Abstract
It has been established that febrile seizures and its extended syndromes like generalized epilepsy with febrile seizures (FS) plus (GEFS+) and Dravet syndrome have been associated with mutations especially in SCN1A and GABRG2 genes. In patients, the onset of FS is likely due to the combined effect of temperature and inflammation in genetically vulnerable individuals because fever is often associated with infection. Much effort has been spent to understand the mechanisms underlying fever induction of seizures. In addition to the role of cytokines in FS, previous studies in Scn1a+/− knockout mice, a model of Dravet syndrome, indicated that temperature elevation alone could result in seizure generation, and the effect of elevated temperature inducing seizures was age-dependent. Here, we report the thermal effect in a different mouse model of Dravet syndrome, the Gabrg2+/Q390X knockin mouse. We demonstrated age-dependent dysregulated temperature control and that temperature elevation produced myoclonic jerks, generalized tonic clonic seizures (GTCSs) and heightened anxietylike symptoms in Gabrg2+/Q390X mice. The study indicated that regardless of other inflammatory factors, brief heat alone increased brain excitability and induced multiple types of seizures in Gabrg2+/Q390X mice, suggesting that mutations like GABRG2(Q390X) may alter brain thermal regulation and precipitate seizures during temperature elevations.
Keywords: temperature, electroencephalography, febrile seizures, myoclonic jerks, generalized tonic clonic seizures, GABRG2(Q390X) mutation
Introduction
Fever is the most common seizure trigger for children between five months and six years of age 1, but the underlying mechanisms by which fevers precipitate seizures are not clear. In humans fever often occurs with infection that could cause release of inflammatory cytokines such as interleukin-1β, tumor necrosis factor (TNF)-α and other cellular mediators. These cytokines could increase brain excitability as seizures are commonly observed in meningitis or encephalitis 2. However, external heat such as a hot bath has also been reported to induce seizures, especially in Dravet syndrome, which is the most severe epileptic encephalopathy with intractable seizures, cognitive impairment and sometimes unexpected death. This suggests that heat alone without infection also increases brain excitability and can lead to seizures in patients with certain genetic predispositions. In previous studies, the peak of the core body temperature has been proposed to be a determining factor for seizure onset during fever 4. In patients, the condition of febrile seizures (FS) is likely due to a combination of temperature and inflammation. In experimental animal models of FS, nearly all animals have a seizure if heated sufficiently, suggesting that the duration of heating correlates with brain excitability 3.
Recent studies indicate that certain genetic factors increase susceptibility to FS. Among all the epilepsy genes, mutations in SCN1A and GABRG2 have been strongly associated with FS and extended epilepsy syndromes like generalized epilepsy with febrile plus (GEFS+) and Dravet syndrome 5–7. In sodium channels, computer simulation has shown that changes in inhibitory ion channels are unable to offset the increased excitability in the brain during a temperature increase 8. Thus, the overall net effect of increased temperature would tilt the brain to a more excitable state, thus making it more prone to seizures. In GABAA receptor channels, it has been demonstrated that mutant γ2 subunit trafficking was impaired during increased temperature in vitro 9. The γ2 subunit is essential for GABAA receptor clustering at synapses 10. It has been demonstrated that the cytokine, TNF-α, caused endocytosis of GABAA receptors through phosphatase-1, thus resulting in decreased inhibitory strength 11. The effect of temperature has also been studied in mouse models carrying mutations in sodium channel Scn1a and Gabrg2 genes. A study of Scn1a+/− mice suggested that age plays a role in temperature-induced seizure susceptibility, and adult Scn1a+/− mice were more prone to thermogenic seizures than younger mice 12. In Gabrg2+/R82Q (also named Gabrg2+/R43Q) mice, the GABRG2(R82Q) mutation increased the susceptibility to thermogenic seizures, but FS could not be induced in the Gabrg2+/- knockout mice13, which represents a condition of simple Gabrg2 haploinsufficiency.
In the current study, we characterized the thermal effect in our recently constructed knockin mouse Gabrg2+/Q390X, which is associated with Dravet syndrome 14. The GABRG2(R82Q) mutation is associated with FS and childhood absence epilepsy 15, while the GABRG2(Q390X) mutation 16 is associated with a more severe epilepsy, Dravet syndrome. It is unknown if temperature elevation has a similar effect on epilepsy syndromes with different severities. Additionally, it is unknown if brief temperature elevation has any effect on epilepsy although it has been reported that prolonged heating could cause seizures in nearly all the experimental animals 17. We hypothesize that the increased age and core body temperature will increase thermogenic seizures and have tested the effect of brief temperature rise in the Gabrg2+/Q390X mice. Because pentylenetetrazol (PTZ) is a known GABAA receptor antagonist that induces seizures by blocking GABAergic neurotransmission, we thus administered PTZ as a control for seizure severity. We compared the severity of seizures induced by temperature elevation with the severity of seizures induced by PTZ in age- and strain-matched mice.
Methods
Mice
The Gabrg2+/Q390X knockin mouse model has been characterized in our previous study 14. All mice were bred for at least 8–10 generations into a C57BL/6J or DBA/2J background, which is a seizure-resistant or seizure-prone mouse background, respectively 18. All procedures were performed in accordance with policies and guidelines set forth by the Vanderbilt University Institutional Animal Care and Use Committee.
ELISA
A standard sandwich ELISA was performed to measure tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) levels in mouse plasma. The ELISA kits were purchased from ThermoFisher and all the cytokine standards were included in the kits. The blood was drawn from mouse tail and plasma was separated. Fifty µl of undiluted plasma was used for reaction. The optical density (OD) of each well was read at 450 nm with an absorbance-based microplate reader. The final concentration was calculated by converting the OD readings against a standard curve.
Electroencephalography (EEG) electrode implant surgery, recording, and analysis
The EEG electrode implant surgery and synchronized video-EEG monitoring have been described before 19;20. Briefly, synchronized video-EEGs were recorded from mice one week after electrode implantation and recorded with a synchronized video-EEG monitoring system from Pinnacle Technology. Mice were anesthetized with 1–3% isoflurane and four epidural electrodes (stainless steel screws affixed to one head mount) were placed on the brain surface and secured in place with dental cement and surgical stiches. EMG leads were inserted into the trapezius muscle. Video-EEG monitoring lasted for 24–48 hrs. Mice were freely moving during EEG recordings, but the headmount was connected to a cable box. At least 24 hours of baseline video-EEG recordings were obtained and analyzed for each mouse.
Seizure induction by temperature elevation
The heating procedure was adapted from a previous study 12. Mice were placed in a Plexiglas cylinder with an infrared heat lamp (250 watt, HL-1, Physitemp Instruments Inc.) kept in a fixed position above. A rectal temperature probe (RET-4, Physitemp Instruments Inc.) was carefully inserted and taped to the tail of the mouse. The temperature probe was connected to a temperature controller (TCAT-2DF, Physitemp Instruments Inc.) to monitor the core body temperature of the animal. Each mouse was allotted a minimum of 10 min for habituation in the Plexiglas cylinder without the heat lamp on. The mouse was then recorded for 30 min to 2 hrs under baseline activity. After baseline recordings, the heat lamp was turned on and the synchronized video-EEG/EMG recording began. The core body temperatures were then elevated approximately 0.5°C every 2 min until 42.5°C was achieved. The rate of temperature rise in the mice was adjusted by the height of the heating lamp. The heating lamp would stop once the mouse core temperature reached 42.5 °C. If the mouse had a GTCS, the heating process would be stopped immediately.
Pentylenetetrazol treatment
Adult mice were injected with a single dose of PTZ (Sigma-Aldrich, St. Louis, MO) 50 mg/kg i.p. to induce seizures and monitored during the first 30 min after administration 20. PTZ has been used for inducing absence seizures at low doses 21 and GTCSs at high doses 22 in rodents because of its GABAA receptor-antagonizing effect.
Statistical analysis
Data were analyzed with GraphPad Prism 5 or the statistical package for the social sciences (SPSS) 23.0 software. Independent-samples t tests or a chi-square (χ2) test were used for the comparisons between genotypes. All analyses used an alpha level of 0.05 to determine statistical significance. Data were presented as Mean ± SEM.
Results
Gabrg2+/Q390X mice had spontaneous GTCSs and myoclonic seizures during baseline activity
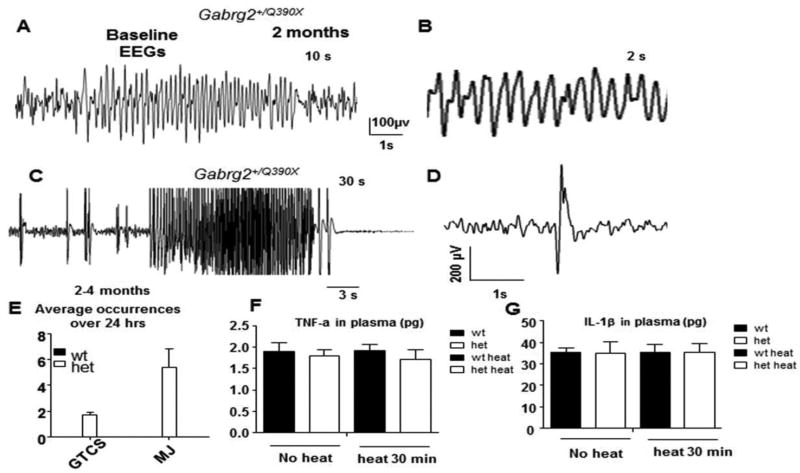
The Gabrg2+/Q390X mice had spontaneous GTCSs in the most seizure-resistant C57BL/6J background (Supplementary video 1). We first characterized the baseline EEGs of Gabrg2+/Q390X knockin mice. We observed spontaneous GTCSs starting from postnatal 16 days in the knockin mice during our routine handling. We could not do EEG recordings on the knockin mice under one month of age because the headmount could not be properly attached to the smaller skulls of the mice. We thus only recorded from mice over 2 months old. We observed sporadic, multiple forms of ictal discharges associated with behavioral seizures. The abnormal EEG activities included 4–7 Hz spike-wave-discharges (SWDs) associated with brief behavioral arrest or without any behavioral changes (Figure 1A and B), prolonged epileptiform discharges associated with GTCSs (Figure 1C), and high amplitude spikes associated with myoclonic jerks (Figure 1D). We also observed behavioral seizures without associated EEG abnormalities of irregular, less rhythmic SWDs. However, the convulsive seizures were rare during baseline activity. The average occurrence was 1.67 ± 0.27 for of GTCSs and 5.4 ± 1.45 for myoclonic jerks over a 24-hr period while no seizures were detected in their wild-type littermates (Figure 1E).
Figure 1. Interictal and ictal EEG s in Gabrg2+/Q390X knockin mice.
(A) Representative EEG recordings show that a 2 month old Gabrg2+/Q390X knockin mouse had spike-wave-discharges (SWDs). (B) The frequency of SWDs in Gabrg2+/Q390X mice was 4–7 Hz. (C) Spontaneous generalized tonic clonic seizures (GTCSs) were associated with epileptiform activity on EEG (D). Spontaneous myoclonic jerks were associated with spike discharges on EEG. (E) Average occurrences of GTCSs and myoclonic jerks (MJ) with noticeable behavioral seizures over 24 hours EEG recordings were measured in 2–4 month old mice (n = 31 for each genotype). (F,G) The pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α) (F) or interleukin-1beta (IL-1β) (G) in 50 µl plasma from peripheral blood of mice untreated or treated at 42.5C for 30 min were measured with Elisa.
We then measured the levels of cytokines in peripheral blood to determine if there was inflammation involved in seizure generation in the Gabrg2+/Q390X mice. We measured TNF-α (Figure 1F) and Il-1β (Figure 1G) prior to and after heat exposure at 42.5°C for 30 min. The baseline level of both cytokines were very low and there was no difference of either cytokines between wild-type and Gabrg2+/Q390X mice before or after heat exposure (TNF- α: 1.89 ± 0.21 for wt vs 1.8 ± 0.14 for het before heat; 1.92 ± 0.14 for wt vs 1.72 ± 0.23 for het after heat; IL-1β: 35.13 ± 2.4 for wt vs 35.08 ± 3.6 for het before heat; 35.28 ± 3.6 for wt vs 35.22 ± 4.2 for het after heat). This thus suggested that there was no systematic inflammation involved in seizure generation in Gabrg2+/Q390X mice.
A brief, rapid temperature rise induced myoclonic jerks and SWDs in Gabrg2+/Q390X mice that were less frequent than seizures induced by PTZ injection
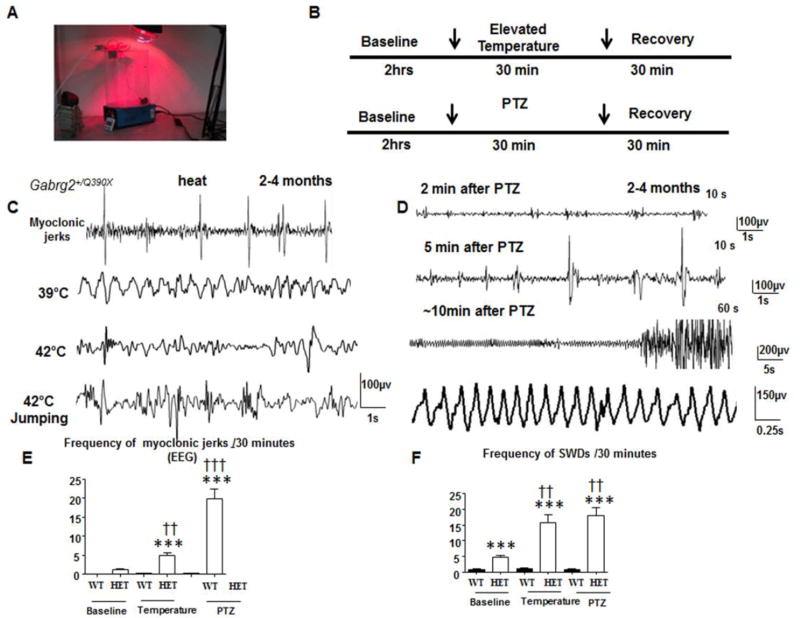
Because sustained high temperature exposure may cause cellular injury, and even cell death, we determined the effects of short temperature elevations on Gabrg2+/Q390X mice using a temperature-controlled heating lamp. The temperature induction protocol was modified based on a previously described study 12 using Scn1a+/- knockout mice (Figure 2A). Heating stopped once the mouse’s core temperature reached 42.5 °C. We first recorded for 2 hrs for baseline EEGs before temperature elevation and 30 minutes after heating for recovery (Figure 2B upper panel). Because PTZ could induce seizures by blocking GABAergic neurotransmission, we administered PTZ as a control for seizure severity (Figure 2B, lower panel). Temperature elevation evoked myoclonic jerks and many SWDs, which tended to be irregular or less rhythmic but were associated with behavioral seizures (Figure 2C). This was thus quantified. With administration of PTZ (50 mg/kg), there were also many myoclonic jerks, which occurred alone or proceeding GTCSs. There were also more rhythmic SWDs in PTZ-induced seizures than heat-induced seizures (Figure 2D). We compared the severity of seizures induced by high temperature and PTZ administration because PTZ is a known GABAA receptor antagonist and our preliminary data indicated that administration of a moderate dose of PTZ (50 mg/kg) could induce GTCS with high mortality in Gabrg2+/Q390X mice. The comparison potentially provides insights into the mechanisms underlying temperature increases and compromised GABAergic inhibition.
Figure 2. Temperature elevation and PTZ induced seizures and abnormal EEGs in Gabrg2+/Q390X knockin mice.
(A) The temperature induction apparatus and heating setup are shown. (B) A diagram of the temperature induction and pentylenetetrazol (PTZ) seizure induction procedures are shown. (C) Sample EEGs traces are shown for 2–4 month old Gabrg2+/(Q390X) knockin mice during temperature elevation. (D) Sample EEGs traces are shown for 2–4 month old Gabrg2+/(Q390X) knockin mice during PTZ seizure induction. (E) Number of myoclonic jerks for 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice are plotted for baseline and for temperature elevation and PTZ seizure induction segments. (F) Frequency of SWDs for 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice are plotted for baseline, temperature induction, and PTZ segments. (Temperature seizure induction, n = 10 for wt and 10 for het; PTZ seizure induction, n = 6 for wt and 10 for het) (***p < 0.001 vs wt); (†† p < 0.01; ††† p < 0.001 vs baseline het).
Gabrg2+/Q390X mice had a few myoclonic jerks (1.18 ± 0.26) while the wild-type mice had no myoclonic jerks during the baseline period. The number of myoclonic jerks was greater for Gabrg2+/Q390X mice following temperature elevation (4.93 ± 0.73) than for wild-type mice, which had almost no myoclonic jerks (0.18 ± 0.12, p <0.0001) following temperature elevation, and Gabrg2+/Q390X mice during a baseline period (1.18 ± 0.26, p = 0.0003; Figure 2E). Similarly, the number of myoclonic jerks was greater for Gabrg2+/Q390X mice following PTZ treatment (19.91 ± 2.60) than for wild-type mice (0.27 ± 0.14, p<0.0001) and a Gabrg2+/Q390X baseline period (1.18 ± 0.26, p <0.0001; Figure 2E).
There was also a greater incidence of SWDs for Gabrg2+/Q390X mice following seizure induction by temperature elevation (15.71 ± 2.46) compared to the wild-type mice (0.67 ± 0.33, p <0.0001) and a Gabrg2+/Q390X baseline period (4.83 ± 0.42, p <0.002). Moreover, there was a greater occurrence of SWDs for Gabrg2+/Q390X mice following PTZ treatment (17.88 ± 2.81) compared to the wild-type mice (0.71 ± 0.36, p <0.0001) and the Gabrg2+/Q390X baseline performance (4.83 ± 0.42 p=0.0019). However, there was not a significant difference in SWDs between Gabrg2+/Q390X mice that were either exposed to temperature elevation (15.71 ± 2.46) or PTZ (17.88 ± 2.81, p = 0.579; Figure 2F).
Seizures induced by brief high temperature were less severe than seizures induced by PTZ in Gabrg2+/Q390X mice
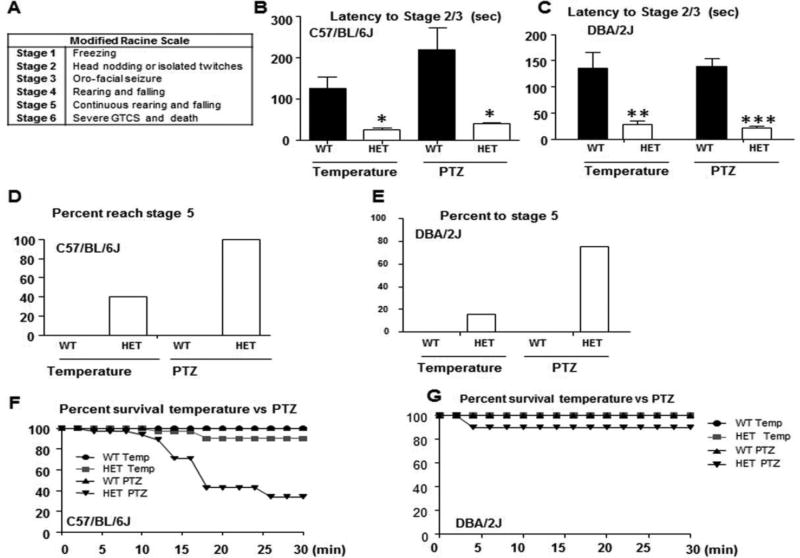
The seizures were scored based on the modified Racine Scale (Figure 3A) 23. The latency to reach stage 2/3 of the modified Racine scale (i.e., head nodding or oro-facial seizure) during seizure induction by heat was significantly reduced for Gabrg2+/Q390X mice in both C57BL/6J (26.0 ± 5.36 sec for Gabrg2+/Q390X vs 125.5 ± 27.70 sec for wild-type, p = 0.028) and DBA/2J (28.1± 6.1 sec for Gabrg2+/Q390X vs 135± 31 sec for wild-type, p <0.0001) (Figure 3B–C). The latency to reach stage 2/3 was also significantly reduced following PTZ administration for Gabrg2+/Q390X mice in both C57BL/6J (39.33 ± 2.65 sec for Gabrg2+/Q390X vs 218.2 ± 53.71 sec for wild-type, p = 0.008; Figure 3B–C) and DBA/2J (21.1 ± 4.0 sec for Gabrg2+/Q390X vs 138 ± 16 sec for wild-type, p = 0.003) backgrounds. All of the Gabrg2+/Q390X, but none of the wild-type, mice reached stage 3 (data not shown), but only 40% of Gabrg2+/Q390X mice in C57BL/6J and 16% in DBA/2J reached stage 5 with temperature induction (Figure 3D–E). However, all of Gabrg2+/Q390X mice in C57Bl/6J and 75% in DBA/2J backgrounds and none of the wild-type mice in either background reached stage 5 with PTZ injection (Figure 3D–E). Three out of 32 Gabrg2+/Q390X mice died after seizure induction by temperature elevation, 22 out of 35 Gabrg2+/Q390X mice died after PTZ induction (Figure 3F), but none of wild-type mice died after seizure induction by either method. The increased mortality of Gabrg2+/Q390X mice during seizure inductions was consistent with our previous report that the Gabrg2+/Q390X mice had spontaneous death with evidence favoring cardiorespiratory failure, which is a putative cause of sudden unexpected death in epilepsy (SUDEP)14;24. However, to our surprise, none of Gabrg2+/Q390X mice in DBA/2J background died during seizure induction by temperature elevation and only 1 out 10 mice died during PTZ seizure induction (Figure 3G), suggesting that some unknown mechanisms modify the phenotype of Gabrg2+/Q390X mice in the two different genetic backgrounds.
Figure 3. Temperature elevation and PTZ induced seizure latency, severity, and survival in Gabrg2+/Q390X knockin mice.
(A) The modified Racine scale used for seizure scoring is shown. (B, C) Latency to reach stages 2 and 3 of the modified Racine scale for 2–4 month old Gabrg2+/Q390X knockin mice in C57BL/6J (B) or DBA/2J (C) was plotted for temperature elevation and PTZ seizure inductions. (D,E) Percentage to reach stage 5 of the modified Racine scale was plotted for 2–4 month old Gabrg2+/Q390X knockin mice in C57BL/6J (D) or DBA/2J (E) during temperature elevation and PTZ seizure inductions. (F,G) Survival percentage following temperature and PTZ induction was plotted for 2–4 month old Gabrg2+/(Q390X) knockin mice in C57BL/6J (F) or DBA/2J (G) (In F, temperature seizure induction, n = 10 for wt and 10 for het; PTZ seizure induction, n = 6 for wt and 6 for het; in G, n = 6 for each genotype for temperature induction and n = 10 for each genotype for PTZ seizure induction).
Adult Gabrg2+/Q390X mice had altered temperature regulation
We noticed that the core body temperature in adult Gabrg2+/Q390X mice rose at a faster rate than wild-type mice during temperature induction under the same heating conditions. We thus compared the rate of temperature elevation using the same heating conditions. We measured the percentage of mice that reached 42.5°C against the heating time (Figure 4A). The rate of body temperature rise was measured by how many minutes it took for a mouse to reach a core body temperature of 42.5°C. We measured core body temperature every 2 min because we used a previous study{Oakley, 2009 235 /id} as reference. In the study, the rate of temperature increase for wild-type mice was set at 0.5 °C per 2 min. For adolescent mice, there was no difference in the rate of body temperature rise for Gabrg2+/Q390X mice (0.46 ± 0.03 °C) and wild-type mice (0.50 ± 0.02 °C, p = 0.290) per 2 minutes, but the heating process raised the heterozygous mouse body temperature at a significantly faster rate (0.78 ± 0.05 °C) than their wild-type littermates (0.43 ± 0.03 °C; p < 0.001; Figure 4B) for adult mice. Regardless of age, all mice had a similar baseline temperature of approximately 36.9°C. However, all adult Gabrg2+/Q390X mice reached 42.5°C within 24 min while only 80% wild-type adult mice, 78% wild-type adolescent mice and 64 % of Gabrg2+/Q390X adolescent mice reached 42.5°C by 24 min (Figure 4B). It is unknown why temperature regulation was altered for Gabrg2+/Q390X mice. It is possible that the specific cells of the hypothalamus involved in temperature regulation may express γ2 subunits and were dysfunctional in the mutant mice, but this needs further study.
Figure 4. Heat induced body temperature changes as well as seizures and anxiety-like phenotypes were differentially expressed in Gabrg2+/Q390X mice at different age.
(A) The percentage of mice to reach 42.5 °C during temperature induction was plotted for P18–20 and 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice. (B) The rate of core body temperature change assessed over 2-minute intervals during temperature induction was plotted for P18–20 and 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice. (C) The number of myoclonic jerks during temperature induction was plotted for P18–20 and 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice. (D) The number of GTCS (GTCSs) during temperature induction was plotted for P18–20 and 2–4 month old wild-type (wt) and Gabrg2+/Q390X (het) mice. (E) Frequency of jumping bouts during temperature induction was plotted for P18–20 and 2–4 month old wild-type (wt) and Gabrg2+/Q390X knockin (het) mice (n = 10 for wt and het for 2–4 month old mice; n = 11 for wt and 14 for het for P18–20); (*p < 0.05 vs wt).
Hyperthermia induced more myoclonic jerks in younger Gabrg2+/Q390X mice, but GTCSs were more frequent in older Gabrg2+/Q390X mice
Previous studies of Scn1a+/− mice indicated that older mice (2 months or older) were more susceptible to temperature-induced seizures than younger mice (1 month or younger)12. We thus compared seizure activity in P18–20 day old (adolescent) and 2–4 month old (adult) mice. For adolescent mice, there was a significant increase in the number of myoclonic jerks for heterozygous Gabrg2+/Q390X mice (5.91 ± 0.96) compared with their wild-type littermates (1.46 ± 1.06, p = 0.040) during heating. For adult mice, there was also a significant increase in the number of myoclonic jerks for the Gabrg2+/Q390X mice (2.10 ± 0.89) compared to their wild-type littermates (0.10 ± 0.10, p = 0.038; Figure 4C). However, the myoclonic jerks were more frequent in the adolescent mice than in the adult mice, regardless of genotype, suggesting a possible role of age in the onset of myoclonic jerks. It has been noticed in epilepsy mouse models carrying Gabra1 mutations that myoclonic seizures are more prominent in older mice 25. Regardless, there were more myoclonic jerks in the Gabrg2+/Q390X mice in both adolescent and adult groups. This indicates the mutation increased myoclonic jerks regardless of age. For heat induced GTCSs in adult mice, there was a significant increase for the Gabrg2+/Q390X mice (0.40 ± 0.16) compared to their wild-type littermates (0.00 ± 0.00, p = 0.025). Four out of 10 adult Gabrg2+/Q390X mice had a GTCS (χ2 = 5.00, p = 0.025; Supplementary video 2), while only 1 out of 11 adolescent Gabrg2+/Q390X mice had a GTCS (χ2 = 1.33, p = 0.250; Figure 4D).
Adult, but not juvenile, Gabrg2+/Q390X mice had heightened anxiety-like symptoms
Severe epilepsies like Dravet syndrome or temporal lobe epilepsy often have high neuropsychiatric comorbidities-including anxiety, hyperactivity, impaired social ability and cognition26. We noticed frequent jumping bouts for all mice during the heating process. Based on a previous study, escape strategies, such as jumping, are closely linked with the psychopathological conditions related to anxiety 27. We thus measured jumping bouts as a behavioral anxiety marker in addition to seizure-related activity. In the evaluation of jumping bouts (i.e., when the mouse was leaping in a vertical direction with all four paws off the ground; Supplementary video 3), adolescent Gabrg2+/Q390X mice (57.45 ± 5.87 bouts) did not jump at a greater rate in comparison to their wild-type littermates (56.43 ± 6.36 bouts, p = 0.909). However, adult Gabrg2+/Q390X mice (99.40 ± 8.75 bouts) jumped at a greater rate than wild-type mice (63.80 ± 5.30 bouts, p = 0.003; Figure 4E). Regardless of genotype, all mice began jumping consistently at a core body temperature of 39 ± 1°C (data not shown). It is important to note, however, that we compared the behavioral characteristics of jumping bouts with GTCSs (Supplementary video 2 for GTCSs and video 3 for jumping bouts). Based on our observations, there was no clear evidence for convulsive seizures or behavioral arrest associated with the jumping bouts.
Discussion
Some seizures in epilepsy patients may be precipitated occasionally by either internal or external stimuli. A rapidly rising and high fever is one of the most measurable parameters among these precipitants. Therefore, the effect of temperature elevation has been investigated in both wild-type and genetically modified mouse epilepsy models 12;13;28. In humans, prolonged and complex FS promote later development of temporal lobe epilepsy associated with hippocampal sclerosis, suggesting prolonged complex FS could cause cellular injury. However, the effect of brief exposure to elevated temperature has never been addressed.
We determined the types of seizures evoked by brief heating in wild-type and in mutant Gabrg2+/Q390X knockin mice associated with GEFS+/Dravet syndrome 14;16. Our data suggest that the GABRG2(Q390X) mutation increases susceptibility to thermogenic seizures, and that a brief temperature elevation alone could induce multiple forms of seizures in Gabrg2+/Q390X mice. Our findings do not necessarily come as a surprise given that multiple mutations in GABRG2 in humans have been linked to FS including R136X, IVS6 + 2T→G 29, K328M 30, Q390X 16, R177G 31, and R82Q 15;32 mutations. Furthermore, the γ2 subunit has been suggested to have temperature-dependent effects due to its importance for receptor trafficking 33, clustering 34, and synaptic maintenance 35. An alteration of temperature could change protein folding and trafficking due to their established temperature-dependent characteristics 9;36.
Our data indicate that the susceptibility to thermogenic seizures is age-dependent as 2–4 month old Gabrg2+/Q390X mice had a more severe seizure phenotype than 18–20 day old mice. A genetic mutation like GABRG2(Q390X) would increase brain excitability and the risk for developing seizures. This is consistent with the previous findings in Scn1a+/- mice12. In Gabrg2+/Q390X mice, hyperthermia induced more GTCSs in adult mice, but more myoclonic jerks in adolescent mice. Our results support a previous study in Gabrg2+/R82Q mice that mutant GABAA receptor γ2(R82Q) subunits increased susceptibility to FS 13. However, the age-dependent seizure phenotypes induced by high temperatures have never been reported. This is the first report that high temperature evokes more myoclonic jerks in adolescent mice, but more GTCSs in adult mice, although both seizure types are seen at either age.
It is intriguing that adult Gabrg2+/Q390X mice had an accelerated temperature increase despite the same heating parameters for all mice. This may be related to age-dependent temperature sensitivity, but needs further elucidation. Because the GABRG2 is ubiquitously expressed in the brain including the brainstem, it is possible that the neurons in the brainstem nuclei responsible for temperature control are dysfunctional. It is also unknown why the temperature regulation was only altered for adult Gabrg2+/Q390X mice. We have previous demonstrated that the mutant γ2(Q390X) subunits accumulate intracellularly over time 14. It is possible that the specific cells in the hypothalamus involving temperature regulation which express GABRG2 gene are only dysfunctional in the older mutant mice, and relatively normal in the young mice, but this needs further elucidation. Nevertheless, elevating the core body temperature for Gabrg2+/Q390X mice revealed the emergence of ictal discharges (unrelated to jumping) leading to a significant increase in myoclonic jerks and GTCSs, as well as evident progressions through the different stages of seizures based on the modified Racine scale 23. This suggests that a brief elevated temperature alone can increase seizure activity in a susceptible host regardless of cellular inflammation.
Seizures induced by high temperature exposure were less severe than seizures produced by PTZ. There were more myoclonic jerks and GTCSs in PTZ-treated Gabrg2+/Q390X mice than the mice exposed to temperature induction. In comparison of the two stimuli (i.e., hyperthermia and PTZ) used to trigger seizures, both were effective in producing SWDs characteristic of epilepsy, but the effect was more robust with PTZ administration, suggesting that there may be some similarities in the mechanistic functions of the stimuli to increase brain excitability.
We first report here that an elevated core body temperature increased the level of anxietylike behavior in Gabrg2+/Q390X mice. It is clear that γ2 subunits are ubiquitously expressed in brain regions associated with anxiety including forebrain, hippocampus and amygdala 37. A disruption in the GABAergic system, particularly genetic mutations, has been known to trigger anxiety 13,37. In addition, anxiety has been viewed as a sign of impaired GABAergic neurotransmission 38;39. Aside from genetic predispositions or mutations, antagonism of GABAergic function resulted in heightened anxiety, whereas enhancing the same system, via benzodiazepines, greatly diminished anxiety 40. Gabrg2+/- knockout mice displayed increased anxiety37. The increased rate of jumping noted for Gabrg2+/Q390X mice could be viewed as an indirect measure of anxiety given that the greater efforts to escape (via jumping) an aversive stimulus has been suggested as a form of intense, panic-like anxiety 27. The anxiety phenotype expressed in Gabrg2+/Q390X mice supports the notion that a GABAA receptor deficiency is a predisposition for anxiety disorders at the clinical level.
Overall, our findings indicated that thermogenic seizures occur in Gabrg2+/Q390X mice and were more severe compared to wild-type mice. Hyperthermia alone was more likely to induce myoclonic jerks in adolescent Gabrg2+/Q390X mice and GTCSs in adult Gabrg2+/Q390X mice. Our data also revealed a more rapid increase in body temperature and signs of anxiety during temperature elevation in Gabrg2+/Q390X mice. Our results demonstrated that the age of the mice played a key role with the aforementioned phenotypes showing an increased severity for the older mice. The thermogenic seizures noted for the Gabrg2+/Q390X mice did display similarities (albeit to a much lesser degree) to that of using PTZ to induce seizures, which could suggest a similar mechanistic pathway or pathways that at least converge for the resulting seizures. Based results from our Gabrg2+/Q390X mice, we suggests that, regardless of any infection related inflammatory mediators, a brief high temperature alone could induce multiple types of seizures and related comorbidities in a subset of the population with a lowered seizure threshold by a mutation like GABRG2(Q390X).
Supplementary Material
Highlights.
A brief temperature rise evoked multiple types of seizures in Gabrg2+/Q390X mice.
A brief temperature rise evoked more myoclonic jerks in adolescent Gabrg2+/Q390X mice and more GTCSs in adult Gabrg2+/Q390X mice.
Gabrg2+/Q390X mice had altered thermoregulation as evidenced by more rapidly increased core temperature in adult than in adolescent Gabrg2+/Q390X mice.
Gabrg2+/Q390X mice had heightened anxiety with temperature elevation.
Seizure severity following temperature elevation was less than seizure severity following PTZ administration.
Acknowledgments
The study was supported by research grants from Citizen United for Research in Epilepsy (CURE), Dravet syndrome foundation (DSF), IDEAleague (Dravet organization) and Vanderbilt Clinical and Translation Science Award (CTSA), NINDS R01 NS082635 to KJQ, and NINDS R01 NS 51590to RLM.
The authors are thankful for the use of the Murine Neurobehavioral Core at the Vanderbilt University Medical Center to generate the data. All the experimental procedures were approved by Vanderbilt University Division of Animal Care. Special thanks to Dr Martin J. Gallagher for his critical review of the manuscript and EEG interpretation and Huancheng Dong for his excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this study is consistent with those guidelines.
Reference List
- 1.Duffner PK, Baumann RJ, Green JL. Febrile seizures: clinical practice guideline for the long-term management of the child with simple febrile seizures. American Academy of Pediatrics. Steering Committee on Quality Improvement and Management. Subcommittee of Febrile Seizures. Pediatrics. 2008;121:1281–6. doi: 10.1542/peds.2008-0939. [DOI] [PubMed] [Google Scholar]
- 2.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta neuropathologica. 2016;131:211–34. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Effect of interleukin-18 on mouse core body temperature. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2002;282:R702–R709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- 4.Millichap JG. STUDIES IN FEBRILE SEIZURES I. Height of Body Temperature as a Measure of the Febrile-seizure Threshold. Pediatrics. 1959;23:76–85. [PubMed] [Google Scholar]
- 5.Mulley JC, Scheffer IE, Petrou S, Berkovic SF. Channelopathies as a genetic cause of epilepsy. Current opinion in neurology. 2003;16:171–6. doi: 10.1097/01.wco.0000063767.15877.c7. [DOI] [PubMed] [Google Scholar]
- 6.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. The Journal of clinical investigation. 2005;115:2010–7. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang JQ, Macdonald RL. GABRG2 Mutations Associated with a spectrum of epilepsy syndromes from Generalized Absence Epilepsy to Dravet syndrome. JAMA Neurology. 2016 doi: 10.1001/jamaneurol.2016.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas EA, Hawkins RJ, Richards KL, Xu R, Gazina EV, Petrou S. Heat opens axon initial segment sodium channels: a febrile seizure mechanism? Annals of neurology. 2009;66:219–26. doi: 10.1002/ana.21712. [DOI] [PubMed] [Google Scholar]
- 9.Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci. 2006;26:2590–7. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct y2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. The Journal of neuroscience. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. The Journal of neuroscience. 2005;25:3219–28. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oakley JC, Kalume F, Frank HY, Scheuer T, Catterall WA. Temperature-and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proceedings of the National Academy of Sciences. 2009;106:3994–9. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid CA, Kim T, Phillips AM, et al. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology. 2013;80:1003–8. doi: 10.1212/WNL.0b013e3182872867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JQ, Shen W, Zhou C, Xu D, Macdonald RL. The human epilepsy mutation GABRG2 (Q390X) causes chronic subunit accumulation and neurodegeneration. Nature neuroscience. 2015;18:988–96. doi: 10.1038/nn.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace RH, Marini C, Petrou S, et al. Mutant GABAA receptor y2-subunit in childhood absence epilepsy and febrile seizures. Nature genetics. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 16.Harkin LA, Bowser DN, Dibbens LM, et al. Truncation of the GABA A-receptor y2 subunit in a family with generalized epilepsy with febrile seizures plus. The American Journal of Human Genetics. 2002;70:530–6. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dube C, Ravizza T, Hamamura M, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. The Journal of neuroscience. 2010;30:7484–94. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson HA. Audiogenic seizures: Increased benzodiazepin receptor binding in a susceptible strain of mice. European journal of pharmacology. 1980;66:249–52. doi: 10.1016/0014-2999(80)90149-1. [DOI] [PubMed] [Google Scholar]
- 19.Chung WK, Shin M, Jaramillo TC, et al. Absence epilepsy in apathetic, a spontaneous mutant mouse lacking the h channel subunit, HCN2. Neurobiology of disease. 2009;33:499–508. doi: 10.1016/j.nbd.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warner TA, Kang JQ, Kennard JA, Harrison FE. Low brain ascorbic acid increases susceptibility to seizures in mouse models of decreased brain ascorbic acid transport and Alzheimer’s disease. Epilepsy research. 2015;110:20–5. doi: 10.1016/j.eplepsyres.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong M, Wozniak DF, Yamada KA. An animal model of generalized nonconvulsive status epilepticus: immediate characteristics and long-term effects. Experimental neurology. 2003;183:87–99. doi: 10.1016/s0014-4886(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 22.El Hamdi Gz, Boutroy MJ, Nehlig A. Effects of pentylenetetrazol-induced seizures on dopamine and norepinephrine levels and on glucose utilization in various brain regions of the developing rat. International journal of developmental neuroscience. 1992;10:301–11. doi: 10.1016/0736-5748(92)90019-v. [DOI] [PubMed] [Google Scholar]
- 23.Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J. Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neuroscience letters. 2004;365:106–10. doi: 10.1016/j.neulet.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 24.Xia G, Pourali S, Warner TA, Zhang C-Q, Macdonald RL, Kang J-Q. Altered GABAA receptor expression in brainstem nuclei and SUDEP in GABRG2+/Q390X mice associated with epileptic encephalopathy. Epilepsy research. 2016 doi: 10.1016/j.eplepsyres.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arain F, Zhou C, Ding L, Zaidi S, Gallagher MJ. The developmental evolution of the seizure phenotype and cortical inhibition in mouse models of juvenile myoclonic epilepsy. Neurobiology of disease. 2015;82:164–75. doi: 10.1016/j.nbd.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Tai C, Westenbroek RE, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–90. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King SM. Escape-related behaviours in an unstable elevated, exposed environment: I. A new behavioural model of extreme anxiety. Behavioural brain research. 1998;98:113–26. doi: 10.1016/s0166-4328(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 28.Dube C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1β contributes to the generation of experimental febrile seizures. Annals of neurology. 2005;57:152–5. doi: 10.1002/ana.20358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kananura C, Haug K, Sander T, et al. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Archives of neurology. 2002;59:1137–41. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- 30.Baulac S, Huberfeld G, Gourfinkel-An I, et al. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the y2-subunit gene. Nature genetics. 2001;28:46–8. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 31.Audenaert D, Schwartz E, Claeys KG, et al. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–90. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- 32.Marini C, Harkin LA, Wallace RH, Mulley JC, Scheffer IE, Berkovic SF. Childhood absence epilepsy and febrile seizures: a family with a GABAA receptor mutation. Brain. 2003;126:230–40. doi: 10.1093/brain/awg018. [DOI] [PubMed] [Google Scholar]
- 33.Keller CA, Yuan X, Panzanelli P, et al. The y2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. The Journal of neuroscience. 2004;24:5881–91. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the y2 subunit and gephyrin. Nature neuroscience. 1998;1:563–71. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 35.Schweizer C, Balsiger S, Bluethmann H, et al. The y2 subunit of GABA A receptors is required for maintenance of receptors at mature synapses. Molecular and Cellular Neuroscience. 2003;24:442–50. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 36.Rusconi R, Scalmani P, Cassulini RR, et al. Modulatory proteins can rescue a trafficking defective epileptogenic Nav1. 1 Na+ channel mutant. The Journal of neuroscience. 2007;27:11037–46. doi: 10.1523/JNEUROSCI.3515-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crestani F, Lorez M, Baer K, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nature neuroscience. 1999;2:833–9. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 38.Lydiard RB. The role of GABA in anxiety disorders. The Journal of clinical psychiatry. 2003;64:1–478. [PubMed] [Google Scholar]
- 39.Ren Z, Sahir N, Murakami S, et al. Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice. Neuropharmacology. 2015;88:171–9. doi: 10.1016/j.neuropharm.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moehler H. GABA A receptors in central nervous system disease: anxiety, epilepsy, and insomnia. Journal of Receptors and Signal Transduction. 2006;26:731–40. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.