Abstract
The emerging mosquito-borne virus, Zika virus (ZIKV), has been causally associated with adverse pregnancy and neonatal outcomes, including miscarriage, microcephaly, serious brain abnormalities, and other birth defects indicative of a congenital ZIKV syndrome. In this review, we highlight work from human and animal studies on routes of infection in pregnancy that lead to adverse fetal and neonatal outcomes. A number of innate and adaptive immune mechanisms and signaling molecules that may have key roles in ZIKV infection pathogenesis are discussed along with putative viral entry pathways. A more granular understanding of pathogenesis of ZIKV infection during pregnancy is critical for developing therapeutics and vaccines and mounting a global public health response to limit ZIKV infections. We also report on new therapeutic interventions that have shown success in preclinical studies.
Keywords: : trophoblast, placenta, Hofbauer, interferon
Zika Virus and Human Pregnancy Outcomes
Zika virus (ZIKV) is a flavivirus in the Flaviviridae family, which includes other globally important pathogens including dengue, West Nile, and yellow fever, and Japanese encephalitis viruses. ZIKV is transmitted predominantly by Aedes aegypti mosquitoes that are common in tropical areas and also by Aedes albopictus, which is prevalent in the upper continental United States and more temperate climates. Following its initial identification in the Zika forest in Uganda in 1947, sporadic outbreaks in parts of Africa and Asia, and high incidence of epidemics in Micronesia and French Polynesia in 2007 and 2013 occurred. Since 2015, ZIKV has emerged as a major cause of adverse fetal outcomes during pregnancy (Petersen and others 2016; Rasmussen and others 2016; van der Eijk and others 2016) and is linked epidemiologically to the Guillain–Barré syndrome in infected adults (Cao-Lormeau and others 2016). ZIKV infection can have devastating effects throughout pregnancy with damage to the fetal brain possible even if the infection occurs in the later stages of pregnancy (Brasil and others 2016). The impact of ZIKV epidemic on human health reflects the devastating fetal and neonatal outcomes, and the possible long-term neurodevelopmental consequences of in utero infection even in those with no overt signs at the time of birth.
Routes and Cellular Sites of ZIKV Infection During Human Pregnancy
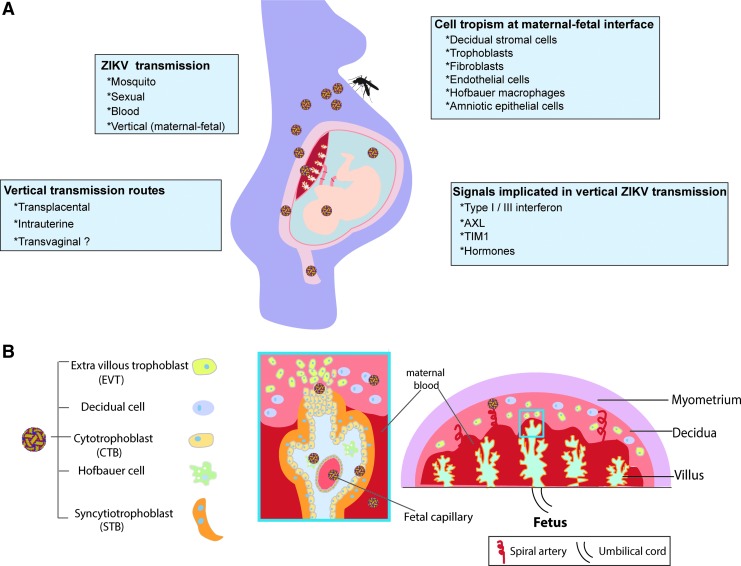
In humans, in addition to mosquito transmission, ZIKV can be spread through sexual contact (male to female, female to male, or male to male) (D'Ortenzio and others 2016; Turmel and others 2016). ZIKV viral RNA has been found in semen for over 6 months following the initial diagnosis of infection (Nicastri and others 2016) and in female vaginal secretions (Davidson and others 2016; Prisant and others 2016). Transmission via blood is also possible (Driggers and others 2016). The route of transmission that has received the most attention in humans is vertical transmission during pregnancy. Infection at any time point during pregnancy has been associated with adverse fetal outcomes, however, the first-trimester infection appears to pose the highest risk for fetal injury (Cauchemez and others 2016; Honein and others 2017) when transmission across the developing placenta and into the amniotic or yolk sacs may occur (Boeuf and others 2016). In the past 12 months, several mouse and human studies have yielded insights into pathogenesis of this viral infection during pregnancy (Fig. 1A).
FIG. 1.
ZIKV infection pathogenesis in pregnancy. (A) Summary of routes of ZIKV transmission in a pregnant woman and the cells and signals implicated in transmission. (B) Structure of human placenta and sites of ZIKV infection. In the human placenta, there exist fetal-derived chorionic villi (blue box), which are tree-like projections lined with 2 layers of trophoblasts and bathed in maternal blood. Villous trophoblasts comprise 2 layers: the STBs and CTBs. The CTBs are highly proliferative and form a monolayer of polarized cells that eventually differentiate via cell–cell fusion into STBs. STBs form a surface covered by a dense network of branched microvilli that are bathed in maternal blood, mediate nutrient and gas exchange between mother and fetus. Fetal-derived macrophages, known as Hofbauer cells, are found in the intervillous spaces. A subset of trophoblasts, termed EVTs, migrates from the chorionic villi, invades into the uterine wall, and remodels maternal spiral arteries to facilitate blood supply of the placental unit. In addition to the EVTs, the decidual compartment also includes maternal immune cells (eg, decidual macrophages, decidual natural killer cells) and stromal cells. CTBs, cytotrophoblasts; EVTs, extravillous cytotrophoblasts; STBs, syncytiotrophoblasts; ZIKV, Zika virus.
Recent studies investigating how ZIKV reaches the intrauterine space and infects the fetus have found broad cell tropism of ZIKV in the human placenta, including infection of placental trophoblasts, endothelial cells, fibroblasts, and fetal macrophages known as Hofbauer cells in the intervillous space (El Costa and others 2016; Jurado and others 2016; Miner and others 2016a; Quicke and others 2016; Tabata and others 2016; Aagaard and others 2017). The placental syncytium comprises undifferentiated cytotrophoblasts (CTBs), which can fuse to form syncytiotrophoblasts (STBs) or migrate as extravillous cytotrophoblasts to invade into the uterine wall and remodel maternal spiral arteries to facilitate blood supply of the placental unit (Red-Horse and others 2004) (Fig. 1B). STBs are refractory to ZIKV infection in primary villous explants (Tabata and others 2016) and primary cultured STBs (Bayer and others 2016). This is consistent with previous studies showing that STBs are resistant to pathogenic infection by parasites (Toxoplasma gondii) and bacteria (Listeria monocytogenes and Escherichia coli) (Robbins and others 2012; Zeldovich and others 2013; Cao and Mysorekar 2014). However, these do not exclude the possibility that cellular damage of the placental syncytium caused by even limited ZIKV replication in STBs could facilitate ZIKV entry into CTBs and further into the intravillous space to infect Hofbauer cells. A number of studies have demonstrated that primary and cultured CTBs (Miner and others 2016a; Quicke and others 2016; Aagaard and others 2017) and Hofbauer cells (Jurado and others 2016; Noronha and others 2016) support ZIKV replication.
A recent study evaluated placentas from a pregnancy complicated by ZIKV infection and demonstrated that infection appeared to induce proliferation of Hofbauer macrophages (Rosenberg and others 2017). In support of this, another human study found ZIKV RNA localized in placental chorionic villi in more than three-quarters of women who were positive for ZIKV RNA during their pregnancies and/or had adverse pregnancy outcomes. ZIKV RNA was predominantly localized to the Hofbauer cells (Bhatnagar and others 2017) (Fig. 1B). Although the importance of ZIKV infection in Hofbauer cells is still unclear, it has been speculated that their infection may promote vertical transmission of ZIKV and pathogenesis of congenital ZIKV symptoms (Simoni and others 2017). It is evident that ZIKV needs to cross a number of cellular protective layers, including those formed by trophoblasts and Hofbauer cells to reach the fetal compartment.
Generating Mouse Models of ZIKV Infection Pathogenesis During Pregnancy
As the Zika viral epidemic started to emerge, it became clear that animal models were needed to better understand the mechanisms of vertical transmission and disease. However, ZIKV did not cause consistent infection in healthy wild-type mice. ZIKV, analogous to other flaviviruses, must overcome type I interferon (IFN) signaling to multiply and cause infection in vertebrates. Activation of IFN signaling via IFN receptors (IFNAR1 and IFNAR2) and subsequent activation of the Jak/Stat pathway (Jak1, TYk2, and STAT1/STAT2), leads to production of IFN-stimulated genes (MacMicking 2012) that restrict infection and modulate cellular and adaptive immunity. Flaviviruses, which require prolonged viremia (viral loads in blood) to maintain their vector–host cycles, efficiently antagonize IFN signaling in humans as some of their nonstructural genes (eg, NS3 and NS5) act as viral IFN antagonists through binding, degradation, and proteasomal targeting of host defense proteins (Versteeg and Garcia-Sastre 2010). In contrast to the human STAT2 ortholog, ZIKV does not promote degradation of murine STAT2 and is thus unable to establish sustained infection and viremia in mice (Grant and others 2016; Kumar and others 2016).
Given these findings, several groups have used mice with deficiencies in IFN signaling to model ZIKV pathogenesis in mice (Cugola and others 2016; Lazear and others 2016; Miner and others 2016b; Tang and others 2016), including during pregnancy (Miner and others 2016a). A contemporary strain of ZIKV from French Polynesia was inoculated subcutaneously in IFNAR-deficient mice to permit a sufficiently high level of viremia in the pregnant dam to infect the placenta. An early time point in pregnancy (embryonic day 6.5) was selected to model the first trimester in human pregnancy. ZIKV infection of pregnant dams led to severe placental and fetal injury, including damage to fetal blood vessels, which in turn led to fetal demise. ZIKV infected trophoblasts and fetal endothelial cells that line fetal capillaries [reviewed in Mysorekar and Diamond (2016)], suggesting a transplacental route of transmission for ZIKV. These observed phenotypes were akin to those noted in pregnant women infected with ZIKV (Parameswaran and others 2010; Brasil and others 2016; Sarno et al., 2016). Particularly noteworthy was that the placental tissue contained ∼1,000-fold higher concentration of ZIKV RNA than was found in maternal serum, suggesting that ZIKV preferentially replicates in cells of the placenta. Thus, maternal ZIKV infection compromises the placental barrier by infecting fetal trophoblasts and thereby enters the fetal circulation and impairs development. A second model of ZIKV infection was also developed that used a monoclonal antibody against IFNAR1 (MAR1-5A3), which transiently blocked IFNAR signaling in wild-type mice (Sheehan and others 2006). Treatment with the anti-IFNAR1 antibody a day before ZIKV infection was sufficient to permit the virus to infect the pregnant dams and result in fetal brain injury and adverse fetal outcomes.
Two additional studies using mouse models also addressed the causal relationship between maternal ZIKV infection in pregnancy and fetal outcomes. Cugola and others (2016) inoculated pregnant SJL dams intravenously with a high dose of a Brazilian strain of ZIKV and demonstrated fetal growth restriction and severe fetal brain injury to cortical neurons in the cerebral cortex, and ocular abnormalities were also noted in human neonates. A third study by Wu and others (2016) injected a contemporary Asian ZIKV strain intraperitoneally into pregnant immunocompetent dams at embryonic day 13.5, which elicited a transient viremia and placental seeding leading to infection of cortical neural progenitors of fetal mice. Together, these studies established that ZIKV infection in pregnancy led directly to fetal brain injury via a transplacental route.
More recent studies have demonstrated that vaginal transmission route can lead to fetal infection as well as direct intrauterine inoculation (Yockey and others 2016). Vaginal infection with an Asian strain of ZIKV of Ifnar1−/− dams at an early pregnancy stage led to embryo reabsorption, intrauterine growth restriction, and infection in fetal brains, suggesting that ZIKV infection in lower female reproductive tract may take a transvaginal ascending route to access the fetus during pregnancy and infect via a placental or paraplacental route (Yockey and others 2016). Most recently, Vermillion and others have established a model of intrauterine infection with ZIKV in wild-type mice. This model has the advantage of using immunocompetent and outbred mice and bypassing the need for ZIKV infection of the periphery. Using intrauterine inoculation with African, contemporary Asian and Brazilian strains of ZIKV directly into the uterine artery of a given fetoplacental unit, they found ZIKV viral RNA localized to the infected uterine horns, placentas, and fetuses (Vermillion and others 2017). Wild-type pregnant mice infected with this strain using the intraperitoneal route did not exhibit these phenotypes. Together, these studies suggest that, similar to ascending intrauterine bacterial infections, if ZIKV reaches the intrauterine compartment via sexual transmission route, vaginal route, or a direct intrauterine route, it poses risk for vertical transmission. Whether a transgenital route is implicated in human vertical transmission of ZIKV remains to be investigated.
Signals Implicated in Vertical ZIKV Transmission in Mouse and Human Pregnancy
Type I/III IFN signaling
As mentioned above, wild-type mice with intact type I IFN do not get infected with ZIKV in the periphery (Lazear and others 2016). However, animal models with compromised type I IFN signaling, including Ifnar1-deficient females crossed to WT males and pregnant WT females treated with an IFNAR-blocking antibody, are susceptible to ZIKV infection and lead to fetal demise (Miner and others 2016a). Intrauterine delivery of ZIKV in pregnancy in immunocompetent mice also upregulates type I IFN signaling and IFN stimulated gene expression (Vermillion and others 2017). These data strongly support an antiviral role of type I IFN in vertical transmission of ZIKV during pregnancy in mice. Similarly, type I IFN signaling has been shown to be critical for infection and prolonged persistence of ZIKV in the female genital tract, as female Ifnar1−/− mice exhibit high-titer ZIKV replication in the vagina (Yockey and others 2016). A recent study also shows dampened induction of type I IFN and various IFN-stimulated genes on ZIKV infection in the vagina in a wild-type mouse relative to what is elicited on systemic administration of ZIKV (Khan and others 2016). This could explain why ZIKV may take a transgenital infection route.
Type III IFN-λ signaling has also been identified as a possible regulator of ZIKV infection (Bayer and others 2016). Primary cultured human trophoblast cells (STBs) isolated from full-term placentas were resistant to ZIKV infection due to production of type III IFNs, especially IFNλ1, which may protect the trophoblasts from ZIKV infection in an autocrine or paracrine manner. Type I and III IFNs have been shown to be induced in response to ZIKV infection in decidual explant cultures, in which ZIKV infection induced transcription of IFNα/β and IFNλ (Weisblum and others 2017). However, another study has reported that type III IFN signals were not induced in CTBs infected with ZIKV (Quicke and others 2016). It remains to be determined whether the differences noted represent different antiviral responses in CTBs and STBs or the experimental conditions of the studies. Antiviral functions of IFN-λ have been shown in viral infections in a number of tissues, including the liver, skin, respiratory, gastrointestinal, and urogenital tracts (Lazear and others 2015a, 2015b).
There is some evidence suggesting a role for IFN-λ in maternal-fetal transmission of placental pathogens. For example, infection of pregnant mice with L. monocytogenes, a vertically transmitted bacterium that causes maternal-fetal listeriosis, induces transcription of IFN-λ2/3 and IFN-responsive genes (IFIT1 and Mx1/2) in their placentas. Furthermore, IFN-λ2 treatment induces robust increase of Mx1 expression in the mouse maternal–fetal unit, including maternal decidua, placental labyrinth, and fetal membranes (Bierne and others 2012). These studies indicate that the maternal–fetal unit responds to IFN-λ and suggest a protective function in placenta to congenital bacterial infections. Further work is needed to provide a complete picture of possible antiviral functions for type III IFN signaling in pregnancy in vivo.
Adaptive immune signals
Deletion of recombination activating gene-2 (Rag2−/−) in mice, which prevents development of mature T and B cells, did not affect ZIKV infectivity in the female genital tract, suggesting that adaptive immune responses are not required to control early ZIKV replication (Yockey and others 2016). However, a recent study has demonstrated a protective function of CD8+ T cells in ZIKV pathogenesis (Elong Ngono and others 2017). ZIKV infection induced CD8+ T cell expansion and activation in mice with compromised type I IFN signaling. CD8+ T cell-deficient (CD8−/−) nonpregnant C57BL/6 were more susceptible to ZIKV infection, and adoptive transfer of ZIKV-immune CD8+ T cells significantly decreased the ZIKV burden. Whether the protective function of CD8+ T cells is true in pregnancy remains to be investigated, especially considering that pregnancy is a naturally immunocompromised state (PrabhuDas and others 2015).
Hormonal signals
The mammalian endocrine system can modulate susceptibility to microbial infections in females. For example, increased levels of the hormone progesterone, which occur during stages of the menstrual cycle or pregnancy, can affect susceptibility to viral infections (eg, HIV). Several studies showed that estradiol upregulates type I IFN production via the canonical estrogen receptor-mediated signaling pathway. This regulatory effect of estrogen on IFNs may explain gender differences of HIV pathogenesis and protective roles of estrogen on in vitro HIV infection. Recently, Tang and others demonstrated that AG129 mice deficient in type I or type II IFN signals systemically or type I IFN in myeloid cells support transgenital transmission when challenged by ZIKV in the diestrus but not estrus phase (Tang and others 2016). This suggests that transgenital transmission of ZIKV may be under hormonal regulation. However, whether different susceptibilities to ZIKV infection at different estrus cycle stages are through estrogen-dependent regulation and involve other IFNs is still unclear. Moreover, whether the hormonal changes that occur during pregnancy play a role in ZIKV susceptibility remains to be elucidated.
Putative receptors for ZIKV entry into placental cells
The TAM receptors (Tyro3, Axl, and Mertk) are a family of receptor tyrosine kinases, activated by soluble ligands Gas6 and Protein S, which recognize phosphatidylserine on the surface of apoptotic cells and enveloped viruses (Meertens and others 2012). TAMs can be exploited by flaviviruses, such as West Nile virus and dengue virus to infect target cells (Meertens and others 2012). TAM receptors activated by viruses can dampen innate immune response, such as inhibition of type I IFN signaling (Bhattacharyya and others 2013). In particular, the TAM receptor Axl has been suggested as a key target for ZIKV attachment for ZIKV in different models (Hamel and others 2015; Ma and others 2016; Nowakowski and others 2016; Savidis and others 2016).
ZIKV infection has been shown to promote Axl kinase activity to enhance infection in glia (Meertens and others 2017). AXL binding but not intracellular kinase activity appears required for ZIKV infection in glial cells (Retallack and others 2016). Similarly, AXL was shown to mediate ZIKV entry via clathrin-mediated endocytosis in glial cells, which requires Gas 6 as a bridge to link ZIKV to glial cells (Meertens and others 2017). Furthermore, blocking AXL activation in endothelial cells by targeting the extracellular domain of the protein has been shown to inhibit ZIKV entry, and viral entry has been shown to require AXL catalytic activity (Liu and others 2016). However, other studies performed in vitro and in vivo do not support the hypothesis that AXL is required for viral entry of ZIKV. For example, in human neural progenitor cells and cerebral organoids, genetic deletion of AXL did not affect ZIKV entry nor limit the cell death caused by ZIKV infection (Wells and others 2016). In addition, mice deficient in Axl, Mertk, or both did not differ from wild-type mice in terms of ZIKV replication or pathogenesis in the brain, eye, or testis, suggesting that Axl and Mertk are not required for infection of these organs in adult mice (Govero and others 2016; Miner and others 2016b). Moreover, ZIKV infection increased Axl kinase activity by promoting Axl phosphorylation and further suppression of innate immunity to enhance infection in glia (Meertens and others 2017).
It is important to note that the majority of mouse models for ZIKV studies were developed by compromising the type I IFN signaling pathway. Inhibition of type I IFN pathways by ZIKV infection through AXL may be not seen in these mouse models. Thus, it is difficult to interpret the effects of TAM receptor on innate immune response in vivo in an innate immunity deficient background. Most recently, Vermillion and others (2017) have shown Axl expression was increased on intrauterine ZIKV infection in placentas from an immunocompetent outbred mouse strain. Tabata and others (2016) demonstrated that inhibiting AXL in primary human trophoblasts led to only a modest reduction of ZIKV infection. However, the human trophoblast cell line, Jeg-3, has low levels of AXL expression but is highly permissive to ZIKV infection, suggesting that additional viral entry mechanisms must exist (Rausch and others 2017). The function of AXL in the context of viral entry, replication, or pathogenesis may vary substantially depending on tissue compartment, cell type, and experimental model. These data together indicate that AXL likely is not the only or dominant entry factor required for ZIKV infection in trophoblasts.
TIM1, a member of the T cell immunoglobulin and mucin domain protein family, has been suggested as an important factor in maternal-fetal transmission of ZIKV. TIM1, like TAM receptors, is widely expressed in different cells at the maternal–fetal interface (Tabata and others 2016). Interestingly, a TIM1 inhibitor, duramycin, reduces ZIKV infection more significantly compared with an AXL inhibitor, indicating perhaps a more important role of TIM1 in ZIKV congenital infection (Tabata and others 2016). There has been no experimental evidence supporting in vivo roles for TAMs or TIM on vertical transmission of ZIKV thus far. Cell-type-specific modulation of TAMs and/or TIM in trophoblasts or other cell types on the maternal–fetal interface may be more reasonable considering the complicated cell-type-specific role of TAM receptors.
Development of Therapeutic Interventions to Block ZIKV Vertical Transmission
Given the lack of effective and safe vaccines against ZIKV, the introduction of immediate interventions to attenuate and stop the maternal-fetal transmission of ZIKV has become an urgent challenge (Pierson and Graham 2016). Systemic administration of convalescent serum from a patient with prior ZIKV infection into the peritoneal cavity of pregnant ICR mice infected with ZIKV successfully protected the fetus from microcephaly and other neurological damage (Wang and others 2017). However, uncertainties and limitations of the convalescent plasma weaken the feasibility of using it as a large-scale therapeutics with certain and proven safety. Remarkably rapid progress has been reported in identifying neutralizing monoclonal antibodies against ZIKV from humans (Sapparapu and others 2016; Stettler and others 2016; Wang and others 2016) and mice (Zhao and others 2016) with the capacity of blocking ZIKV transmission. In vivo passive transfer of these antibodies protects adult mice from ZIKV infection providing avenues of use as prophylaxis or treatment against ZIKV infections. However, prevention and mitigation of ZIKV congenital infections require that any ZIKV therapeutic developed should be amenable to be given to pregnant women. Thus, the efficacy and safety of these treatments against ZIKV infection should be tested in pregnant animal models at the preclinical stage. To this end, a neutralizing mAb, ZIKV-117, worked as both prophylaxis and therapy in ZIKV-infected pregnant mice, as evidenced by reductions in ZIKV titers in maternal organs and the feto-placental units. ZIKV-117 treatment improved or completely rescued pregnancy complications caused by maternal-fetal transmission of ZIKV in mouse, including placental insufficiency, fetal growth restriction, and fetal demise (Sapparapu and others 2016).
Summary
Since the appreciation of ZIKV congenital syndrome in 2015, an unprecedented level of collaborative, global, rapid progress has been made to understand the routes of ZIKV maternal-fetal transmission and develop new therapeutic interventions. Ongoing and future investigations into the impact of ZIKV infection at different stages of pregnancy and the identification of ZIKV entry mechanisms into the placenta will undoubtedly yield further insights into its unique pathogenesis.
Acknowledgments
This work was supported by a Preventing Prematurity Initiative grant from the Burroughs Wellcome Fund and a Prematurity Research Initiative Investigator award from the March of Dimes (to I.U.M.), NIH/NICHD grant R01HD091218 (to I.UM. and M.S.D.), and R01 AI073755, R01 AI104972, and P01 AI106695 to M.S.D.
Author Disclosure Statement
No competing financial interests exist.
References
- Aagaard KM, Lahon A, Suter MA, Arya RP, Seferovic MD, Vogt MB, Hu M, Stossi F, Mancini MA, Harris RA, Kahr M, Eppes C, Rac M, Belfort MA, Park CS, Lacorazza D, Rico-Hesse R. 2017. Primary human placental trophoblasts are permissive for Zika virus (ZIKV) replication. Sci Rep 7:41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr., Cherry S, Sadovsky Y, Coyne CB. 2016. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19(5):705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, Gary J, Muehlenbachs A, Lambert A, Lanciotti R, Oduyebo T, Meaney-Delman D, Bolanos F, Saad EA, Shieh WJ, Zaki SR. 2017. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis 23(3):405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Zagorska A, Lew ED, Shrestha B, Rothlin CV, Naughton J, Diamond MS, Lemke G, Young JA. 2013. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe 14(2):136–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierne H, Travier L, Mahlakoiv T, Tailleux L, Subtil A, Lebreton A, Paliwal A, Gicquel B, Staeheli P, Lecuit M, Cossart P. 2012. Activation of type III interferon genes by pathogenic bacteria in infected epithelial cells and mouse placenta. PLoS One 7(6):e39080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeuf P, Drummer HE, Richards JS, Scoullar MJ, Beeson JG. 2016. The global threat of Zika virus to pregnancy: epidemiology, clinical perspectives, mechanisms, and impact. BMC Med 14(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baiao AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375(24):2321–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Mysorekar IU. 2014. Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta 35(2):139–142 [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Despres P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawche F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387(10027):1531–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, Salje H, Van Kerkhove MD, Abadie V, Garel C, Fontanet A, Mallet HP. 2016. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: a retrospective study. Lancet 387(10033):2125–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria Dde P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534(7606):267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, Descamps D, Damond F, Leparc-Goffart I. 2016. Evidence of sexual transmission of Zika virus. N Engl J Med 374(22):2195–2198 [DOI] [PubMed] [Google Scholar]
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. 2016. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep 65(28):716–717 [DOI] [PubMed] [Google Scholar]
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, Rosenberg A, Hill DA, DeBiasi RL, Vezina G, Timofeev J, Rodriguez FJ, Levanov L, Razak J, Iyengar P, Hennenfent A, Kennedy R, Lanciotti R, du Plessis A, Vapalahti O. 2016. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374(22):2142–2151 [DOI] [PubMed] [Google Scholar]
- El Costa H, Gouilly J, Mansuy JM, Chen Q, Levy C, Cartron G, Veas F, Al-Daccak R, Izopet J, Jabrane-Ferrat N. 2016. ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep 6:35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A, Vizcarra EA, Tang WW, Sheets N, Joo Y, Kim K, Gorman MJ, Diamond MS, Shresta S. 2017. Mapping and role of the CD8+ T cell response during primary Zika virus infection in mice. Cell Host Microbe 21(1):35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. 2016. Zika virus infection damages the testes in mice. Nature 540(7633):438–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, Garcia-Sastre A. 2016. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 19(6):882–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. 2015. Biology of Zika virus infection in human skin cells. J Virol 89(17):8880–8896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, Ellington SR, Shapiro-Mendoza CK, Oduyebo T, Fine AD, Brown CM, Sommer JN, Gupta J, Cavicchia P, Slavinski S, White JL, Owen SM, Petersen LR, Boyle C, Meaney-Delman D, Jamieson DJ; US Zika Pregnancy Registry Collaboration. 2017. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 317(1):59–68 [DOI] [PubMed] [Google Scholar]
- Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, Fikrig E. 2016. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 1(13):pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Woodruff EM, Trapecar M, Fontaine KA, Ezaki A, Borbet TC, Ott M, Sanjabi S. 2016. Dampened antiviral immunity to intravaginal exposure to RNA viral pathogens allows enhanced viral replication. J Exp Med 213(13):2913–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Hou S, Airo AM, Limonta D, Mancinelli V, Branton W, Power C, Hobman TC. 2016. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep 17(12):1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Jr., Klein RS, Diamond MS. 2015a. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med 7(284):284ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19(5):720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Nice TJ, Diamond MS. 2015b. Interferon-lambda: Immune functions at barrier surfaces and beyond. Immunity 43(1):15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, DeLalio LJ, Isakson BE, Wang TT. 2016. AXL-mediated productive infection of human endothelial cells by Zika virus. Circ Res 119(11):1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M, Yan J, Li W, Qin C, Han D, Qin C, Wang N, Li X, Gao GF. 2016. Zika virus causes testis damage and leads to male infertility in mice. Cell 167(6):1511–1524.e10 [DOI] [PubMed] [Google Scholar]
- MacMicking JD. 2012. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol 12(5):367–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. 2012. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12(4):544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meertens L, Labeau A, Dejarnac O, Cipriani S, Sinigaglia L, Bonnet-Madin L, Le Charpentier T, Hafirassou ML, Zamborlini A, Cao-Lormeau VM, Coulpier M, Misse D, Jouvenet N, Tabibiazar R, Gressens P, Schwartz O, Amara A. 2017. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep 18(2):324–333 [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016a. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165(5):1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Ruckert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. 2016b. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep 16(12):3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysorekar IU, Diamond MS. 2016. Modeling Zika virus infection in pregnancy. N Engl J Med 375(5):481–484 [DOI] [PubMed] [Google Scholar]
- Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. 2016. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill 21(32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. 2016. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz 111(5):287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. 2016. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 18(5):591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, Heissmeyer V, Einav S, Jackson W, Doukas T, Paranjape S, Polacek C, dos Santos FB, Jalili R, Babrzadeh F, Gharizadeh B, Grimm D, Kay M, Koike S, Sarnow P, Ronaghi M, Ding SW, Harris E, Chow M, Diamond MS, Kirkegaard K, Glenn JS, Fire AZ. 2010. Six RNA viruses and forty-one hosts: viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog 6(2):e1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. 2016. Zika virus. N Engl J Med 374(16):1552–1563 [DOI] [PubMed] [Google Scholar]
- Pierson TC, Graham BS. 2016. Zika virus: immunity and vaccine development. Cell 167(3):625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Bonney E, Caron K, Dey S, Erlebacher A, Fazleabas A, Fisher S, Golos T, Matzuk M, McCune JM, Mor G, Schulz L, Soares M, Spencer T, Strominger J, Way SS, Yoshinaga K. 2015. Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nat Immunol 16(4):328–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisant N, Bujan L, Benichou H, Hayot PH, Pavili L, Lurel S, Herrmann C, Janky E, Joguet G. 2016. Zika virus in the female genital tract. Lancet Infect Dis 16(9):1000–1001 [DOI] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. 2016. Zika virus infects human placental macrophages. Cell Host Microbe 20(1):83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374(20):1981–1987 [DOI] [PubMed] [Google Scholar]
- Rausch K, Hackett BA, Weinbren NL, Reeder SM, Sadovsky Y, Hunter CA, Schultz DC, Coyne CB, Cherry S. 2017. Screening bioactives reveals nanchangmycin as a broad spectrum antiviral active against Zika virus. Cell Rep 18(3):804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. 2004. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest 114(6):744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, Pollen AA, Mandel-Brehm C, Nowakowski TJ, Kriegstein AR, DeRisi JL. 2016. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 113(50):14408–14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Zeldovich VB, Poukchanski A, Boothroyd JC, Bakardjiev AI. 2012. Tissue barriers of the human placenta to infection with Toxoplasma gondii. Infect Immun 80(1):418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. 2017. Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med 141(1):43–48 [DOI] [PubMed] [Google Scholar]
- Sapparapu G, Fernandez E, Kose N, Bin C, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE. 2016. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540(7633):443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarno M, Sacramento GA, Khouri R, do Rosário MS, Costa F, Archanjo G, Santos LA, Nery N, Jr, Vasilakis N, Ko AI, de Almeida AR. 2016. Zika virus infection and stillbirths: a case of hydrops fetalis, hydranencephaly and fetal demise. PLoS Negl Trop Dis 10(2):e0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, John SP, Aker AM, Renzette N, Robbins DR, Guo Z, Green S, Kowalik TF, Brass AL. 2016. Identification of Zika virus and dengue virus dependency factors using functional genomics. Cell Rep 16(1):232–246 [DOI] [PubMed] [Google Scholar]
- Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res 26(11):804–819 [DOI] [PubMed] [Google Scholar]
- Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S. 2017. Zika virus infection of Hofbauer cells. Am J Reprod Immunol 77(2). [Epub ahead of print]; DOI: 10.1111/aji.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353(6301):823–826 [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. 2016. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 20(2):155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. 2016. A mouse model of Zika virus sexual transmission and vaginal viral replication. Cell Rep 17(12):3091–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, Leparc-Goffart I. 2016. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 387(10037):2501. [DOI] [PubMed] [Google Scholar]
- van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mogling R, van Kampen JJ, Widagdo W, Aron GI, GeurtsvanKessel CH, Pas SD, Raj VS, Haagmans BL, Koopmans MP. 2016. Miscarriage associated with Zika virus infection. N Engl J Med 375(10):1002–1004 [DOI] [PubMed] [Google Scholar]
- Vermillion MS, Lei J, Shabi Y, Baxter VK, Crilly NP, McLane M, Griffin DE, Andrew Pekosz A, Klein SL, Burd I. 2017. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun 8:14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg GA, Garcia-Sastre A. 2010. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol 13(4):508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, Li S, Song J, Liu J, He J, Yuan H, Xiong Y, Liao Y, Li J, Yang J, Tong Z, Griffin BD, Bi Y, Liang M, Xu X, Qin C, Cheng G, Zhang X, Wang P, Qiu X, Kobinger G, Shi Y, Yan J, Gao GF. 2016. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med 8(369):369ra179. [DOI] [PubMed] [Google Scholar]
- Wang S, Hong S, Deng YQ, Ye Q, Zhao LZ, Zhang FC, Qin CF, Xu Z. 2017. Transfer of convalescent serum to pregnant mice prevents Zika virus infection and microcephaly in offspring. Cell Res 27(1):158–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, Bronstein M, Stockheim D, From I, Eisenberg I, Lewkowicz AA, Yagel S, Panet A, Wolf DG. 2017. Zika virus infects early- and mid-gestation human maternal-decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J Virol 91(4):pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MF, Salick MR, Wiskow O, Ho DJ, Worringer KA, Ihry RJ, Kommineni S, Bilican B, Klim JR, Hill EJ, Kane LT, Ye C, Kaykas A, Eggan K. 2016. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from Zika virus infection. Cell Stem Cell 19(6):703–708 [DOI] [PubMed] [Google Scholar]
- Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. 2016. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res 26(6):645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. 2016. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 166(5):1247–1256.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldovich VB, Clausen CH, Bradford E, Fletcher DA, Maltepe E, Robbins JR, Bakardjiev AI. 2013. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog 9(12):e1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. 2016. Structural basis of Zika virus-specific antibody protection. Cell 166(4):1016–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]