Abstract
Traumatic brain injury (TBI) leads to a series of pathological events that can have profound influences on motor, sensory and cognitive functions. Conversely, TBI can also stimulate neural stem/progenitor cell proliferation leading to increased numbers of neuroblasts migrating outside their restrictive neurogenic zone to areas of damage in support of tissue integrity. Unfortunately, the factors that regulate migration are poorly understood. Here, we examine whether ephrinB3 functions to restrict neuroblasts from migrating outside the subventricular zone (SVZ) and rostral migratory stream (RMS).We have previously shown that ephrinB3 is expressed in tissues surrounding these regions, including the overlying corpus callosum (CC), and is reduced after controlled cortical impact (CCI) injury. Our current study takes advantage of ephrinB3 knockout mice to examine the influences of ephrinB3 on neuroblast migration into CC and cortex tissues after CCI injury. Both injury and/or ephrinB3 deficiency led to increased neuroblast numbers and enhanced migration outside the SVZ/RMS zones. Application of soluble ephrinB3-Fc molecules reduced neuroblast migration into the CC after injury and limited neuroblast chain migration in cultured SVZ explants. Our findings suggest that ephrinB3 expression in tissues surrounding neurogenic regions functions to restrict neuroblast migration outside the RMS by limiting chain migration.
Keywords: Traumatic brain injury, Neuroblast, Migration, EphrinB3
1. Introduction
In the adult SVZ, slow dividing type B stem cells give rise to rapidly dividing type C neural progenitor cells (or transit amplifying cells) that eventually differentiate into slow dividing type A neuroblasts (Doetsch et al., 1997, 1999; Aguirre et al., 2004). Neuroblasts are capable of migrating long distances, through a neurogenic region called the rostral migratory stream (RMS), to eventually terminally differentiate into interneurons in the olfactory bulb (Belluzzi et al., 2003). The RMS is a specialized migratory route that is ensheathed by astrocytes to form a tube-like structure, and is thought to provide instructive signals to the migrating neuroblasts (Sun et al., 2010). Migrating neuroblasts move in chains, where gap and adherens junctions between neighboring neuroblasts form connected movements (Lois et al., 1996; Ohab et al., 2006; Yamashita et al., 2006; Belvindrah et al., 2007; Kojima et al., 2010). After CNS injury and disease, neuroblasts have been shown to exit the RMS and migrate ectopically to sites of tissue damage (Arvidsson et al., 2002; Parent et al., 2002; Dixon et al., 2015); however, little is known about the cues that regulate ectopic migration. Outside the neurogenic regions neuroblasts have the capacity to differentiate into both neural and glial cell fates (Miragall et al., 1990; Bonfanti and Theodosis, 1994; Doetsch et al., 1997). In models of traumatic brain injury (TBI), migrating neuroblasts have been shown to provide beneficial effects to damaged tissues, such as the cortex, where they express trophic factors that support residential cell survival (Li et al., 2010; Dixon et al., 2015). In the absence of these cells, increased tissue damage and reduced functional recovery is observed (Dixon et al., 2015). Thus, expanding this population of cells and increasing their numbers in injured tissues can benefit functional recovery.
Recent studies have shown that a family of receptor tyrosine kinase receptors, Eph receptors, and their ephrin ligands can regulate neural stem/progenitor cell (NSPC) and neuroblast proliferation and survival (Ricard et al., 2006; Furne et al., 2009; Theus et al., 2010; Theus et al., 2014). The ligand ephrinB3 is expressed in tissues surrounding the neurogenic niche, including by astrocytes that ensheathe the RMS (Ricard et al., 2006; Zhuang et al., 2010), whereas NSPCs express the EphB3 and EphA4 receptors but not ephrinB3 (Furne et al., 2009; Theus et al., 2010). Ephrins and Eph receptors are membrane-bound proteins that require direct cell-cell interaction to induce receptor signaling (Gale et al., 1996). B-class ephrins represent a subclass of ephrins that contain an intracellular domain also capable of signaling, such that interactions with their respective Eph receptor(s) can lead to bidirectional signals. Ephrins and Eph receptors are historically known to facilitate developmental tissue patterning and axon pathfinding through modulating actin cytoskeleton dynamics (Boyd et al., 2014). Activation of EphB3 and EphA4 by ephrinB3 can also induce anti-proliferative signs to negatively regulate the neurogenic response in both naïve and TBI conditions (Ricard et al., 2006; Furne et al., 2009; Theus et al., 2010; Theus et al., 2014). Here, we examine whether ephrinB3 regulates neuroblast migration in the TBI brain using loss-of-function and gain-of-function approaches, and demonstrate that ephrinB3 functions to restrict neuroblast movement to damaged tissues by limiting chain migration following injury.
2. Material & methods
2.1. Animals
Male C57Bl/6 mice, aged 2 to 4 months, were used for the current study. The generation and genotyping of wildtype and ephrinB3−/− mice has been previously described (Yokoyama et al., 2001). Animals were housed in a 12-hour light/dark cycle with food and water ad libitum. Procedures related to animal use conducted at the University of Miami were approved by the University of Miami Animal Use and Care Committee (in accordance with NIH care and use of laboratory animals). Those conducted at the University of Melbourne were approved by the University of Melbourne Animal Ethics Committee and in strict accordance with the National Health and Medical Research Council of Australia guidelines.
2.2. Controlled cortical impact (CCI) injury, cortical cannula injury and ephrinB3-Fc infusion
Mice were anaesthetized with ketamine (75 mg/kg body weight; VEDCO, Missouri USA) and xylazine (14 mg/kg body weight; LLOYD Laboratories, Philippines) by intraperitoneal injection and positioned in a stereotaxic frame. Body temperature was monitored with a rectal probe and maintained at 37 °C with a controlled heating pad set. A 5 mm craniotomy was made using a portable drill over the right parieto-temporal cortex (–2.5 mm caudal and 2.0 mm lateral from bregma). The CCI injury was generated using a 3 mm beveled stainless steel tip attached to an eCCI-6.3 device (Custom Design & Fabrication, VA USA), at a velocity of 4.0m/s, depth of 0.5 mm deep and 150 ms impact duration. After CCI injury the skin was sutured using 5-0 silk sutures, and the animals allowed recover on a heat pad. For the cannula injury, a cannula (0.3 mm wide, Alzet, CA USA) with 3 spacers was lowered into the craniotomy using a stereotaxic holder, and glued to the skull surface using trace amounts of Loctite 454 glue. Tubing 1-inch-long joined the cannula to mini-osmotic pumps pre-filled 18 h in advance with clustered ephrinB3-Fc or human Fc control molecules (R&D Systems, MN USA). 140 μg/mL of ephrinB3-Fc or Fc molecules were pre-clustered with goat anti-human Fc antibody (Jackson Research Laboratories, PA USA) at a ratio of 10:1 in PBS for 2 h prior to loading pump. Following the attachment of the cannula, pumps were placed under the skin of the dorsal neck region for an infusion over a 2-day period (0.5 μl/h). Skin was sutured using 5-0 silk sutures, and the animals allowed recovery on a heat pad.
2.3. SVZ dissection and immunohistochemistry
To analyze neuroblast migration in the SVZ of naïve or CCI-injured mice, brains were removed, cut sagittally, and the SVZ of each hemisphere was dissected out and placed in 4% paraformaldehyde (PFA, pH 7.4, Sigma, MO USA). After 30 min each SVZ was placed in phosphate buffered saline (PBS) for a maximum of 3 days until immunohistochemistry was performed. The SVZ was blocked in 2% fish gel (Sigma, MO USA) containing 0.2% triton-X100 (Sigma, MO USA) for 24 h at 4 °C, then incubated in primary antibody (goat anti-DCX 1:100, Santa-Cruz, TX USA; rat anti-CD31 1:500, BD Pharmingen, CA USA) diluted in blocking solution for 48 h at 4 °C, followed by washing in PBS for 2 h. The SVZs were then incubated in fluorescent secondary antibody (1:300, Molecular Probes, NY USA) diluted in PBS for 24 h at 4 °C. Samples were then washed in PBS overnight at 4 °C and carefully cover-slipped in mounting medium.
2.4. Histological preparation and immunohistochemistry
To analyze neuroblast migration in the RMS, CC and cortex at 3 days after CCI-injury or at 2 days after cannula injury, mice were perfused with PBS and 4% PFA (pH 7.4, Sigma). The brains were stored in 4% PFA for 2 h, 30% sucrose for 48 h and then frozen in OCT on dry-ice and stored at–80 °C. Either 30 μmthick serial sagittal sections (CCI-injured brains) or 20 μm thick serial sagittal sections (cannula-injured brains) were cut and stored at –80 °C. Sections were permeabilized with 0.2% triton-X100 (Sigma, MO USA) and immunohistochemically labeled with the primary antibodies (goat anti-DCX 1:100, Santa-Cruz, TX USA; rabbit anti-glucose transporter-1 (GT-1) 1:500, Millipore, MA USA) overnight at 4 °C. All sections were washed 3 times in PBS, incubated in fluorescent secondary antibodies (1:300, Molecular Probes) for 30 min at room temperature, washed an additional 3 times in PBS and cover slipped in mounting medium containing DAPI (Invitrogen, NY USA).
2.5. Human brain injured tissue collection and western blot analysis
Collection of human brain injured tissue was approved by the University of Miami Institutional Review Board (protocol #20030154 and 20080609), and informed consent was obtained for each patient. De-identified patient demographics are identified in Table 1. Tissues from control individuals #15 and #16 were obtained following terminal heart disease and not TBI, while all other tissues were from TBI patients. In accordance with standard neurosurgical practice, brain tissues were collected from patients using a rongeur from amorphous contusion and peri-contusional cortical tissue, mostly at the tips of pulverized temporal and frontal lobes that would otherwise have been discarded at the time of decompression surgery for removal of an intracranial hematoma. The tissue samples were placed into sterile Hibernate A media (Brainbits LLC, IL USA) and frozen at–80 °C. The samples were lysed in modified RIPA buffer, and equal amounts of protein were resolved on 10% SDS-PAGE gels, transferred to PVDF membranes and probed using rabbit anti-ephrinB3 (1:80, Invitrogen) and mouse anti-β-actin (1:8000, Sigma) in 5% milk diluted in TBST overnight at 4 °C. Membranes were washed in TBST then incubated in HRP-conjugated secondary antibodies for 30 min before being incubated in ECL (Thermo Scientific, NY USA) and imaged.
Table 1.
Patient demographics.
| De-identified patient # | 15 | 16 | 7 | 3 | 9 | 4 | 12 | 10 | 13 |
|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | M | M | M | M | F |
| Race | C | C | B | C | B | C | Unknown | C | H |
| Age | 55 | 57 | 25 | 30 | 41 | 31 | 45 | 18 | 41 |
| TBI | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reason | Heart | Heart | GSW | MVA | GSW | MVA | BFT | MVA | GSW |
| GCS (arrival at hospital) | n/a | n/a | 6 | 7 | 13 | 9 | Unknown | 6 | 7 |
| Time in hours (hospital admittance to surgery) | n/a | n/a | 3 | 3.5 | 4.5 | 8.5 | 17.5 | 122 | 4.25 |
| Tissue location | T | T | LT | RT | RO | RF | LT | LRFT | RO |
| GCS (at discharge or death) | n/a | n/a | 3 | 3 | 14 | 10 | Unknown | 3 | 9 |
| GOS-E (at 3 year follow-up) | n/a | n/a | 1 | 1 | 3 | 8 | Unknown | 1 | n/a |
GCS = Glasgow Coma Scale; GOS-E= Extended Glasgow Outcome Scale; n/a= not available; Heart=heart disease; GSW= gun shot wound; MVA = motor vehicle accident; BFT = blunt force trauma; T=temporal; LT=left temporal; RT=right temporal; RO=right occipital; RF=right frontal; LRFT=left & right frontal & temporal.; M=male; F=female; C=Caucasian; B = Black or Americans of African decent; H = Hispanic.
2.6. Stereological cell counts
For in vivo analysis of neuroblast migration, lateral sections containing the SVZ and CCI injury site, as well as more medial sections containing the SVZ, RMS and cannula injury site were collected. Stereology of DCX-positive neuroblasts was performed using a motorized Zeiss Axiovert 200 M microscope, Qimaging camera and Micro Bright Field StereoInvestigator 11.03 software package. For the CCI-injury studies, non-biased cell number estimations were performed on 2 areas, the caudal peri-lesional region (as determined by disorganization of DAPI-labeling in the cortex) and the RMS (as determined by intensity of DCX-labeling). At both locations 5 sections, which were 30 μm thick and 180 μm apart were counted. For cannula injury studies, non-biased cell number estimations were performed on the 4 most central sections containing the RMS (as determined by intensity of DCX-labeling), that were 20 μm thick and 120 μm apart. The number of DCX-positive cells with an identifiable nucleus was counted using the optical fractionator at 63× magnification.
Using lateral sections, the entire CC was outlined and a contour of 66,436 μm2 was placed at the ventral edge of the caudal CC, just rostral to the beginning of the hippocampus, at 5×magnification, and a grid of 40 × 40 μm was placed over this area, containing a counting frame of 20 × 20 μm. For counts in the peri-lesional region, the entire injury site was outlined using DAPI-labeling and a contour of 35,160 μm2 wide was placed over intact cortical tissue at the ventral edge of the injury site, above the caudal CC contour at 5× magnification, and a grid of 50 × 50 μm was placed over this area, containing a counting frame of 20 × 20 μm.
Using medial sections, the area of the RMS was outlined and 2 contours of 6351 μm2 wide were placed alongside each other on the RMS approximately at the caudal genu (i.e. below the cannula injury location) at 5× magnification, and a grid of 40 × 40 μm was placed over this area, containing a counting frame of 20 × 20 μm. For counts in the rostral CC, the entire area of the CC was outlined and 2 contours of 29,180 μm2 wide were placed alongside each other at the dorsal edge of the CC directly in line with the RMS contours (i.e. below the cannula injury location) at 5× magnification, and a grid of 40 × 40 μm was placed over this area, containing a counting frame of 20 × 20 μm. For counts in the rostral cortex, a contour of 47,486 μm2 wide was placed immediately above the CC contours (i.e. below the cannula injury location) at 5×magnification, and a grid of 40 × 40 μm was placed over this area, containing a counting frame of 20 × 20 μm.
2.7. Histological analysis
For additional in vivo analysis, photomicrographs of sections containing the rostro-caudal extent of the corpus callosum (CC) and RMS were taken at 10× magnification on a Zeiss Axiovert 20 M microscope using a Zeiss AxioCam MRm camera with Axiovision software (version 4.8). Using ImageJ photographs containing the rostral CC the mode of neuroblast migration was assessed by manually counting the number of DCX-positive chains or cell clusters. The chains or clusters consisted of at least 3 DCX-positive cells aligned in a row (chains) or touching each other in a ball shape (clusters). Additionally, in the same photographs the distance between each DCX-positive chain/cluster and GT1-positive blood vessels was measured. For chain and cluster quantitation, as well as for neuroblast-vessel distance, cell counts and distances were averaged for each photograph then averaged across each section, animal and group.
2.8. SVZ explants
To assess neuroblast migration, cultures from SVZ explants were established from postnatal 1–2 day old C57BL/6 wildtype mice. SVZ tissue at –2.10 to –2.20 mm Bregma was carefully dissected, placed in ice-cold Hanks Balanced Salt Solution (Gibco-Invitrogen) and sliced into coronal sections. SVZ was dissected from the wall of the lateral ventricle and cut into explants. Explants were mixed with growth factor reduced Matrigel (Becton-Dickinson, Australia) diluted 3:1 in Neurobasal medium supplemented with B-27, glutamine and penicillin/streptomycin (all from Life Technologies, Australia), and cultured in four-well 3 mm dishes. Explants were cultured under four conditions: basal (Matrigel alone);Matrigel containing 1 μg/mL human anti-Fc-molecules (Vector Laboratories, USA); Matrigel containing 10 μg/mL ephrinB3-Fc (R&D Systems Inc., USA) or Matrigel containing clustered ephrinB3-Fc (10 μg/mL ephrinB3 pretreated with 1 μg/mL human anti-Fc molecules for 30 min at room temperature). After polymerization (20 min), 2 mL of supplemented Neurobasal media was added. Cultures were maintained in a humidified, 5% CO2, 37 °C incubator for 72 h. Explants were fixed with pre-warmed 4% PFA in PBS for 1 h, and washed with PBS containing DAPI. They were then incubated with blocking buffer (5% donkey serum, 0.5% Triton X-100 in PBS), followed by goat anti-double cortin (DCX) antibody (1:500; Santa Cruz Biotechnology) diluted in blocking buffer overnight at 4 °C. Explants were washed with PBS and incubated with secondary antibody, Cy3-conjugated donkey anti-goat (1:500; Jackson ImmunoResearch) for 48 h at 4 °C, and washed again with PBS. All explant images for migration analysis were captured on an Olympus IX81 inverted fluorescent microscope at 4× magnification. The observed cell migration from SVZ explants was categorized into three groups: explants that gave rise to compact chains, explants that exhibited mixed cell outgrowth (both individual cells migrating and cells migrating in chains), and explants that only exhibited individual cell migration. The extent of chain and cell outgrowth was assessed using a semi-quantitative scale: 0 = no outgrowth; 1 ≤ 10 chains/cells; 2 = ~10–50 chains/cells; 3 = ~50–100 chains/cells; 4 = extensive (>100 chain/cell) growth. The area of migration per explant was assessed using Axiovision software v4.1 (Zeiss, Thornwood, NY). The area of the explant body was subtracted from the area of outgrowth and then normalized for explant size by expressing as the ratio of area of outgrowth (i.e. explant size). Results are from 39–48 explants combined from 3 individual experiments and graphs show mean ± SEM.
2.9. Statistical analysis
Data were assessed for homogeneity of variance, after which statistical analysis was performed using GraphPad Prism (v4.03). Data in figures is expressed as mean ± SEM. Histological differences were assessed using Student’s t-test for comparisons between two groups or one-way Anova with Student-Newman-Keuls post-hoc test for comparisons between multiple groups. For CCI injury and cannula studies, it should be noted that comparisons were made between non-injured and injured groups within a genotype and not between genotypes. This rationale is based on our observations in current and previous studies that ephrinB3−/− mice are developmental different from WT mice in NSPC proliferation and survival in the subventricular zone and olfactory bulb (Ricard et al., 2006; Theus et al., 2010) and thus non-injured WT mice are not comparable with CCI injured ephrinB3−/− mice or vice versa. For the SVZ explants, group statistics were analyzed using one-way ANOVA and individual comparisons made using the Bonferroni post-hoc test.
3. Results
3.1. EphrinB3 restricts neuroblast migratory streams in the SVZ
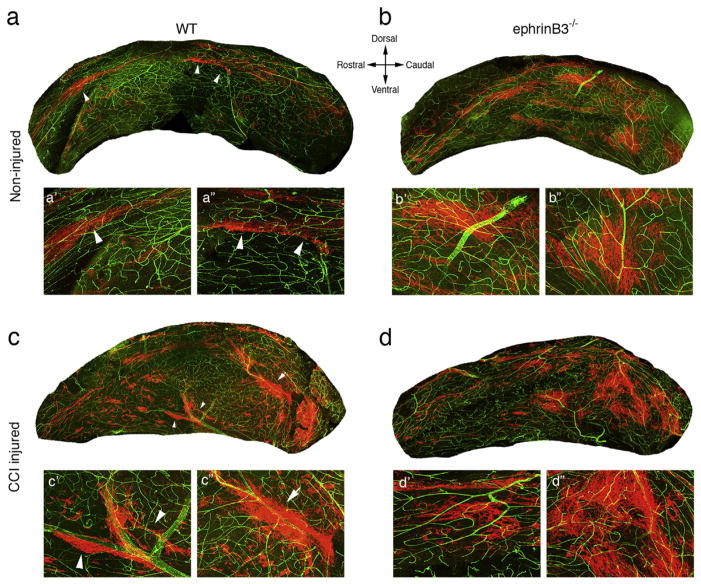
Eph receptors are expressed by neural stem/progenitor cells (NSPCs) and neuroblasts in the adult subventricular zone (SVZ) and interact with ephrinB3 in the surrounding tissues to regulate proliferation and survival (Ricard et al., 2006; Theus et al., 2010; Theus et al., 2014). To initially evaluate whether ephrinB3 also plays a role in migration in the adult SVZ, we examined whether neuroblast migration patterns (or streams) were altered in the presence and absence of ephrinB3. Whole mount tissue preparations of the SVZ from wild type (WT) and ephrinB3−/− mice were immunolabeled with anti-DCX antibodies that recognize neuroblasts, as well as vascular anti-CD31 antibodies to provide tissue perspective and orientation. In adult WT mice, DCX-positive neuroblasts were observed in tight streams (arrow heads) mainly on the dorsal SVZ regions (Fig. 1a, a′, a″). In ephrinB3−/− mice, there were greater numbers of neuroblasts throughout the SVZ, and migratory streams showed a less compact sheet-like appearance (Fig. 1b, b′, b″). These findings provide a visible perspective to support our previously published studies showing increased neuroblast numbers in the SVZ in the absence of ephrinB3 (Ricard et al., 2006).
Fig. 1.
EphrinB3 and CCI injury alter neuroblast migration in the subventricular zone (SVZ). The SVZ was dissected from non-injured (a,b) and CCI injured (c,d) wild type (WT) (a,c) and ephrinB3−/− (b,d) mice, and labeled with anti-DCX (red) and anti-CD31 (green) antibodies. In non-injured WT, neuroblasts can be seen predominantly in thick streams (arrowheads) across the dorsal aspect of the SVZ (a). High magnification insets (a′,a″). CCI injury leads to a mixture of tight (arrowheads) and broader streams (arrows) (c). High magnification insets (c′,c″). In ephrinB3−/− mice, neuroblasts are mainly observed in broad streams (b) and little change after CCI injury (d). High magnification insets (b′,b″,d′,d″).
Controlled cortical impact (CCI) injury also upregulates neuroblast numbers in the SVZ within the first week post-injury (Theus et al., 2010; Dixon et al., 2015), which is thought to result from reduced ephrinB3 expression in the CCI injured brain (Theus et al., 2010; Theus et al., 2014). Thus, we examined neuroblast migratory patterns in the SVZ after CCI injury in both WT and ephrinB3−/− mice. We observed increased DCX-positive neuroblast numbers and a mixture of tight neuroblast streams (arrow heads) and broader neuroblast sheets (arrow) in the WT (Fig. 1c, c′, c″) and ephrinB3−/− SVZ (Fig. 1d, d′, d″) at 3 days post-CCI injury (dpi). These observations were similar to the non-injured ephrinB3−/− SVZ, and together support the ability of CCI injury and/or ephrinB3 deletion to alter neuroblast migration patterns in the SVZ.
3.2. CCI injury disrupts neuroblast migration
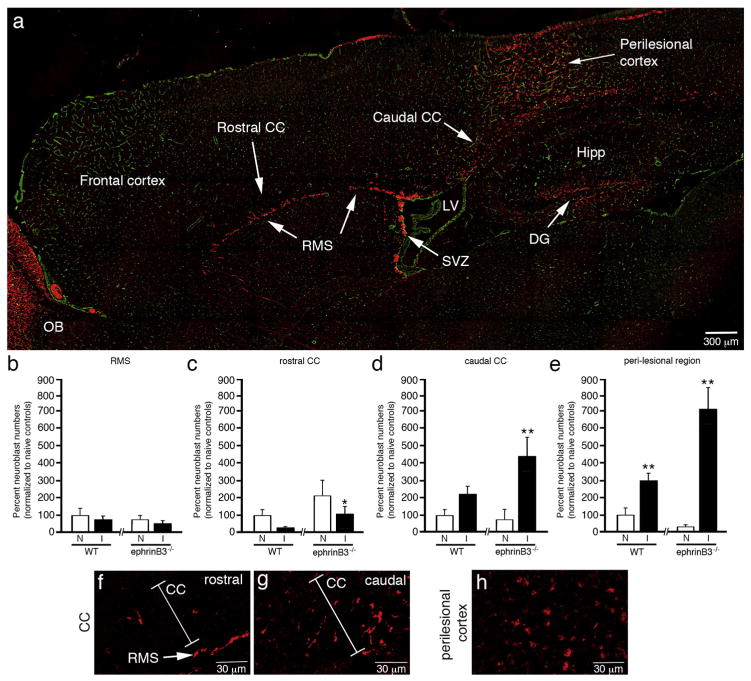
To examined whether neuroblast migration through the rostral migratory stream (RMS) is altered following CCI injury, and examined whether ephrinB3 influenced their ability to migrate outside the neurogenic zones. The CCI injury cortical epicenter was contained to the right parieto-temporal cortex directly above the hippocampus, and neuroblasts numbers were quantified in the RMS, CC, and the perilesional cortex using non-biased stereology (Fig. 2a). In addition, the CC was separated into two divisions based on whether the tissue being analyzed was rostral or caudal to the SVZ-RMS interface.
Fig. 2.
EphrinB3 increases neuroblast migration outside the SVZ and RMS. Immunolabeled sagittal brain section from a WT mouse showing anti-DCX labeled neuroblasts (red) in RMS, CC and perilesional cortex 3 days after CCI injury, while anti-CD31 (green) labeled vessels were used for tissue referencing (a). Stereological cell counts of the RMS (b), rostral CC (c), caudal CC (d) and peri-lesional cortex (e) show a rostral-to-caudal gradient of neuroblasts from the rostral CC towards the caudal CC and injury site. Increased neuroblasts numbers were observed in the rostral CC (c), caudal CC (d) and perilesional cortex (e) in ephrinB3−/− as compared to WT mice. High-magnification images of neuroblasts in the rostral CC (f), caudal CC (g) and perilesional cortex (h). CC, corpus callosum; DG, dentate gyrus; Hipp, Hippocampus; LV, lateral ventricle; OB, olfactory bulb; RMS, rostral migratory stream; SVZ, subventricular zone. *p < 0.05, **p < 0.01 as compared with their respective non-injured controls.
In non-injured mice, we observed no significant difference in RMS neuroblasts numbers between ephrinB3−/− mice and WT controls. Small numbers of DCX-labeled neuroblasts were also observed in the rostral CC, caudal CC and cortex in non-injured conditions, suggesting that neuroblasts may not be strictly confined to the RMS in C57Bl/6 mice. After CCI injury, we did not observe differences in RMS neuroblast numbers between WT and ephrinB3−/− mice at 3 dpi (n=6; Fig. 2b), although differences were observed in the CC and cortex. In the non-injured rostral CC we observed a 2.1-fold increase in the number of neuroblasts in the absence of ephrinB3, suggesting the ephrinB3 may restrict migration outside the RMS (Fig. 2c). However, CCI injury led to approximately 70% and 50% fewer neuroblasts in the rostral CC for WT and ephrinB3−/− mice, respectively. Reduced neuroblast numbers in the rostral CC but not RMS after CCI injury suggests that neuroblasts may be retained in the RMS or quickly diverted to another location. Increased neuroblast numbers in the caudal CC and perilesional cortex support this later possibility and suggest neuroblasts are directed towards injured tissues (Fig. 2d, e). In the absence of ephrinB3, neuroblast numbers in the CCI injured caudal CC and perilesional cortex were significantly increased (Fig. 2d,e). High-magnification images show DCX-positive cells in the rostral CC (Fig. 2f), caudal CC (Fig. 2g) and perilesional cortex (Fig. 2h).
3.3. Neuroblasts migrate towards injured tissues using a cannula cortical injury model, and ephrinB3 functions to restrict migration
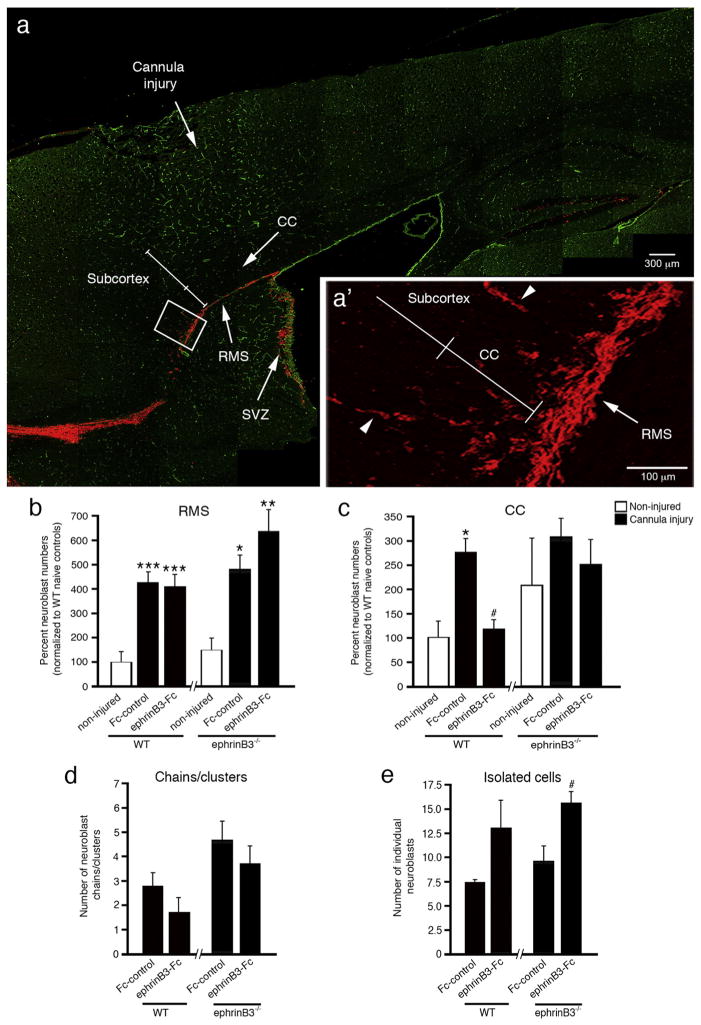
We next examine whether a more localized and focal injury would influence migration from the rostral RMS. A cannula injury dorsal to the rostral CC results in focal cortical damage but also a means to introduce agents into this local environment through Alzet pump infusion (Fig. 3a). At 2 dpi we examined neuroblast numbers in the RMS to determine whether a cannula injury influenced overall neuroblast numbers similar to that observed for CCI injury. In fact, cannula injury led to a significant 4 to 6-fold increase in neuroblast numbers in the rostral RMS in both WT and ephrinB3−/− mice (Fig. 3b). This supports the possibility that neuroblasts migrate to the site of injury and not that caudal tissues provide a more permissive environment for migration. Infusion of ephrinB3-Fc did not significantly influence neuroblast numbers in the RMS.
Fig. 3.
EphrinB3-Fc infusion reduces neuroblast migration into the overlying CC at 2 days following cannula injury. Immunolabeled sagittal brain section from a WT mouse shows anti-DCX labeled neuroblasts (red) in the RMS, CC and subcortical tissues at 2 days post-injury, while anti-CD31 (green) labeled vessels were used for tissue referencing (a). High-magnification inset of neuroblasts migrating from RMS to CC and subcortical tissues (arrowheads depict neuroblast chains) (a′). Stereology shows increased numbers of neuroblasts in the RMS (b) and CC (c) in Fc-control WT mice as compared with non-injured WT mice. Infusion of ephrinB3-Fc reduced neuroblast numbers in the CC of WT mice (c). EphrinB3−/− mice show increased chain (d) and isolated cell (e) migration in the rostral CC as compared with WT mice. Infusion of clustered ephrinB3-Fc reduced the number of neuroblast chains/clusters but increased the number of isolated neuroblasts. CC, corpus callosum; RMS, rostral migratory stream; SVZ, subventricular zone. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with their respective non-injured controls; #p < 0.05 as compared with their respective Fc controls.
In the rostral CC, WT mice showed a significant 2.7-fold increase in neuroblast numbers in cannula-injured mice receiving vehicle Fc-control molecules as compared with non-injured WT controls (Fig. 3c), demonstrating that focal cortical injury stimulates neuroblast migration outside the RMS. Following 2 days infusion of 140 μg/mL pre-clustered ephrinB3-Fc molecules, we observed a significant 2.3-fold reduction in neuroblast numbers in the CC as compared to the vehicle Fc-control. In the absence of ephrinB3, there was a trend towards greater neuroblast numbers observed in the non-injured ephrinB3−/− CC as compared to non-injured WT mice, which was not significantly enhanced after CCI injury (Fig. 3c). These trends were also observed in the caudal CC after CCI injury and support our previous conclusions that increased neuroblast migration may, in part, result from reduced ephrinB3 expression after CCI injury (Theus et al., 2014). Infusion of ephrinB3-Fc showed no significant difference from non-injured or vehicle Fc-control mice but showed a reduced trend similar to that observed in WT mice. These findings suggest that administration of the ephrinB3 can influence neuroblast migration outside the RMS, potentially by stimulating Eph receptor signaling in neuroblasts that limits chain formation and in turn their migratory potential.
3.4. EphrinB3 restricts neuroblast chain migration in vivo and in vitro
In the RMS, neuroblasts are known to migrate in chains (Lois et al., 1996); however, less is known about their migration outside the RMS. As DCX-positive neuroblasts exit the RMS and enter the CC, neuroblasts were observed as isolated cells or as groups of cells defined as chains or clusters (Fig. 3a′, arrowhead). To evaluate whether infusion of ephrinB3-Fc alters neuroblast migration patterns in the CC, we quantified the number of neuroblasts as either chains/clustered or isolated cells (Fig. 3d,e). After cannula injury, we observed increased numbers of neuroblast chains/clusters in ephrinB3−/− mice as compared to WT mice (p < 0.05). Infusion of pre-clustered ephrinB3-Fc tended to reduce the number of neuroblast chains/clusters in both WT and ephrinB3−/− mice. Conversely, infusion of ephrinB3-Fc showed a tendency for increased numbers of isolated neuroblasts, which was significant in the ephrinB3−/− mice (p < 0.05). No differences in the number of isolated neuroblasts were observed between WT and ephrinB3−/− mice. These findings support the possibility that ephrinB3 could restrict neuroblast migration by limiting cell-cell interactions involved in chain migration outside the RMS.
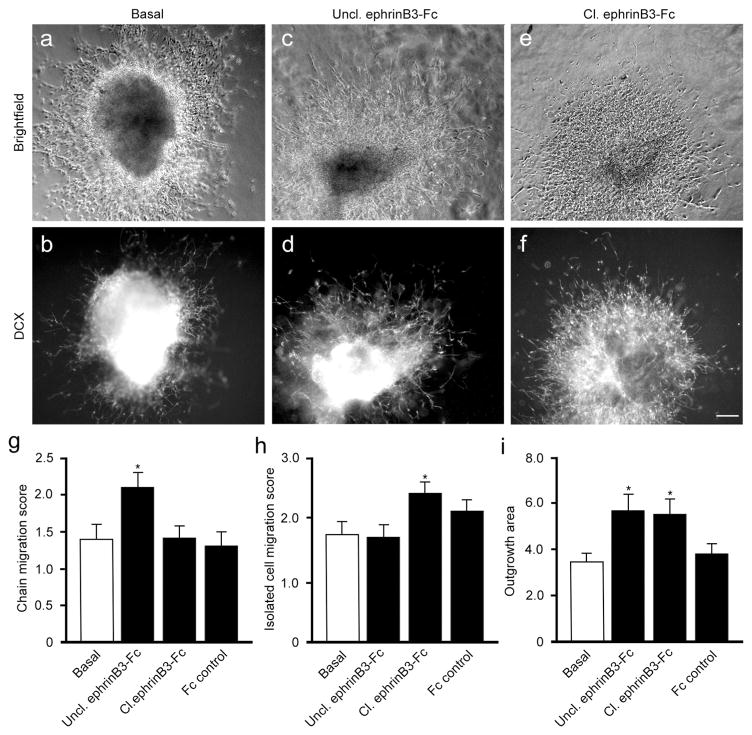
To further evaluate the cell autonomous role of ephrinB3 on neuroblast chain migration, we examined neuroblast migration in cultured WT SVZ explants for 72 h under basal conditions or with clustered ephrinB3-Fc molecules (i.e. Cl. ephrinB3-Fc), Unclustered ephrinB3-Fc molecules (Uncl. ephrinB3-Fc), or Fc controls (Fig. 4). Unclustered ephrinB3-Fc can function as a competitive inhibitor since explants express both ephrinB3 and Eph receptors, and Eph signaling often requires higher order clustering (Davis et al., 1994). The extent of chain migration, single cell migration and area of explant outgrowth were measured. In enriched growth conditions, DCX-labeled cells migrated outside the explants as both isolated cells and in chains, but the extent of each type of outgrowth varied depending on treatment (Fig. 4a–f). We observed greater numbers of migrating chains following the inhibition of Eph signaling with unclustered ephrinB3-Fc molecules (p < 0.05) as compared with basal and Fc controls (Fig. 4g). We also observed a greater percent of explants that showed only chain migration from 15.4% under basal conditions to 20.5% with unclustered ephrinB3-Fc. Activation of EphB3 using clustered ephrinB3-Fc showed greater migration of isolated cells (p < 0.05; Fig. 4h) and a decrease in the percentage of explants containing only chain migration to 8.3%. Both unclustered and clustered ephrinB3-Fc promoted increased outgrowth compared to controls (p < 0.05; Fig. 4i). These findings suggest that ephrinB3 stimulation of Eph-expressing neuroblasts does not alter their ability to migrate on laminin-enriched Matrigel but does effect whether neuroblasts migrate in chains or as isolated cells.
Fig. 4.
Application of ephrinB3 regulates neuroblast chain migration from cultured SVZ explants. Brightfield (a,c,e) and DCX-immunolabeled (b,d,f) SVZ explants show migrating neuroblast in basal (a,b) conditions, unclustered (Uncl.) ephrinB3-Fc (c,d) to block Eph signaling, and clustered (Cl.) ephrinB3-Fc (e,f) to activate Eph signaling. The extent of chain migration and single cell migration were measured using a semi-quantitative scale: 0 = no outgrowth; 1 ≤ 10 chains/cells; 2 = 10–50 chains/cells; 3 = 50–100 chains/cells; 4 = extensive growth. The area of explant outgrowth was measured and expressed as a ratio of outgrowth area (i.e. explant size). Graphs show increased numbers of chains migrating out of the explants after treatment with Uncl. ephrinB3-Fc (g), while isolated cells were increased after treating with Cl. ephrinB3-Fc (h). Both Cl. and Uncl. ephrinB3-Fc increased the total outgrowth of the explants (i). Results in all graphs show mean ± SEM of 39–48 explants per treatment; *p < 0.05 compared to basal conditions.
3.5. Expression of ephrinB3 in the human brain after traumatic injury
To examine whether our observations have the potential to translate to human TBI patients, we sought to examine ephrinB3 expression in human cortical tissues. Resected cortical tissue samples were obtained from patients at acute periods (between 3 and 24 h; n = 6), or > 5 days (122 h; n = 1) after insult (Table 1). EphrinB3 expression levels were examined using Western blot analysis and compared to uninjured cortical tissue (n=2). We found ephrinB3 to be expressed in naïve and TBI cortices of adult humans (Fig. 5). No differences were observed between acute TBI samples and uninjured controls; however, in the one sample collected from a patient 122 h after injury a reduced level of ephrinB3 was observed. Although n-values are too small to provide an accurate assessment of temporal expression, these findings support the presence of ephrinB3 in the adult human cortex.
Fig. 5.
EphrinB3 is expressed in the human control and TBI brains. Expression of ephrinB3 in uninjured (n = 2) or traumatized (n = 7) human brain samples as detected by Western blotting. The traumatized samples were grouped into tissue collection between 3 and 24 h (acute; n= 6) or >5 days (122 h; n = 1). No differences were observed between acute TBI samples and uninjured controls; however, in the one sample collected from a patient 122 h after injury a reduced level of ephrinB3 was observed despite a high level of expression of β-actin in this sample.
4. Discussion
This study examines the effects of ephrinB3 on neuroblast migration after cortical brain injury. In the non-injured SVZ, neuroblasts migrate from the subventricular zone (SVZ) through the rostral migratory stream (RMS) to the olfactory bulb. When cortical tissues are damaged, neuroblasts tend to migrate outside the RMS into adjacent tissues that include the corpus callosum(CC) and eventually the perilesional cortex. Here, we show that ephrinB3 may restrict neuroblast migration into the injured cortex by reducing migratory chain formations. In the absence of ephrinB3, increased numbers of neuroblasts and chain formations are observed in tissues between the SVZ/RMS and injury epicenter. These findings are confirmed in SVZ explants where blocking Eph signaling with non-aggregated ephrinB3 increase neuroblast chain migration, while stimulating Eph signals reduce chain numbers. Together, these findings support a role for ephrinB3 in limiting the migratory potential of neural progenitor cells that may have important implications for stabilizing the injured human brain.
4.1. TBI leads to increased neurogenesis and migration outside neurogenic regions
Following TBI in both mice and humans, neural stem/progenitor cells (NSPCs) in the SVZ increase their proliferation by approximately 3-fold leading to increased neuroblast pools (Kernie and Parent, 2010; Theus et al., 2010; Zheng et al., 2013). Injury and ablation studies suggest that the time-course of NSPC proliferation may be at least 2 days, with an additional 2.5 days required to differentiate into neuroblasts (Doetsch et al., 1999; Theus et al., 2010). EphB3 signaling in NSPCs has been implicated in neuroblast differentiation by inducing cell cycle arrest through a p53 dependent pathway following ephrinB3 activation (Theus et al., 2010). The absence of ephrinB3 or EphB3 led to expansion of the NSPC and neuroblast pools that was further augmented following TBI (Theus et al., 2010; Theus et al., 2014).
In the naïve brain, neuroblast migration occurs in a caudal-to-rostral direction within the RMS. In a sagittal perspective, these cells migrate with the leading edge of one neuroblast contacting the tailing edge of another neuroblast, while in the coronal perspective they form clusters that are typically one to four cells wide (Doetsch et al., 1997). Trophic factors and their receptors are present in the environment that promote neuroblast migration, including stromal cell-derived factor 1 (SDF)/C-X-C motif chemokine 4 (CXCR4), brain-derived neurotrophic factor (BDNF)/tropomyosin receptor kinase B (TrkB) and vascular endothelial growth factor (VEGF)/VEGF receptor (Snapyan et al., 2009; Kokovay et al., 2010). Factors that regulate chain migration are known to include β1-integrin/laminin and Ephs/ephrins. Direct infusion of ephrinB2-Fc or EphB2-Fc into the lateral ventricle, which directly bind or competitively antagonize EphB2 and EphA4, results in altered chain migration, as does blocking an EphA4 downstream signaling molecule, Rho-dependent kinase (Conover et al., 2000; Emsley and Hagg, 2003; Belvindrah et al., 2007; Leong et al., 2011). Following cortical injury many neuroblasts migrate outside the SVZ and RMS into the striatum and overlying CC, and many of these regulatory factors are altered within the changing injury landscape (Saha et al., 2013). In the CCI injured brain, we show that neuroblast numbers in the CC reflect a rostral-to-caudal gradient towards the damaged cortex, including numerous DCX-positive neuroblasts in peri-lesional tissues. Migration patterns of neuroblasts after brain injury are thought to respond to tropic signals released by cells within damaged tissues (Xue et al., 2014). Neuroblasts in cortical tissues have been shown to release trophic molecules to play a beneficial role in minimizing progressive loss of residential cells (Dixon et al., 2015).
4.2. EphrinB3 restricts neuroblast migration after TBI
Although neuroblasts can migrate long distances within CNS to sites of injury (Arvidsson et al., 2002; Parent et al., 2002; Dixon et al., 2015), the molecular mechanisms that control and direct neuroblast migration have not been clearly defined. Tracking neuroblast migration is inherently difficult as labeling agents (e.g. viruses, BrdU or EdU) necessitate injection through the cortex creating an unintended secondary injury and/or off target labeling. Alternate transgenic reporter methods can also lead to off target labeling and/or labeling of a subset of cells within the NSPC and neuroblast populations (Dixon et al., 2015). For this reason, our studies focused on the early migratory responses where neuroblasts migrate from the RMS into the CC. The short-time courses of the cannula and CCI models provide a focal injury where these early migratory signals can be evaluated in the CC.
EphrinB3 has the potential to interact with several B-class and A-class Eph receptors, although recent studies suggest that ephrinB3 may regulate adult neurogenesis through preferentially binding with EphB3 and/or EphA4 receptors (Ricard et al., 2006; Furne et al., 2009; Theus et al., 2010; Theus et al., 2014). EphB3 and EphA4 receptors, but not ephrinB3 are expressed by NSPCs, whereas ephrinB3 is expressed in cells that surround the neurogenic niches (Ricard et al., 2006; Furne et al., 2009; Theus et al., 2010). In the CCI injured brain, reduced ephrinB3 expression coincides with increased neuroblast migration. Ephs and ephrins are also known to regulate cytoskeletal dynamics involved in cellular and subcellular motility, mainly through repulsive cues that destabilize the cytoskeletal matrix (Carter et al., 2002; Santiago and Erickson, 2002; Puschmann and Turnley, 2010). Our findings demonstrate that application of ephrinB3-Fc molecules can restrict neuroblast migratory potential possibly through reducing cell-cell interactions important for chain migration. Other factors that may regulate neuroblast chain migration include the β-integrins and laminins (Emsley and Hagg, 2003; Belvindrah et al., 2007; Mobley and McCarty, 2011). Integrins are known to regulate cell motility and neuroblasts upregulate β1-integrin and interact with laminin in the extracellular environment to initiate neuroblast chain migration (Emsley and Hagg, 2003; Belvindrah et al., 2007). Interestingly, EphB2 and EphB3 receptors have been shown to inhibit integrin activity through R-Ras and Rac inhibition, respectively (Zou et al., 1999; Miao et al., 2005). It is therefore plausible, that ephrinB3 expression may be restricting migration due to its ability to inhibit β1-integrin-induced chain migration.
After CCI injury, the down-regulation of ephrinB3 expression in CC and cortical tissues could be responsible for the enhanced neuroblast migration by limiting Eph receptor signaling. Like many ephrins, ephrinB3 can bind multiple Eph receptors including EphA4, which is known to regulate cell movements (Fukai et al., 2008; Parmentier-Batteur et al., 2011). Activation of EphA4 has been shown to promote Rho-dependent kinase (ROCK) signaling, whereas reduced ROCK activity leads to enhanced cell migration into ectopic locations (Leong et al., 2011). Disruption in neuroblast migration was observed following Eph-Fc and ephrin-Fc infusion into the lateral ventricle (Conover et al., 2000).
4.3. Relevance to human traumatic brain injury
NSPCs and neuroblasts play beneficial roles after TBI to stabilize the injury site by preventing neuronal cell death and regulating the glial response (Dixon et al., 2015). Similarly, the human brain also contains a neurogenic niche where, under naïve conditions, NSPCs proliferate and neuroblasts migrate along specific routes (van Strien et al., 2011). Most recently the presence of neural stem-like markers were found in cells from resected tissue from injured human brain (Zheng et al., 2013), suggesting that TBI in humans can induce neurogenesis. In the current study we found that naïve and TBI brains both express similar levels of ephrinB3 in the first week after injury. To provide confidence and extend the temporal profile, additional expression studies in humans are needed.
Acknowledgments
We thank Melissa Carballosa-Gautam in the Miami Project to Cure Paralysis Imaging Core. This design, experimentation, data analysis and manuscript writing was supported by the Miami Project to Cure Paralysis; NIH/NINDS (DJL: NS049545 and NS30291); and an NH&MRC fellowship (AMT: 628344).
References
- Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Belluzzi O, Benedusi M, Ackman J, LoTurco JJ. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Hankel S, Walker J, Patton BL, Muller U. Beta1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci. 2007;27:2704–2717. doi: 10.1523/JNEUROSCI.2991-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT. Expression of polysialylated neural cell adhesion molecule by proliferating cells in the subependymal layer of the adult rat, in its rostral extension and in the olfactory bulb. Neuroscience. 1994;62:291–305. doi: 10.1016/0306-4522(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Dixon KJ, Theus MH, Nelersa CM, Mier J, Travieso LG, Yu TS, Kernie SG, Liebl DJ. Endogenous neural stem/progenitor cells stabilize the cortical microenvironment after traumatic brain injury. J Neurotrauma. 2015;32:753–764. doi: 10.1089/neu.2014.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. alpha6beta1 integrin directs migration of neuronal precursors in adult mouse forebrain. Exp Neurol. 2003;183:273–285. doi: 10.1016/s0014-4886(03)00209-7. [DOI] [PubMed] [Google Scholar]
- Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, Liebl DJ. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta. 2009;1793:231–238. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SY, Faux CH, Turbic A, Dixon KJ, Turnley AM. The Rho kinase pathway regulates mouse adult neural precursor cell migration. Stem Cells. 2011;29:332–343. doi: 10.1002/stem.577. [DOI] [PubMed] [Google Scholar]
- Li B, Piao CS, Liu XY, Guo WP, Xue YQ, Duan WM, Gonzalez-Toledo ME, Zhao LR. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res. 2010;1327:91–102. doi: 10.1016/j.brainres.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of rho family small GTPases. J Biol Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Faissner A, Antonicek H, Schachner M. Retention of J1/tenascin and the polysialylated form of the neural cell adhesionmolecule (N-CAM) in the adult olfactory bulb. J Neurocytol. 1990;19:899–914. doi: 10.1007/BF01186818. [DOI] [PubMed] [Google Scholar]
- Mobley AK, McCarty JH. beta8 integrin is essential for neuroblast migration in the rostral migratory stream. Glia. 2011;59:1579–1587. doi: 10.1002/glia.21199. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Finger EN, Krishnan R, Rajapakse HA, Sanders JM, Kandpal G, Zhu H, Moore KP, Regan CP, Sharma S, Hess JF, Williams TM, Reynolds IJ, Vacca JP, Mark RJ, Nantermet PG. Attenuation of scratch-induced reactive astrogliosis by novel EphA4 kinase inhibitors. J Neurochem. 2011;118:1016–1031. doi: 10.1111/j.1471-4159.2011.07375.x. [DOI] [PubMed] [Google Scholar]
- Puschmann TB, Turnley AM. Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J Neurochem. 2010;113:881–894. doi: 10.1111/j.1471-4159.2010.06655.x. [DOI] [PubMed] [Google Scholar]
- Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Saha B, Peron S, Murray K, Jaber M, Gaillard A. Cortical lesion stimulates adult subventricular zone neural progenitor cell proliferation and migration to the site of injury. Stem Cell Res. 2013;11:965–977. doi: 10.1016/j.scr.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, Parent A, Saghatelyan A. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Kim H, Moon Y. Control of neuronal migration through rostral migration stream in mice. Anat Cell Biol. 2010;43:269–279. doi: 10.5115/acb.2010.43.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theus MH, Ricard J, Bethea JR, Liebl DJ. EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem Cells. 2010;28:1231–1242. doi: 10.1002/stem.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theus MH, Ricard J, Glass SJ, Travieso LG, Liebl DJ. EphrinB3 blocks EphB3 dependence receptor functions to prevent cell death following traumatic brain injury. Cell Death Dis. 2014;5:e1207. doi: 10.1038/cddis.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien ME, van den Berge SA, Hol EM. Migrating neuroblasts in the adult human brain: a stream reduced to a trickle. Cell Res. 2011;21:1523–1525. doi: 10.1038/cr.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Wang J, Wang W, Yang Z, Hu Z, Hu M, Ding P. The effect of stromal cell-derived factor 1 in the migration of neural stem cells. Cell Biochem Biophys. 2014;70:1609–1616. doi: 10.1007/s12013-014-0103-5. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Zheng W, ZhuGe Q, Zhong M, Chen G, Shao B, Wang H, Mao X, Xie L, Jin K. Neurogenesis in adult human brain after traumatic brain injury. J Neurotrauma. 2013;30:1872–1880. doi: 10.1089/neu.2010.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Yang B, Theus MH, Sick JT, Bethea JR, Sick TJ, Liebl DJ. EphrinBs regulate D-serine synthesis and release in astrocytes. J Neurosci. 2010;30:16015–16024. doi: 10.1523/JNEUROSCI.0481-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci U S A. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]