Abstract
Powassan/Deer Tick Virus (POWV/DTV) is an emerging cause of arboviral neuroinvasive disease in the upper Midwest. These studies describe the prevalence and geographic distribution of Wisconsin ticks carrying POWV/DTV as well as the high frequency of Ixodes scapularis ticks coinfected with both POWV/DTV and Borrelia burgdorferi, the causative agent of Lyme disease. These findings suggest that concurrent transmission of POWV/DTV and B. Burgdorferi from coinfected ticks is likely to occur in humans.
Keywords: : arbovirus, Borrelia, encephalitis, Lyme, Powassan, ticks
Introduction
Minnesota and Wisconsin rank in the top 12 states with incident-reported cases of Lyme disease (LD) at ∼17 cases/100,000 population annually (CDC Lyme website). A recent report (CDC Ticks website) indicates that clinician-diagnosed LD in the U.S. is underreported by a factor of 11, suggesting that the true rate of Lyme borreliosis in the upper Midwest is closer to 187 cases/100,000 population. Additionally, Minnesota and Wisconsin rank in the top nine states for reported cases of anaplasmosis, the top seven for babesiosis, and are ranked second and third only behind New York with reported cases of POWV neuroinvasive disease (Wisconsin DHS Tickborne website). Tick-borne diseases represent a major public health concern in Wisconsin, but little is known regarding the frequency of transmissible pathogens harbored by Wisconsin ticks and the distribution of those infected ticks. The goal of these studies was to provide a broad geographical survey of the frequency and distribution of Ixodes scapularis ticks infected with B. burgdorferi and POWV/DTV.
Materials and Methods
The Wisconsin Department of Natural Resources (DNR) and civilian volunteers provided I. scapularis and D. variabilis ticks removed from their clothing, skin, and family pets. Tick recovery instructions, supplies, and prepaid postage mailers were provided to all participants to standardize collection and transport. Ticks were frozen and stored at −20°C upon receipt at our laboratory. The large majority of ticks submitted by the volunteers were adult females from each species, and data presented in this study represent analysis of these ticks only.
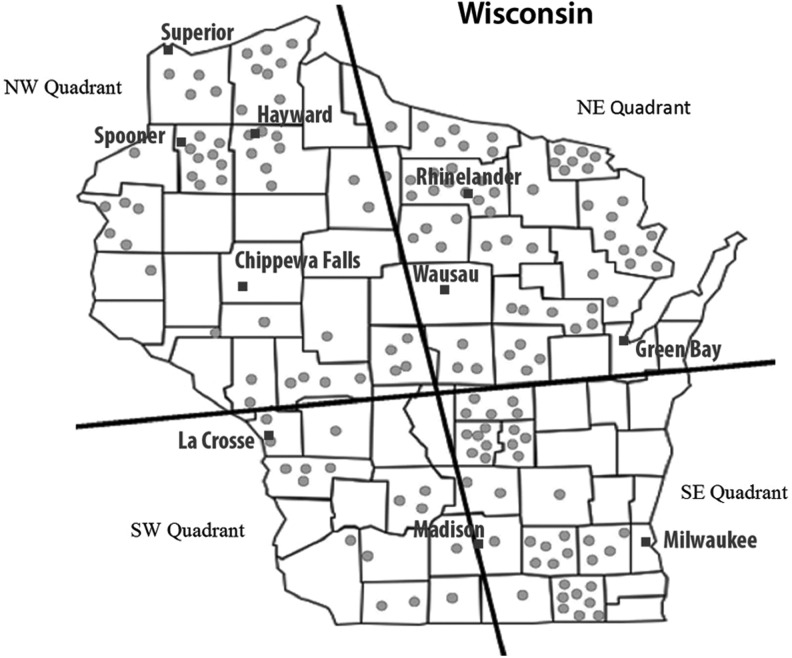
Tick epidemiology studies were divided into two processing stages or harvests. Harvest One occurred during the period September 2011 through July 2012 and consisted primarily of adult Ixodes and Dermacentor ticks collected from the northwest quadrant of the state (Fig. 1). The collection area for this harvest included land surrounding the towns of Spooner and Hayward, the same territory as described in earlier studies (Ebel et al. 1999, Brackney et al. 2008). Eighty-six adult Ixodes (54 female, 32 male) and 17 adult Dermacentor (6 female, 11 male) ticks were selected for analysis from 112 ticks submitted. This region historically contains the highest density of I. scapularis ticks harboring B. burgdorferi within Wisconsin and also reports the highest frequency of LD (Wisconsin DHS Tickborne website). For Harvest Two, greater than 2000 adult I. scapularis and D. variabilis ticks were submitted by civilian volunteers. Adult female ticks of both species were selected for analysis due to ease of identification and efficiency of pooling. Harvest Two consisted of 56 pools of I. scapularis (n = 353 ticks) and 59 pools of D. variabilis (n = 278 ticks) collected from 33 counties across the state from September 2013 through June 2015. This provided a broad geographic survey of pathogen prevalence across the state.
FIG. 1.
Map of tick collection locations across Wisconsin.
All ticks were thawed, visually examined, and classified by life cycle stage, gender, and species. Single ticks from Harvest One were washed in 70% ethanol, rinsed with deionized water, crushed, homogenized using QIAshredder (Qiagen #79656), and nucleic acid extracted with the QIAamp Viral RNA Mini Kit (Qiagen #52904). PCR testing was performed for conserved genomic segments from the 16S rRNA of B. burgdorferi (Bor sense 5′-cagataagactgccggtgataag-3′, Bor anti 5′-ccggactgagacctgcttta-3″, product 156 bp). RT-PCR for the NS5 gene of Powassan/Deer-tick virus (POWV/DTV) (sense 5′-aacatgatgggaaagagagag-3″, anti 5′-cagatccttcggtacatggaa-3″, product 318 bp) was also performed. PCR products were visually confirmed by agarose gel electrophoresis. For Harvest Two, single adult ticks and tick pools of 2–5 ticks of the same species and sex were tested. Tubes of washed and rinsed tick pools were placed into liquid nitrogen and disposable pestles were used for homogenization. The homogenate was lysed and total nucleic acid extracted using the GenElute™Plant Genomic DNA Miniprep Kits (Sigma #G2 N70). Real-time PCR was performed to assess each pool for the presence of 16S rRNA of B. burgdorferi, using the Rotor-Gene SYBR® Green PCR Kits (Qiagen #204074). RT-PCR for the NS5 gene of POWV/DTV was performed using the Rotor-Gene SYBR Green RT-PCR Kits (Qiagen # 204174). For visual confirmation on agarose gel, all positive SYBR Green RT-PCR samples were subjected to a second RT-PCR utilizing the NS5 sequencing primers described for Harvest One. Four representative POWV/DTV amplicons were verified by sequence analysis (Alpha Biolabs, Burlingame, California). National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) analysis found the NS5 amplicons to be 85–88% identical to DTV strains (taxid: 58535) as opposed to 76–80% identical to POWV (taxid: 11083), confirming the viruses to be most consistent with POWV lineage II (DTV). B. burgdorferi and POWV infection frequencies in both I. scapularis and D. variabilis ticks were calculated for minimal infection rate (MIR). Infection of I. scapularis tick pools were also analyzed for maximum likelihood estimation (MLE) utilizing PooledInfRate v. 4.0 software (Biggerstaff 2005).
Results
The distribution of I. scapularis and D. variabilis tick collection sites are categorized by geographic quadrant (QNW, QNE, QSW, & QSE) of the state (Fig. 1, Table 1). Nearly 80% of adult female I. scapularis ticks analyzed were collected from the northern half of the state (QNW and QNE) and accounted for 85% of POWV-positive ticks. While only 90 I. scapularis ticks were collected from the southern two quadrants, POWV-positive ticks were identified in both QSE and QSW. QNW I. scapularis ticks revealed the highest MLE of infection for both POWV and B. burgdorferi (4.67% and 23.42%, respectively). A separate analysis of I. scapularis collections from Harvest One endemic zone (Spooner/Hayward) QNW demonstrated a frequency of infection for both POWV (4.65%) and B. burgdorferi (27.91%) that is comparable to the total QNW (Fisher's exact, p = 1.00 and p = 0.35, respectively). QSE contained the lowest MLE for POWV (1.53%), but B. burgdorferi-infected ticks were high with a MLE of 15.69%. Of the 295 D. variabilis ticks analyzed from both harvests, none (0%) had evidence of POWV infection; however, B. burgdorferi infection in D. variabilis ticks was seen in both QNW (3.1%) and QSW (2.86%), consistent with the high B. burgdorferi infection rate observed in I. scapularis ticks in these same quadrants.
Table 1.
Infection Rates of Ixodes scapularis by State Quadrant
| Pooled | Tick MIR | % MLE (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quadrant | Single (N) | Pools (N) | Total Pools (N) | Total Ticks (N) | POWV Pos N (%) | B. burgdorferi Pos N (%) | POWV Pos N (%) | B. burgdorferi Pos N (%) | % POWV Pos | % B. burgdorferi Pos |
| NW | 50 | 46 | 96 | 173 | 8 (8.33) | 38 (39.58) | 8 (4.62) | 38 (21.97) | 4.67 (2.23–8.62) | 23.42 (17.72–30.05) |
| NE | 21 | 36 | 57 | 176 | 3 (5.26) | 5 (8.77) | 3 (1.70) | 5 (2.84) | 1.72 (0.46–4.57) | 2.92 (1.10–6.33) |
| SW | 0 | 6 | 6 | 26 | 1 (16.67) | 0 (0.00) | 1 (3.85) | 0 (0) | 3.85 (0.23–18.10) | 0 |
| SE | 10 | 14 | 24 | 64 | 1 (4.17) | 9 (37.50) | 1 (1.56) | 9 (14.06) | 1.53 (0.09–7.12) | 15.69 (8.28–26.73) |
MIR, minimum infection rate; MLE, maximum likelihood estimates; NE, Northeast, NW, Northwest; POWV, Powassan virus, SE, Southeast; SW, Southwest.
Discussion
In this survey, I. scapularis ticks collected from QNW demonstrated a POWV/DTV infection frequency of 4.67% (Table 1), similar in frequency to a survey conducted in 1998 (Ebel et al. 1999), but significantly higher rate than 1.3% reported from a more recent survey of adult deer ticks collected from the northwestern part of the state (Brackney et al. 2008). The tick collections described in earlier studies relied on the technique of “flagging” to capture ticks from vegetative undergrowth, whereas ticks collected in our studies were primarily harvested from the clothing and skin of humans and hair of domestic pets. Studies show that adult, host-seeking female I. scapularis ticks are more likely to carry POWV (Ebel et al. 1999, Anderson and Armstrong 2012) than are adult male I. scapularis ticks (Ebel et al. 2000), and that I. scapularis ticks removed from a blood host are more likely to harbor a transmissible pathogen (Han et al. 2016) than are questing ticks pulled from vegetation. Consistent with previous reports (Brackney et al. 2008) our studies demonstrated a lack of POWV/DTV infection in the common dog tick D. variabilis. Our finding of borrelia DNA in D. variabilis ticks is consistent with reports that these ticks can become infected with B. burgdorferi, from feeding on bacteremic rodents and small animals in Lyme endemic regions. While D. variabilis can acquire the infection, it is generally accepted that this tick species is not a competent vector for B. burgdorferi, and human transmission of the bacteria by the tick is unlikely (Mukolwe et al. 1992, Soares et al. 2006).
Unlike other tick-borne bacteria and protozoa, which require a blood meal and 24–48 h for pathogen replication and transmission, effective transmission of POWV has been reported within 15 min of tick attachment to the blood host (Ebel and Kramer 2004). Tick saliva appears capable of enhancing the transmission of POWV to the host (Hermance and Thangamani 2015) during even casual tick attachment and suggests a higher infectious risk than what is noted for other tick-borne pathogens. Interestingly, 4 of 8 POWV-positive single ticks from QNW were coinfected with B. burgdorferi (50%), demonstrating the potential for concurrent transmission of LD and POWV to humans.
Conclusion
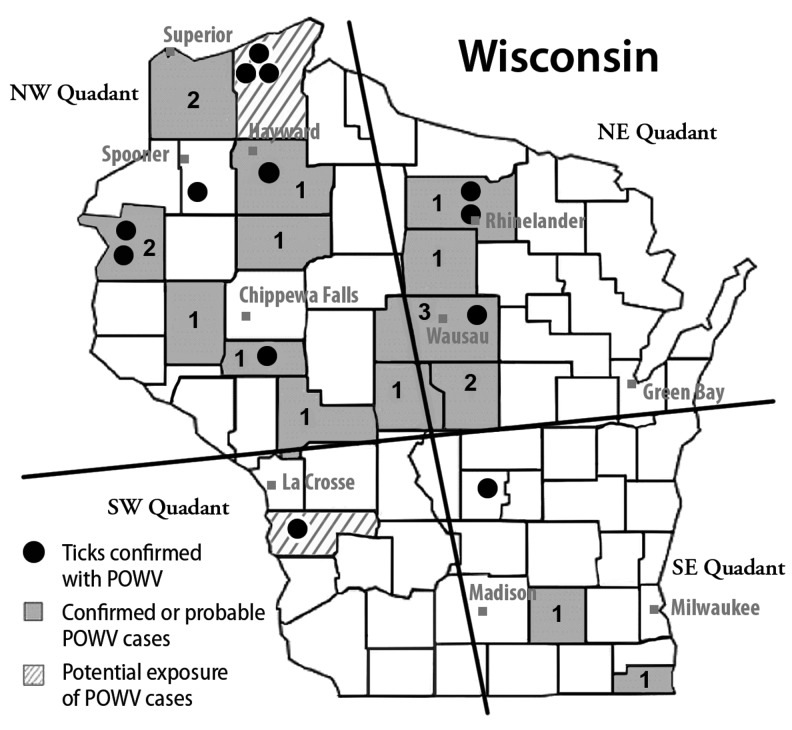
In these studies we show that the distribution of POWV-positive ticks closely mirrors the Wisconsin counties reporting confirmed cases of POWV encephalitis or suspected POWV exposure (Fig. 2). A surveillance study conducted in a northern Ontario community revealed seroreactivity to POWV/DTV in up to 3% of the population (Dhama et al. 2014), suggesting that POWV infection without encephalitis is likely in humans. Recent studies by CDC (Eisen et al. 2016) demonstrate the rapid geographical expansion of I. scapularis, which would provide the permissive vector necessary for expanding the territory of both B. burgdorferi and POWV/DTV and increase the likelihood for human infection with both of these agents. The frequency, clinical presentation, and outcomes of POWV infection and potential concurrent POWV and B. burgdorferi infection are understudied. In the LD endemic regions of the upper Midwest, North Atlantic states and expanding I. scapularis territories, epidemiologic studies of POWV infection and coinfection with other tick-borne pathogens are warranted.
FIG. 2.
Map of positive cases of POWV Encephalitis, Wisconsin 2003–2014. (Wisconsin DHS Tickborne website).
Acknowledgments
The authors thank Sue C. Kehl, Medical College of Wisconsin, Anna M. Schotthoefer, Marshfield Clinic Research Foundation, and Phillip F. Pratt, Coppe Laboratories, for review of this article and insightful discussions. They also thank the many civilian volunteers and the Wisconsin Department of Natural Resources for their support in the tick collection efforts.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson JF, Armstrong PM. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg 2012; 87:754–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff BJ. PooledInfRate software. Vector Borne Zoonotic Dis 2005; 5:420–421 [DOI] [PubMed] [Google Scholar]
- Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, et al. Short Report: Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 2008; 79:971–973 [PMC free article] [PubMed] [Google Scholar]
- CDC. Lyme. Website. Available at: www.cdc.gov/lyme/stats/tables.html
- CDC. Ticks. Website. Available at: www.cdc.gov/ticks/diseases/index.html
- Dhama K, Pawaiya RSV, Chakraborty S, Tiwari R, et al. Powassan virus (POWV) infection in animals and humans: A review. Asian J Anim Vet Adv 2014; 9:177–189 [Google Scholar]
- Ebel GD, Campbell EN, Goethert HK, Spielman A, et al. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg 2000; 63:36–42 [DOI] [PubMed] [Google Scholar]
- Ebel GD, Foppa I, Spielman A, Telford SR. A focus of deer tick virus transmission in the Northcentral United States. Emerg Infect Dis 1999; 5:570–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Kramer LD. Short report: Duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 2004; 71:268–271 [PubMed] [Google Scholar]
- Eisen R, Eisen L, Beard C. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 2016; 53:349–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Hickling GJ, Tsao JI. High prevalence of Borrelia miyamotoi among adult blacklegged ticks from white-tailed deer. Emerg Infect Dis 2016; 22:316–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermance ME, Thangamani S. Tick saliva enhances Powassan virus transmission to the host influencing its dissemination and the course of disease. J Virol 2015; 89:7852–7860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukolwe SW, Kocan AA, Barker RW, Kocan KM, et al. Attempted transmission of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) (JDI strain) by Ixodes scapularis (Acari: Ixodidae), Dermacentor variabilis, and Amblyomma americanum. J Med Entomol 1992; 29:673–677 [DOI] [PubMed] [Google Scholar]
- Soares CA, Zeidner NS, Beard CB, Dolan MC, et al. Kinetics of Borrelia burgdorferi infection in larvae of refractory and competent tick vectors. J Med Entomol 2006; 43:61–67 [DOI] [PubMed] [Google Scholar]
- Wisconsin Department Health Services. Tickborne. Website. Available at: www.dhs.wisconsin.gov/tickborne/powassan.htm
- Wisconsin Department Health Services. Website. Tickborne. Available at: www.dhs.wisconsin.gov/tickborne/powassan-data.htm