 mM EDTA, 1% NP-40). The cell lysate was centrifuged at 7,300 × g for 15 min at 4°C to pellet the nuclei. The supernatant was transferred to a new tube and supplemented with 10 mM CaCl2–12 mM MgCl2 and digested with 10 U of DNase I (TaKaRa, Dalian, China) and 40 U of mung bean nuclease (TaKaRa) at 37°C for 1 h to degrade the transfected plasmid DNA. A 256-μl volume of polyethylene glycol (PEG) solution (1.2 M NaCl, 60 mM EDTA, 30% sucrose, 26% PEG) was added to each tube and incubated at 4°C for at least 1 h before pelleting of the core particles by centrifugation at 7,300 × g for 20 min at room temperature. The pellet was resuspended in 180 μl of resuspension solution (10 mM Tris at pH 7.5, 6 mM MgCl2, 8 mM CaCl2) and further treated with 5 U of DNase I and 20 U of mung bean nuclease for 30 min at 37°C to further degrade transfected plasmid DNA. The DNase activity was stopped by the addition of 50 μl of stop solution (10 mM Tris at pH 7.5, 50 mM EDTA), and the HBV DNA from the intracellular core particle was further extracted with a viral genome purification kit (Cwbiotech, Beijing, China). Quantitative PCR analysis of HBV DNA was performed with an HBV diagnostic kit (Kehua Biotech, Shanghai, China) in accordance with the manufacturer's instructions. For Southern blot analysis, extracted HBV DNA was separated on a 1.0% agarose gel, blotted onto a nylon membrane, and probed with a digoxigenin (DIG)-labeled full-length HBV probe synthesized with the DIG Probe synthesis kit (Roche Diagnostics, Mannheim, Germany).
qRT-PCR analysis of HBV RNAs and preC-pgRNA.
Cells were harvested and total RNA was isolated with RNAiso Plus (TaKaRa). cDNA was synthesized from 0.5 μg of RNA with the PrimeScript RT reagent kit with gDNA Eraser (Perfect Real Time; TaKaRa). Prior to reverse transcription (RT), gDNA Eraser was used to remove contaminating DNA in accordance with the manufacturer's instructions. Quantitative RT (qRT)-PCR analysis of cDNA targets was performed with a LightCycler 480 and the SYBR green system (TaKaRa) in the absence or presence of reverse transcriptase to rule out transfected HBV-DNA contamination. The primers used to amplify HBV RNAs and precore-pregenomic RNA (preC-pgRNA) were taken from a previous investigation (71), and their sequences are as follows: HBV RNAs, 5′-GCACTTCGCTTCACCTCTGC-3′ (forward) and 5′-CTCAAGGTCGGTCGTTGACA-3′ (reverse); HBV preC-pgRNA, 5′-TGTTCAAGCCTCCAAGCT-3′ (forward) and 5′-GGAAAGAAGTCAGAAGGCAA-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-CGGGTGGGAATGTTGAGG-3′ (forward) and 5′-TGGCGGGAGATGTGGGTAC-3′ (reverse). The level of HBV RNA or preC-pgRNA expression was calculated following normalization to the GAPDH level by the comparative ΔΔ threshold cycle method.
Statistical analysis.
All values are expressed as the mean ± the standard error of the mean of at least three independent experiments. Statistical analysis was performed with the SPSS software (version 18.0). The Student t test was used to compare differences between two groups, whereas comparison of multiple groups was performed by analysis of variance with post hoc tests to compare differences between individual groups. A P value of <0.05 was considered statistically significant for all analyses.
ACKNOWLEDGMENTS
This work was supported by grants from the Major State Basic Research Development Program of China (2013CB530501), the National Natural Science Foundation of China (31470839, 21334001), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Jiangsu Provincial Innovative Research Team and the Program for Changjiang Scholars and Innovative Research Team in University of the Ministry of Education of China (PCSIRT-IRT1075).
REFERENCES
- 1.Chisari FV, Ferrari C. 1995. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 13:29–60. [DOI] [PubMed] [Google Scholar]
- 2.Trépo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 3.Feitelson MA, Bonamassa B, Arzumanyan A. 2014. The roles of hepatitis B virus-encoded X protein in virus replication and the pathogenesis of chronic liver disease. Expert Opin Ther Targets 18:293–306. doi: 10.1517/14728222.2014.867947. [DOI] [PubMed] [Google Scholar]
- 4.Tsuge M, Hiraga N, Akiyama R, Tanaka S, Matsushita M, Mitsui F, Abe H, Kitamura S, Hatakeyama T, Kimura T, Miki D, Mori N, Imamura M, Takahashi S, Hayes CN, Chayama K. 2010. HBx protein is indispensable for development of viraemia in human hepatocyte chimeric mice. J Gen Virol 91:1854–1864. doi: 10.1099/vir.0.019224-0. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard MJ, Wang LH, Schneider RJ. 2001. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science 294:2376–2378. doi: 10.1126/science.294.5550.2376. [DOI] [PubMed] [Google Scholar]
- 6.Bertoletti A, Rivino L. 2014. Hepatitis B: future curative strategies. Curr Opin Infect Dis 27:528–534. doi: 10.1097/QCO.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertoletti A, Ferrari C. 2012. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut 61:1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 8.Grimm D, Thimme R, Blum HE. 2011. HBV life cycle and novel drug targets. Hepatol Int 5:644–653. doi: 10.1007/s12072-011-9261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen DH, Ludgate L, Hu J. 2008. Hepatitis B virus-cell interactions and pathogenesis. J Cell Physiol 216:289–294. doi: 10.1002/jcp.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti LG, Isogawa M, Chisari FV. 2015. Host-virus interactions in hepatitis B virus infection. Curr Opin Immunol 36:61–66. doi: 10.1016/j.coi.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi AM, Ryter SW, Levine B. 2013. Autophagy in human health and disease. N Engl J Med 368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 12.Deretic V, Saitoh T, Akira S. 2013. Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13:722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubinsztein DC, Codogno P, Levine B. 2012. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gracia-Sancho J, Guixe-Muntet S, Hide D, Bosch J. 2014. Modulation of autophagy for the treatment of liver diseases. Expert Opin Investig Drugs 23:965–977. doi: 10.1517/13543784.2014.912274. [DOI] [PubMed] [Google Scholar]
- 15.Mizushima N, Levine B. 2010. Autophagy in mammalian development and differentiation. Nat Cell Biol 12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher LE, Williamson LE, Chan EY. 2016. Advances in autophagy regulatory mechanisms. Cells 5:E24. doi: 10.3390/cells5020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 18.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. 2005. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 19.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B. 2012. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattingre S, Bauvy C, Codogno P. 2003. Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem 278:16667–16674. doi: 10.1074/jbc.M210998200. [DOI] [PubMed] [Google Scholar]
- 21.Belaid A, Ndiaye PD, Klionsky DJ, Hofman P, Mograbi B. 2013. Signalphagy: scheduled signal termination by macroautophagy. Autophagy 9:1629–1630. doi: 10.4161/auto.25880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webber JL, Tooze SA. 2010. Coordinated regulation of autophagy by p38alpha MAPK through mAtg9 and p38IP. EMBO J 29:27–40. doi: 10.1038/emboj.2009.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldsmith J, Levine B, Debnath J. 2014. Autophagy and cancer metabolism. Methods Enzymol 542:25–57. doi: 10.1016/B978-0-12-416618-9.00002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine B, Kroemer G. 2008. Autophagy in the pathogenesis of disease. Cell 132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orvedahl A, Levine B. 2009. Autophagy in mammalian antiviral immunity. Curr Top Microbiol Immunol 335:267–285. doi: 10.1007/978-3-642-00302-8_13. [DOI] [PubMed] [Google Scholar]
- 27.Zhou L, He X, Gao B, Xiong S. 2015. Inhibition of histone deacetylase activity aggravates coxsackievirus B3-induced myocarditis by promoting viral replication and myocardial apoptosis. J Virol 89:10512–10523. doi: 10.1128/JVI.01028-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sir D, Tian Y, Chen WL, Ann DK, Yen TS, Ou JH. 2010. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc Natl Acad Sci U S A 107:4383–4388. doi: 10.1073/pnas.0911373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Liu Y, Wang Z, Liu K, Wang Y, Liu J, Ding H, Yuan Z. 2011. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J Virol 85:6319–6333. doi: 10.1128/JVI.02627-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian Y, Sir D, Kuo CF, Ann DK, Ou JH. 2011. Autophagy required for hepatitis B virus replication in transgenic mice. J Virol 85:13453–13456. doi: 10.1128/JVI.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sir D, Ann DK, Ou JH. 2010. Autophagy by hepatitis B virus and for hepatitis B virus. Autophagy 6:548–549. doi: 10.4161/auto.6.4.11669. [DOI] [PubMed] [Google Scholar]
- 32.Zhong L, Hu J, Shu W, Gao B, Xiong S. 2015. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis 6:e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li F, Wang Y, Tiollais P, Li T, Zhao M. 2009. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 49:60–71. doi: 10.1002/hep.22581. [DOI] [PubMed] [Google Scholar]
- 34.Zhang HT, Chen GG, Hu BG, Zhang ZY, Yun JP, He ML, Lai PB. 2014. Hepatitis B virus X protein induces autophagy via activating death-associated protein kinase. J Viral Hepat 21:642–649. doi: 10.1111/jvh.12191. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Fang M, Hu Y, Huang B, Li N, Chang C, Huang R, Xu X, Yang Z, Chen Z, Liu W. 2014. Hepatitis B virus X protein inhibits autophagic degradation by impairing lysosomal maturation. Autophagy 10:416–430. doi: 10.4161/auto.27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Guo QS, Wang ZW, Qian HX. 2013. HBx induces HepG-2 cells [sic] autophagy through PI3K/Akt-mTOR pathway. Mol Cell Biochem 372:161–168. doi: 10.1007/s11010-012-1457-x. [DOI] [PubMed] [Google Scholar]
- 37.Polivka JJ, Janku F. 2014. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 142:164–175. doi: 10.1016/j.pharmthera.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Zeng H, Chi H. 2014. mTOR signaling and transcriptional regulation in T lymphocytes. Transcription 5:e28263. doi: 10.4161/trns.28263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou YY, Li Y, Jiang WQ, Zhou LF. 2015. MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci Rep 35:e00199. doi: 10.1042/BSR20140141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Luo P, Wang J, Dai J, Yang X, Wu H, Yang B, He Q. 2014. Autophagy blockade sensitizes the anticancer activity of CA-4 via JNK-Bcl-2 pathway. Toxicol Appl Pharmacol 274:319–327. doi: 10.1016/j.taap.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Han M, Yan W, Guo W, Xi D, Zhou Y, Li W, Gao S, Liu M, Levy G, Luo X, Ning Q. 2008. Hepatitis B virus-induced hFGL2 transcription is dependent on c-Ets-2 and MAPK signal pathway. J Biol Chem 283:32715–32729. doi: 10.1074/jbc.M806769200. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Bai X, Zhang Q, Wen L, Su W, Fu Q, Sun X, Lou Y, Yang J, Zhang J, Chen Q, Wang J, Liang T. 2016. The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett 380:98–105. doi: 10.1016/j.canlet.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Chung TW, Lee YC, Kim CH. 2004. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: involvement of invasive potential. FASEB J 18:1123–1125. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 44.Benn J, Su F, Doria M, Schneider RJ. 1996. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol 70:4978–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nijhara R, Jana SS, Goswami SK, Rana A, Majumdar SS, Kumar V, Sarkar DP. 2001. Sustained activation of mitogen-activated protein kinases and activator protein 1 by the hepatitis B virus X protein in mouse hepatocytes in vivo. J Virol 75:10348–10358. doi: 10.1128/JVI.75.21.10348-10358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki M, Bandoski C, Bartlett JD. 2015. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic Biol Med 89:369–378. doi: 10.1016/j.freeradbiomed.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou H, Shen T, Shang C, Luo Y, Liu L, Yan J, Li Y, Huang S. 2014. Ciclopirox induces autophagy through reactive oxygen species-mediated activation of JNK signaling pathway. Oncotarget 5:10140–10150. doi: 10.18632/oncotarget.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim AD, Kang KA, Kim HS, Kim DH, Choi YH, Lee SJ, Kim HS, Hyun JW. 2013. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis 4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scherz-Shouval R, Elazar Z. 2011. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Ha HL, Yu DY. 2010. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J Gastroenterol 16:4932–4937. doi: 10.3748/wjg.v16.i39.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YI, Hwang JM, Im JH, Lee YI, Kim NS, Kim DG, Yu DY, Moon HB, Park SK. 2004. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem 279:15460–15471. doi: 10.1074/jbc.M309280200. [DOI] [PubMed] [Google Scholar]
- 52.Surviladze Z, Sterk RT, DeHaro SA, Ozbun MA. 2013. Cellular entry of human papillomavirus type 16 involves activation of the phosphatidylinositol 3-kinase/Akt/mTOR pathway and inhibition of autophagy. J Virol 87:2508–25017. doi: 10.1128/JVI.02319-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joubert PE, Werneke SW, de la Calle C, Guivel-Benhassine F, Giodini A, Peduto L, Levine B, Schwartz O, Lenschow DJ, Albert ML. 2012. Chikungunya virus-induced autophagy delays caspase-dependent cell death. J Exp Med 209:1029–1047. doi: 10.1084/jem.20110996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma J, Sun Q, Mi R, Zhang H. 2011. Avian influenza A virus H5N1 causes autophagy-mediated cell death through suppression of mTOR signaling. J Genet Genomics 38:533–537. doi: 10.1016/j.jgg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Yen CJ, Lin YJ, Yen CS, Tsai HW, Tsai TF, Chang KY, Huang WC, Lin PW, Chiang CW, Chang TT. 2012. Hepatitis B virus X protein upregulates mTOR signaling through IKKbeta to increase cell proliferation and VEGF production in hepatocellular carcinoma. PLoS One 7:e41931. doi: 10.1371/journal.pone.0041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HY, Yang SL, Liang HF, Li CH. 2014. HBx protein promotes oval cell proliferation by up-regulation of cyclin D1 via activation of the MEK/ERK and PI3K/Akt pathways. Int J Mol Sci 15:3507–3518. doi: 10.3390/ijms15033507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berryman S, Brooks E, Burman A, Hawes P, Roberts R, Netherton C, Monaghan P, Whelband M, Cottam E, Elazar Z, Jackson T, Wileman T. 2012. Foot-and-mouth disease virus induces autophagosomes during cell entry via a class III phosphatidylinositol 3-kinase-independent pathway. J Virol 86:12940–12953. doi: 10.1128/JVI.00846-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. 2007. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol 170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin-Vilchez S, Lara-Pezzi E, Trapero-Marugan M, Moreno-Otero R, Sanz-Cameno P. 2011. The molecular and pathophysiological implications of hepatitis B X antigen in chronic hepatitis B virus infection. Rev Med Virol 21:315–329. doi: 10.1002/rmv.699. [DOI] [PubMed] [Google Scholar]
- 60.Tarn C, Lee S, Hu Y, Ashendel C, Andrisani OM. 2001. Hepatitis B virus X protein differentially activates RAS-RAF-MAPK and JNK pathways in X-transforming versus non-transforming AML12 hepatocytes. J Biol Chem 276:34671–34680. doi: 10.1074/jbc.M104105200. [DOI] [PubMed] [Google Scholar]
- 61.Ren JH, Chen X, Zhou L, Tao NN, Zhou HZ, Liu B, Li WY, Huang AL, Chen J. 2016. Protective role of Sirtuin3 (SIRT3) in oxidative stress mediated by hepatitis B virus X protein expression. PLoS One 11:e0150961. doi: 10.1371/journal.pone.0150961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L, Wang H, Xu J, Zhu J, Ding K. 2014. Inhibition of cathepsin S induces autophagy and apoptosis in human glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K and JNK signaling pathways. Toxicol Lett 228:248–259. doi: 10.1016/j.toxlet.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, Shi Y, Yang K. 2016. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 341-343:28–40. doi: 10.1016/j.tox.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Xu J, Yun X, Jiang J, Wei Y, Wu Y, Zhang W, Liu Y, Wang W, Wen Y, Gu J. 2010. Hepatitis B virus X protein blunts senescence-like growth arrest of human hepatocellular carcinoma by reducing Notch1 cleavage. Hepatology 52:142–154. doi: 10.1002/hep.23613. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Liu H, Chen L, Wang S, Zhou L, Yun X, Sun L, Wen Y, Gu J. 2012. Hepatitis B virus X protein confers resistance of hepatoma cells to anoikis by up-regulating and activating p21-activated kinase 1. Gastroenterology 143:199-212.e4. doi: 10.1053/j.gastro.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 66.Ni Y, Urban S. 2017. Hepatitis B virus infection of HepaRG cells, HepaRG-hNTCP cells, and primary human hepatocytes. Methods Mol Biol 1540:15–25. doi: 10.1007/978-1-4939-6700-1_2. [DOI] [PubMed] [Google Scholar]
- 67.Gao B, Xu W, Zhong L, Zhang Q, Su Y, Xiong S. 2013. p300, but not PCAF, collaborates with IRF-1 in stimulating TRIM22 expression independently of its histone acetyltransferase activity. Eur J Immunol 43:2174–2184. doi: 10.1002/eji.201343308. [DOI] [PubMed] [Google Scholar]
- 68.Lu M, Xu W, Gao B, Xiong S. 2016. Blunting autoantigen-induced FOXO3a protein phosphorylation and degradation is a novel pathway of glucocorticoids for the treatment of systemic lupus erythematosus. J Biol Chem 291:19900–19912. doi: 10.1074/jbc.M116.728840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao B, Duan Z, Xu W, Xiong S. 2009. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located [sic] RING domain. Hepatology 50:424–433. doi: 10.1002/hep.23011. [DOI] [PubMed] [Google Scholar]
- 70.Parekh S, Zoulim F, Ahn SH, Tsai A, Li J, Kawai S, Khan N, Trépo C, Wands J, Tong S. 2003. Genome replication, virion secretion, and e antigen expression of naturally occurring hepatitis B virus core promoter mutants. J Virol 77:6601–6612. doi: 10.1128/JVI.77.12.6601-6612.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. 2015. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]