ABSTRACT
Human cytomegalovirus (HCMV) is the most common viral infection acquired by the developing human fetus and can result in damage to the developing central nervous system. Although vaccine development to modify this congenital infection is ongoing, the unique epidemiology of maternal HCMV infections appears discordant with strategies for vaccine development. Several characteristics of congenital HCMV infections suggest that the efficacy of vaccines designed to induce responses similar to those that follow natural infection will be limited.
KEYWORDS: human cytomegalovirus infection, congenital viral infection
INTRODUCTION
Congenital (present-at-birth) infection with human cytomegalovirus (HCMV) has been well documented as the most common viral infection acquired in utero, yet awareness of this relatively common cause of disease in infants and children remains surprisingly limited (1, 2). More recently, the description of the Zika virus outbreak in Brazil significantly increased awareness of the potential of intrauterine viral infections to lead to severe central nervous system (CNS) damage (3–6). Fortunately, results from nearly 5 decades of natural history studies of congenital HCMV infections have provided a roadmap for studies of the consequences of Zika virus infections that occur during pregnancy.
The prevalences of congenital HCMV infection vary widely depending on the characteristics of maternal populations, such as age and maternal HCMV seroprevalence. However, a reasonable estimate of the overall birth prevalence of congenital HCMV infection is about 4 to 5/1,000 live births based on recent findings from a large, multicenter study performed in the United States (7, 8). When the prevalence of congenital HCMV infections is compared to those of other causes of disease and disability in infants, such as congenital heart malformations (10/1,000) and cystic fibrosis (0.3/1,000), or those of chromosomal abnormalities, such as trisomy 21 (1.2/1,000), the significance of congenital HCMV infection as a cause of disease in infants and children can be appreciated. In the United States, it is estimated that approximately 20,000 to 30,000 infants are born infected with HCMV each year, and in countries such as India, this number may exceed 250,000. Because up to 10% of infected infants exhibit some type of neurological sequelae associated with this infection, the magnitude of the burden of disease associated with congenital HCMV infection is significant. Hearing loss is by far the most frequent long-term sequela in congenitally infected infants and occurs in 8 to 15% of infected infants (9). It has been reported that approximately 25% of all cases of sensorineural hearing loss in children in the United States can be attributed to congenital HCMV infection (9, 10).
Currently, there is no approved treatment that can be used to prevent the transmission of HCMV from mother to fetus. Treatment of infants with congenital HCMV infections with the antiviral drug ganciclovir was shown to modestly reduce the incidence of hearing loss in infected infants, and although encouraging, this study must be viewed as incomplete because of the limited duration of follow-up of treated patients (11). Similarly, there is no licensed vaccine to prevent infection in pregnant women or transmission to a developing fetus or to limit disease in infants infected in utero. Development of an efficacious vaccine remains a high priority for federal agencies, and several pharmaceutical companies are actively pursuing vaccine development. Candidate vaccines, including attenuated live-virus vaccines and adjuvanted viral proteins, have provided insight into the development of an effective vaccine but not definitive evidence of efficacy (12–14). Efforts to develop vaccines to limit disease associated with congenital HCMV infections have identified several hurdles that continue to challenge vaccine development. (i) We have limited information on the components and the specificities of the protective adaptive immune response, including the utilization of potentially noninformative in vitro surrogates of protective immunity in clinical studies. (ii) There are concerns surrounding the use of replication-competent vaccines in women of childbearing age. (iii) An enormous number of enrollees is required if the efficacy of a candidate vaccine will be defined by its capacity to modify outcomes of infants born to women with HCMV infection during pregnancy, and this is perhaps the most vexing concern. In contrast, if a vaccine could prevent maternal acquisition of HCMV and/or inhibit virus transmission to the developing fetus and if these endpoints were considered evidence of HCMV vaccine efficacy by regulatory agencies, then clinical trials would become feasible. However, the unique epidemiology of HCMV infections in immunocompetent women suggests that even these endpoints may be difficult to achieve and that vaccines that induce adaptive immune responses to HCMV that are similar to those that follow natural infection will likely fail to significantly modify the outcome of maternal HCMV infections during pregnancy in the vast majority of women.
HCMV INFECTIONS DURING PREGNANCY. (i) MATERNAL IMMUNITY AND FETAL INFECTION
Although the risks of HCMV infection in women of childbearing age can vary depending on the HCMV seroprevalence within maternal populations, the annualized rate of HCMV infection during pregnancy has been estimated to be 2 to 3% in the United States (15). HCMV infection during pregnancy in nonimmune women (primary maternal infection) is associated with an approximately 30% transmission rate of HCMV to the developing fetus, a rate that is dependent on gestational period at the time of maternal infection (16–18). However, in contrast to the situation with other congenital infections, such as rubella, parvovirus infection, and toxoplasmosis, preconceptional maternal immunity to HCMV does not prevent transmission to the fetus, and even women with long-standing immunity to HCMV can transmit virus to their fetuses (19–24). This unique feature of the natural history of maternal HCMV infection provides an explanation for the observation that as HCMV seroprevalence increases in maternal populations, the rate of congenital HCMV infection also increases such that the highest rates of congenital HCMV infections are found in populations in which women of childbearing age have the highest prevalence of serological immunity to HCMV (25).
In contrast to women with primary HCMV infection during pregnancy, pregnant women with preexisting immunity to HCMV (nonprimary maternal infection) have a rate of intrauterine transmission that is frequently stated to be 1%, based on the rate of congenital HCMV infection observed in populations of seroimmune women (26). Thus, the difference in transmission rates between women undergoing primary infection and women undergoing nonprimary infection has frequently been cited as evidence that maternal adaptive immunity can significantly reduce rates of intrauterine transmission. However, the transmission rate of 1% following nonprimary maternal infection is based on dated findings as well as several untested assumptions. The first assumption is that congenital infection following nonprimary maternal infections results from the recurrence of an existing persistent maternal infection that is by definition present in 100% of seroimmune women. This dogma was derived largely from studies initiated in the late 1970s that utilized restriction enzyme digestion of viral DNA to compare viruses isolated from infected women and their infants (27). In addition to the limited sampling of genomic diversity inherent in restriction fragment length polymorphism (RFLP) analyses, the extensive in vitro amplification of viral isolates required prior to these analyses introduced a range of potential biases secondary to passage of clinical viral isolates. As DNA sequencing of individual viral genes became more commonplace and the genetic diversity of isolates of HCMV were observed, reinfection (superinfection) of immunocompetent women with new strains of HCMV were reported (28–30). Reinfection with an antigenically distinct strain of virus in these studies could be demonstrated by development of corresponding serologic reactivity to the new viral strain and confirmed by recovery of viruses encoding new antigenic determinants (28, 30). Importantly, reinfection of seroimmune pregnant women was also associated with transmission of the newly acquired virus to the fetus (28, 30). Similarly, more controlled studies of rhesus macaques have clearly demonstrated that macaques with robust preexisting adaptive immunity to rhesus CMV could be readily reinfected with laboratory and wild strains of rhesus CMV (31–33). Thus, current literature strongly argues that existing adaptive immunity to HCMV cannot prevent infection with a new strain of HCMV and that the assumption that all women with nonprimary HCMV infection during pregnancy are at similar risks secondary to recurrence of an existing persistent infection cannot be supported with available data.
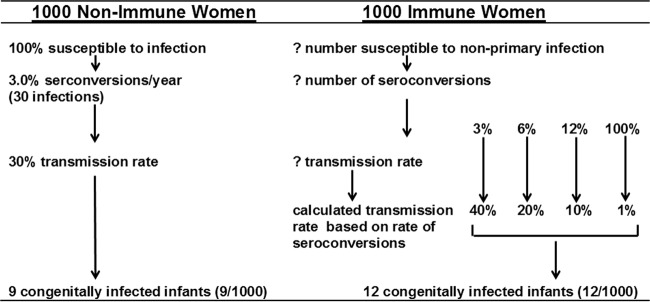
A second major assumption that is often implicit in comparisons of rates of transmission between women with nonprimary HCMV infections and women undergoing primary infection during pregnancy is that the two populations have similar risks for HCMV reinfection (or even recurrence). This assumption is unproven. Rates of exposure to HCMV, including exposure to new strains of the virus, and perhaps more importantly, the breadth of protective immunity in HCMV-immune women that may reduce the risk of reinfection with new strains of virus, are unknown. Thus, direct comparison of rates of HCMV intrauterine transmission between women with nonprimary infections and HCMV-nonimmune women will likely be noninformative. To more fully illustrate the potential fallacies in estimates of the importance of adaptive immunity in limiting intrauterine transmission of HCMV in pregnant women undergoing nonprimary infections, I compared the rates of intrauterine transmission of HCMV in 1,000 women undergoing primary and nonprimary HCMV infection that would yield the expected number of congenitally infected infants born to these populations based on the prevalence of congenital HCMV in different populations (Fig. 1) (2). As can be seen, 1,000 nonimmune women are uniformly susceptible to infection, and approximately 3% will acquire HCMV during pregnancy, resulting in 30 maternal infections. Assuming the consistently reported 30% transmission rate, 9 infants (9/1,000) will be congenitally infected with HCMV. In contrast, the number of immune women susceptible to nonprimary infection during pregnancy is unknown, as is the number of seroconversions (reinfections); thus, the rates of transmission cannot be accurately calculated. However, assuming several different rates of reinfection (seroconversion to a new strain of HCMV) in immune women, transmission rates can also be estimated based on the expected outcomes of 12 congenitally infected infants (Fig. 1). Intrauterine transmission rates in these women might range between 1 and 40%. As an example, if immune women have the same risk of acquisition of HCMV as nonimmune women (3% seroconversion rate to a new viral strain), then the rate of intrauterine transmission in these women undergoing nonprimary infection would be 40% (Fig. 1). Thus, the assumption that all HCMV-immune women have the same risk of recurrence of a persistent infection during pregnancy and thus the same risk for intrauterine transmission has likely led to an overestimate of the effect of preexisting HCMV adaptive immunity on the prevention of intrauterine transmission.
FIG 1.
Estimated rates of HCMV intrauterine transmission as a function of maternal infection (seroconversion) in nonimmune women (primary infections) and reinfection (seroconversions) in immune women (nonprimary infections). Several possible seroconversion rates in immune women are shown, with transmission rates calculated based on the prevalence of congenitally infected infants from seroimmune maternal populations.
(ii) MATERNAL IMMUNITY AND THE OUTCOME OF CONGENITAL INFECTION
Although many investigators concede that maternal immunity cannot completely prevent intrauterine transmission of HCMV, dogma in this area of research continues to be that maternal immunity can prevent disease in infected infants. However, as in the case of the impact of maternal immunity on intrauterine transmission, this concept has remained in the absence of data from well-controlled studies. Studies from Sweden beginning in the 1980s suggested that there was little difference between the outcomes of infants born to women with primary HCMV infections during pregnancy and the outcomes of infected infants born to women with nonprimary infection (34). Results from natural history studies at several different institutions have shown that congenital HCMV infections that follow nonprimary maternal infections can result in a spectrum of disease in infected infants that is similar to that observed in infants infected following primary maternal infections (35–37) (Table 1). These outcomes include both the stigmata of clinically apparent congenital HCMV infection in the newborn period (symptomatic infections) and the presence of neurological sequelae in infected infants (Table 1). It is important to note that limitations in serological assays utilized to define the type of maternal infections in these studies may have resulted in misclassifications of the type of maternal infection (primary versus nonprimary) in some of the pregnant women enrolled in these studies. However, even if strict criteria, such as de novo IgG seroconversion during pregnancy, are utilized to classify the type of maternal infection, the outcomes of infected offspring of women undergoing primary and nonprimary infection during pregnancy are strikingly similar, albeit with smaller sample sizes (35). More-recent studies in populations with near-universal serological immunity before pregnancy demonstrated that overall outcomes in congenitally infected infants were similar following both primary and nonprimary maternal HCMV infections during pregnancy and, perhaps more importantly, were similar to the outcomes of infants born to women with primary infections that have been reported over the last 3 decades (24, 38).
TABLE 1.
Type of maternal HCMV infection and outcome of congenitally infected infants
| Outcome | Maternal infection typea |
Study location (reference[s]) | |
|---|---|---|---|
| Primary | Nonprimary | ||
| Symptomatic congenital infection | 10 (8/82) | 23 (7/30) | Sweden, U.K. (34, 35) |
| 11 (14/124) | 11 (19/176) | USA (36) | |
| 50 (1/2) | 5 (2/39) | Brazil (24) | |
| Total | 11 (23/208) | 11 (28/245) | |
| Neurological sequelae | 6 (5/82) | 30 (9/30) | Sweden, U.K. (34, 35) |
| 38 (3/8) | 75 (6/8) | USA (37)b | |
| Total | 9 (8/90) | 39 (15/38) | |
| Hearing loss | 0 (0/8) | 29 (2/7) | USA (37) |
| 33 (1/3) | 27 (6/22) | Brazil (38) | |
| 11 (19/176) | 10 (13/124) | USA (36) | |
| Total | 11 (20/187) | 14 (21/153) | |
Values are percentages (numbers of infants with the specific outcome over the total number of infected infants in the study).
Only infants with symptomatic infections were analyzed in this study.
It has been argued by some investigators that even if maternal immunity cannot prevent intrauterine transmission of HCMV infection or significantly modify long-term outcomes in congenitally infected infants, it can limit or prevent intrauterine infections that are associated with severe CNS damage that often includes structural brain abnormalities. The phenotype of severe CNS damage following intrauterine HCMV infection is observed in only a fraction of newborn infants infected in utero, likely on the order of 3 to 5% of congenitally infected infants (39, 40). Thus, the frequency of infants with such CNS disease is low in most clinical series, and sufficient numbers of such cases are not available to either confirm or reject this hypothesis. Lastly, older publications describing congenitally infected infants with clinically apparent CNS disease invariably included significant numbers of infants identified by clinical presentation and not through universal screening of newborn populations. Thus, the presence of bias in case ascertainment has rendered much of the existing literature less reliable in terms of the estimate of the incidence of congenitally infected infants who have severe CNS disease (39). Larger and more-complete data sets will help define the importance of preconceptional immunity and prevention of severe infections of the developing brain following intrauterine HCMV infection.
(iii) IMPLICATIONS FOR PROPHYLACTIC INTERVENTIONS
The importance of nonprimary maternal HCMV infections as a source of congenital HCMV infections has been appreciated for decades. It has been estimated that between 70 and 80% of all infants with congenital HCMV infections are born to women undergoing nonprimary infection during pregnancy (41). The contribution of nonprimary maternal HCMV infections to the overall incidence of congenital HCMV infections is even more striking in Africa, Asia, and South America, where maternal immunity to HCMV is often >95% in women of childbearing age and rates of congenital HCMV infections are often higher than in most other regions of the world (42). Findings from a study in Brazil demonstrated that 88.6% (39/44) of congenitally infected infants were born to women with nonprimary infections compared to 4.5% (2/44) born to women with primary infections (24). Maternal immunity to HCMV in this maternal population as measured by serum HCMV-specific IgG antibodies was nearly universal (>96%), and as expected from this high maternal HCMV seroprevalence, the rate of congenital HCMV infection was 1.1% (87/8,047). Findings from this study strongly suggest that providing natural immunity to HCMV by early exposure to HCMV or by prophylactic vaccines that induce immunity as measured by surrogate assays of naturally acquired immunity to HCMV would have little impact on the prevalence of congenital HCMV infections. Modeling of the impact of maternal HCMV immunity on the natural history of congenital HCMV infection has also suggested a limited role of maternal immunity, as measured by current surrogates of protective immunity, in the modification of the natural history of congenital HCMV infections (43). As was noted earlier, the rate of congenital HCMV infections continues to increase as the rates of maternal seroimmunity increase, an epidemiology that is in direct contrast to that observed for rubella virus infections in that the incidence of congenital rubella virus infection falls as the rate of maternal rubella immunity increases (25, 44–46). This relationship between rubella immunity and prevention of congenital rubella virus infections underpins the success of rubella vaccines that induce antibody responses similar to those measured following infection with wild rubella virus.
In summary, there is a lack of convincing evidence that maternal immunity to HCMV as commonly measured provides solid protection from maternal infection, intrauterine transmission, and perhaps most importantly, long-term sequelae of congenital HCMV infections. Thus, investigators must consider several possibilities, including that either (i) HCMV-induced immunity plays little if any role in the natural history of this common congenital infection or (ii) the metrics of immunity currently used as surrogates of protective maternal responses to HCMV that might modify congenital infections are inaccurate and of limited predictive value, which is perhaps a more appealing alternative explanation.
REFERENCES
- 1.Boppana S, Britt WJ. 2013. Synopsis of clinical aspects of human cytomegalovirus disease, p 1–25. In Reddehase M. (ed), Cytomegaloviruses: from molecular pathogenesis to intervention, vol 2 Caister Academic Press, Norfolk, United Kingdom. [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 17:253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 3.Brito CA, Cordeiro MT. 2016. One year after the Zika virus outbreak in Brazil: from hypotheses to evidence. Rev Soc Bras Med Trop 49:537–543. doi: 10.1590/0037-8682-0328-2016. [DOI] [PubMed] [Google Scholar]
- 4.Coyne CB, Lazear HM. 2016. Zika virus—reigniting the TORCH. Nat Rev Microbiol 14:707–715. doi: 10.1038/nrmicro.2016.125. [DOI] [PubMed] [Google Scholar]
- 5.Klase ZA, Khakhina S, Schneider Ade B, Callahan MV, Glasspool-Malone J, Malone R. 2016. Zika fetal neuropathogenesis: etiology of a viral syndrome. PLoS Negl Trop Dis 10:e0004877. doi: 10.1371/journal.pntd.0004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martinez-Vega R, Porgo TV, Haefliger A, Broutet NJ, Low N. 2017. Zika virus infection as a cause of congenital brain abnormalities and Guillain-Barre syndrome: systematic review. PLoS Med 14:e1002203. doi: 10.1371/journal.pmed.1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, Sanchez PJ, Bernstein DI, Tolan RW Jr, Novak Z, Chowdhury N, Britt WJ, Fowler KB, National Institute on Deafness and Other Communication Disorders CHIMES Study. 2011. Saliva polymerase-chain-reaction assay for congenital cytomegalovirus screening in newborns. N Engl J Med 364:2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinninti SG, Ross SA, Shimamura M, Novak Z, Palmer AL, Ahmed A, Tolan RW Jr, Bernstein DI, Michaels MG, Sanchez PJ, Fowler KB, Boppana SB. 2015. Comparison of saliva PCR assay versus rapid culture for detection of congenital cytomegalovirus infection. Pediatr Infect Dis J 34:536–537. doi: 10.1097/INF.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler KB. 2013. Congenital cytomegalovirus infection: audiologic outcome. Clin Infect Dis 57(Suppl 4):S182–S184. doi: 10.1093/cid/cit609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morton CC, Nance WE. 2006. Newborn hearing screening—a silent revolution. N Engl J Med 354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, Ashouri N, Englund JA, Estrada B, Jacobs RF, Romero JR, Sood SK, Whitworth MS, Abzug MJ, Caserta MT, Fowler S, Lujan-Zilbermann J, Storch GA, DeBiasi RL, Han JY, Palmer A, Weiner LB, Bocchini JA, Dennehy PH, Finn A, Griffiths PD, Luck S, Gutierrez K, Halasa N, Homans J, Shane AL, Sharland M, Simonsen K, Vanchiere JA, Woods CR, Sabo DL, Aban I, Kuo H, James SH, Prichard MN, Griffin J, Giles D, Acosta EP, Whitley RJ. 2015. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 372:933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 360:1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein DI, Munoz FM, Callahan ST, Rupp R, Wootton SH, Edwards KM, Turley CB, Stanberry LR, Patel SM, McNeal MM, Pichon S, Amegashie C, Bellamy AR. 2016. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: a randomized clinical trial. Vaccine 34:313–319. doi: 10.1016/j.vaccine.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause PR, Bialek SR, Boppana SB, Griffiths PD, Laughlin CA, Ljungman P, Mocarski ES, Pass RF, Read JS, Schleiss MR, Plotkin SA. 2013. Priorities for CMV vaccine development. Vaccine 32:4–10. doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyde TB, Schmid DS, Cannon MJ. 2010. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol 20:311–326. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 16.Boppana SB, Ross SA, Fowler KB. 2013. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 57(Suppl 4):S178–S181. doi: 10.1093/cid/cit629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, Ayoubi JM, Grangeot Keros L, Benachi A. 2013. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 33:751–758. doi: 10.1002/pd.4118. [DOI] [PubMed] [Google Scholar]
- 18.Enders G, Daiminger A, Bader U, Exler S, Enders M. 2011. Intrauterine transmission and clinical outcome of 248 pregnancies with primary cytomegalovirus infection in relation to gestational age. J Clin Virol 52:244–246. doi: 10.1016/j.jcv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Stagno S, Reynolds DW, Huang E-S, Thames SD, Smith RJ, Alford CA. 1977. Congenital cytomegalovirus infection: occurrence in an immune population. N Engl J Med 296:1254–1258. doi: 10.1056/NEJM197706022962203. [DOI] [PubMed] [Google Scholar]
- 20.Dar L, Pati SK, Patro AR, Deorari AK, Rai S, Kant S, Broor S, Fowler KB, Britt WJ, Boppana SB. 2008. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr Infect Dis J 27:841–843. doi: 10.1097/INF.0b013e3181723d55. [DOI] [PubMed] [Google Scholar]
- 21.Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM. 2002. Congenital cytomegalovirus infection: review of the epidemiology and outcome. Obstet Gynecol Surv 57:245–256. doi: 10.1097/00006254-200204000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Schopfer K, Lauber E, Krech U. 1978. Congenital cytomegalovirus infection in newborn infants of mothers infected before pregnancy. Arch Dis Child 53:536–539. doi: 10.1136/adc.53.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello C, Whittle H. 1991. Cytomegalovirus infection in Gambian mothers and their babies. J Clin Pathol 44:366–369. doi: 10.1136/jcp.44.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mussi-Pinhata MM, Yamamoto AY, Moura-Britto RM, Lima-Issacs M, Boppana S, Britt WJ. 2009. Birth prevalence and natural history of congenital cytomegalovirus (CMV) infection in highly seroimmune population. Clin Infect Dis 49:522–528. doi: 10.1086/600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stagno S, Pass RF, Dworsky ME, Alford CA. 1983. Congenital and perinatal cytomegaloviral infections. Semin Perinatol 7:31–42. [PubMed] [Google Scholar]
- 26.Stagno S, Pass RF, Dworsky ME, Henderson RE, Moore EG, Walton PD, Alford CA. 1982. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med 306:945–949. doi: 10.1056/NEJM198204223061601. [DOI] [PubMed] [Google Scholar]
- 27.Huang ES, Alford CA, Reynolds DW, Stagno S, Pass RF. 1980. Molecular epidemiology of cytomegalovirus infections in women and their infants. N Engl J Med 303:958–962. doi: 10.1056/NEJM198010233031702. [DOI] [PubMed] [Google Scholar]
- 28.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 29.Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. 2010. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis 201:386–389. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto AY, Mussi-Pinhata MM, Boppana SB, Novak Z, Wagatsuma VM, Oliviera PD, Duarte G, Britt WJ. 2010. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 202:297.e1–297.e8. doi: 10.1016/j.ajog.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, Tassone F, Hertz-Picciotto I. 2011. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 22:476–485. doi: 10.1097/EDE.0b013e31821d0e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. 2010. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlfors K, Ivarsson SA, Harris S. 2001. Secondary maternal cytomegalovirus infection—a significant cause of congenital disease. Pediatrics 107:1227–1228. doi: 10.1542/peds.107.5.1227. [DOI] [PubMed] [Google Scholar]
- 35.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. 2013. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis 56:1232–1239. doi: 10.1093/cid/cit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross SA, Fowler KB, Ashrith G, Stagno S, Britt WJ, Pass RF, Boppana SB. 2006. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J Pediatr 148:332–336. doi: 10.1016/j.jpeds.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. 1999. Symptomatic congenital cytomegalovirus infection in infants born to mothers with preexisting immunity to cytomegalovirus. Pediatrics 104:55–60. doi: 10.1542/peds.104.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto AY, Mussi-Pinhata MM, Isaac MDL, Amaral FR, Carvalheiro CG, Aragon DC, da Silva Mafredi AK, Boppana SB, Britt WJ. 2011. Congenital cytomegalovirus infection as a cause of sensorineural hearing loss in a highly seropositive population. Pediatr Infect Dis J 30:1043–1046. doi: 10.1097/INF.0b013e31822d9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dreher AM, Arora N, Fowler KB, Novak Z, Britt WJ, Boppana SB, Ross SA. 2014. Spectrum of disease and outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr 164:855–859. doi: 10.1016/j.jpeds.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, Voigt RG, Demmler-Harrison G. 6 April 2017. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J Perinatol doi: 10.1038/jp.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C, Zhang X, Bialek S, Cannon MJ. 2011. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 52:e11–. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 42.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 26:86–102. doi: 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vries JJ, van Zwet EW, Dekker FW, Kroes AC, Verkerk PH, Vossen AC. 2013. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol 23:241–249. doi: 10.1002/rmv.1744. [DOI] [PubMed] [Google Scholar]
- 44.Cutts FT, Vynnycky E. 1999. Modelling the incidence of congenital rubella syndrome in developing countries. Int J Epidemiol 28:1176–1184. doi: 10.1093/ije/28.6.1176. [DOI] [PubMed] [Google Scholar]
- 45.Cooper LZ, Preblud SR, Alford CA. 1995. Rubella, p 268–311. In Remington JS, Klein JO (ed), Infectious diseases of the fetus and newborn infant, 4th ed WB Saunders, Philadelphia, PA. [Google Scholar]
- 46.Britt W. 2015. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol 204:263–271. doi: 10.1007/s00430-015-0399-9. [DOI] [PubMed] [Google Scholar]