Abstract
Sepsis is one of the main causes of morbidity and mortality in critically ill patients despite the use of modern antibiotics and resuscitation therapies. Outcomes in sepsis have improved overall, probably because of an enhanced focus on early diagnosis and other improvements in supportive care, but mortality rates still remain unacceptably high. The diagnosis and definition of sepsis is a critical problem due to the heterogeneity of this disease process. Although it is apparent that much more needs to be done to advance our understanding, sepsis and related terms remain difficult to define. A 1991 consensus conference developed initial definitions that systemic inflammatory response syndrome (SIRS) to infection would be called sepsis. Definitions of sepsis and septic shock were revised in 2001 to incorporate the threshold values for organ damage. In early 2016, the new definitions of sepsis and septic shock have changed dramatically. Sepsis is now defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. The consensus document describes organ dysfunction as an acute increase in total Sequential Organ Failure Assessment (SOFA) score two points consequently to the infection. A significant change in the new definitions is the elimination of any mention of SIRS. The Sepsis-3 Task Force also introduced a new bedside index, called the qSOFA, to identify outside of critical care units patients with suspected infection who are likely to develop sepsis. Recently updated the consensus definitions improved specificity compared with the previous descriptions.
Keywords: Sepsis, definitions, septic shock, SIRS
Introduction
Sepsis is the most expensive health-care problem in the USA, with a cost of more than $20 billion (1). Sepsis is one of the most prevalent causes of mortality in intensive care units (ICUs), and its incidence increased by more than double over the last 10 years (2). According to data from the Surviving Sepsis Campaign, mortality rates from sepsis are 41% in Europe and 28.3% in the USA (3). In Australia and New Zealand, a multi-centre study which included 101,064 critical patients revealed that mortality rates decreased over the years and finally reached the 18–20% interval (4).
Sepsis-related mortality risk factors vary according to the size and technological level of the centre. Among the predisposing factors which may cause sepsis, patients staying for longer periods in ICUs where advanced technologies are used, the increasing elderly population, immune suppression resulting from malignant diseases and their aggressive treatment, increasing transplantation practices and the use of related immunosuppressive drugs, invasive procedures, antibiotic resistance and society-sourced and nosocomial infections can be listed (1).
As there is no gold standard in the definition of sepsis, clinicians have attempted to diagnose sepsis by combining non-specific physiological and laboratory anomalies. Thus, definitions of sepsis were proposed at international conferences which were held in 1991, 2001 and finally in 2016 (Table 1). Guidelines provide efficient utilisation of knowledge as presented by companies and prevents different implementation among the user groups (5).
Table 1.
Definitions of sepsis
| Older definitions | Newer definition: Sepsis 3 | ||
|---|---|---|---|
|
| |||
| Sepsis 1 | Sepsis 2 | Definition | Clinical Criteria |
Systemic inflammatory response syndrome (SIRS) = the systemic inflammatory response to a variety of severe clinical insults.
|
Diagnostic criteria for sepsis Infection Documented or suspected and some of the following: General parameters Fever (core temperature >38.3°C) Hypothermia (core temperature <36°C Heart rate >90 bpm or >2 SD above the normal value for age Tachypnea: >30 bpm Altered mental status Significant edema or positive fluid balance (>20 mL kg−1 over 24 h) Hyperglycemia (plasma glucose >110 mg dL−1 or 7.7 mM L−1) in the absence of diabetes |
Screening for Sepsis qSOFA (quick Sequential Organ Failure Assessment) scoring system Accordingly, an increase of 2 or more in the qSOFA score should create a suspicion of sepsis and organ dysfunction. |
qSOFA
|
| Sepsis = the systemic response to infection, manifested by two or more of the SIRS criteria as a result of infection |
Inflammatory parameters Leukocytosis (white blood cell count >12,000/μL) Leukopenia (white blood cell count <4,000/μL) |
||
| Normal white blood cell count with >10% immature forms Plasma C reactive protein >2 SD above the normal value Plasma procalcitonin >2 SD above the normal value | Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection | Suspected or documented infection and an acute increase of ≥2 SOFA points (a proxy for organ dysfunction as shown in Table 2) | |
| Severe sepsis = sepsis associated with organ dysfunction, hypoperfusion, or hypotension. Hypoperfusion and perfusion abnormalities may include, but are not limited to lactic acidosis, oliguria, or an acute alteration in mental status. |
Hemodynamic parameters Arterial hypotension (systolic blood pressure <90 mmHg, mean arterial pressure <70, or a systolic blood pressure decrease >40 mmHg in adults or <2 SD below normal for age) Mixed venous oxygen saturation >70% Cardiac index >3.5 l min−1 m−2 |
||
| Septic shock = sepsis-induced with hypotension despite adequate fluid resuscitation along with the presence of perfusion abnormalities that may include, but are not limited to, lactic acidosis, oliguria, or an acute alteration in mental status. Patients who are receiving inotropic or vasopressor agents may not be hypotensive at the time that perfusion abnormalities are measured. |
Organ dysfunction parameters Arterial hypoxemia (PaO2/FiO2 <300) Acute oliguria (urine output <0.5 ml kg−1 h−1 or 45 mM L−1 for at least 2 h) Creatinine increase ≥0.5 mg dL−1 Coagulation abnormalities (international normalized ratio >1.5 or activated partial thromboplastin time >60 s) Ileus (absent bowel sounds) Thrombocytopenia (platelet count <100,000/μL) Hyperbilirubinemia (plasma total bilirubin >4 mg dL−1 or 70 mmol L−1) Tissue perfusion parameters Hyperlactatemia (>3 mmol L−1) Decreased capillary refill or mottling |
Septic shock is a subset of sepsis in which underlying circulatory and cellular/metabolic abnormalities are profound enough to substantially increase mortality | Sepsis and vasopressor therapy needed to elevate MAP ≥65 mm Hg and lactate >2 mmol L−1 (18 mg dL−1) despite adequate fluid resuscitation |
Treatment guidelines which recommend the use of definitions for sepsis were published in the 2000s. The treatment guidelines for sepsis and septic shock by Levy et al. (6) allowed sepsis awareness campaigns to be launched and caused a marked decrease in mortality rates. After the first Surviving Sepsis Campaign guidelines in 2004, revisions were made in 2008 and 2012. The fourth updated guideline was published recently to improve the outcome with new recommendations (7).
The difficulty in diagnosis is due to the complexity of the disease. Today, the information obtained from scientific studies in intensive care, microbiology, biochemistry, immunology and other medical fields and from technological advances allows a better understanding of the pathophysiology of sepsis. The better understanding of the pathophysiology and its increasing importance in public health makes it necessary to have reliable and valid diagnosis criteria for sepsis (8).
Recently, it was suggested that sepsis develops by immune suppression (9, 10). In light of these developments, it became necessary to revise the current definitions for sepsis and septic shock to ensure early diagnosis and treatment, in addition to providing the ability to select suitable patients for future clinical studies.
Although common and associated with high morbidity and mortality, sepsis and related terms remain difficult to define. Three international consensus conferences used expert opinion to generate the current definitions. However, advances in the understanding of the pathobiology may cause outdated definitions, and inaccuracy can lead to re-examination of the current definitions in the future.
The purpose of this review is to discuss the current understanding of the definition of sepsis, with particular attention to changes over time. Precise definitions of sepsis ensure accurate diagnosis and appropriate treatment. An important aim is also reducing risks for misclassification bias. We will explore the evolution of the definition of sepsis, the limitations and consider directions for future clinical definitions. This article will start by discussing the old definitions, sepsis 1 and sepsis 2. The continuation of the article will include the current clinical definitions of sepsis 3, including recent considerations for revision and diagnostic criteria for sepsis and septic shock with the application of the new definition in clinical practice. The final section will discuss difficulties with the new definition and diagnostic criteria and the future of definitions of sepsis.
History of sepsis
Sepsis is a word derived from the ancient Greek [σηψις], which means the decomposition of animal- or plant-based organic materials by bacteria. The word ‘sepsis’ was used in Homeric poems as ‘sepo’ [σηπω], meaning ‘I rotted.’ Hippocrates represented the term sepsis with the word ‘sepidon,’ which meant ‘distortion, dissolution of a web structure’ between 460-370 BC. The term was used by Aristotle, Plutarch and Galen with this meaning, and it has been in use with virtually no change in meaning for over 2700 years (11, 12).
Older definitions by consensus conference
Most clinicians believe that there is no consensus on the definition of sepsis in clinical practice, that treatment starts late, that more sensitive indicators must be identified for early sepsis diagnosis and that the public is not well-informed about sepsis. The first consensus reports published since the 1990s until today have tried to mitigate these deficiencies.
Sepsis 1
The American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) convened in Chicago in 1991 and emphasised that sepsis was an ‘ongoing process.’ Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock and multiple organ dysfunction syndrome began to be used in clinical practice (13). Sepsis was defined as the identification of two or more SIRS criteria, in addition to known or suspected infection (Table 1), while severe sepsis was defined as clinical sepsis accompanied by organ dysfunction, hypoperfusion or hypotension. According to this definition, simultaneous multiple organ involvement is observed in septic shock (such as cardiovascular [hypotension or hypoperfusion], renal [oliguria], respiratory [PaO2/FiO2 <300], hepatic [plasma total bilirubin >4 mg dL−1], haematologic [thrombocyte count <100.000/μL], central nervous system [mental changes], unexplained metabolic acidosis, etc). Septic shock is defined as a clinical tableau in which fluid/vasopressor-resistant hypotension (average artery blood pressure ≤70 mmHg) and hypoperfusion is observed.
Deficiencies related to the definition in sepsis 1
Although the 1991 North American consensus definition considers the combination of infection and SIRS response as sepsis, a sepsis-like clinical picture may be observed without infection. The current significance of inflammation is non-specific and may manifest in many conditions. A hyperkinetic state after cardiac surgery without any infection is a good example of the sepsis-like statement which shows a very different prognosis and therapeutic approach from those of real sepsis (14). Moreover, sepsis is a complex interplay of pro-inflammatory and anti-inflammatory responses and now evolve into two phases: hyper-inflammation and hypo-inflammation. Therefore, the inflammation itself carries little meaning, because inflammation is a very non-specific response to any insult from minor trauma to complicated autoimmune disease (15).
Systemic inflammatory response syndrome findings are rather sensitive, and under stressful conditions in which tachycardia, hyperventilation and leukocytosis are observed, these criteria may not be sensitive enough (16). Moreover, as SIRS also includes infection findings, clinicians may sometimes perceive infection as sepsis according to this definition. It is already known that SIRS is just a primary host response against infection. According to this definition and diagnostic criteria, almost all infection accompanied by symptoms of systemic inflammatory reaction will be diagnosed as sepsis, most of which, in fact, can be easily cured. While all patients with sepsis have infections, not all infected patients can be diagnosed with sepsis. This definition and diagnostic criteria cannot accurately present the essence of sepsis.
Systemic inflammatory response syndrome has been incorporated as inclusion criteria in many sepsis trials, and the consensus definitions were used for study purposes. The major problem was the high inconsistency of the definitions (17).
Sepsis 2
In 2001, the SCCM, the European Society of Intensive Care Medicine (ESICM), the ACCP, the American Thoracic Society and the Surgical Infection Society held the second consensus meeting and updated the criteria for sepsis. Definitions of sepsis, severe sepsis and septic shock which were ratified at the consensus meeting 10 years previously were modified. Signs and symptoms of sepsis were much greater in number and detail in comparison to the criteria identified at the consensus meeting in 1991, and the 2001 conference attempted to determine these signs and symptoms. The definitions cannot adequately define the response of the patient to infection. The documented or suspected infection-specific findings were categorised as general, inflammatory, hemodynamic, organ dysfunction and tissue perfusion variations, biochemical indicators were considered and their roles in early diagnosis were emphasised in the update (18).
Deficiencies related to definition in sepsis 2
In 1992, sepsis was defined as a clinical syndrome by the presence of both infection and systemic inflammatory response. The 2001 consensus conference proposed a new term for sepsis which was described as a clinical syndrome combined with organ injury, but the old diagnostic criteria for sepsis was kept in use (Table 1). This conference did not make a corresponding revision to the definition of sepsis. Severe sepsis was defined as ‘sepsis complicated by organ dysfunction.’ As a result, there was no difference in diagnostic criteria compared with old definitions. Similar characteristics of two definitions made a confusion of sense for to diagnose ‘sepsis’ by the new diagnostic criteria or ‘severe sepsis’ by the old diagnostic criteria. This high-level disagreement led to mismatching bias for researchers and physicians.
Goals of sepsis 3: why did the definition of sepsis needed to be updated?
In light of current knowledge, a new definition was required due to advances in sepsis epidemiology and management. Published in February 2016, the new consensus report, which emphasises organ dysfunction, considers the fact that SIRS criteria may change depending on many factors in intensive care patients and that SIRS has low sensitivity and specificity in discriminating sepsis and non-complicated infection. The international consensus definitions of sepsis and septic shock were updated, and the qSOFA (quick Sequential Organ Failure Assessment) scoring system was developed for the early identification of simultaneous organ dysfunction in sepsis. The latest sepsis guide emphasised the requirement for early diagnosis and quick application of treatment procedures during the ongoing sepsis process.
Trauma, burns, pancreatitis and ischaemia-reperfusion injury are among the non-infection causes of SIRS. Since inflammation at the molecular level triggered by the infection in the clinical tableau has similarities with sterile inflammation, it is difficult to discriminate between the two in the early stages of the disease. Both infection-triggered inflammation and sterile inflammation activate coagulation, inflammation and tissue repair pathways (19). According to the old definition, sepsis was diagnosed in the presence of SIRS criteria and infection. Thus, since most patients who presented with simple infection also exhibited SIRS criteria, all infections were diagnosed as sepsis. However, while all sepsis patients exhibit infection, it would be wrong to diagnose all patients presenting infection with sepsis (Figure 1). Similarly, sepsis and severe sepsis are generally used interchangeably in clinical practice; to resolve this confusion, organ dysfunction must be included in the definition (14). SIRS criteria have low specificity in the first 24 hours of admission to intensive care. Haemodialysis patients have high sepsis risk. The disrupted immune system functions may vary in response to the pathogen depending on the chronic process. Cloudy consciousness may be the first sign of sepsis in these patients, even in the absence of SIRS criteria (20). Similarly, elderly people and people who use medication for heart rate control may not present with SIRS criteria, even if they have infection and organ dysfunction. The response of the host and the development of severe organ dysfunction must be important parameters in the definition. At the Merinoff Symposium in 2010, sepsis was defined as ‘the life-threatening process due to organ and tissue damage caused by the body’s response to infection’ (21). The infection may be of a heterogeneous nature depending on the pathogen type and amount and the locus and duration of exposure. The response may also vary according to the different organs and tissue types in the organs of the host. Sepsis treatment varies according to all these factors (22). In their retrospective analysis which included 172 centres and 1,171,797 patients, Kaukonen et al. (16) concluded that 12.1% of patients diagnosed with severe sepsis did not exhibit SIRS symptoms and that SIRS criteria have low specificity and low sensitivity.
Figure 1.
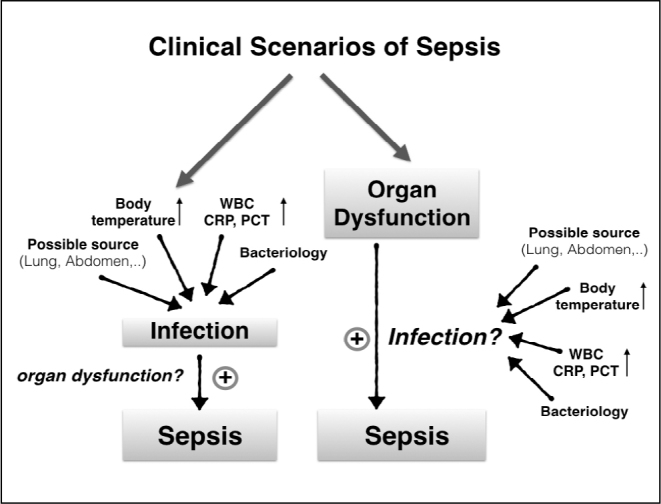
Clinical scenarios of sepsis
Newer definition
Sepsis 3
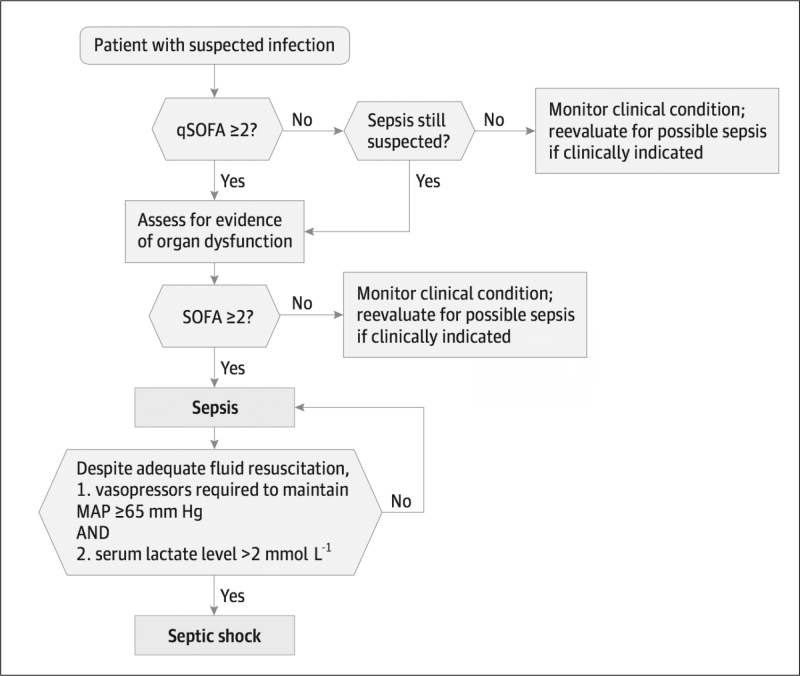
The working group’s SEPSIS 3 report defines sepsis as a ‘life-threatening organ dysfunction caused by a dysregulated host response to infection.’ Septic shock is defined as lactate levels rising above 2 mmol L−1 without hypovolemia and initiation of vasopressor treatment to keep mean arterial pressure above 65 mmHg (Figure 2) (23). Organ dysfunction is defined as an increase of two or more in the Sequential Organ Failure Assessment (SOFA) scoring system, and it was determined that this caused a more than 10% increase in hospital mortality (Table 2). Accordingly, when newly developing and unexplained organ dysfunction is identified, it should be kept in mind that the patient may be experiencing sepsis.
Figure 2.
Operationalization of Clinical Criteria Identifying Patients with Sepsis and Septic Shock
The baseline Sequential [Sepsis-related] Organ Failure Assessment (SOFA) score should be assumed to be zero unless the patient is known to have preexisting (acute or chronic) organ dysfunction before the onset of infection. qSOFA: indicates quick SOFA; MAP: mean arterial pressure. From Singer et al. (23)
Table 2.
The Sequential Organ Failure Assessment (SOFA) score
| SOFA score | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Respiration | -----with respiratory support----- | |||
| PaO2/FiO2 (mm Hg) | <400 | <300 | <200 | <100 |
|
| ||||
| Coagulation | ||||
| Platelets ×103/mm3 | <150 | <100 | <50 | <20 |
|
| ||||
| Liver | ||||
| Bilirubin (mg dL−1) | 1.2–1.9 | 2.0–5.9 | 6.0–11.9 | >12.0 |
|
| ||||
| Cardiovascular | ||||
| Hypotension | MAP <70 | Dopamine ≤5 or dobutamine (any) | Dopamine >5 or norepinephrine ≤0.1 | Dopamine >15 or norepinephrine >0.1 |
|
| ||||
| Central Nervous System | ||||
| Glasgow Coma Score | 13–14 | 10–12 | 6–9 | <6 |
|
| ||||
| Renal | ||||
| Creatinine (mg dL−1) or urine output (mL) | 1.2–1.9 | 2.0–3.4 | 3.5–4.9 or <500 | >5.0 or <200 |
MAP: mean arterial pressure; vasoactive mediations administered for at least 1 hr (dopamine and norepinephrine μg kg−1 min−1).
Clinical infection was retrospectively identified in 148,907 patients among 1,300,000 patients in a total of 12 hospitals between 2010 and 2012, and they were included in the analysis to determine new criteria. The power of the SOFA in predicting hospital mortality in an ICU was equivalent to Logistic Organ Dysfunction Score (LODS) and higher than SIRS. The choice of the SOFA to measure organ dysfunction is due to its broad contents. The reason for not choosing LODS is that it became obsolute and requires many measurements (24). The consensus introduced the rapid bedside qSOFA tool to determined patients at emergency departments or floor of the hospitals who likely develop sepsis from the retrospectively derived databases. The qSOFA was developed to prevent overlooking sepsis-related organ dysfunction outside the ICU and in the emergency department, to begin the appropriate treatment early, to speed up admission to intensive care and to increase monitoring frequency. It was determined that this scoring system was more successful and more easily applied than other systems. An increase of two or more in a qSOFA score corresponds to a 3 to 14-fold increase in hospital mortality. Accordingly, an increase of two or more in the qSOFA score should create a suspicion of sepsis, and organ dysfunction assessment should be conducted (Figure 2).
A qSOFA score is not a diagnostic criterion for sepsis and not a part of the new definition of sepsis. Rather, it can be regarded as a warning of an increased risk of deterioration for patients with suspected infections. The Sepsis Definitions Task Force proposed that among ICU encounters, the qSOFA had statistically worse predictive validity in identifying organ dysfunction compare with the SOFA in ICU, likely related effects of ongoing organ support. Thus, the 2016 consensus report claims that use of the qSOFA in ICU settings reduces to consider the possibility of sepsis.
There is little data for the predictive performance of the qSOFA as a screening tool for early and accurate prediction of sepsis and mortality at (ICU) admission. Additionally, it was suggested that the qSOFA has a lower prognostic accuracy for predicting hospital mortality who admitted to the ICU with suspected infection. The SOFA was more accurate in predicting in-hospital mortality than both SIRS and the qSOFA (25). In a new retrospective analysis, which corresponds with the findings of the Sepsis-3 Task Force, it was shown the qSOFA is not a good assessment tool among patients admitted to the ICU (26). However, the qSOFA requires prospective, real-world validation before entering routine clinical practice.
The SOFA
The SOFA is a scoring system used to determine the pervasiveness and speed of sepsis-related organ dysfunction. It calculates the level of dysfunction in five systems (respiratory, cardiovascular, coagulation, renal, neurologic) (Table 2). The SOFA is simply a scale which focuses on organ dysfunction among bedside clinical variables and calculates morbidity rather than mortality. This scoring system is calculated at admission and once every 24 hours. The calculation, based on the average and worst scores during a stay in an ICU, predicts clinical expectations. The minimum score is 0, and the maximum score is 24. However, this scoring system should not be used to determine the success of treatment or the clinical method.
According to the SOFA, mortality rates can only be valuable during the stay in intensive care. If the score is between 0 and 6, mortality should be expected to be <10%; for scores of 13–14, 50% mortality should be expected, and for scores above 15, mortality of 90% should be expected.
Seymour et al. (24) compared the predictive value of the SOFA, SIRS, LODS and the recently developed qSOFA in their analyses of 148,907 suspected sepsis patients among 706,399 patients in 165 hospitals. This analysis found that the SOFA and LODS provided similar results for possible ICU infections and hospital mortality, and both scoring systems were found to be superior to SIRS. For patients outside the ICU, the qSOFA was more meaningful than SIRS in predicting hospital mortality.
Application of the new definition in clinical practice
Systemic inflammatory response syndrome criteria were revised in 2001; however, a controversy ensued as the signs and symptoms of sepsis were published as a very long list. In 2014, ideas for a new consensus on sepsis were discussed in a meeting held by the SSCM and ESICM. While it was suggested that a new definition was required, it was also stated that the new definition could be based on the old criteria. This consensus report defined the clinical definition of sepsis, which has been accepted in recent years, as ‘at least a 2 point increase in the SOFA score along with life-threatening organ dysfunction due to an inappropriate response of the host to infection.’ Septic shock is the most severe form of sepsis and is an emerging acute circulation dysfunction. Septic shock requires a vasopressor dose to remedy hypotension, discoloration of the skin, reduced urine output and altered states of consciousness, tissue perfusion symptoms and lactate levels exceeding 2 mmol L−1.
Potential deficiencies related to definition in sepsis 3
There is still no perfect method to discriminate between sepsis and non-sepsis, including the new definition of sepsis (24). The term ‘organ dysfunction’ used in the new definition is unclear, since organs may have more than one function. Again, it is unclear how the organ dysfunction or how the other concept of ‘inappropriate host response’ will be measured. Organ dysfunction may emerge for reasons other than sepsis, and it is difficult to discriminate between sepsis-related organ dysfunction. When the infection is not certain and organ dysfunction is present, it is difficult to exclude a sepsis diagnosis (27). One of the important points is to be able to diagnose sepsis early. In this regard, while the SOFA is a valuable method to identify organ dysfunction, it has limited value outside the ICU since it is not practical to use. Database scans in recent years indicate that three simple elements may be alarming. This definition included tachypnea, hypotension and altered states of consciousness and was named the qSOFA (23). This definition is a simple and readily applicable model for nurses and other health-care employees. Positive qSOFA and laboratory tests including lactate for patients with suspected infection are thought to speed up the identification of organ dysfunction and the patients’ transfer to the ICU. As qSOFA and SOFA values may vary according to both acute and chronic organ dysfunction, the variation in SOFA scores over time is more important (28).
Patients with infection and organ dysfunction may be heterogeneously distributed regarding demographics, characteristics, underlying causes and microbiological and other clinical factors (29). The new definition does not conduct a sub-group analysis of microbiological, pathophysiological and cellular variables like the previous consensus reports. American hospitals were used as a basis for the new definition, and large databases were used. The fact that non-developed or developing countries do not have accumulated data may be considered a limitation. The lactate parameter used to define septic shock is not widely used in these countries. There is also no data from the paediatric population in the new definition (30).
One of the main components of sepsis and septic shock is the existence of infection. The number of patients diagnosed with sepsis despite negative culture is significantly high (29, 31). In intensive care practice, organ dysfunction due to clinical conditions (such as trauma, pancreatitis) is frequently observed and difficult to distinguish from simultaneous infection. There is a limited number of good-quality studies for the rapid detection of pathogens for the early diagnosis sepsis. Rapid testing of the microorganisms is needed for reducing the time to targeted therapy for ICU patients (32). In this topic, polymerase chain reaction is an excellent technique for the rapid detection of pathogens for the early diagnosis of culture negative sepsis (33).
The new definition considers sepsis as a syndrome, rather than a specific disease. The new approach defines it along with diseases with similar diagnostic processes, such as cancer, based on anatomic localisation, cell type, specific biomarkers, cellular receptors, intra-cellular pathways and genomic modifications. Patient-specific treatment principles were developed based on this type of identification.
The patient group exhibiting infection and organ dysfunction is heterogeneous. The newly developed definition of sepsis cannot categorise the underlying pathobiological and demographic information. The new definition does not allow for specific treatment based on the patient-specific underlying cause. This may be stated to be a global weakness of the sepsis concept, apart from of the definition itself.
Although both the SOFA and SIRS are indicators of organ dysfunction and mortality, they do not determine the cause of the dysfunction. The qSOFA system, which was created based on retrospective data, needs prospective studies for validation before being used clinically. In patients with infection risk, the qSOFA can be used as a secondary screening tool to determine organ dysfunction. Sepsis treatment bundles should begin after organ dysfunction is identified.
The SOFA scoring system is complex and needs to be updated because the Glasgow Coma Scale is problematic and current guidelines do not recommend dopamine as first-line vasopressor support for sepsis. The scoring system also does not show previous organ system dysfunction. Another fear for the SOFA scoring system is a handicap for the late recognition of deterioration of the organ perfusion. It is now well-known that potentially septic patients may benefit from early intervention (34).
The future of definitions of sepsis
To make the diagnostic criteria for a disease clearer and more helpful, the following criteria are required:
It must be reliable.
It must contain information which corresponds to scientific facts.
The results of different tests must abide by the definition.
The terms which apply to the definition of the disease should also apply during the advanced stages of the disease.
The definition must be up to date.
The measurement methods must be practical (35).
We should remember the underlying physiological and terminological rules while examining the definition of sepsis. The current definition of sepsis considers sepsis conceptually based on infection and emphasises organ dysfunction and the response of the host, but it does not measure the interaction between these symptoms. Thus, it is evident that clinical criteria are more important for sepsis than theory. These six fundamental rules should be applied to the definition to achieve the desired goal (35).
The next consensus report will include the definition of the early phase of developing sepsis and the involvement of fundamental sciences to identify this process. The biggest expectation in this new period will be not only the identification of infection in the patient but the identification of organ dysfunction, which is one of the basic characteristics of sepsis and in providing patient-specific treatment. One of the most limiting factors in sepsis is that there is no gold standard to identify infection. A difficult point is the fact that SIRS can be used to predict infection but is excluded from the new definition.
The basic factor in the management of sepsis is its early diagnosis and treatment. The priority is to identify infections based on signs and symptoms, obtain the necessary cultures and blood samples and prevent sepsis-related organ dysfunction through antibiotic therapy.
Understanding the underlying primary pathogenesis along with immunologic responses will make early diagnosis and treatment more comprehensive (36). The definitions will continue to evolve along with these developments. Discussion of the classification of the syndrome and the characterisation of cellular features will be beneficial for future definitions of sepsis. The next consensus report will define the cellular changes due to sepsis and septic shock and provide a biomarker-based definition instead of syndrome-based definition and will help to quickly understand organ dysfunction and mortality. The applicability of these developments to millions of people around the world should be considered as a measure of success.
Roadmap for the future
It is not currently realistic to have a gold standard definition for sepsis. However, using a methodological approach, it can be realistic to aim for different definitions and criteria.
What are our expectations for the future?
Maybe the most important point is that a single definition of sepsis is not sufficient to raise awareness.
When the definitions are standardised, the limitations of terminology may be lifted.
Many elements which function as a catch-all for sepsis are not well-established yet. Future clinical studies must detail the relationships between sepsis, infection, organ dysfunction and natural or inappropriate host response.
Future sepsis criteria need to develop molecular indicators. Although there are more than 2000 biomarkers for sepsis, sharp, reliable and realistic parameters such as those used in diagnosing myocardial infarction do not exist yet.
Prospective studies are needed for new sepsis criteria (37).
It would be a good idea to refined the SOFA, and prospective validation of the qSOFA is necessary.
There is a limited number of good-quality studies for the rapid detection of pathogens for the early diagnosis sepsis. Rapid testing of the microorganisms is needed for reducing the time to targeted therapy for ICU patients (32).
There may be a need for new, simple, reliable and clinically available measurements to contribute to the definition of the sepsis. ETCO2 levels, a good example of this, have been shown as a reliable measurement to identifying sepsis (38, 39) and an accurate predictor of mortality. It was found that an ETCO2 concentration less than 25 mmHg are strongly associated with serum lactate levels >4 mmol L−1 (38). In addition, a significant correlation was found between ETCO2 levels and SOFA scores. It was established that an ETCO2 of <35 has a high sensitivity to predict SOFA scores >2 (39).
Early diagnosis of sepsis is one of the most important points to keep expectations high. The role of early recognition of organ system dysfunction should be studied in detail. For instance, dysfunction of the coagulation system is an early manifestation of sepsis and is seen commonly with this disease. The haematologic organ system is a major element in the response to a septic insult, and in the majority of patients, systemic activation of coagulation is present (17, 40). Increasing evidence suggests that molecular mechanisms which cause inflammation-induced coagulation play an important role in the pathogenesis of sepsis. Prolongation of the PT and/or activated partial thromboplastin time is prevalent and detectable in 15–30% of septic patients (41).
Conclusion
As noted, sepsis is the most common cause of mortality in the ICUs of the developed world with a consistently increasing incidence over the last few decades. Appropriate definitions of sepsis are critically important for correct clinical management and effective clinical sepsis research. Definitions of sepsis have evolved since the syndrome was recognised over a century ago. Most recently, several international societies have sponsored consensus definitions of sepsis, septic shock and related conditions. The most recent version of these consensus definitions was published within the last year. Updated consensus definitions are designed to address specific deficiencies identified in previous published versions of consensus sepsis definitions. Despite the best efforts of the members of the expert panel that developed them, certain problems with the definitions persist. Nonetheless, it is hoped that these new definitions will help advance clinical sepsis research and clinical care. However, it remains to be seen as to whether this hope will translate into reality.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - İ.C.; Design - İ.C., F.G., M.K.A.; Supervision - İ.C., A.K.; Resources - İ.C., F.G., M.K.A.; Materials - İ.C., F.G., M.K.A.; Data Collection and/or Processing - İ.C., F.G., M.K.A.; Analysis and/or Interpretation - İ.C., F.G., M.K.A., A.K.; Literature Search - İ.C., F.G., M.K.A.; Writing Manuscript - İ.C., F.G., M.K.A., A.K.; Critical Review - İ.C., A.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. https://doi.org/10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley, et al. Nationwide trends of severe sepsis in the 21st century (2000–2007) Chest. 2011;140:1223–31. doi: 10.1378/chest.11-0352. https://doi.org/10.1378/chest.11-0352. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12:919–24. doi: 10.1016/S1473-3099(12)70239-6. https://doi.org/10.1016/S1473-3099(12)70239-6. [DOI] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–16. doi: 10.1001/jama.2014.2637. https://doi.org/10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger RP, Schorr CA, Levy MM. A users’ guide to the 2016 Surviving Sepsis Guidelines. Intensive Care Med. 2017;43:299–303. doi: 10.1007/s00134-017-4681-8. [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;38:367–74. doi: 10.1097/CCM.0b013e3181cb0cdc. https://doi.org/10.1097/ccm.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes A, Evans L, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for the management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 8.Iskander KN, Osuchowski MF, Stearns-Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, et al. Sepsis: Multiple Abnormalities, Heterogeneous Responses, and Evolving Understanding. Physiol Rev. 2013;93:1247–88. doi: 10.1152/physrev.00037.2012. https://doi.org/10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Gullaume M, Didier P. Immunosupression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–8. doi: 10.1016/S1473-3099(13)70001-X. https://doi.org/10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus DC, Opal S. Immunosuppression and Secondary Infection in Sepsis Part, Not All, of the Story. JAMA. 2016;315:1457–9. doi: 10.1001/jama.2016.2762. https://doi.org/10.1001/jama.2016.2762. [DOI] [PubMed] [Google Scholar]
- 11.Geroulanos S, Douka ET. Historical perspective of the word “sepsis”. Intensive Care Med. 2006;32:2077. doi: 10.1007/s00134-006-0392-2. https://doi.org/10.1007/s00134-006-0392-2. [DOI] [PubMed] [Google Scholar]
- 12.Johnson GB, Brunn GJ, Samstein B, Platt JL. New insight into the pathogenesis of sepsis and the sepsis syndrome. Surgery. 2005;137:393–5. doi: 10.1016/j.surg.2004.08.001. https://doi.org/10.1016/j.surg.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. https://doi.org/10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774–5. doi: 10.1016/S0140-6736(12)61815-7. https://doi.org/10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent JL. Dear SIRS, I’m sorry to say that I don’t like you. Crit Care Med. 1997;25:372–4. doi: 10.1097/00003246-199702000-00029. https://doi.org/10.1097/00003246-199702000-00029. [DOI] [PubMed] [Google Scholar]
- 16.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic Inflammatory Response Syndrome Crıterıa in Defining Severe Sepsis. N Engl J Med. 2015;372:1629–38. doi: 10.1056/NEJMoa1415236. https://doi.org/10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 17.Trzeciak S, Zanotti-Cavazzoni S, Parrillo JE, Dellinger RP. Inclusion criteria for clinical trials in sepsis: did the American College of Chest Physicians/Society of Critical Care Medicine consensus conference definitions of sepsis have an impact? Chest. 2005;127:242–5. doi: 10.1378/chest.127.1.242. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. https://doi.org/10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. https://doi.org/10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 20.Drewry AM, Hotchkiss RS. Sepsis: Revising definitions of sepsis. Nat Rev Nephrol. 2015;11:326–8. doi: 10.1038/nrneph.2015.66. https://doi.org/10.1038/nrneph.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czura CJ. “Merinoff Symposium 2010: sepsis”–speaking with one voice. Mol Med. 2011;17:2–3. doi: 10.2119/molmed.2010.00001.commentary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankar-Hari M, Deutschman CS, Singer M. Do we need a new definition of sepsis? Intensive Care Med. 2015;41:909–1. doi: 10.1007/s00134-015-3680-x. [DOI] [PubMed] [Google Scholar]
- 23.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. https://doi.org/10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–74. doi: 10.1001/jama.2016.0288. https://doi.org/10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, et al. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328. https://doi.org/10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 26.Lamontagne F, Harrison DA, Rowan KM. qSOFA for Identifying Sepsis Among Patients With Infection. JAMA. 2017;317:267–8. doi: 10.1001/jama.2016.19684. https://doi.org/10.1001/jama.2016.19684. [DOI] [PubMed] [Google Scholar]
- 27.Verdonk F, Blet A, Mebazaa A. The new sepsis definition: limitations and contribution to research and diagnosis of sepsis. Curr Opin Anaesthesiol. 2017;30:200–4. doi: 10.1097/ACO.0000000000000446. https://doi.org/10.1097/ACO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. doi: 10.1001/jama.286.14.1754. https://doi.org/10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. https://doi.org/10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E. New definitions for sepsis and septic shock, continuing evolution but with much still to be done. JAMA. 2016;315:757–9. doi: 10.1001/jama.2016.0290. https://doi.org/10.1001/jama.2016.0290. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture Negative Severe Sepsis – Nationwide Trends and Outcomes. Chest. 2016;150:1251–9. doi: 10.1016/j.chest.2016.08.1460. https://doi.org/10.1016/j.chest.2016.08.1460. [DOI] [PubMed] [Google Scholar]
- 32.Jessica HP, Michael DS, Kenneth A, Joseph H. Culture Negative Sepsis and Systemic Inflammatory Response Syndrome in Neonates. Neoreviews. 2013;14:294. https://doi.org/10.1542/neo.14-6-e294. [Google Scholar]
- 33.Buehler SS, Madison B, Snyder SR, Derzon JH, Cornish NE, Saubolle MA, et al. Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. 2016;29:59–103. doi: 10.1128/CMR.00053-14. https://doi.org/10.1128/CMR.00053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charles LS, Roland MHS, Robert AB. The new sepsis consensus definitions: the good, the bad and the ugly. Intensive Care Med. 2016;42:2024–6. doi: 10.1007/s00134-016-4604-0. https://doi.org/10.1007/s00134-016-4604-0. [DOI] [PubMed] [Google Scholar]
- 35.Angus DC, Seymour CW, Coopersmith CM, Deutschman CS, Klompas M, Levy MM, et al. A Framework for the Development and Interpretation of Different Sepsis Definitions and Clinical Criteria. Crit Care Med. 2016;44:113–21. doi: 10.1097/CCM.0000000000001730. https://doi.org/10.1097/CCM.0000000000001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent JL, Mira JP, Antonelli M. Sepsis: older and newer concepts. Lancet Respir Med. 2016;4:237–40. doi: 10.1016/S2213-2600(15)00522-6. https://doi.org/10.1016/S2213-2600(15)00522-6. [DOI] [PubMed] [Google Scholar]
- 37.Seymour CW, Coopersmith CM, Deutschman CS, Gesten F, Klompas M, Levy M, et al. Application of a Framework to Assess the Usefulness of Alternative Sepsis Criteria. Crit Care Med. 2016;44:122–30. doi: 10.1097/CCM.0000000000001724. https://doi.org/10.1097/CCM.0000000000001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter CL, Silvestri S, Dean M, Falk JL, Papa L. End-tidal carbon dioxide is associated with mortality and lactate in patients with suspected sepsis. Am J Emerg Med. 2013;31:64–71. doi: 10.1016/j.ajem.2012.05.034. https://doi.org/10.1016/j.ajem.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 39.McGillicuddy DC, Tang A, Cataldo L, Gusev J, Shapiro NI. Evaluation of end-tidal carbon dioxide role in predicting elevated SOFA scores and lactic acidosis. Intern Emerg Med. 2009;4:41–4. doi: 10.1007/s11739-008-0153-z. https://doi.org/10.1007/s11739-008-0221-4. [DOI] [PubMed] [Google Scholar]
- 40.Marcel L. The coagulant response in sepsis. Clin Chest Med. 2008;29:627–42. doi: 10.1016/j.ccm.2008.06.006. https://doi.org/10.1016/j.ccm.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 41.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. https://doi.org/10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]