Abstract
Phthalates are a class of plasticizing chemicals produced in high volume and widely found in consumer products. Evidence suggests that phthalates may have non-monotonic effects on reproductive hormone activity. With exposure to phthalates virtually ubiquitous among industrialized populations, identifying unexposed and/or minimally exposed human populations is essential for understanding the effects of low level exposures. Our primary objective was to quantify urinary phthalate metabolite concentrations in the Tsimane’, a remote population of Bolivian forager-horticulturalists. Our secondary objectives were to determine if phthalate metabolite concentrations vary in relation to access to market goods; and to explore relationships between phthalate and reproductive hormone metabolite concentrations. Given that phthalate exposure is of particular concern during fetal development, we focused on reproductive age women in the current analyses. Phthalate metabolites were assayed in urine samples from 59 naturally cycling, reproductive age Tsimane’ women. Market access was assessed as: (1) distance from residence to the largest nearby town (San Borja, Bolivia) and (2) Spanish fluency. Urinary reproductive hormone metabolite concentrations were quantified using enzyme immunoassays. We fit linear models to examine: (1) predictors of phthalate exposure; and (2) relationships between urinary phthalate and reproductive hormone metabolite concentrations. Eight phthalate metabolites were detectable in at least 75% of samples. Median concentrations were up to an order of magnitude lower than industrialized populations. Proximity to San Borja and Spanish fluency were strong predictors of exposure. In exploratory analyses, the sum of the di-2-ethylhexyl phthalate metabolites (ΣDEHP) and Mono-isobutyl phthalate (MiBP) were significantly associated with altered concentrations of urinary reproductive hormone metabolites. Remote, subsistence populations, like the Tsimane’, offer a unique window into the health effects of endocrine active compounds because: (1) exposures are low and likely to be first generation; (2) a natural fertility lifestyle allows for exploration of reproductive effects; and (3) ever-increasing globalization will result in increasing exposure in the next decade.
Keywords: phthalates, global marketplace, toxicoanthropology, endocrine disrupting chemicals, low concentration exposure
Introduction
Phthalates are a large class of synthetic chemicals with demonstrated endocrine-disrupting properties. In both animal models and humans, exposure to di-2-ethylhexyl phthalate (DEHP) and other phthalates, is associated with a range of adverse health consequences including impaired reproductive function, asthma, preterm birth, obesity, and altered neurodevelopmental outcomes (Engel et al., 2010; Ferguson et al., 2014; Just et al., 2012; Meeker et al., 2009; Stahlhut et al., 2007; Swan et al., 2010; Swan et al., 2005; Whyatt et al., 2011; Whyatt et al., 2012). Given phthalates’ potential to disrupt endocrine signaling, prenatal exposure, during which organ systems and endocrine axes are established, is of particular concern (Hauser and Calafat, 2005; Huang et al., 2009; Rider et al., 2009). Although it is worth noting that exposure during adulthood may potentially also impact circulating steroid reproductive hormone concentrations (Duty et al., 2005; Meeker and Ferguson, 2014; Sathyanarayana et al., 2014).
Despite these health concerns, because of their utility as plasticizers, solvents and emulsifiers, phthalates are produced in high volume (over 470 million pounds per year) for use in manufactured goods including personal care products, food, pharmaceuticals and medical devices, pesticides, and cleaning products (U.S.E.P.A, 2012). Phthalates do not covalently bond to substrates, thus they can leach into the environment, where they can then be inhaled, ingested, or dermally absorbed (Schettler, 2006). It is no surprise that among industrialized populations, therefore, exposure to phthalates is virtually ubiquitous (Koch and Calafat, 2009). However, little is known about body burden of phthalates in non-industrialized populations, who often live traditional lifestyles with relatively limited use of and access to manufactured goods. To date, only a handful of studies have examined phthalate exposure in non-industrialized populations. In Old Order Mennonite (OOM) women living a traditional lifestyle in upstate New York (n=10), urinary concentrations of most phthalate metabolites were significantly lower than in the general U.S. population (as measured in the National Health and Nutrition Examination Survey [NHANES]), however all OOM women still had detectable levels of at least one metabolite of di-2-ethylhexyl phthalate (DEHP), a commonly used phthalate. The lower phthalate levels among OOM may be due to lifestyle differences, including the relatively limited use of store-bought foods, personal care products, and cars (Martina et al., 2012). Despite leading a traditional lifestyle by Western standards, the OOM still live in close proximity to industrialized populations and have relatively easy access to typical American culture, products, and environments.
Two additional studies have examined exposure to phthalates in urban and rural populations in Peru and Egypt. The first examined phthalate exposure among pregnant women living in a large, developing Peruvian city (Trujillo, population ~800,000) and found that phthalate metabolite concentrations were measurable in most samples (Irvin et al., 2010). However concentrations in Peruvian women were typically lower than those documented in NHANES, suggesting that phthalate exposures may be lower in developing populations. A second study measured phthalate metabolite concentrations in premenstrual girls living in rural and urban communities around Gharbiah, Egypt (a province of >4 million people). Levels of most (but not all) metabolites were again lower than those of age- and sex-matched samples from NHANES, and no differences were found between the urban and rural subpopulations, reflecting increasing urbanization and access to manufactured goods even among more rural Egyptians (Colacino et al., 2011).
Assessing exposure to phthalates in traditional populations may provide an opportunity to examine health effects at low concentrations (and in the absence of hormonal contraception use), which is particularly important for understanding human risk because emergent data suggests that many endocrine disrupting chemicals (EDCs), including phthalates, have non-monotonic dose response effects on reproductive health outcomes (Andrade et al., 2006; Do et al., 2012; Kortenkamp, 2008). Mechanistic studies in animals have identified reproductive deficits at both low and high phthalate doses, whereby influences at high doses are not necessarily predictive of low dose influences (and vice versa) (Andrade et al., 2006; Christiansen et al., 2010; Vandenberg et al., 2012). Considering the increasing recognition of low concentration effects in animal studies, there is an urgent need to examine low-dose and non-monotonic environmental chemical exposures in an epidemiological setting (Birnbaum, 2012). However, in the case of plasticizers like phthalates, identifying unexposed and minimally exposed “low dose” human populations has proven difficult. One unexplored option is building upon established anthropological field sites studying remote, locally-subsistent populations with limited access to the global marketplace. As global marketplace usage and acculturation increases worldwide, these traditional populations may provide a unique opportunity to assess consequences of low concentration exposure to phthalates and other EDCs. Additionally, anthropological field sites often have long-term access to biological specimens (blood, urine or stool samples) and detailed knowledge of their study subject’s lives which provides the ability to assess biomarkers of physiological function that can be directly correlated with hypothesized environmental and lifestyle variables.
The extent to which globalization has resulted in environmental chemical exposure among very remote populations is unknown. Around the world, many locally-subsistent populations, including hunter-gatherers and horticulturalists, still live in small villages and follow traditional lifestyles, but exposures and interactions with the market economy are increasing due to greater socioeconomic transformation in recent decades. Determining whether these populations are “pristine” with respect to environmental chemical exposure, and assessing the ‘proof of concept’ for collaborations with anthropological study populations for such studies, is of great interest. With this in mind, we assessed phthalate exposure in the Tsimane’, a group of traditional, non-industrialized Bolivian Amazon forager-horticulturalists. We focused on reproductive age women, given the concern about adverse health effects associated with prenatal exposures. The objectives of this study were to: (1) quantify urinary phthalate concentrations in the Tsimane’; (2) determine the extent to which Tsimane’ phthalate metabolite concentrations vary in relation to access to market goods; and (3) given their known mode of action as endocrine disrupting chemicals, to explore relationships between phthalate metabolite levels and reproductive hormone concentrations.
Materials and Methods
Study population
The Tsimane’ and their lifestyle have been described in greater detail elsewhere (Gurven, 2004; Veile et al., 2014). Briefly, the Tsimane’ engage in a small-scale traditional economy based mostly on slash-and-burn horticulture, supplemented by fishing, hunting, and gathering fruits. A very small subset of Tsimane’ couples now use birth control methods (IUD or injectable Depo Provera), however, it is largely a natural fertility population, defined as individuals that do not use birth control including, but not limited to, hormonal contraception. There is limited access to modern medical care and the average woman has nine births over her reproductive life span (McAllister et al., 2012). Most households are constructed from available forest materials, with thatched palm roofs and dirt floors. As of 2016, few villages have electricity. In surveys conducted across 66 communities from 2013–2015 (n =1016), only 24% of respondents reported regularly using latrines. Seventy-three percent of respondents reported relying exclusively on rivers, streams, or lakes for water, with the remaining 27% reporting exclusive or additional use of unprotected community wells and, more rarely, household groundwater pumps (M. Martin, personal communication).
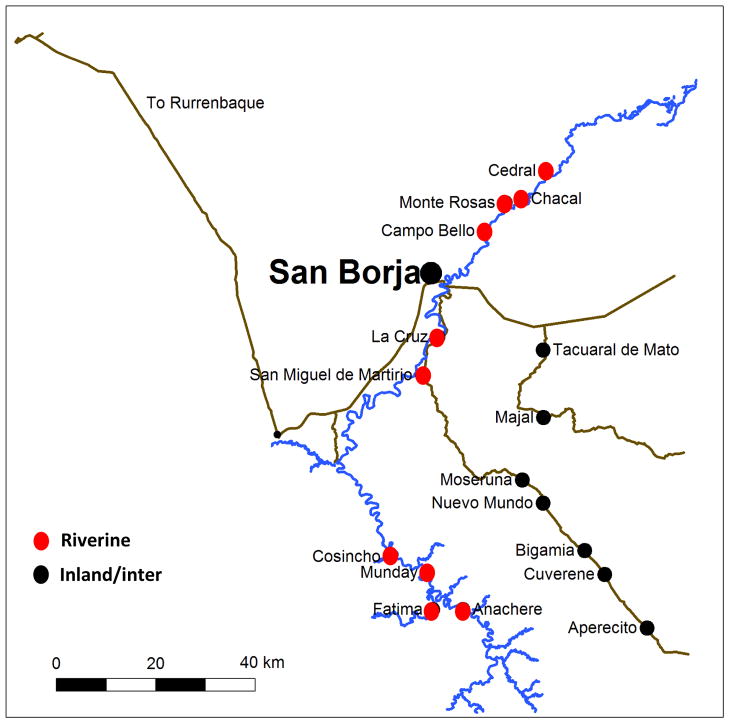
Market integration and material wealth varies by proximity and access to San Borja, Bolivia. The dirt roads leading to San Borja are frequently unnavigable during the rainy season (~November to ~April), and travel by foot or boat from more distant villages can take upwards of 1–2 days. Still, living in closer proximity to San Borja, enables more regular contact with other Bolivian nationals and increased participation in agricultural labor and the local economy (Veile et al., 2014). Historically, market access has been minimal; roughly a third of Tsimane’ adults have had no schooling, and ~40% speak no or poor Spanish, speaking only their native Tsimane’ language. However, in the last decade, changes in politics, infrastructure, and regional development have promoted local integration, while income derived from wage labor and crop sales have allowed for increased expenditures on market goods in recent years. In 2003–2005, about 90% of the Tsimane’ diet was derived from horticulture, hunting, and foraging, (Martin et al., 2012), though market food consumption increased 6.35% per year from 2002 – 2006 (Rosinger et al., 2012). In 2006, Tsimane adults spent roughly the equivalent of $131 US per year on market goods (Masferrer-Dodas et al., 2011). However, market expenditures are highly variable. In surveys done in 2006 and 2008, 39% and 63% of individuals reported no market food expenditures in the previous week (Masferrer-Dodas et al., 2011; Rosinger et al, 2012). In the 2006 survey, weekly expenditures on market goods were significantly higher for males and positively associated with age, frequency of travel to town, years of schooling, and household cash income (Masferrer-Dodas et al., 2011), the latter three of which are generally correlated with distance to town. Rapid integration into and usage of the global marketplace suggests that Tsimane’ will be for the first-time, increasingly exposed to EDCs, such as phthalates.
Thus to summarize, Tsimane’ villages have considerable variation in river access, surrounding game densities, access to market goods, and integration into the larger Bolivian society, making them an ideal population in which to examine the relationship between global market integration and phthalate body burden.
Study overview
The Tsimane’ Health and Life History Project (THLHP), which began in 2002, is a long-term study aimed at understanding the impacts of ecology, economy and evolution of the human life course (http://www.umn.edu/~Tsimane'). Tsimane’ participants contributed biospecimens (including urine samples), participated in interviews, and underwent basic medical exams. From 2008–2009, the study visited 19 villages covering a census population of 1,860 females (38% of which were aged 15–44). All women age 40+ and a random, age-stratified sample of women under age 40 were recruited to participate in the larger THLHP study. A total of 1,374 women (74% of the census population) were seen by the medical team during this period.
Urine samples were collected from 30% (414/1374) of the women seen by the medical team. Funding for phthalate analysis in the current sub-study was limited to sixty samples, which were randomly selected from the available banked urine samples, and represented reproductive-aged women (age 13–51) across a range of 17 villages with known reproductive status (cycling, pregnant, or lactational amenorrhea) at the time of urine collection. Additional participant data gathered at time of urine collection included highest level of education (grade 0–12), Spanish fluency (non-Spanish speaker, moderate Spanish, fluent), and reproductive history including menstrual status, date of last menstrual period, and date of last birth. Basic anthropometric measures (height, weight, skinfold thickness) were measured in all subjects. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Cycle phase (follicular or luteal) was estimated based on date of last menstruation, either self-reported or determined by the attending physician during medical exam. This phase determination was later confirmed through our measurement of progesterone metabolite concentrations. Store-bought alcohol and smoking rates are rare among Tsimane’ woman, however no data were available for these subjects. Additionally, income differences among Tsimane’ families are minor relative to income disparities between the Tsimane’ and Bolivian nationals living in San Borja. Differences in standard of living among Tsimane’ families would also have been negligible. At the time of sample collection, all Tsimane’ were living without electricity, plumbing, access to clean water. Prior to data collection, research protocols were approved by all participating institutions including the University of California-Santa Barbara (UCSB), University of New Mexico (UNM), and University of Rochester. The Tsimane’ government (Gran Consejo Tsimane), village leaders and all individual participants consented to participate in the study.
Phthalate metabolite measurement
Participants provided urine samples at their medical visits. An aliquot of each sample was taken, stored in liquid nitrogen, and transported to the Centro Nacional de Enfermedades Tropicales (CENETROP) in Santa Cruz, where they were stored at −20°C. Samples were transported on dry ice from Bolivia to UNM or UCSB for long term storage in −80°C freezers. For the current analysis, samples were shipped from UNM to the Division of Laboratory Sciences, National Center for Environmental Health and Centers for Disease Control and Prevention (CDC) where phthalate metabolites were measured: Mono-2-ethylhexyl phthalate (MEHP), Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), Mono-2-ethyl-5-oxohexyl phthalate (MEOHP), Mono-2-ethyl-5-carboxypentyl phthalate (MECPP), Mono-n-butyl phthalate (MBP), Mono-isobutyl phthalate (MiBP), Mono-benzyl phthalate (MBzP), Mono-ethyl phthalate (MEP), Mono-carboxy-isooctyl phthalate (MCOP), Mono-3-carboxy-propyl phthalate (MCPP), Mono-carboxy-isononyl phthalate (MCNP) (Table 1). Urine samples from 59 women were analyzed using a standard approach involving enzymatic deconjugation of metabolites from their glucuronidated form, automated on-line solid phase extraction, high performance liquid chromatography (HPLC) separation, and finally, detection by isotope-dilution tandem mass spectrometry (Silva et al., 2007). Isotopically labeled internal standards and conjugated internal standards were used to improve precision and accuracy. Because samples were not originally collected with exposure to environmental chemicals in mind, materials used to collect and process urine samples were screened for phthalates, including three types of urine cups used to collect urine samples during the study and plastic bags used to send the samples from UNM to the CDC. This was done by incubating HPLC grade water in the cups and bags and then assaying the water as a typical, unknown sample using the methods described above. The cups were found to be phthalate free, but the zip-lock bags used for shipment to CDC showed low levels (2–3 ng/mL) of one metabolite, MBP.
Table 1.
Specific-gravity adjusted phthalate metabolite concentrations (in ng/mL) in reproductive-age Tsimane’ women (n=59)1.
| Phthalate (abbreviation) | Metabolite (abbreviation) | LOD (%detected) | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|
| Di-2-ethylhexyl phthalate (DEHP) | Mono-2-ethylhexyl phthalate (MEHP) | 0.5 (75) | 0.55 | 1.03 | 2.46 |
| Mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP) | 0.2 (97) | 2.22 | 4.31 | 10.86 | |
| Mono-2-ethyl-5-oxohexyl phthalate (MEOHP) | 0.2 (98) | 1.56 | 3.13 | 6.50 | |
| Mono-2-ethyl-5-carboxypentyl phthalate (MECPP) ΣDEHP | 0.2 (100) | 4.69 | 9.71 | 15.37 | |
|
| |||||
| Dibutyl phthalate (DBP) | Mono-n-butyl phthalate (MBP) | 0.4 (80) | 0.68 | 6.14 | 24.38 |
|
| |||||
| Di-isobutyl phthalate (DiBP) | Mono-isobutyl phthalate (MiBP) | 0.2 (93) | 1.28 | 7.53 | 16.44 |
|
| |||||
| Butylbenzyl phthalate (BBzP) | Mono-benzyl phthalate (MBzP) | 0.3 (81) | 0.63 | 0.97 | 3.18 |
|
| |||||
| Diethyl phthalate (DEP) | Mono-ethyl phthalate (MEP) | 0.6 (54) | 0.43 | 1.01 | 6.44 |
|
| |||||
| Di-isononyl phthalate (DINP) | Mono-carboxy-isooctyl phthalate (MCOP) | 0.2 (73) | 0.33 | 0.66 | 1.78 |
|
| |||||
| Di-n-octyl phthalate (DnOP), dibutyl phthalate (DBP), and other high molecular weight phthalates | Mono-3-carboxy-propyl phthalate (MCPP) | 0.2 (10) | 0.11 | 0.16 | 0.21 |
|
| |||||
| Di-isodecyl phthalate (DIDP) | Mono-carboxy-isononyl phthalate (MCNP) | 0.2 (22) | 0.13 | 0.20 | 0.29 |
Another metabolite measured, mHINCH, was not detectable in any subjects.
Any analyte below the limit of detection (LOD) in over 50% of samples was excluded from further analysis. For the remaining metabolites, following conventions used for data that are not highly skewed, metabolite concentrations under the LOD were assigned to be the LOD divided by the square root of 2 (Hornung and Reed, 1990). The specific gravity (SpG) of each urine sample was measured using a hand-held refractometer in order to adjust for urine dilution. We adjusted phthalate metabolite concentrations for variation in urine concentration according to the following formula: Pc = P [(1.015-1)/SpG-1)]. In this equation, Pc is the SpG-adjusted phthalate concentration (ng/ml), P is the measured phthalate concentration (ng/ml), 1.015 is the mean SpG for all study samples, and SpG is the specific gravity of the individual urine sample (Boeniger et al., 1993). Four of the metabolites measured (MEHP, MEOHP, MEHHP, MECCP) derive from the same parent compound of interest (DEHP), so to approximate total exposure to DEHP, we calculated the molar sum of those metabolites as follows: ΣDEHP (in nmol/ml) = (MEHP*(1/278)) + (MEHHP*(1/294)) + (MEOHP*(1/292)) + (MECPP*(1/308)) (Wolff et al., 2008).
Hormone measurement
Urine samples were analyzed at the University of Rochester using the commercially available Quansys multiplex female hormone array for adiponectin, free cortisol, follicle stimulating hormone beta (FSH-beta), human chorionic gonadotropin beta (hCG-beta), and estrone-3-glucuronide (E1G; an estradiol metabolite) (Quansys Biosciences, Logan, UT). The Q-plex technology simultaneously identifies and quantifies multiple biomarkers in the same sample by spotting individually specific antibodies into single wells of a 96-well plate. Pregnanediol-3-glucuronide (PdG; a progesterone metabolite) was measured using a single enzyme immunoassay also developed by Quansys Biosciences (Logan, Utah). The Quansys Q-view Imager, which includes a digital camera, was used to quantify the intensity of the chemiluminescence of each spot. These multiplex assays have been shown to significantly correlate with single ELISA kits for female urinary hormone concentrations (Salvante et al., 2012). For this analysis, our analytes of interest were the reproductive hormone metabolites E1G and PdG. All samples were run on the same day for each kit. Samples that fell beyond the limits of quantification for the assay or CV values for duplicates above 15% were not included in statistical analyses. The average CV value for duplicates of PdG was 5.9% and for E1G was 5.5%. For Pdg, no samples were removed and for E1G one sample was removed due to CV error. These hormone concentrations were log transformed and adjusted for specific gravity.
Statistical Analysis
Phthalates and market access
Descriptive statistics were calculated for each phthalate metabolite and phthalate metabolite concentrations were then log-transformed to better approximate a normal distribution. Pearson’s correlations were conducted to assess correlations among the various phthalate metabolites. For our main analyses, our primary outcome variables were phthalate metabolite concentrations. We selected three exposure variables as potential proxies for market access to or environmental exposure from San Borja: distance from village of residence to San Borja, location of village (riverine versus inland), and Spanish fluency. Distance from San Borja was calculated as a linear distance using global positioning system (GPS) units from San Borja to the respective town. The ecological location of the village was also taken into consideration because the location on the river system could influence access to San Borja, particularly during the rainy season. Spanish fluency allows for increased communication with individuals living in San Borja and proposes increased integration into the global market place. Several potential covariates were selected for consideration as we hypothesized that they might have an influence on market access or phthalate metabolism: reproductive state (cycling, pregnant or lactating), education, age, and BMI. We fit linear multivariable models to examine associations between phthalate metabolite concentrations and market access measures. In each model, specific-gravity adjusted, log-transformed phthalate metabolite concentrations were the outcome of interest. The market access variables were all included together in models. None of the potential covariates considered predicted phthalate metabolite concentrations or appreciably changed estimates, thus they were not included in final models.
Phthalates and hormone concentrations
In exploratory analyses, we fit a series of linear models to examine associations between hormone concentrations and phthalate metabolite concentrations. In these models, our outcome measures were log transformed hormone concentrations (E1G or PdG), as adjusted hormone concentrations were not normally distributed. Our primary predictors were specific-gravity adjusted, log-transformed phthalate metabolite concentrations. Five phthalate metabolites (ΣDEHP, MCOP, MBzP, MEP, and MiBP) were included in the model based on their prevalence in the population and to minimize using multiple correlated metabolites. To address the shared covariance of phthalates directly, variance inflation factors were calculated for each factor to ensure that all phthalates could be assessed in the same analysis. For Model 1, age and skinfold thickness, a measure of body fat, were selected for a priori inclusion in all models given the well-documented age- and weight-related patterns in female reproductive hormones (Burger, 2008; Nelson et al., 1995; Noth and Mazzaferri, 1985; Ziomkiewicz et al., 2008). Because the hormonal profiles of lactating and pregnant women differ radically from cycling women, we only considered cycling women (n= 25; one woman was excluded for Depo Provera usage) in these analyses. Cycle phase (follicular or luteal phase, as estimated based on cycle day at urine collection) was included as a covariate, with luteal phase as the referent. In a second model, we also included distance from San Borja and parity. Distance from San Borja was also included in models because of the close relationship between distance and phthalate exposure. Parity (nulliparous or parous) was included because as it may be a predictor of sex steroid hormone concentrations (Barrett et al., 2014). Both models were presented (Table 6). All statistical analyses were conducted using JMP Pro 12.0 (SAS Institute Inc., Cary, N.C.) and an alpha-level of 0.05 was considered statistically significant.
Table 6.
Results of a Linear model analysis examining the association between reproductive hormone concentrations and phthalate metabolite concentrations (n=25). Bold indicates p ≤ 0.05 for both models, Italics indicates p ≤ 0.05 for one model.
| Phthalate metabolite | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | Estimate | 95% Confidence Interval | Predictor | Estimate | 95% Confidence Interval | |
|
| ||||||
| PdG | Log ΣDEHP_SG | 0.87 | (0.25, 1.48) | Log ΣDEHP_SG | 0.79 | (0.01, 1.56) |
| Log MEP_SG | 0.058 | (−0.21, 0.32) | Log MEP_SG | 0.07 | (−0.24, 0.38) | |
| Log MCOP_SG | −0.39 | (−0.90, 0.12) | Log MCOP_SG | −0.33 | (−0.96, 0.31) | |
| Log MBzP_SG | 0.38 | (0.01, 0.76) | Log MbZP_SG | 0.34 | (−0.13, 0.80) | |
| Log MiBP_SG | −0.26 | (−0.59, 0.06) | Log MiBP_SG | −0.30 | (−0.74, 0.13) | |
| Body Fat | −0.01 | (−0.05, 0.03) | Body Fat | −0.01 | (−0.07, 0.04) | |
| Cycle Phase | −0.49 | (−0.86, −0.10) | Cycle Phase | −0.48 | (−0.96, −0.001) | |
| Age (Years) | 0.01 | (−0.03, 0.05) | Age (Years) | 0.01 | (−0.05, 0.07) | |
| Parity | −0.02 | (−0.54, 0.50) | ||||
| Distance from SB | −0.01 | (−0.02, 0.01) | ||||
|
| ||||||
| E1G | Log ΣDEHP_SG | 0.31 | (−0.74, 1.37) | Log ΣDEHP_SG | 0.72 | (−0.22, 1.67) |
| Log MEP_SG | 0.32 | (−0.14, 0.78) | Log MEP_SG | 0.28 | (−0.10, 0.67) | |
| Log MCOP_SG | −0.095 | (−0.91, 0.72) | Log MCOP_SG | −0.43 | (−1.15, 0.28) | |
| Log MBzP_SG | 0.53 | (−0.20, 1.25) | Log MBzP_SG | 0.83 | (0.20, 1.46) | |
| Log MiBP_SG | −0.58 | (−1.09, −0.06) | Log MiBP_SG | −0.46 | (−0.92, −0.00) | |
| Body Fat | 0.017 | (−0.05, 0.08) | Body Fat | 0.05 | (−0.01, 0.10) | |
| Cycle Phase | −0.79 | (−1.38, −0.2) | Cycle Phase | −0.69 | (−1.19, −0.18) | |
| Age (Years) | 0.012 | (−0.04, 0.07) | Age (Years) | −0.01 | (−0.08, 0.05) | |
| Parity | −0.22 | (−0.80, 0.36) | ||||
| Distance from SB | 0.014 | (0.00, 0.03) | ||||
Results
On average, the 59 women who provided urine samples for the current analysis were 29 years old and had a BMI of 23.8 kg/m2 (Table 2). Of the 59 women, 26 of the women were cycling, 23 were not cycling due to lactational amenorrhea, and 10 were pregnant. Subjects resided in 17 different villages ranging from 17–144 km from San Borja. Fifty-eight percent of women lived in riverine villages compared to 42% in inland villages. Spanish fluency was distributed roughly evenly, with one-third of women in each category (non-Spanish speaker, moderate Spanish, fluent). Of the urinary phthalate metabolites measured as part of the standard CDC panel, most were measureable in the majority of women and every woman had measureable levels of at least one metabolite (Table 1). The DEHP metabolites, MEOHP, MEHHP, MEHP and MECPP, were detectable in virtually all subjects (≥97%). MiBP was similarly ubiquitous with 93% of women having measurable levels. Only three metabolites in the panel were not detectable in most samples., mHINCH, the metabolite of DINCH, a phthalate replacement, was not measurable in any of the 59 subjects, and two phthalate metabolites, MCNP and MCPP, were present at measurable levels in less than 50% of subjects’ samples. These three metabolites were excluded from subsequent analysis (Table 1). Phthalate metabolite levels were considerably lower among the Tsimane’ as compared to industrialized populations, such as NHANES participants (Table 3). Additionally, phthalate concentrations (ΣDEHP, MBP, MiBP, MBzP, MEP, MCOP) were significantly correlated with each other (Table 4: with significant Pearson’s correlation values ranging from .29 to .88). For the multiple metabolites of DEHP, metabolite correlation, as expected, was higher (average r correlation value of .90; data not shown).
Table 2.
Characteristics of the study population (n=59)1.
| Characteristic | Mean (SD) |
|---|---|
| Age (yrs) | 29 (4.4) |
|
| |
| Age at menarche(yrs) | 14 (1.4) |
|
| |
| BMI (kg/m2) | 23.8 (3.1) |
|
| |
| Highest grade of education | 3.1 (3.1) |
|
| |
| Distance from residence to San Borja (km) | 65.3 (38.0) |
|
| |
| Population of village of residence | 358 (184) |
|
| |
| Urinary hormone concentrations | Mean (SE) |
| E1G (ng/ml sg-adjusted) | 231.3 (54.9) |
| PdG (pg/ml sg adjusted) | 5277.8 (534.9) |
| FSH (mlU/ml sg adjusted) | 4.1 (0.84) |
|
| |
| N (%) | |
|
| |
| Parous2 | 39 (66) |
|
| |
| Reproductive status | |
| Cycling | 26 (44) |
| Lactational amenorrhea | 23 (39) |
| Pregnant | 10 (17) |
|
| |
| Spanish fluency | |
| None | 22 (38) |
| Some | 18 (31) |
| Fluent | 18 (31) |
|
| |
| Village location relative to San Borja | |
| Riverine | 34 (58) |
| Interior (inland) | 25 (42) |
Exact n may vary due to missing values
Of the 20 nulliparous women, 10 were currently pregnant
Table 3.
Unadjusted concentrations of phthalate metabolites (μg/L) in females from several global populations.
| Tsimane’, Bolivia (n=59) | Trujillo, Peru (n=79)1 | Egyptian, rural (n=29)2 | Egyptian, urban (n=28) 2 | US Mennonite (n=10)3 | US NHANES (n=1310)4 | |
|---|---|---|---|---|---|---|
| Population and year | Reproductive age women (2008–2009) | Pregnant women (2004) | Adolescent girls (2009) | Adolescent girls (2009) | Pregnant women (2009) | All females (2007–2008) |
| MEHP | 1.0 | 1.6 | 3.5 | 4.7 | 0.9 | 2.0 |
| MEOHP | 2.1 | 3.1 | 16.0 | 18.8 | 7.4 | 11.7 |
| MEHHP | 3.6 | 4.1 | 23.0 | 29.1 | 9.3 | 19.9 |
| MECPP | 8.1 | 10.5 | 60.9 | 58.0 | 9.9 | 31.0 |
| MBP | 5.0 | 9.3 | 47.5 | 53.3 | 13.6 | 20.8 |
| MBzP | 0.9 | 1.1 | 0.4 | 2.2 | 7.4 | 8.0 |
| MEP | 1.1 | 32.2 | 43.2 | 98.8 | 7.9 | 82.4 |
| MiBP | 4.1 | 1.2 | 17.6 | 25.4 | 1.1 | 7.4 |
Irvin et al (2010). Geometric means are shown; medians not provided.
CDC Fourth report updated tables (2013).
Table 4.
Specific-gravity adjusted log-transformed phthalate metabolite concentrations (in ng/mL) correlations for ΣDEHP, MBP, MiBP, MBzP, MEP, MCOP. Bold indicates p < 0.05.
| ΣDEHP | MBP | MiBP | MBzP | MEP | MCOP | |
|---|---|---|---|---|---|---|
| ΣDEHP | - | |||||
| MBP |
r = 0.72 P < 0.001 |
- | ||||
| MiBP |
r = 0.71 P < 0.001 |
r = 0.88 P < 0.001 |
- | |||
| MBzP |
r = 0.62 P < 0.001 |
r = 0.60 P < 0.001 |
r = 0.64 P < 0.001 |
- | ||
| MEP |
r = 0.37 P = 0.01 |
r = 0.43 P < 0.001 |
r = 0.48 P < 0.001 |
r = 0.29 P = 0.03 |
- | |
| MCOP |
r = 0.83 P < 0.001 |
r = 0.59 P < 0.001 |
r = 0.61 P < 0.001 |
r = 0.61 P = 0.055 |
r = 0.37 P = 0.01 |
- |
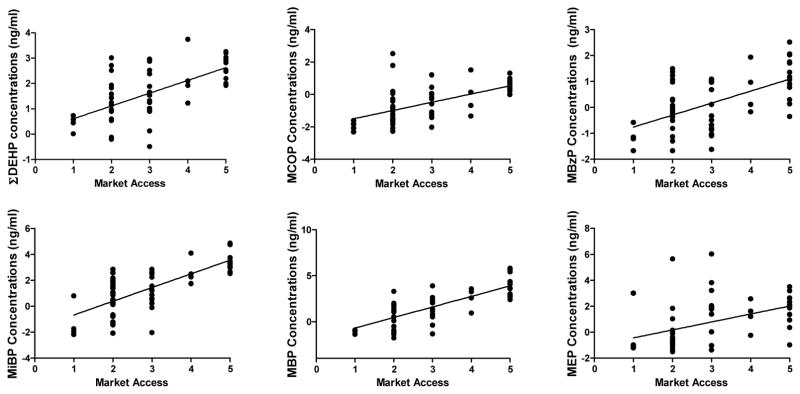
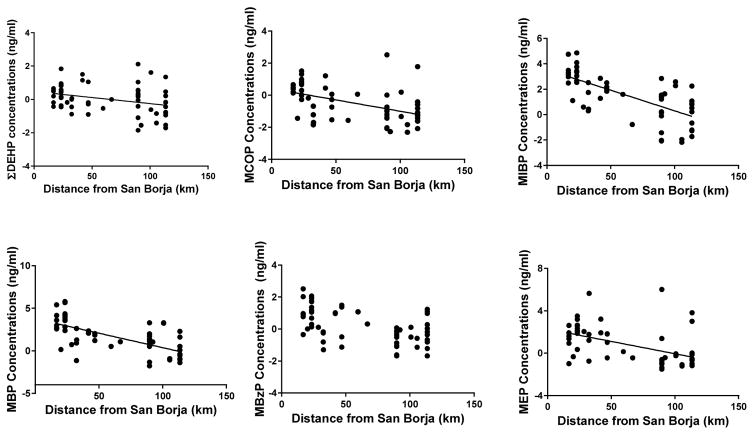
Using linear models, we examined predictors of urinary phthalate metabolite concentrations (and ΣDEHP) among Tsimane’ women (Table 5). Distance from village of residence to San Borja was significantly and inversely associated with nearly all individualphthalate metabolites and ΣDEHP (Figure 3, ΣDEHP: F(1,1) = 19.1, p < 0.001; MBP: F(1,1) = 18.2, p < 0.001; MiBP: F(1,1) = 16.9, p < 0.001; MEP: F(1,1) = 15.3, p < 0.001; MCOP: F(1,1) = 11.7, p = 0.002). Spanish fluency was significantly associated with higher concentrations of several metabolites including ΣDEHP (F(1,1) = 4.46; p = 0.04); MBP (F(1,1) = 8.67; p = 0.005); MiBP (F(1,1) = 12.14; p = 0.001); MBzP (F(1,1) = 6.22; p = 0.02); and MCOP (F(1,1) = 4.14; p = 0.03). However, ecological location of the village of residence relative to San Borja was not significantly associated with any phthalate metabolites, although it was marginally significant for MEP concentrations (F(1,1) = 3.60; p = 0.06). Taken together, distance from San Borja and Spanish fluency variables were significantly associated with phthalate metabolite concentrations in nearly all models. As a representation of this combined influence, we created a market access metric (Spanish Fluency (0 = no Spanish, 1 = moderately fluent, and 2 = most fluent) + Distance from San Borja (1 to 3; with 1 being furthest from San Borja (mean distance plus 1 SD) and 3 being closest (mean minus on SD). Six phthalates (ΣDEHP, MCOP, MBzP, MEP, MBP and MiBP) metabolite levels were plotted against this summary market access metric (Figure 3).
Table 5.
Linear models examining phthalate metabolite concentrations in relation to markers of Market Access (n=59). Bold indicates p≤0.05. Reference group is Riverine.
| Phthalate metabolite | Predictor | Estimate | t-Ratio | Prob> F | 95% Confidence Interval |
|---|---|---|---|---|---|
|
| |||||
| ΣDEHP | Distance from San Borja | −0.02 | −4.37 | 0.001 | (−0.03, −0.01) |
| Interior location | −0.28 | −1.71 | 0.09 | (−0.61, 0.04) | |
| Spanish Fluency | 0.26 | 1.82 | 0.04 | (0.01, 0.56) | |
|
| |||||
| MEHP | Distance from San Borja | −0.02 | −3.73 | 0.005 | (−0.02, −0.007) |
| Interior location | −0.12 | −0.85 | 0.40 | (−0.41, 0.16) | |
| Spanish Fluency | 0.32 | 2.68 | 0.01 | (0.08, 0.56) | |
|
| |||||
| MEHHP | Distance from San Borja | −0.02 | −4.50 | 0.001 | (−0.04, −0.01) |
| Interior location | −0.35 | −1.82 | 0.07 | (−0.74, 0.04) | |
| Spanish Fluency | 0.27 | 1.72 | 0.09 | (−0.04, 0.59) | |
|
| |||||
| MEOHP | Distance from San Borja | −0.03 | −4.53 | 0.001 | (−0.03, −0.01) |
| Interior location | −0.35 | −1.64 | 0.11 | (−0.68, 0.07) | |
| Spanish Fluency | 0.28 | 1.85 | 0.07 | (−0.02, 0.59) | |
|
| |||||
| MECPP | Distance from San Borja | −0.02 | −4.05 | 0.002 | (−0.03, −0.009) |
| Interior location | −0.27 | −1.69 | 0.09 | (−0.61, 0.05) | |
| Spanish Fluency | 0.27 | 2.01 | 0.049 | (0.001, 0.54) | |
|
| |||||
| MBP | Distance from San Borja | −0.03 | −4.27 | 0.001 | (−0.04, −0.02) |
| Interior location | −0.15 | −0.54 | 0.59 | (−0.72, 0.41) | |
| Spanish Fluency | 0.68 | 2.95 | 0.08 | (0.22, 1.15) | |
|
| |||||
| MiBP | Distance from San Borja | −0.03 | −4.12 | 0.001 | (−0.04, −0.02) |
| Interior location | −0.07 | −0.29 | 0.77 | (−0.59, 0.44) | |
| Spanish Fluency | 0.73 | 3.48 | 0.001 | (0.31, 1.16) | |
|
| |||||
| MBzP | Distance from San Borja | −0.01 | −1.65 | 0.10 | (−0.05, 0.01) |
| Interior location | 0.19 | 1.07 | 0.28 | (−0.17, 0.55) | |
| Spanish Fluency | 0.37 | 2.49 | 0.02 | (0.07, 0.70) | |
|
| |||||
| MEP | Distance from San Borja | −0.03 | −3.92 | 0.003 | (−0.05, 0.01) |
| Interior location | −0.61 | −1.90 | 0.06 | (−1.25, −0.034) | |
| Spanish Fluency | 0.29 | 1.09 | 0.28 | (−0.24, 0.81) | |
|
| |||||
| MCOP | Distance from San Borja | −0.02 | −3.43 | 0.001 | (−0.03, −0.01) |
| Interior location | −0.30 | −1.54 | 0.13 | (−0.69, 0.09) | |
| Spanish Fluency | 0.35 | 2.19 | 0.03 | (0.03, 0.67) | |
Figure 3.
Phthalates and Market Access. Representation of urinary phthalate metabolite concentrations in relation to Market Access Metric (Spanish fluency + distance from San Borja: 1 = least market access and 5 = most market access).
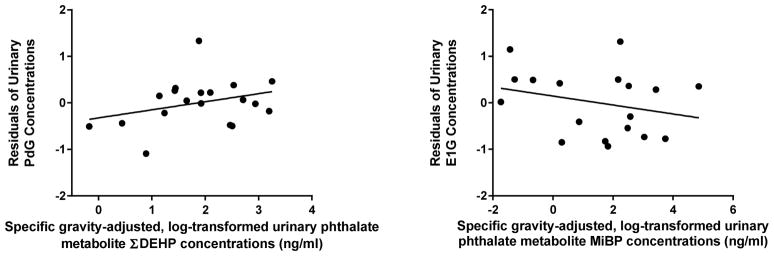
In exploratory analyses, we observed several associations between reproductive hormone levels and urinary phthalate metabolite concentrations. For PdG and E1G models, variance inflation factors for individual phthalates average to 4.3 (range: 2.2 to 8.1) and 4.7 (range: 2.3 to 8.0), respectively, indicated that these variables may be assessed simultaneously in our models (O’Brien, 2007). Among cycling women, there were significant associations between urinary phthalate and reproductive hormone metabolite concentrations (Figure 4). Cycle phase and ΣDEHP significantly associated with variation in PdG concentrations in both models (Table 6: Model 1; cycle phase: F(1,1) = 8.18; p = 0.02; ΣDEHP: F(1,1) = 9.9; p = 0.01), and as predicted PdG levels were higher during the luteal phase. Cycle phase and MiBP concentrations were significantly associated with E1G concentrations (Table 6: Model 1; cycle phase: F(1,1) = 9.3; p = 0.01; MiBP: F(1,1) = 6.30; p = 0.03). Considering only parsimonious findings across models, only ΣDEHP and MiBP phthalates showed persistent associations with variation in either PdG or E1G concentrations.
Figure 4.
Phthalates and Reproductive Hormones. The Residual Values of PDG and E1G models adjusted for age, cycle phase, other phthalates, and body fat (Model 1) graphed against phthalate metabolites with significant associations.
Discussion
In this study, we found evidence of measureable urinary phthalate metabolite concentrations among the Tsimane’, despite their traditional lifestyle and remote geographic location with limited usage of manufactured goods. For most phthalate metabolites, levels were considerably lower among the Tsimane’ as compared to industrialized populations (Table 3). With the exception of MiBP, phthalate metabolite levels among the Tsimane’ were also lower than among other developing and traditional-living populations such as rural Egyptians and OOM living in New York State (Table 3, Colacino et al., 2011; Martina et al., 2012). Distance from village of residence to the nearest city, San Borja, was a significant predictor of most phthalate metabolites measured, with women living closer to the city having higher levels. Spanish fluency was also predictive of phthalate metabolite levels, with greater fluency associated with increased exposure. Ecological location was not a significant predictor of phthalate metabolite levels. Considering a combined market access metric using both distance from San Borja and Spanish fluency, increased market access is associated with increasing urinary phthalate metabolite concentrations (Figure 3). In this isolated population, these measures may be proxies for market access. As a result, individuals who have better geographic access through shorter distance to San Borja and/or who can communicate in Spanish with non-Tsimane’ Bolivians may more readily able to obtain manufactured products containing phthalates, including foodstuffs, plastic kitchenware (bags, bottles, cups, bowls), personal care products, plastic tarps, and medications. These items have all been observed in Tsimane’ households in present day; however were in very limited supply at the time of urine collection. Among the Tsimane’, there is also some (albeit very limited) use of pesticides, insecticides, and medications, all of which may contain phthalates. Ultimately, structured household and community inventories of manufactured goods, along with water and soil sampling, are needed to better understand the potential sources of exposure in this remote population.
In exploratory analyses, we also found preliminary evidence that in this subsistence population, low-dose exposure to phthalates may be associated with altered hormone activity. Due to the significant differences in characteristic hormone profiles between lactating and cycling women, we focused on cycling woman only for this analysis, reducing our sample size to 25 women. Nevertheless, we observed significant associations between certain phthalate metabolites and reproductive axis hormone metabolites. ΣDEHP was significantly positively associated with PdG concentrations in both models, suggesting parsimonious support for the relationship in this limited set of samples. Additionally, MiBP was negatively associated with E1G levels and also significant in both models. These findings echo work in animal models and in vitro suggesting that phthalate exposure can alter ovarian development, function, and steroidogenesis (reviewed in Hannon and Flaws, 2015). However, given the small sample size and the natural variation present in circulating sex hormones, these results need to be confirmed and replicated. A number of studies, in human females, have examined associations between phthalate metabolites and testosterone levels, and both inverse (Meeker and Ferguson, 2014; Sathyanarayana et al., 2014) and positive associations (Watkins et al., 2014) have been noted. Much less is known about phthalates in relation to estradiol and progesterone activity in humans. A study of Taiwanese girls found that serum MEHP and MBzP levels were positively associated with serum progesterone (Su et al., 2014). In a study of pregnant women in Puerto Rico, MEP concentrations were inversely associated with progesterone across pregnancy (Johns et al., 2015). In that study, as well as a U.S. pregnancy cohort, no associations were observed between phthalate metabolite levels and estradiol (Johns et al., 2015; Sathyanarayana et al., 2014). Inconsistent findings across studies may be attributable to differences in timing of exposure, phthalate metabolites considered, reproductive status, and population. Indeed, phthalates may act in a non-monotonic manner (Beausoleil et al., 2013; Vandenberg, 2014), thus the relationship between phthalates and ovarian steroid activity may also differ in low exposure populations (like the Tsimane’) compared to more highly exposed industrialized populations. Additionally, recent research has shown that EDCs, particularly phthalates, have been shown to act as mixtures (Gray et al., 2000; Howdeshell et al., 2008; Rider et al., 2009; Sobolewski et al., 2014). This creates a situation where a combination of phthalates, or concurrent toxicant exposures, may be responsible for alterations in hormone concentrations. Future studies should focus on identifying the possibility that several chemicals, with the same mechanism of action or those that converge on a single physiological pathway, may lead to cumulative effects on reproductive hormone function.
There are several limitations of note. The statistical power is limited by the small sample size, increasing the likelihood of chance or missed associations. As a result, this work requires replication in a larger study population. Also, potential unmeasured sampling differences between contacted and uncontacted women, and women that provided urine, may have influenced results. As mentioned, a total of 1,374 women (74% of the census population) were seen by the medical team during this period and, roughly half of the women not observed (~13%) were absent from their villages during the team visit. The Tsimane’ are semi-nomadic and absences during medical team visits are common. Absences during the study period are unlikely to have been biased towards any specific activity, however, there is still the possibility that unmeasured confounds associated with such sampling and urine collection profiles may have biased hormone profiles or phthalate concentrations. For example, the remaining 13% of unobserved women declined medical examination for unreported reasons. Women who agreed to medical examinations may have been more likely to be ill or have ill young dependents. Conversely, women declining examinations may have had other household obligations. There is the possibility that, although we adjusted for known confounders, there could be residual confounding or other, unmeasured factors that could attenuate the noted associations with hormone profiles. Furthermore, we focused on a single class of chemicals, phthalates, when in reality the Tsimane’ (and all populations) are exposed to complex mixtures. Phthalate exposure may be correlated with other exposures and it is possible that those unmeasured environmental exposures could be driving the endocrine associations we observed. Whether exposure to phthalates and other environmental chemicals may contribute to adverse reproductive health effects in Tsimane’ women are unknown. These results highlight the need for more studies of environmental chemicals in cycling women (both Western and non-Western) in order to better understand their effects on the reproductive and endocrine system. Finally, studies of unique populations like the Tsimane’ may limit our ability to extrapolate findings to other populations. As a result, these studies should be replicated in multiple locally-subsistent, traditional communities to extend the generalizability of data from low levels of human exposure.
Our results clearly demonstrate that exposure to phthalates occurs among the Tsimane’, and most likely, other traditional populations as there is increasing utilization of the global marketplace. This presents a novel context in which to explore the adverse health effects of phthalates and other EDCs. Unlike industrialized populations in which the use of plastics has been widespread for a century, such products have only trickled down to populations like the Tsimane’ in the recent past. In just the few years since the collection of these urine samples (2008–2009), there has been increasing usage of global marketplace goods. In public health surveys conducted from 2013–2015, five years after the current samples were collected, 99% of households reported owning one or more mosquito nets, 61% radios, 31% plastic watches, 15% mobile phones, and 8% motorcycles (n=1016). Eight percent of households surveyed were in current possession of insecticide or fertilizer for use in crop fields (Tsimane Health and Life History Project, unpublished data; survey methodology described in Gurven et al., 2015). This trend of increasing utilization of global market goods will, very likely, result in increased exposure to environmental chemicals for the Tsimane’ over the next decade.
Nevertheless, today’s Tsimane’ adults most likely represent the first generation with exposure to phthalates, and in all likelihood, did not experience in utero exposure, in contrast to industrialized populations. This raises the previously impossible option of studying the adult effects of EDC exposure separate from transgenerational or in utero effects in humans. Following this population and subsequent generations may yield important information on inter-generational effects that are no longer possible to study in industrialized countries. This setting is also an ideal one in which to explore the extent to which phthalates (and other EDCs) demonstrate non-monotonic dose-response relationships, whereby adverse health effects at low doses are potentially unpredicted by known influences at higher doses (Vandenberg, Colborn, 2012). The suggested health influence of low-dose EDCs on reproductive and behavioral health is a public health issue of critical importance (Birnbaum, 2013).
Studying the Tsimane’, a natural fertility population, may offer valuable insight on fertility-related outcomes as well. For instance, recent data from a large study of women undergoing assisted reproductive technology found that women with the highest levels of exposure to ΣDEHP were less likely to have clinical pregnancies or live births (Hauser et al., 2015). The Tsimane’ and other similar populations offer additional opportunities to study phthalates in relation to time to pregnancy, pregnancy loss, and total fertility, all of which are extremely difficult to study in industrialized populations in which use of hormonal contraception and family planning are standard.
Conclusions
Overall, remote, subsistence populations like the Tsimane’ offer a unique window into the health effects of endocrine disrupting chemicals (like phthalates) because: (1) exposures are low-dose and likely to be first generation; (2) a natural fertility lifestyle allows for better exploration of downstream reproductive effects; (3) large family sizes provides an opportunity to assess influences on fertility.
Figure 1.
Map of San Borja and neighboring Tsimane’ villages.
Figure 2.
Phthalates and Distance from Town. Log-transformed, specific-gravity adjusted urinary phthalate metabolite concentrations in relation to distance from village of residence to nearest city (San Borja) among Tsimane’ women (n=59).
Highlights.
Phthalates are a class of plasticizing chemicals associated with adverse health effects.
Identifying no or low exposure individuals is necessary to discern low dose effects.
Tsimane’ live locally subsistent lifestyles in remote villages in Bolivia.
Phthalate exposure is significantly associated with market access in Tsimane’ women.
Phthalates (ΣDEHP and MiBP) are associated with altered urinary hormone levels.
Acknowledgments
The authors would like to thank Drs. Melissa Emery-Thompson and Ben Trumble for their logistical and laboratory assistance. We thank Antonia Calafat and Xiaoyun Ye (Centers for Disease Control) for urinary phthalate metabolite analyses and Drs. Shanna Swan, Joshua Allen and Deborah Cory-Slechta for their comments on the manuscript. Funding for the current analysis was provided by the URMC IHSFC, which is supported by NIH grant P30ES001247. The Tsimane Health and Life History Project was funded by NIH grant R01AG024119-01. Additional support for E. Barrett was provided by K12ES019852 and P30ES005022. M. Sobolewski was supported by T32ES007026.
Footnotes
The authors have no competing financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose–response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227:185–192. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Windham GC, Swan SH. Differences in ovarian hormones in relation to parity and time since last birth. Fertility and sterility. 2014;101:1773–1780. e1771. doi: 10.1016/j.fertnstert.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil C, Ormsby JN, Gies A, Hass U, Heindel JJ, Holmer ML, Nielsen PJ, Munn S, Schoenfelder G. Low dose effects and non-monotonic dose responses for endocrine active chemicals: science to practice workshop: workshop summary. Chemosphere. 2013;93:847–856. doi: 10.1016/j.chemosphere.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. Environmental chemicals: evaluating low-dose effects. Environ Health Perspect. 2012;120:A143–144. doi: 10.1289/ehp.1205179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS. State of the science of endocrine disruptors. Environmental health perspectives. 2013;121:a107. doi: 10.1289/ehp.1306695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Burger H. The menopausal transition--endocrinology. The journal of sexual medicine. 2008;5:2266–2273. doi: 10.1111/j.1743-6109.2008.00921.x. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U. Low-dose perinatal exposure to di (2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reproductive Toxicology. 2010;30:313–321. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Colacino JA, Soliman AS, Calafat AM, Nahar MS, Van Zomeren-Dohm A, Hablas A, Seifeldin IA, Rozek LS, Dolinoy DC. Exposure to phthalates among premenstrual girls from rural and urban Gharbiah, Egypt: A pilot exposure assessment study. Environ Health. 2011;10:40. doi: 10.1186/1476-069X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do RP, Stahlhut RW, Ponzi D, vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di (2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reproductive toxicology. 2012;34:614–621. doi: 10.1016/j.reprotox.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Human reproduction. 2005;20:604–610. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental health perspectives. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA pediatrics. 2014;168:61–67. doi: 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Price M, Veeramachaneni DR, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gurven M. Economic games among the Amazonian Tsimane: Exploring the roles of market access, costs of giving, and cooperation on pro-social game behavior. Experimental Economics. 2004;7:5–24. [Google Scholar]
- Gurven M, Jaeggi AV, von Rueden C, Hooper PL, Kaplan H. Does market integration buffer risk, erode traditional sharing practices and increase inequality? A test among Bolivian forager-farmers. Human ecology: an interdisciplinary journal. 2015;43:515–530. doi: 10.1007/s10745-015-9764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Flaws JA. The effects of phthalates on the ovary. Frontiers in endocrinology. 2015;6:8. doi: 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat A. Phthalates and human health. Occupational and environmental medicine. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing Fertilization: Results from the EARTH Study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicological Sciences. 2008;105:153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC. Association between prenatal exposure to phthalates and the health of newborns. Environment International. 2009;35:14–20. doi: 10.1016/j.envint.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Irvin EA, Calafat AM, Silva MJ, Aguilar-Villalobos M, Needham LL, Hall DB, Cassidy B, Naeher LP. An estimate of phthalate exposure among pregnant women living in Trujillo, Peru. Chemosphere. 2010;80:1301–1307. doi: 10.1016/j.chemosphere.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-Gonzalez LO, Del Toro LV, Calafat AM, Ye X, Alshawabkeh AN, Cordero JF, Meeker JD. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reproductive biology and endocrinology : RB&E. 2015;13:4. doi: 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AC, Whyatt RM, Perzanowski MS, Calafat AM, Perera FP, Goldstein IF, Chen Q, Rundle AG, Miller RL. Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environmental health perspectives. 2012;120:1475–1480. doi: 10.1289/ehp.1104544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. International journal of andrology. 2008;31:233–240. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- Martin MA, Lassek WD, Gaulin SJ, Evans RW, Woo JG, Geraghty SR, Davidson BS, Morrow AL, Kaplan HS, Gurven MD. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Maternal & child nutrition. 2012;8:404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina CA, Weiss B, Swan SH. Lifestyle behaviors associated with exposures to endocrine disruptors. Neurotoxicology. 2012 doi: 10.1016/j.neuro.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L, Gurven M, Kaplan H, Stieglitz J. Why do women have more children than they want? Understanding differences in women’s ideal and actual family size in a natural fertility population. American journal of human biology : the official journal of the Human Biology Council. 2012;24:786–799. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Urinary Phthalate Metabolites Are Associated With Decreased Serum Testosterone in Men, Women, and Children From NHANES 2011–2012. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-2555. jc20142555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiology of aging. 1995;16:837–843. doi: 10.1016/0197-4580(95)00072-m. discussion 855–836. [DOI] [PubMed] [Google Scholar]
- Noth RH, Mazzaferri EL. Age and the endocrine system. Clinics in geriatric medicine. 1985;1:223–250. [PubMed] [Google Scholar]
- O’brien RM. A caution regarding rules of thumb for variance inflation factors. Quality & Quantity. 2007;41:673–690. [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, Gray LE. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicologic pathology. 2009;37:100–113. doi: 10.1177/0192623308329478. [DOI] [PubMed] [Google Scholar]
- Salvante KG, Brindle E, McConnell D, O’Connor K, Nepomnaschy PA. Validation of a new multiplex assay against individual immunoassays for the quantification of reproductive, stress, and energetic metabolism biomarkers in urine specimens. American journal of human biology : the official journal of the Human Biology Council. 2012;24:81–86. doi: 10.1002/ajhb.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang CW, Swan S. Phthalate Exposure and Reproductive Hormone Concentrations in Pregnancy. Reproduction (Cambridge, England) 2014;147:401–409. doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. International journal of andrology. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Deborah AC-S. Sex-Specific Enhanced Behavioral Toxicity Induced by Maternal Exposure to a Mixture of Low Dose Endocrine-Disrupting Chemicals. Neurotoxicology. 2014 doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult US males. Environmental health perspectives. 2007:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PH, Chen JY, Lin CY, Chen HY, Liao PC, Ying TH, Wang SL. Sex steroid hormone levels and reproductive development of eight-year-old children following in utero and environmental exposure to phthalates. PloS one. 2014;9:e102788. doi: 10.1371/journal.pone.0102788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S, Liu F, Hines M, Kruse R, Wang C, Redmon J, Sparks A, Weiss B. Prenatal phthalate exposure and reduced masculine play in boys. International journal of andrology. 2010;33:259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental health perspectives. 2005;113:1056. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.E.P.A. Phthalates Action Plan. U.S.E.P.A; Washington, D.C: 2012. [Google Scholar]
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study. Dose-response : a publication of International Hormesis Society. 2014;12:259–276. doi: 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veile A, Martin M, McAllister L, Gurven M. Modernization is associated with intensive breastfeeding patterns in the Bolivian Amazon. Social Science & Medicine. 2014;100:148–158. doi: 10.1016/j.socscimed.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DJ, Tellez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, Peterson KE, Meeker JD. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res. 2014;134:233–241. doi: 10.1016/j.envres.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt R, Arias F, Calafat A, Rauh V, Factor-Litvak P. Associations between maternal prenatal phthalate urinary metabolite concentrations and child mental and motor development at age 3 years. Epidemiology. 2011;22:S126–S127. [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP. Research| Children’s Health. Environmental health perspectives. 2012;120:291. doi: 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Hum Reprod. 2008;23:2555–2563. doi: 10.1093/humrep/den213. [DOI] [PubMed] [Google Scholar]