Abstract
Significance: Military service members are susceptible to traumatic extremity injuries that often result in limb loss. Tremendous efforts have been made to improve medical treatment that supports residual limb function and health. Despite recent improvements in treatment and novel prosthetic devices, many patients experience a wide range of clinical problems within residual limbs that can negatively impact the progress of rehabilitation programs while also impairing functional capacity and overall quality of life.
Recent Advances: In addition to existing standard imaging modalities that are used for clinical evaluation of patients suffering from traumatic extremity injury, novel noninvasive imaging techniques are in development that may facilitate rapid and sensitive assessment of various aspects of traumatic extremity injuries and residual limb health.
Critical Issues: Despite recent advances, there remains a clinical need for noninvasive quantitative imaging techniques that are capable of providing rapid objective assessments of residual limb health at the time of initial presentation as well as after various forms of medical treatment.
Future Directions: Ongoing development of imaging techniques that allow for assessment of anatomical and physiological characteristics of extremities exposed to traumatic injury should greatly enhance the quality of patient care and assist in optimizing clinical outcomes.
Keywords: : imaging, extremity trauma, amputation, military medicine

Mitchel R. Stacy, PhD
Scope and Significance
The following review discusses a variety of imaging modalities that are currently available and used clinically for assessing traumatic extremity injuries, while also addressing relative benefits and limitations associated with each modality. In addition, several imaging modalities that have been more recently developed and are in the process of validation are discussed in the context of evaluating patients with traumatic extremity injuries.
Translational Relevance
A variety of noninvasive imaging techniques that could have potential application in the assessment of traumatic extremity injuries continue to be developed and validated in the preclinical setting. Numerous animal models of limb ischemia and skeletal muscle tissue injury are available for initial testing; however, before obtaining FDA approval and widespread clinical application, these imaging modalities must undergo rigorous testing and validation. Ongoing efforts by imaging scientists should facilitate the development of noninvasive quantitative indices that will one day assist clinicians with improved assessment and tracking of medical treatments in patients suffering from traumatic extremity injuries.
Clinical Relevance
Advances in protective body armor, vehicles, and medical treatment have improved combat survival rates; however, survivors often suffer traumatic extremity injuries.1,2 As of October 1, 2016, there are 1,703 service members who have sustained traumatic limb loss due to Operations: Enduring Freedom, Iraqi Freedom, New Dawn, Inherent Resolve, and Freedom's Sentinel (Source: Extremity Trauma and Amputation Center of Excellence, Walter Reed National Military Medical Center). Though less apparent, the civilian population also suffers from traumatic extremity injuries; an estimated 185,000 Americans undergo limb amputation annually,3 and an estimated 900,000 will be living with traumatic limb loss in 2020.4
Background
The integrity of the vasculature, nerves, and soft tissue within the extremities is of high importance, as an impairment or deficiency to any of these tissues in isolation or combination can lead to issues with residual limb pain, impair the progress of rehabilitation programs, and/or result in prosthesis abandonment, thus reducing mobility, function, and overall quality of life for patients.5 Residual limb pain, in particular, may occur due to numerous reasons, such as neuroma, chronic inflammation, infection, retained foreign bodies, heterotrophic bone formation, and vascular abnormalities.6 Therefore, effective diagnosis can be critical in directing the medical treatment of patients. Standard noninvasive imaging modalities such as ultrasound, X-ray computed tomography (CT) imaging, magnetic resonance (MR) imaging, single photon emission computed tomography (SPECT), and positron emission tomography (PET) are currently available for assessing various aspects of extremity health. Specifically, X-rays, CT, and MR are used for imaging suspected anatomical complications associated with extremity trauma, such as vascular (e.g., pseudoaneurysm, vascular stenosis or occlusion, hematoma) and nonvascular injuries (e.g., bone fracture, soft tissue defect or trauma). Alternatively, SPECT and PET are the primary modalities for physiological imaging of molecular and cellular processes (e.g., inflammation, metabolism, angiogenesis). However, with the development of hybrid systems such as SPECT/CT, PET/CT, and PET/MR, clinicians can now co-register anatomical images with functional images. Despite the breadth of currently available modalities, all clinical imaging modalities possess relative benefits and limitations related to their ability to provide comprehensive noninvasive assessment of extremity health (Table 1). Other imaging modalities are still in developmental stages and have yet to be validated as clinically useful tools; however, recently, there has been an increased focus on the development of noninvasive imaging approaches that are capable of assessing tissue viability in patients with limb loss. The ability to assess tissue viability through the evaluation of vascular supply as well as tissue blood flow, perfusion, and/or oxygenation within residual limbs could provide novel insight into physiological changes that occur after surgical or medical treatment while also allowing for improved assessment of next-generation prosthetic devices. Therefore, sensitive, quantitative imaging approaches that could provide an objective assessment of residual limb health should have increased roles in the future and advance the standard of care for patients suffering from traumatic extremity injuries and extremity amputation.
Table 1.
Characteristics of imaging modalities available for assessing extremity trauma
| Modality | Sensitivity | Penetration Depth | Spatial Resolution |
|---|---|---|---|
| Ultrasound | Moderate | Low | 1 mm |
| CT imaging | Limited | No limit | <1 mm3 |
| MR imaging | Moderate | No limit | <1–3 mm3 |
| SPECT | High | No limit | ∼5–8 mm3 |
| PET | High | No limit | ∼3–5 mm3 |
Modified from Stacy and Sinusas.7
CT, computed tomography; MR, magnetic resonance; PET, positron emission tomography; SPECT, single photon emission computed tomography.
Ultrasound
Within the clinical setting, ultrasound is one of the most frequently used imaging modalities due to its relatively low cost and easy portability. Ultrasound systems utilize the principle of wave reflection and echo from oscillating sound waves in tissues to produce two-dimensional (2D) and three-dimensional (3D) real-time images of structure, function, and blood flow, thus making this modality particularly relevant and valuable as a tool for quick, noninvasive assessment of a variety of injuries associated with extremity trauma.7
In patients who have experienced traumatic lower extremity injuries and undergone amputation, ultrasound imaging has been shown to be a valuable tool for assessing nerve-related complications.6,8–10 Specifically, ultrasound has been shown to be useful for visualizing sciatic nerves and residual limb neuromas, which are caused by growing proximal axons from the amputated nerve that lead to the formation of painful bulbous overgrowths.9 Neuromas represent a frequent cause of residual limb pain after amputation and appear on ultrasound images as oval, hypoechoic masses that are in contact with the nerve.11 Due to the relationship between the nerve and neuroma, ultrasound imaging along the path of the nerve can be used to localize the source of pain for guidance of fine needle aspiration or biopsy. In addition, ultrasound imaging of neuromas has been shown to allow for real-time guidance of various treatments, including local anesthetic injections with and without steroids, neurolytic injections, radiofrequency ablation, and surgical revision.6,8,9 Figure 1 demonstrates the value of ultrasound imaging of a neuroma before and after treatment with a steroid anesthetic mixture, where the needle placement is visualized in real time for guidance of the therapeutic injection.8 In addition to image guidance for treatment of neuromas, color flow Doppler ultrasound has also been shown to be useful for visualization of vascularity around the site of neuroma formation, thus adding further value to ultrasound by allowing clinicians to avoid specific vascular structures during therapeutic injections, as well as permitting evaluation of the relationship between various therapies and associated changes in neuroma blood flow and pain.6
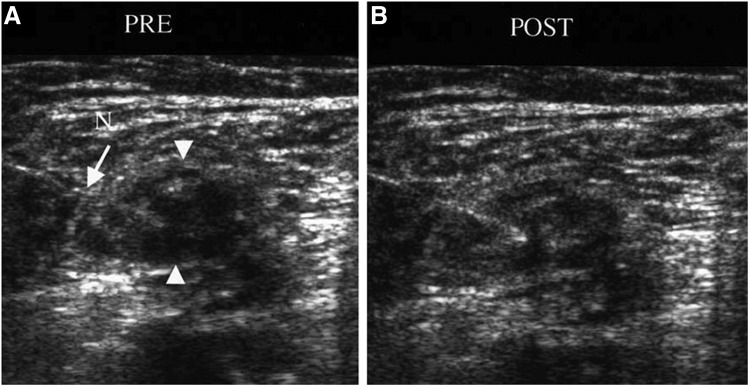
Figure 1.
Ultrasound imaging of a neuroma with the needle in position (A) before and (B) after anesthetic steroid injection (arrowheads represent location of neuroma; arrow denotes location of needle). (B) After the injection, increased echogenicity is noticed within the neuroma, demonstrating effective delivery of the steroid anesthetic. Reprinted with permission from Ernberg et al.8 N, needle.
Along with assessing residual limb neuromas, ultrasound imaging has been found to be a useful tool for evaluating structural changes that occur in the patellar tendon of patients with traumatic transtibial amputations.12 Since the patellar tendon can be a significant weight-bearing structure for prosthetic use in individuals with transtibial limb loss, ultrasound can possess significant value for designing prosthetic devices that allow for optimal load transfer between the prosthesis and residual limb. Indeed, prior work has already demonstrated that ultrasound is capable of assisting in the development of prosthetic sockets through the measurement and modeling of the residual limb-to-prosthetic socket interface.13 Improved assessment and understanding of the complex biomechanical interactions between the residual limb and prosthetic socket should allow for improved next-generation designs that facilitate optimal pressure distribution over the residual limb. Research in the field of finite element analysis has shown that ultrasound imaging can be useful for modeling of the limb-to-socket interface by developing quantitative indices to predict the quality of prosthetic fit. This finite element modeling of the limb-to-socket interface is critical not only at the time of prosthetic development but also over time, as residual limbs can undergo serial changes due to muscle atrophy, edema, and weight gain or loss, among others.13 Any of these changes in residual limb structure and health can contribute to the future development of limb pain or skin damage. Therefore, the use of ultrasound imaging to precisely model and predict changes in residual limb characteristics should facilitate future efforts that are directed at optimizing limb-to-socket fitting while also improving the long-term outlook for patients with lower extremity limb loss.
In addition to being a useful noninvasive tool for assessing the lower extremities, ultrasound imaging has been extensively applied in the evaluation of traumatic upper extremity injuries. In the setting of traumatic extremity injury, ultrasound has been utilized to assess tendons, peripheral nerves, vascular structures, bone fractures, and foreign bodies.14 For tendon-specific injuries, ultrasound can identify partial or full tendon rupture, swelling, and effusion in the tendon sheath. In addition, ultrasound imaging can be applied to noninvasively assess superficial peripheral nerves and identify damage to their normal fasicular pattern, nerve swelling or thickening, loss of nerve bundle integrity, and development of neuromas. Due to the superficial location of some upper extremity peripheral nerves, ultrasound can be applied for quick noninvasive assessment after traumatic injury, therefore assisting in diagnosis of nerve injuries that necessitate immediate surgical repair. Along with nerves, vessels of the upper extremities are susceptible to traumatic injury due to their tendency to be superficial and/or close to the bone,15 with penetrating trauma being the most frequent cause of traumatic upper extremity vascular injury.16 In instances of traumatic vascular injury, ultrasound can be extremely useful for evaluating the patency of the affected artery or vein as well as the integrity of the vascular wall.14 Although potential bone fractures are commonly assessed and identified by using standard radiography, CT imaging, or MR imaging, superficial bones of the upper extremities can also be identified by using ultrasound imaging and can provide an alternative approach for quick assessment of traumatic bone and joint injuries.17
One of the most relevant applications of ultrasound for military medicine can be in the identification of foreign bodies following instances of penetrating wounds, which can result in pain and tissue infection. Since ultrasound allows for identification of both opaque and radiolucent foreign bodies, this modality offers some advantages over standard radiography, which can only identify radiopaque materials.18 Fast and accurate identification of foreign bodies can be critical for directing surgical removal and can provide the anatomical location of the foreign body in relation to tendons, nerves, and vessels.14 In addition to providing the location of the foreign body, ultrasound imaging can also assist in characterizing wound tracts after traumatic extremity injuries such as soft tissue gunshot wounds.19
X-Ray and Ct Imaging
In the setting of traumatic extremity injury, digital subtraction angiography (DSA) and CT angiography have been the imaging modalities of choice for evaluating patients with possible vascular injuries. Traditionally, DSA was the primary imaging approach for evaluating vascular integrity after traumatic extremity injury; however, the development of modern-day CT scanners has resulted in rapid image acquisition times and whole-body imaging that possesses high accuracy and excellent penetration depth at sub-millimeter isovolumetric voxels, therefore offering high spatial resolution of vascular anatomy, bone, and surrounding soft tissue.20,21 Although CT imaging requires the use of X-rays and exposes patients to ionizing radiation,7 many CT scanners now possess the ability to modulate radiation exposure to patients through various attenuation-based techniques.20 CT imaging has become such a vital component of clinical care for patients with traumatic injuries that we have now reached an era where almost every emergency department has at least one CT scanner available at any given time.22
In the evaluation of patients exposed to blast injuries, both X-ray and CT imaging are fast and effective imaging techniques that are capable of detecting bone fractures. In addition, metallic and/or glass fragments, which possess a higher relative density than soft tissue, can be identified as radiopaque objects in the extremities after traumatic injury.23 No matter the cause of traumatic injury (e.g., blast injury, stab wound, or gunshot wound), CT imaging on 16- and 64-slice scanners offer unique opportunities to have the combination of high spatial and temporal resolution, along with fast image reconstruction and data processing techniques, all of which are critical in the emergency diagnosis and surgical planning for patients suffering from traumatic injury.22
Although CT imaging provides value in the noninvasive assessment of bone, soft tissue defects, and fragments in soft tissue after extremity trauma (Fig. 2), an important capability of CT imaging remains the rapid assessment of vascular structures, since vascular injuries significantly contribute to morbidity and mortality associated with traumatic injuries.20 In patients with suspected vascular injuries, CT angiography is the initial modality of choice and has been shown to have high sensitivity and specificity for diagnosis of extremity vascular injuries.24,25 Specifically, Soto et al.26 demonstrated a sensitivity of 95.1% and specificity of 98.7% when applying CT angiography for the detection of focal arterial injuries in patients suffering from penetrating and blunt traumatic injuries, whereas Rieger et al.21 found that CT angiography had 95% sensitivity and 87% specificity for detection of peripheral vascular lesions. Recent research also suggests that CT angiography predicts limb salvage rates in patients suffering from lower extremity vascular injury, where the need for surgical intervention and amputations was found to increase as the number of patent vessels to the lower extremity decreased.27 Direct evidence of vascular injury on CT angiography can be indicated by extravasation of the intravenous iodinated contrast agent, localization of extraluminal contrast (suggestive of pseudoaneurysm), vascular stenosis or occlusion, or arteriovenous fistulae, whereas more indirect evidence of vascular injury may appear as a perivascular hematoma or a projectile in close proximity to an artery.28 In instances when CT angiography findings are inconclusive, as in cases of potential vascular dissection, patients may require conventional DSA under fluoroscopic guidance to assist with diagnosis and guidance of endovascular treatment or surgical planning.29 Specific vascular injuries that may be better identified by secondary inspection on DSA include vascular dissection, occlusion, and spasm. Aside from instances when significant CT image artifacts are anticipated due to metallic shrapnel from blast injuries or gunshot wounds, DSA remains a second-line tool to CT angiography for the noninvasive assessment of vascular injuries at a majority of trauma centers.20
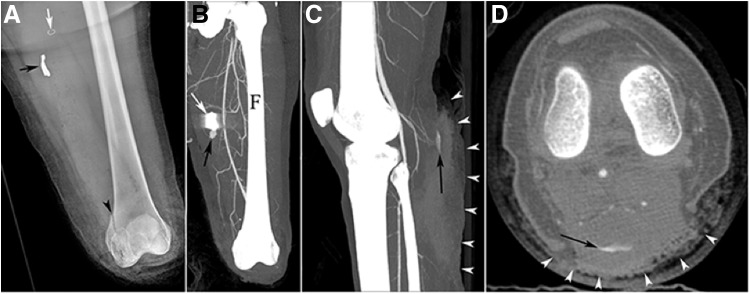
Figure 2.
CT imaging in a patient after primary and secondary blast injuries to the lower extremities and an emergency below-the-knee amputation of the left limb. (A) Radiography postamputation identified the presence of a metallic foreign body (black arrow), surgical staple (white arrow), and distal femoral fracture (arrowhead) in the left limb. (B) A maximum intensity projection (MIP) of CT angiography revealed a pseudoaneurysm (black arrow) in the immediate proximity of shrapnel (white arrow). (C) A sagittal MIP of a CT angiogram of the right limb identified the presence of a second pseudoaneurysm (black arrow) that is also apparent in the (D) axial view, in combination with a prominent soft tissue defect on the posterior aspect of the limb (arrowheads). Reprinted with permission from Guermazi et al.23 CT, computed tomography; F, femur.
Despite the numerous advantages that CT angiography and DSA provide in the noninvasive evaluation of extremity trauma, there are also limitations that exist for both modalities. Two primary limitations of DSA and CT imaging unrelated to image quality include the use of X-rays that emit ionizing radiation that is capable of damaging DNA, and the use of iodinated contrast agents that can be nephrotoxic for patients with impaired renal function.7 Additional pitfalls associated with CT image quality in trauma patients include metal fragments and other foreign objects associated with trauma that can produce high attenuation image artifacts that prevent visualization of specific vessel segments.22 Therefore, radiologists should carefully review cross-sectional images to optimize evaluation of vascular injuries that are not be as readily detected on 3D-rendered images due to metallic streak artifacts, motion artifacts, and nonenhanced vascular segments.28
MR Imaging
MR imaging utilizes magnetic fields of varying strengths (1.5–9.4 Tesla for human use and greater than 10 Tesla for research purposes) to send and receive radio frequency pulse sequences that produce high-resolution images that are capable of assessing anatomy and physiology without the need for ionizing radiation. Since MR possesses good penetration depth, superior soft tissue contrast, and does not require ionizing radiation, this imaging modality has been widely applied for evaluating anatomical and functional characteristics of the extremities.7 However, despite the recognized advantages of MR imaging, MR is not indicated in the acute stages of trauma after blast injury due to the likelihood of metallic foreign bodies being present in the body, as well as the length of time required for acquisition of MR images compared to CT imaging. In addition, MR imaging is more expensive than ultrasound and CT imaging and, therefore, not always as readily available as an initial tool for diagnosing complications associated with blast trauma.23
In instances of extremity trauma not associated with blast injury, MR imaging is becoming a preferred modality for assessing injuries to extremity soft tissue. In particular, MR imaging is a valuable noninvasive tool for identifying and characterizing the extent of neural injuries and nerve impairment, as well as associated issues such as muscle edema and denervation (Fig. 3).30 Extremity trauma can result in neuropathy due to direct injury to nerves, or from injury to adjacent anatomical structures. High-resolution 2D fast spin echo sequences are one MR-based approach that can be utilized to detect numerous nerve-related injuries in the extremities, such as traumatic or iatrogenic injuries, nerve entrapment, inflammation, and tumor-like lesions.30 In addition, volumetric MR imaging has been shown to be a useful tool for quantifying sensory neuron loss within dorsal root ganglia after nerve transection.31 A more recent emerging tool for assessment of peripheral nerves is 3D diffusion tensor imaging (DTI) with MR tractography, which allows for visualization of nerve orientation and course.32 DTI permits visualization of nerve tractography and microstructural characteristics by utilizing the anisotropic diffusion of water molecules through axons, thereby producing quantifiable parameters (e.g., fractional anisotropy, mean diffusivity, eigenvalues) that offer insight into characteristics such as axon density and myelin thickness.33,34 DTI-derived measures of fractional anisotropy have been shown to correlate well with histological analyses of axons and myelin35 while also demonstrating the ability to noninvasively identify serial changes in nerve characteristics after peripheral nerve injury in patients36 and animal models.34,35,37,38 Collectively, MR-based imaging of peripheral nerves possesses significant clinical potential as a tool for quantitatively assessing the effects of microsurgical repair of nerves as well as neuroprotective therapies after extremity trauma.
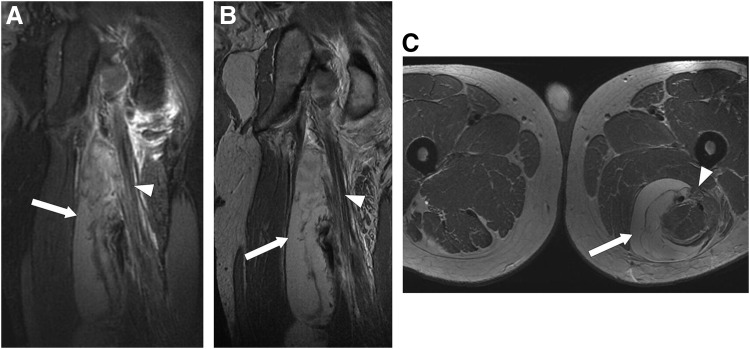
Figure 3.
(A) Coronal views of inversion recovery and (B) fast spin echo, as well as (C) axial view of fast spin echo MR images after traumatic injury to the lower extremities reveal the presence of a large hematoma (white arrow) that is responsible for compression of the left sciatic nerve (white arrowheads). Reprinted with permission from Burge et al.30 MR, magnetic resonance.
Along with established techniques for assessing extremity nerves, MR imaging has also demonstrated utility in characterizing numerous other extremity complications, including neuromas, bursitis, soft tissue inflammation, abscesses, osteomyelitis, stress fractures, bone bruises, cutaneous lesions, and neoplastic recurrences.39 In patients who undergo amputation of a limb, MR imaging is particularly useful for identifying bursitis, adventitious bursae, and regions of localized soft tissue inflammation, resulting from an improper interaction between the residual limb stump and prosthetic device. In the noninvasive diagnosis of residual limb stump bursitis, MR assists in differentiating inflammation between cutaneous and subcutaneous tissue, as well as identifies differences between bone and muscle inflammation caused by abnormal levels of mechanical stress on the residual limb.40 In addition to an evaluation of structural and inflammatory consequences associated with improper socket fitting, nuclear MR spectroscopy has also been applied in patients with lower extremity amputation to assess exercise-induced changes in skeletal muscle metabolism, demonstrating further applicability and relevance of MR-based approaches in the assessment of patients after traumatic extremity injury and limb loss.41
In the assessment of vascular abnormalities in the extremities, multiple MR angiography approaches are available that do not require the use of iodinated or gadolinium-based contrast agents, which is particularly valuable in assessing patients with renal insufficiency. Specifically, time-of-flight and phase-contrast imaging are capable of producing dynamic images of blood vessels, but are limited in their application in extremity trauma due to long acquisition times and their tendency to overestimate vessel stenosis.42 More recent MR techniques such as quiescent-interval single-shot MR angiography, cardiac-gated 3D-fast spin echo MR angiography, and flow-sensitive dephasing sequences have been developed that allow for relatively faster acquisition times, and they are capable of producing diagnostic-level images that possess similar sensitivity as contrast-enhanced MR angiography.43,44 In addition to noncontrast approaches, contrast-enhanced MR angiography with gadolinium-based contrast agents remains a viable option that produces fast, dynamic, and high-temporal resolution angiographic images, which is valuable in the setting of extremity trauma by allowing for differentiation of high-flow and low-flow vascular abnormalities.28
Aside from assisting with diagnosis of complications associated with extremity trauma, MR imaging has also proved to be a useful tool for evaluating the prosthesis-to-residual limb interface and has been utilized to identify extremity characteristics for modification and optimization of prosthetic device fit. Specifically, Douglas et al.45 previously developed algorithms that allow for the automatic extraction of the skin and bone boundaries from MR images of individuals with lower extremity limb loss to facilitate biomechanical modeling (i.e., finite element analysis) of the residual limb-to-prosthetic interactions, whereas additional work by Buis et al.46 has used MR images to establish a reference grid of residual limbs to quantify differences in volume and shape of soft tissues. Taken together, this information related to the residual limb could provide valuable information to guide individualized design of prosthetic devices that allow for ideal comfort and optimize patient mobility.
Radiotracer Imaging
SPECT and PET imaging are the standard clinical imaging modalities for radiotracer-based imaging that allows for high-sensitivity 3D assessment of a wide range of physiological processes via detection of gamma rays and photons emitted from the radioactive decay of isotopes. Though SPECT and PET provide high-sensitivity functional images, both offer low spatial resolution and are, therefore, typically paired with high-resolution anatomical images produced by CT or MR systems for accurate radiotracer localization and quantification. In addition, both SPECT and PET imaging expose patients to ionizing radiation due to the use of isotopes that possess varying half-lives.7
SPECT/CT imaging has been applied in the clinical environment for many years for the assessment of myocardial perfusion in patients with coronary artery disease; however, SPECT/CT may also have value in the assessment of extremity trauma through its ability to evaluate skeletal muscle perfusion under conditions of rest or stress.47 In addition, SPECT/CT imaging has already demonstrated potential for assessing a wide range of other physiological processes, such as bone and tissue infection,48–51 heterotrophic ossification,52 and skeletal muscle angiogenesis,47 which could be useful in the evaluation of extremity trauma and tracking the response to medical treatment. In the assessment of infection, multiple technetium-99 m (99mTc)-labeled radiotracers have been applied in the extremities. Specifically, Filippi and Schillaci50 utilized 99mTc-hexamethylpropylene amine oxime-labeled leukocytes to localize and define the extent of infection in patients with suspected osteomyelitis and joint infections, whereas Erdman et al.51 have applied 99mTc-labeled white blood cells to assess infections and developed a standardized scoring system for rating the severity of wound infections. In addition, 99mTc-hydroxydiphosphonate SPECT/CT imaging has been utilized to evaluate painful knee prostheses and has demonstrated the ability to identify instances of prosthesis loosening or associated tissue infection.49 Aside from SPECT/CT imaging of infection, 99mTc-methyl diphosphonate (MDP)53 as well as gallium-67 (67Ga) citrate48 have been applied in instances of suspected osteomyelitis of the extremities, and 99mTc-MDP has been used for the additional noninvasive identification of heterotrophic ossification at the site of residual limb stumps.52
In addition to potential clinical applications of SPECT/CT imaging in the noninvasive assessment of extremity trauma, PET/CT imaging also possesses capabilities that could have considerable value in patients. Specifically, a frequently used PET tracer, fluorine-18 (18F)-fluorodeoxyglucose (FDG), has been applied in the assessment of suspected osteomyelitis and demonstrated excellent sensitivity (100%), specificity (93%), and accuracy (96%) in a lesion-based analysis.54 18F-FDG PET/CT imaging has also shown value for assessing exercising skeletal muscle metabolism in the lower extremities of patients with transfemoral amputation by characterizing variations in metabolic activity between specific muscle groups of the lower extremities, indicating potential utility of FDG imaging in the evaluation of patients undergoing exercise rehabilitation programs.55 Along with imaging of muscle metabolism, FDG PET imaging has also been paired with high-resolution anatomical MR imaging to noninvasively assess metabolic activity within peripheral nerves and has demonstrated increased radiotracer uptake within injured sciatic nerves (Fig. 4).56 In addition to FDG PET/CT imaging, PET imaging with oxygen-15 (15O)-water has proved to be useful for quantifying muscle blood flow and identifying areas of tissue ischemia with sensitivity and specificity levels similar to those of laser Doppler imaging and transcutaneous oxygen (TcPO2) measurements, leading the authors to suggest that quantitative PET imaging could be useful for future assessment of lower extremity tissue viability and in determining the appropriate level of amputation before surgical intervention.57
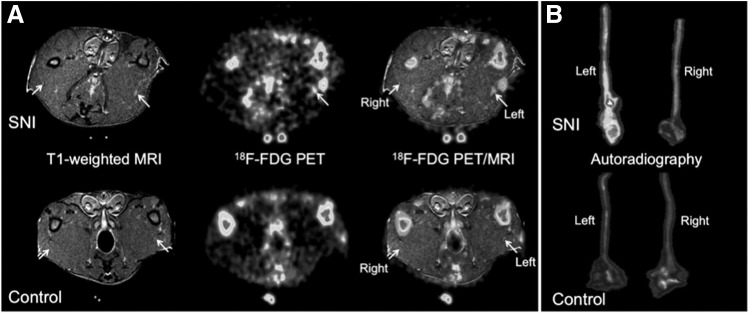
Figure 4.
PET/MR imaging in a rat model of unilateral spared-nerve injury of the left sciatic nerve (A, top row) compared with a control uninjured animal (A, bottom row) demonstrates increased 18F-FDG uptake in the limb (left) with nerve injury. Autoradiography of the excised sciatic nerves from injured (B, top row) and uninjured (B, bottom row) animals reveals dramatically higher radiotracer uptake in the injured (left) sciatic nerve compared with control sciatic nerves. This research was originally published in J Nucl Med.56 © by the Society of Nuclear Medicine and Molecular Imaging, Inc. FDG, fluorodeoxyglucose; PET, positron emission tomography; SNI, spared-nerve injury.
Although both SPECT and PET imaging have demonstrated efficacy for evaluating skeletal muscle perfusion and blood flow, both imaging approaches have relative benefits and limitations, and thus one modality may be more favorable depending on the clinical scenario. For example, SPECT is performed by using radioisotopes that possess longer half-lives, which can be beneficial when combining lower extremity perfusion imaging with clinically indicated myocardial perfusion imaging. However, longer half-life tracers can be unfavorable due to the resultant higher doses of ionizing radiation for patients. SPECT imaging is more widely available than PET due to higher costs associated with PET imaging, which remains costly for medical centers due to the need for more expensive instrumentation, including an onsite cyclotron or portable generator for isotope production. PET scanners already have tools in place for quantitative assessment of skeletal muscle blood flow, though, whereas conventional SPECT systems are limited to evaluation of relative perfusion. Therefore, both SPECT and PET possess relative benefits and limitations that should be taken into account during evaluation of extremity trauma.
Summary
Along with the more established clinical imaging modalities already discussed, additional noninvasive approaches continue to emerge that could one day reach widespread application for the assessment of extremity trauma. Specifically, ultrasound systems continue to evolve and can now be incorporated with other imaging approaches such as near-infrared spectroscopy and photoacoustic imaging, thus creating hybrid systems that may lead to additional applications for ultrasound systems in the future. Additional progress in the field of ultrasound contrast agents and nanoparticles may also facilitate targeted molecular imaging for the evaluation of various regenerative medicine treatments for extremity trauma. Other modalities such as TcPO2,58 laser Doppler imaging,59 laser speckle flowmetry,60 and hyperspectral imaging61 are also available and could possess potential utility in the evaluation of residual limb tissue health; however, these approaches are traditionally limited by their ability to assess measures of tissue perfusion and blood flow at a superficial level and may present issues with regard to a reproducible serial assessment of specific anatomical sites due to their limited field of views.
Currently, a variety of imaging modalities are available that offer a wide range of diagnostic information on patients who have suffered extremity trauma. Although many modalities exist, all of these imaging approaches still come with relative strengths and limitations. Therefore, clinicians who are responsible for the noninvasive assessment and fast care of patients and military service members after traumatic extremity injury should give careful consideration of the pros and cons associated with each modality to facilitate and optimize evaluation and medical treatment that will lead to the most favorable clinical outcomes.
Abbreviations and Acronyms
- 2D
two-dimensional
- 3D
three-dimensional
- 15O
oxygen-15
- 18F
fluorine-18
- 67Ga
gallium-67
- 99mTc
technetium-99 m
- CT
computed tomography
- DSA
digital subtraction angiography
- FDG
fluorodeoxyglucose
- MR
magnetic resonance
- DTI
diffusion tensor imaging
- MDP
methyl diphosphonate
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- TcPO2
transcutaneous oxygen
Acknowledgments and Funding Sources
This work was supported in part by funding from the Department of Defense through the Orthotics and Prosthetics Outcomes Research Program (Award #W81XWH-15-1-0669 to CLD and MRS), the American Heart Association (Award #14CRP20480404 to MRS), the National Institute of Biomedical Imaging and Bioengineering (Award # 1R03EB018889-01A1 to CLD), the DoD-VA Extremity Trauma and Amputation Center of Excellence (Public Law 110-417, National Defense Authorization Act 2009 to CLD), and the BADER Consortium via Congressionally Designated Medical Research Program (CDMRP) award W81XWH-11-2-0222.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article. The views expressed in this article are those of the authors, and do not necessarily reflect the official policy of the Departments of the Army, Navy, Defense, nor the United States Government.
About the Authors
Mitchel R. Stacy, PhD, is a faculty member in the Department of Internal Medicine (Cardiology) at Yale University School of Medicine, where his research focuses on the development and validation of translational imaging techniques and novel approaches for medical image analysis. Specifically, Dr. Stacy's research in preclinical models and patients is directed at noninvasive quantitative imaging of pathophysiology associated with extremity trauma, peripheral vascular disease, diabetes, and myocardial infarction. Dr. Stacy's work incorporates a wide variety of imaging modalities, including SPECT, PET, CT angiography, MR imaging, and ultrasound. Christopher L. Dearth, PhD, serves as the Facility Research Director for the Extremity Trauma and Amputation Center of Excellence, Director of Research for the Department of Rehabilitation at Walter Reed National Military Medical Center, and the Founding Director of the Regenerative Rehabilitation Laboratory at the Uniformed Services University of the Health Sciences. Dr. Dearth leads a multidisciplinary team of clinicians and researchers whose collective focus is on the mitigation, treatment, and rehabilitation of traumatic extremity injuries and amputations by implementation of clinically relevant research aimed at optimizing the quality of life of service members and veterans.
Take-Home Messages.
• Traumatic extremity injuries are clinical problems that require fast diagnosis and treatment to facilitate limb salvage and positive patient outcomes.
• Multiple imaging modalities are available for noninvasive assessment of extremity complications after traumatic events; however, certain modalities are favored depending on the form of traumatic injury.
• Ultrasound imaging offers the ability to quickly assess real-time images of structure, function, and blood flow, but it is limited by its penetration depth.
• CT imaging offers rapid image acquisition times, excellent penetration depth, and high-spatial resolution for assessment of vascular anatomy, bone, and surrounding soft tissue. However, limitations of CT include the use of X-rays that expose patients to ionizing radiation, as well as image artifacts that can be created from metallic foreign bodies.
• MR imaging provides high-resolution images that are capable of assessing anatomy and function without the need for ionizing radiation; however, limitations of MR imaging in the assessment of traumatic extremity injuries include long image acquisition times, high cost, and its contraindication when there is the suspected presence of metallic foreign bodies from blast-related injuries.
• Radiotracer imaging with SPECT and PET offers high-sensitivity functional assessment of a wide range of physiological processes, but it is limited by poor spatial resolution that often requires the pairing of SPECT and PET with high-resolution CT or MR systems for optimal radiotracer localization and quantification.
References
- 1.Galarneau MR, Hancock WC, Konoske P, et al. The Navy-Marine Corps combat trauma registry. Mil Med 2006;17:691–697 [DOI] [PubMed] [Google Scholar]
- 2.Melcer T, Sechriest VF, Walker J, Galarneau M. A comparison of health outcomes for combat amputee and limb salvage patients injured in Iraq and Afghanistan wars. J Trauma Acute Care Surg 2013;75:S247–S254 [DOI] [PubMed] [Google Scholar]
- 3.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Heal Stat 13 1998;1–119 [PubMed] [Google Scholar]
- 4.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89:422–429 [DOI] [PubMed] [Google Scholar]
- 5.Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol 2014;32:670–677 [DOI] [PubMed] [Google Scholar]
- 6.Shankar H. Ultrasound demonstration of vascularity changes with changes in pain perception in a stump neuroma. Clin J Pain 2009;25:253–255 [DOI] [PubMed] [Google Scholar]
- 7.Stacy MR, Sinusas AJ. Emerging imaging modalities in regenerative medicine. Curr Pathobiol Rep 2015;3:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernberg LA, Adler RS, Lane J. Ultrasound in the detection and treatment of a painful stump neuroma. Skelet Radiol 2003;32:306–309 [DOI] [PubMed] [Google Scholar]
- 9.Kesikburun S, Yasar E, Dede I, Goktepe S, Tan AK. Ultrasound-guided steroid injection in the treatment of stump neuroma: pilot study. J Back Musculoskelet Rehabil 2014;27:275–279 [DOI] [PubMed] [Google Scholar]
- 10.Goktepe AS, Ozcakar L, Komurcu E, Safaz I, Yazicioglu K. Sonographic evaluation of the sciatic nerve in patients with lower-limb amputations. Muscle Nerve 2010;41:763–766 [DOI] [PubMed] [Google Scholar]
- 11.Provost N, Bonaldi VM, Sarazin L, Cho KH, Chhem RK. Amputation stump neuroma: ultrasound features. J Clin Ultrasound 1997;25:85–89 [DOI] [PubMed] [Google Scholar]
- 12.Ozcakar L, Komurcu E, Safaz I, Goktepe AS, Yazicioglu K. Evaluation of the patellar tendon in transtibial amputees: a preliminary sonographic study. Prosthet Orthot Int 2009;33:324–328 [DOI] [PubMed] [Google Scholar]
- 13.Douglas T, Solomonidis S, Sandham W, Spence W. Ultrasound imaging in lower limb prosthetics. IEEE Trans Neural Syst Rehabil Eng 2002;10:11–21 [DOI] [PubMed] [Google Scholar]
- 14.Karabay N. US findings in traumatic wrist and hand injuries. Diagn Interv Radiol 2013;19:320–325 [DOI] [PubMed] [Google Scholar]
- 15.Bianchi S, Martinoli C, Abdelwahab IF. High-frequency ultrasound examination of the wrist and hand. Skelet Radiol 1999;28:121–129 [DOI] [PubMed] [Google Scholar]
- 16.Joshi V, Harding GE, Bottoni DA, Lovell MB, Forbes TL. Determination of functional outcome following upper extremity arterial trauma. Vasc Endovasc Surg 2007;41:111–114 [DOI] [PubMed] [Google Scholar]
- 17.Herneth AM, Siegmeth A, Bader TR, et al. Scaphoid fractures: evaluation with high-spatial-resolution US initial results. Radiology 2001;220:231–235 [DOI] [PubMed] [Google Scholar]
- 18.Manthey DE, Storrow AB, Milbourn JM, Wagner BJ. Ultrasound versus radiography in the detection of soft-tissue foreign bodies. Ann Emerg Med 1996;28:7–9 [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Deng D, Tao J, et al. Ultrasonic imaging of gunshot wounds in pig limb. Genet Mol Res 2015;14:4291–4302 [DOI] [PubMed] [Google Scholar]
- 20.Uyeda JW, Anderson SW, Sakai O, Soto JA. CT angiography in trauma. Radiol Clin North Am 2010;48:423–438 [DOI] [PubMed] [Google Scholar]
- 21.Rieger M, Mallouhi A, Tauscher T, Lutz M, Jaschke WR. Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography. AJR Am J Roentgenol 2006;186:656–664 [DOI] [PubMed] [Google Scholar]
- 22.Fishman EK, Horton KM, Johnson PT. Multidetector CT and three-dimensional CT angiography for suspected vascular trauma of the extremities. Radiographics 2008;28:653–665 [DOI] [PubMed] [Google Scholar]
- 23.Guermazi A, Hayashi D, Smith SE, Palmer W, Katz JN. Imaging of blast injuries to the lower extremities sustained in the Boston Marathon bombing. Arthritis Care Res 2013;65:1893–1898 [DOI] [PubMed] [Google Scholar]
- 24.Soto JA, Munera F, Cardoso N, Guarin O, Medina S. Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr 1999;23:188–196 [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Branco BC, Reddy S, et al. Prospective evaluation of multidetector computed tomography for extremity vascular trauma. J Trauma 2011;70:808–815 [DOI] [PubMed] [Google Scholar]
- 26.Soto JA, Munera F, Morales C, et al. Focal arterial injuries of the proximal extremities: helical CT arteriography as the initial method of diagnosis. Radiology 2001;218:188–194 [DOI] [PubMed] [Google Scholar]
- 27.Branco BC, Linnebur M, Boutrous ML, et al. The predictive value of multidetector CTA on outcomes in patients with below-the-knee vascular injury. Injury 2015;46:1520–1526 [DOI] [PubMed] [Google Scholar]
- 28.Nagpal P, Maller V, Garg G, et al. Upper extremity runoff: pearls and pitfalls in computed tomography angiography and magnetic resonance angiography. Curr Probl Diagn Radiol 2016. [Epub ahead of print; DOI: 10.1067/j.cpradiol.2016.01.002] [DOI] [PubMed] [Google Scholar]
- 29.Fleiter TR, Mervis S. The role of 3D-CTA in the assessment of peripheral vascular lesions in trauma patients. Eur J Radiol 2007;64:92–102 [DOI] [PubMed] [Google Scholar]
- 30.Burge AJ, Gold SL, Kuong S, Potter HG. High-resolution magnetic resonance imaging of the lower extremity nerves. Neuroimaging Clin North Am 2014;24:151–170 [DOI] [PubMed] [Google Scholar]
- 31.West CA, Ljungberg C, Wiberg M, Hart A. Sensory neuron death after upper limb nerve injury and protective effect of repair: clinical evaluation using volumetric magnetic resonance imaging of dorsal root ganglia. Neurosurgery 2013;73:632–639 [DOI] [PubMed] [Google Scholar]
- 32.Khalil C, Budzik JF, Kermarrec E, Balbi V, Le Thuc V, Cotten A. Tractography of peripheral nerves and skeletal muscles. Eur J Radiol 2010;76:391–397 [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Kumaravel M, Patel VS, Sheikh KA, Narayana PA. Diffusion tensory imaging of forearm nerves in humans. J Magn Reson Imaging 2012;36:920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyer RB, Kelm ND, Riley DC, et al. 4.7-T diffusion tensor imaging of acute traumatic peripheral nerve injury. Neurosurg Focus 2015;39:E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takagi T, Nakamura M, Yamada M, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage 2009;44:884–892 [DOI] [PubMed] [Google Scholar]
- 36.Meek MF, Stenekes MW, Hoogduin HM, Nicolai JP. In vivo three-dimensional reconstruction of human median nerves by diffusion tensor imaging. Exp Neurol 2006;198:479–482 [DOI] [PubMed] [Google Scholar]
- 37.Lehmann HC, Zhang J, Mori S, Sheikh KA. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol 2010;223:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Chen J, Hong G, et al. In vivo DTI longitudinal measurements of acute sciatic nerve traction injury and the association with pathological and functional changes. Eur J Radiol 2013;82:e707–e714 [DOI] [PubMed] [Google Scholar]
- 39.Henrot P, Stines J, Walter F, Martinet N, Paysant J, Blum A. Imaging of the painful lower limb stump. Radiographics 2000;20:S219–S235 [DOI] [PubMed] [Google Scholar]
- 40.Foisneau-Lottin A, Martinet N, Henrot P, Paysant J, Blum A, Andre JM. Bursitis, adventitious bursa, localized soft-tissue inflammation, and bone marrow edema in tibial stumps: the contribution of magnetic resonance imaging to the diagnosis and management of mechanical stress complications. Arch Phys Med Rehabil 2003;84:770–777 [DOI] [PubMed] [Google Scholar]
- 41.Dulieu V, Casillas JM, Maillefert JF, et al. Muscle metabolism changes with training in the nonamputated limb after vascular amputation: interest of phosphorous 31 NMR spectroscopy. Arch Phys Med Rehabil 1997;78:867–871 [DOI] [PubMed] [Google Scholar]
- 42.Stepansky F, Hecht EM, Rivera R, et al. Dynamic MR angiography of upper extremity vascular disease: pictorial review. Radiographics 2008;28:e28. [DOI] [PubMed] [Google Scholar]
- 43.Wheaton AJ, Miyazaki M. Non-contrast enhanced MR angiography: physical principles. J Magn Reson Imaging 2012;36:286–304 [DOI] [PubMed] [Google Scholar]
- 44.Knobloch G, Gielen M, Lauff MT, et al. ECG-gated quiescent-interval single-shot MR angiography of the lower extremities: initial experience at 3T. Clin Radiol 2014;69:485–491 [DOI] [PubMed] [Google Scholar]
- 45.Douglas TS, Solomonidis SE, Lee VS, Spence WD, Sandham WA, Hadley DM. Automatic segmentation of magnetic resonance images of the trans-femoral residual limb. Med Eng Phys 1998;20:756–763 [DOI] [PubMed] [Google Scholar]
- 46.Buis AW, Condon B, Brennan D, McHugh B, Hadley D. Magnetic resonance imaging technology in transtibial socket research: a pilot study. J Rehabil Res Dev 2006;43:883–890 [DOI] [PubMed] [Google Scholar]
- 47.Stacy MR, Sinusas AJ. Novel applications of radionuclide imaging in peripheral vascular disease. Cardiol Clin 2016;34:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aslangul E, M'Bemba J, Caillat-Vigneron N, et al. Diagnosing diabetic foot osteomyelitis in patients without signs of soft tissue infection by coupling hybrid 67Ga SPECT/CT with bedside percutaneous bone puncture. Diabetes Care 2013;36:2203–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Nabhani K, Michopoulou S, Allie R, et al. Painful knee prosthesis: can we help with bone SPECT/CT? Nucl Med Commun 2014;35:182–188 [DOI] [PubMed] [Google Scholar]
- 50.Filippi L, Schillaci O. Usefulness of hybrid SPECT/CT in 99mTc-HMPAO-labeled leukocyte scintigraphy for bone and joint infections. J Nucl Med 2006;47:1908–1913 [PubMed] [Google Scholar]
- 51.Erdman WA, Buethe J, Bhore R, et al. Indexing severity of diabetic foot infection with 99mTc-WBC SPECT/CT hybrid imaging. Diabetes Care 2012;35:1826–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hassan UI, Enayat M, Mohammed F, Vijayanathan S, Gnanasegaran G. Heterotrophic ossification in a patient suspected of having osteomyelitis: additional value of SPECT/CT. Clin Nucl Med 2012;37:170–171 [DOI] [PubMed] [Google Scholar]
- 53.Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol 2008;5:881–886 [DOI] [PubMed] [Google Scholar]
- 54.Kagna O, Srour S, Melamed E, Militianu D, Keidar Z. FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imaging 2012;39:1545–1550 [DOI] [PubMed] [Google Scholar]
- 55.Shinozaki T, Suzuki K, Yamaji T, et al. Evaluation of muscle metabolic activity in the lower limb of a transfemoral amputee using a prosthesis by using (18)F-FDG PET imaging-an application of PET imaging to rehabilitation. J Orthop Res 2004;22:878–883 [DOI] [PubMed] [Google Scholar]
- 56.Behera D, Jacobs KE, Behera S, Rosenberg J, Biswal S. (18)F-FDG PET/MRI can be used to identify injured peripheral nerves in a model of neuropathic pain. J Nucl Med 2011;52:1308–1312 [DOI] [PubMed] [Google Scholar]
- 57.Scremin OU, Figoni SF, Norman K, et al. Preamputation evaluation of lower-limb skeletal muscle perfusion with (15)O H2O positron emission tomography. Am J Phys Med Rehabil 2010;89:473–486 [DOI] [PubMed] [Google Scholar]
- 58.Yip WL. Evaluation of the clinimetrics of transcutaneous oxygen measurement and its application in wound care. Int Wound J 2015;12:625–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul DW, Ghassemi P, Ramella-Roman JC, et al. Noninvasive imaging technologies for cutaneous wound assessment: a review. Wound Repair Regen 2015;23:149–162 [DOI] [PubMed] [Google Scholar]
- 60.Nadort A, Kalkman K, van Leeuwen TG, Faber DJ. Quantitative blood flow velocity imaging using laser speckle flowmetry. Sci Rep 2016;6:25258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care 2009;32:2056–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]