Abstract
Objective
Postoperative cognitive dysfunction (POCD) is common after anaesthesia in elderly patients. However, it may appear in patients of all ages. The main pathogenesis of cognitive dysfunction remains unclear, although there is some evidence that brain inflammation may alter cognitive abilities. In the present study, we aim to evaluate short-term and long-term effects of dexamethasone on cognitive dysfunction induced by sevoflurane anaesthesia in adult rats.
Methods
Seven-month-old 30 male Wistar albino rats were randomised into three groups: sevoflurane group (exposure to sevoflurane), sevoflurane + dexamethasone group (exposure to sevoflurane and dexamethasone injection), and control group (exposure to 100% oxygen). Spatial learning and short-term (7 days after exposure) and long-term (30 days after exposure) memory were evaluated using Morris water maze test.
Results
Sevoflurane induced significant deficit in spatial learning and short-term and long-term memory in adult rats. Dexamethasone-treated animals exposed to sevoflurane had equivalent performance as control animals in training and probe trials.
Conclusion
Sevoflurane may impair spatial learning and short-term and long-term memories in adult rats. The co-administration of dexamethasone and sevoflurane may ameliorate short-term and long-term cognitive dysfunctions induced by sevoflurane in adult rats.
Keywords: Sevoflurane, dexamethasone, cognitive disorders
Introduction
Postoperative cognitive dysfunction (POCD) is a term used to describe the cognitive impairment of memory, attention, concentration, and information processing occurring after surgery (1). Although elderly adults seem to be more sensitive to its long-term effects, POCD may appear in patients of all ages (2). The main reason for POCD is not clearly understood, but animal studies have shown that anaesthetic agents can play a role in causing neurodegenerative changes in the brain (3). The cognitive impairment effects of sevoflurane on both aged and young rats have been shown by several researchers, but there is a paucity of data on adult rats (4, 5).
Dexamethasone is a well-known steroid agent that regulates inflammation by inhibiting anti-inflammatory mediators and other mechanisms such as induction of the expression of dual specificity phosphatase-1 (6). The anti-inflammatory effects of dexamethasone in brain cells have been shown in several studies (7, 8). Furthermore, researchers have implied that dexamethasone may support the treatment of brain inflammatory diseases (7, 8). Based on these findings, we hypothesised that dexamethasone could mitigate the cognitive impairment induced by sevoflurane in adult rats.
Methods
Animals
All experiments were approved by the Gaziosmanpasa University Animal Ethics Committee (2015 HAYDEK-34) and were performed in Gaziosmanpasa University Experimental Medicine Research Unit. Thirty male Wistar albino rats aged 7 months and weighing 200–250 g were purchased from the Gaziosmanpasa University Experimental Medicine Research Unit. Animals were divided into three groups: control (C), sevoflurane (S) and sevoflurane + dexamethasone (SD). Animals were housed in clean acrylic cages under 12-h light/dark cycle (lights on 07.00–19.00) with controlled temperature (21+1°C) and humidity (45%-65%) and had ad libitum access to food and water. All behavioural tests were performed during the light phase of the light/dark cycle. The timeline of all experiments is shown in Figure 1.
Figure 1.
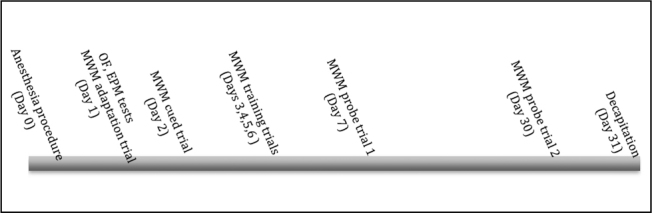
Experimental timeline. OF, EPM and Morris MWM tests were conducted
OF: open field; EPM: elevated plus maze; MWM: Morris water maze
Anaesthesia
Rats were randomly allocated to one of the three groups: C (n=10), S (n=10) or SD (n=10). All rats were placed in an anaesthetic chamber. Rats randomly placed in S or SD groups received a 5% concentration of sevoflurane for 1 min for anaesthesia induction, and then anaesthesia was maintained with a 2%-4% concentration of sevoflurane in 100% oxygen at a flow rate of 2 L min−1 for 4 h via an anaesthetic system (Prisma SP Alpa, Penlon Limited, Oxon, UK). The sevoflurane, oxygen and carbon dioxide concentrations inside the chamber were monitored (Datex-Ohmeda, USA). During anaesthetic treatment, rats spontaneously breathed and the investigator monitored the respiratory frequency and skin colour of rats. In addition, the depth of anaesthesia was evaluated by clamping the tail with an alligator clamp at 30-min intervals. Anaesthesia was maintained by adjusting the sevoflurane concentration (2%–4%) according to the respiratory frequency, skin colour or response to tail clamping. Rats randomized to the C group received 100% oxygen at a flow rate of 2 L min−1 during 4 h.
Before the induction of anaesthesia, 0.1 mg kg−1 dexamethasone in 0.25 mL/100 g of body weight volume and 0.9% saline were administered via the tail vein to rats in the SD group. Plain 0.9% saline in 0.25 mL/100 g of body weight volume was administered to rats in the C and S groups.
Behavioural tests
Behavioural tests were delayed until 24 h after the induction of anaesthesia to avoid the residual effects of sevoflurane. Rats were placed in a quiet light-controlled test room at least 30 min before the tests for adaptation to room conditions. All tests were performed by the same investigator who was blinded to the groups and were recorded by a video tracking system (ANY-maze version 4.82).
The Open Field (OF) Test
The OF test was performed as described by Brotto et al. (9) on day 1 after the induction of anaesthesia to evaluate the behavioural activity of rats. The arena for the OF test was made of wood and was 100 × 100 cm2 in size with 40-cm-high walls and was divided into 16 equal squares. The rats were placed on an OF area and observed for 5 min. The total distance travelled by rats was measured to evaluate the general activity. The OF arena was cleaned after each test with 70% ethanol.
The Elevated Plus Maze (EPM) Test
The EPM test was used to assess anxiety-related behaviours of the rats according to previously described methods with some modifications (10). The maze was constructed with two open (45 × 10 cm2) and two enclosed (45 × 10 × 45 cm2) arms and elevated 50 cm from the floor. The rat was placed on the centre of the maze facing towards the enclosed arm, and the time spent on the open arm was observed for 5 min.
The Morris Water Maze (MWM) Test
The MWM test, which was first introduced by Morris et al. (11) to exhibit deficits in spatial learning and memory depending on hippocampal lesions, was performed as previously described with minor modifications (12). A round (diameter: 180 cm, height: 50 cm), black-painted pool was filled with clean water at 26±1°C to a height of 30 cm. The pool was divided into four imaginary quadrants: north-east (NE), south-east (SE), north-west (NW) and south-west (SW). Rats were placed in the pool facing towards the wall by the same investigator for all trials. In the trials, if the rats could not find the platform in 60 s, the investigator guided them to the platform. When the rats arrived at the platform, they stayed there for 15 s. They performed four trials in a day with a 60-s rest between each trial. At the end of the trials, rats were dried with a towel and put back in their cages.
Twenty-four hours after the induction of anaesthesia, rats were allowed to swim freely in the pool for 60 s to adapt to the test condition.
On the following day, a cued trial was performed. In the cued trial, rats were placed in one part of the pool with the same randomisation (SE, SW, NW and SE). They were released for free swimming to find the black escape platform (diameter: 10 cm) with a collared ball that was placed 1 cm above the water at the centre of each quadrant of the pool (NE, NW, SE and SW). On days 3–6 after anaesthesia, training trials were conducted. The escape platform was submerged 2 cm below the water surface in the same position (NE). The starting position of the rats was changed in each trial with the assigned randomisation that was the same for all rats. On days 7 and 30 after anaesthesia, probe trials were executed. In the probe trials, the rats started the test from the opposite side of the hidden platform quadrant in training trials (SW) and swam in the pool without the escape platform for 30 s.
The time to find the hidden platform (latency to platform) and swim speed of the rats during training trials, time spent and the distance travelled on zone (previous platform area) during the probe trials were measured. In addition, the time taken to reach the target zone (latency to zone) and crossing over the target zone during the probe trials were recorded.
Statistical analysis
All analyses were performed using IBM Statistical Package for the Social Sciences (IBM SPSS Statistics; Armonk, NY, USA) software (version 20). The normality was tested using Kolmogorov-Smirnov test, whereas homogeneity of variance was calculated using Levene’s test. The normally distributed data were expressed as mean (±standard deviation) and compared between groups by analysis of variance (ANOVA) with Tukey’s post hoc test or Games-Howell test, whereas not normally distributed data were expressed as median (range) and compared using the Kruskal-Wallis test. Repeated measured data were analysed using repeated measures ANOVA test. A p value of <0.05 was considered statistically significant.
Results
All animals survived and participated in all stages of the experiment. The general motor activity of the rats was the same in the OF test. The total distances on the OF arena were 4.27±2.47 m in the S group, 5.49±3.05 m in the SD group and 6.51±1.19 m in the control group (p=0.13). The time spent on the open arm during EPM test was significantly different between the groups, and exposure to SD caused lower anxiety-like behaviour than that in the control group (p=0.02, Table 1).
Table 1.
Time spent on open arm (OA) in elevated plus maze (EPM) test
| Control | Sevoflurane | Sevoflurane+ dexamethasone | p | |
|---|---|---|---|---|
| Time spent on OA (s) | 157.48±121,99 | 232.7±63.40* | 264.0±36.79 | 0.022 |
ANOVA test is used for comparisons. Data are presented as mean±standard deviation.
p<0.05, compared with sevoflurane + dexamethasone group.
In the MWM test training trials, the time spent by the rats to find the hidden platform (latency to platform) decreased over the trials in all groups. However there was a significant difference among the groups (p=0.004; Figure 2). However, the comparison of the swim speed of the rats did not show significant difference among the groups (p=0.62; Figure 3).
Figure 2.
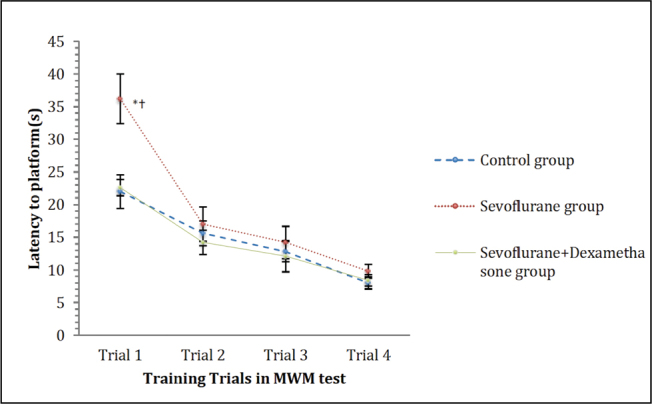
Latency to platform in MWM training trials. Data are presented as mean ± standard error of mean (SEM). Repeated measures ANOVA (RMA) is used for comparisons.
*p<0.05, compared with control group and †:p<0.05, compared with the sevoflurane + dexamethasone group
MWM: Morris water maze
Figure 3.
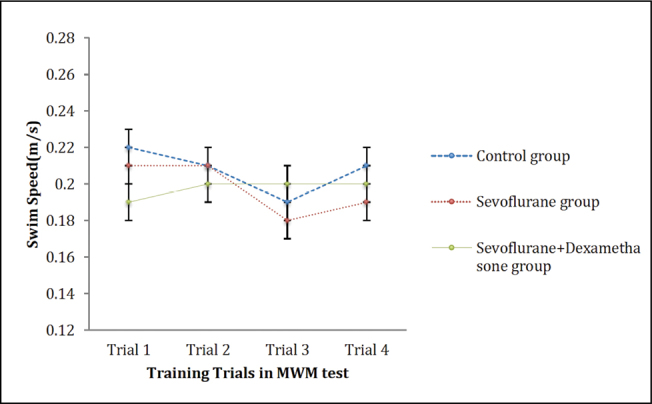
Swim speed of the rats during MWM training trials. Data are presented as mean ± standard error of mean (SEM). Repeated measures ANOVA (RMA) is used for comparisons. There is no effect of sevoflurane or dexamethasone on the swim speed of the rats during training trials (p>0.05)
MWM: Morris water maze
In the MWM test probe trials 1 (on day 7 after the induction of anaesthesia) and 2 (on day 30 after the induction of anaesthesia), there were significant differences in the time spent, distance travelled and the latency to the zone that the platform was submerged during the training trials between the groups (p=0.00, p=0.003 and p=0.002, respectively; Figures 4–6). However, the number of crossings over the previous platform zone was not significantly different between the groups in probe trials 1 and 2 [p=0.914 (P1) and p=0.144 (P2); Figure 7].
Figure 4.
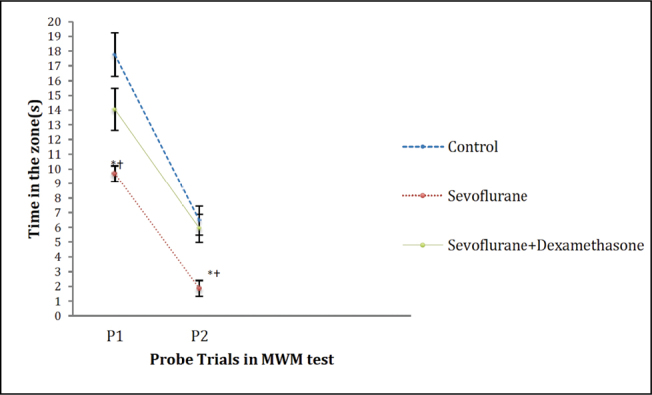
Time travelled on the zone in MWM probe trials. Data are presented as mean ± standard error of mean (SEM). Repeated measures ANOVA (RMA) is used for comparisons. *: p<0.05, compared with Control group and †: p<0.05, compared with the sevoflurane + dexamethasone group
MWM: Morris water maze
Figure 5.
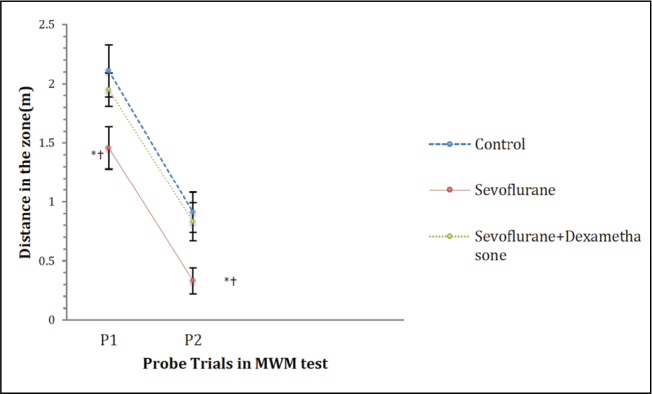
Distance travelled on the zone in MWM probe trials. Data are presented as mean ± standard error of mean (SEM). Repeated measures ANOVA (RMA) is used for comparisons. *: p<0.05, compared with Control group and †: p<0.05, compared with the sevoflurane + dexamethasone group
MWM: Morris water maze
Figure 6.
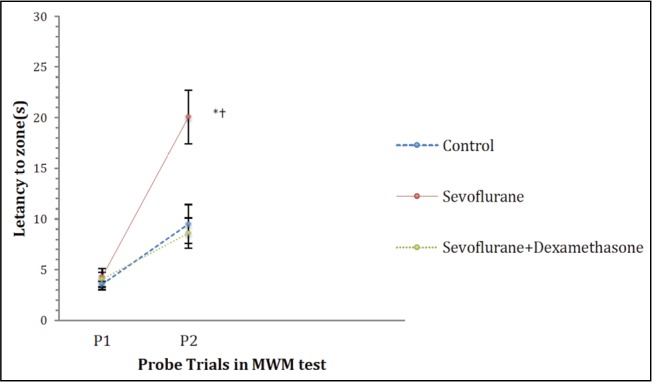
Latency to the zone in MWM probe trials. Data are presented as mean ± standard error of mean (SEM). Repeated measures ANOVA (RMA) is used for comparisons. *: p<0.05, compared with Control group and †: p<0.05, compared with the sevoflurane + dexamethasone group
MWM: Morris water maze
Figure 7.
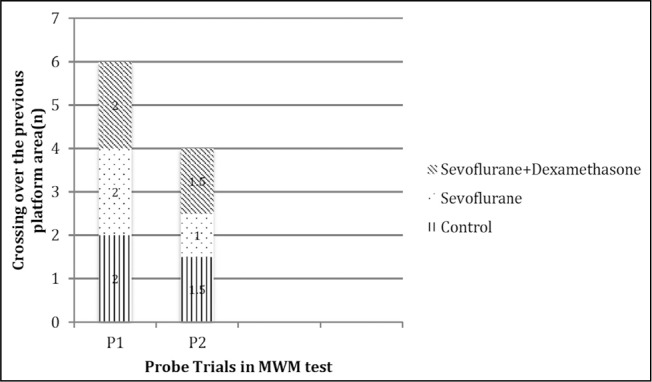
Number of the crossings over the zone in MWM probe trials. Data are presented as median. Kruskal-Wallis test is used for comparisons. There are no significant differences between the groups at any time
MWM: Morris water maze
Discussion
The findings of the present study showed that a 2%–4% concentration of sevoflurane exposure during 4 h had significant negative effects on the learning abilities of adult rats. In addition, there was an impaired long-term memory retention capacity in young adult rats in the S group compared with the SD and C groups. Furthermore, combining a single low-dose dexamethasone (0.1 mg kg−1) with sevoflurane caused lesser anxiety-related behaviour.
Sevoflurane is a safe inhalation anaesthetic agent that is commonly preferred by anaesthesiologist for patients of all ages due to its low blood-gas partition coefficients and low metabolic breakdown. These properties imply that sevoflurane provides rapid and smooth induction and recovery from anaesthesia (13). However, it is known that sevoflurane may lead to cognitive dysfunction, despite the sub-anaesthetic dose usage in adults (14). On the other hand, the knowledge about cognitive impairment related to sevoflurane in adult animals is controversial and associated with the small number of results of experimental studies (5, 15). Learning and memory are the researched aspects of cognitive functions in animals. The MWM test is a reliable method of assessing spatial learning and reference memory in rodents (16). Using the MWM test, we measured the latency to find a hidden platform and swim speed in training trials to determine spatial learning ability of rats. Short-term and long-term memory deficits are assessed by the probe trials of MWM test analysing the time spent, distance travelled, latency and number of crossings over the previous platform area. Our study demonstrated that the adult rats have deficits in spatial learning and memory on days 7 and 30 after the induction of anaesthesia with 2%–4% sevoflurane in 100% oxygen during 4 h.
Neuroinflammation is a cornerstone of degenerative changes and cognitive disease (3, 17). However, the cognitive impairment induced by anaesthetics may depend on different factors, and it was recently shown that sevoflurane leads to increased interleukin-6 (IL-6) levels and induces neuroinflammation and cognitive disorders in young mice (18). In contrast, Gong et al. (4) investigated the effects of a nonsteroid anti-inflammatory drug (parecoxib) on cognitive impairments and they concluded that parecoxib attenuated the nugatory effects induced by sevoflurane in rats. Dexamethasone, which is a widely used agent in the perioperative course to prevent postoperative nausea, vomiting, and inflammatory events, can inhibit the production of IL-6 (19). Many studies have revealed that dexamethasone can make alterations in memory and learning processes (20, 21). Although the popular opinion is that dexamethasone diminishes cognitive function, some researches presented evidence that indicated otherwise (22). Venturella et al. (22) showed that dexamethasone reverses induced memory impairment. To our best knowledge, this is the first study to evaluate the effect of dexamethasone on sevoflurane-induced cognitive dysfunction. In our study, there were significant differences in spatial learning and short-term and long-term memory in the S group. In addition, the rats in the SD group had better performance on latency to platform on day 3 after the induction of anaesthesia (training trial 1) than those in the S group. Moreover, in the short-term and long-term memory tasks (probe trials 1 and 2), the rats that received dexamethasone had significantly less reduction in the time spent on and distance travelled in previous platform area compared with the rats in the S groups. Moreover, the latency time to the previous platform zone was significantly higher in the S group. In addition, according to our study results, general motor activity was not significantly dissimilar in the rats between groups. Thus, we suggested that the impaired abilities in the MWM test may have been based on changes in the learning and memory functions of rats.
The memory and learning processes require a basal level of cortisol production; however, a high level of secretion can inhibit this mechanism (23). Corticosteroids bind to glucocorticoid receptors, and activated glucocorticoid receptors conduct transcription of the various genes and lead to several events in brain, including intracellular signal transduction, metabolism and synaptic plasticity which are related with cognitive functions. Intermediate activation of the receptors is related to memory consolidation (24). In the present study, a low-dose dexamethasone was used. Thus, intermediate activation with low doses may play a role in this memory enhancement.
Anxiety is a disorder caused by a neurobiological imbalance in the brain. The primary inhibitory neurotransmitter gamma-aminobutyric acid (GABA) seems to be an important regulator in anxiety-like behaviours (25). Like other anaesthetics, sevoflurane acts on the GABAergic system and modulates GABA (26). In addition, N-methyl-D-aspartate (NMDA) antagonism can result in anxiety-related disorders (27). Moreover, Haseneder et al. (28) showed that sevoflurane elevates the expression of the NMDA receptor type 1 and 2B subunits in the hippocampus leading to less anxious behaviour. Another study conducted by Luo et al. (29) suggested that sevoflurane inhibits pain-related anxiety, along with ERK activation.
However, the relationship between dexamethasone and anxiety is complicated. The peripheral administration of high-dose dexamethasone can lead to anxiogenic reaction, whereas lower doses can produce anxiolytic effects due to the increased GABAergic neurons (30, 31). In our study, rats anaesthetised with sevoflurane showed less anxious behaviour (not statistically significant) than non-anaesthetised rats. However, the addition of dexamethasone to sevoflurane caused significantly reduced anxiety. In the present study, the usage of sevoflurane with dexamethasone may have generated more anxiolytic influence in rats due to the synergistic effect of the drugs on GABAergic system.
This study has some limitations. First, we did not evaluate the dexamethasone-alone group in this study because the primary goal was to determine the effect of sevoflurane on cognitive function and possible relationship with inflammation in adult rats. Second, we did not investigate the possible mechanisms related to cognitive dysfunction histopathologically. Finally, this study had an experimental design that restricts the possibility of generalising the results to humans.
Conclusion
Sevoflurane may impair spatial learning and short-term and long-term memory in adult rats. The administration of dexamethasone along with sevoflurane anaesthetics may reduce anxiety without leading to any alteration in general motor activity and ameliorate the short-term and long-term cognitive dysfunctions induced by sevoflurane in adult rats.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Gaziosmanpasa University Animal Ethics Committee (2015 HAYDEK-34).
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - T.K., S.K.; Design - T.K., S.K., H.T.; Supervision - T.K., S.K.; Resources - T.K., S.K., H.T., S.D., A.Ş., M.S.; Materials - T.K., S.K., S.D., A.Ş.; Data Collection and/or Processing - T.K., S.K., H.T.; Analysis and/or Interpretation - T.K., S.K., S.D., M.S.; Literature Search - T.K., A.Ş., M.S.; Writing Manuscript - T.K., S.K.; Critical Review - T.K., S.K., H.T., S.D., A.Ş., M.S.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anesthesia. 2009;103:41–6. doi: 10.1093/bja/aep291. https://doi.org/10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. https://doi.org/10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 3.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. https://doi.org/10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 4.Gong M, Chen G, Zhang XM, Xu LH, Wang HM, Yan M. Parecoxib mitigates spatial memory impairment induced by sevoflurane anesthesia in aged rats. Acta Anaesthesiol Scand. 2012;56:601–7. doi: 10.1111/j.1399-6576.2012.02665.x. https://doi.org/10.1111/j.1399-6576.2012.02665.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu XS, Xue QS, Zeng QW, Li Q, Liu J, Feng XM. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3β in the hippocampus. Neurobiol Learn Mem. 2010;94:461–7. doi: 10.1016/j.nlm.2010.08.011. https://doi.org/10.1016/j.nlm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, et al. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–9. doi: 10.1084/jem.20060336. https://doi.org/10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczepanik AM, Ringheim GE. IL-10 and glucocorticoids inhibit Abeta (1–42)- and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J Alzheimers Dis. 2003;5:105–17. doi: 10.3233/jad-2003-5205. https://doi.org/10.3233/JAD-2003-5205. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Ling EA, Dheen ST. Dexamethasone suppresses monocyte chemoattractant protein-1 production via mitogen activated protein kinase phosphatase-1 dependent inhibition of Jun N-terminal kinase and p38 mitogen-activated protein kinase in activated rat microglia. J Neurochem. 2007;102:667–78. doi: 10.1111/j.1471-4159.2007.04535.x. https://doi.org/10.1111/j.1471-4159.2007.04535.x. [DOI] [PubMed] [Google Scholar]
- 9.Brotto LA, Barr AM, Gorzalka BB. Sex differences in forced-swim and open-field test behaviours after chronic administration of melatonin. Eur J Pharmacol. 2000;402:87–93. doi: 10.1016/s0014-2999(00)00491-x. https://doi.org/10.1016/S0014-2999(00)00491-X. [DOI] [PubMed] [Google Scholar]
- 10.Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn-Schmiedeberg’s Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. https://doi.org/10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 11.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. https://doi.org/10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 12.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. https://doi.org/10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Delgado-Herrera L, Ostroff RD, Rogers SA. Sevoflurance: approaching the ideal inhalational anesthetic: a pharmacologic, pharmacoeconomic, and clinical review. CNS Drug Rev. 2001;7:48–120. doi: 10.1111/j.1527-3458.2001.tb00190.x. https://doi.org/10.1111/j.1527-3458.2001.tb00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galinkin JL, Janiszewski D, Young CJ, Klafta JM, Klock PA, Coalson DW, et al. Subjective, psychomotor, cognitive, and analgesic effects of subanesthetic concentrations of sevoflurane and nitrous oxide. Anesthesiology. 1997;87:1082–8. doi: 10.1097/00000542-199711000-00012. https://doi.org/10.1097/00000542-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Callaway JK, Jones NC, Royse AG, Royse CF. Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology. 2012;117:1091–101. doi: 10.1097/ALN.0b013e31826cb228. https://doi.org/10.1097/ALN.0b013e31826cb228. [DOI] [PubMed] [Google Scholar]
- 16.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. https://doi.org/10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain Pathol. 2012;22:530–46. doi: 10.1111/j.1750-3639.2011.00550.x. https://doi.org/10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hen XS, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15. doi: 10.1097/ALN.0b013e3182834d77. https://doi.org/10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waage A, Slupphaug G, Shalaby R. Glucocorticoids inhibit the production of IL6 from monocytes, endothelial cells and fibroblasts. Eur J Immunol. 1990;20:2439–43. doi: 10.1002/eji.1830201112. https://doi.org/10.1002/eji.1830201112. [DOI] [PubMed] [Google Scholar]
- 20.Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: an interaction with opiate system. Behav Brain Res. 2004;154:193–8. doi: 10.1016/j.bbr.2004.02.007. https://doi.org/10.1016/j.bbr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Yao YY, Liu DM, Xu DF, Li WP. Memory and learning impairment induced by dexamethasone in senescent but not young mice. Eur J Pharmacol. 2007;574:20–8. doi: 10.1016/j.ejphar.2007.07.021. https://doi.org/10.1016/j.ejphar.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Venturella R, Lessa D, Luft T, Roozendaal B, Schwartsmann G, Roesler R. Dexamethasone reverses the memory impairment induced by antagonism of hippocampal gastrin-releasing peptide receptors. Peptides. 2005;26:821–5. doi: 10.1016/j.peptides.2004.12.010. https://doi.org/10.1016/j.peptides.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999;56:527–33. doi: 10.1001/archpsyc.56.6.527. https://doi.org/10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- 24.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. https://doi.org/10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26:36–43. doi: 10.1016/j.tips.2004.11.004. https://doi.org/10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa K, Harrison NL. The actions of sevoflurane and desflurane on the gamma-aminobutyric acid receptor type A: effects of TM2 mutations in the alpha and beta subunits. Anesthesiology. 2003;99:678–84. doi: 10.1097/00000542-200309000-00024. https://doi.org/10.1097/00000542-200309000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM. Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion. Eur J Pharmacol. 2010;626:49–56. doi: 10.1016/j.ejphar.2009.10.014. https://doi.org/10.1016/j.ejphar.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haseneder R, Starker L, Berkmann J, Kellermann K, Jungwirth B, Blobner M, et al. Sevoflurane anesthesia improves cognitive performance in mice, but does not influence in vitro long-term potentation in hippocampus CA1 stratum radiatum. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0064732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo C, Zhang YL, Luo W, Zhou FH, Li CQ, Xu JM, et al. Differential effects of general anesthetics on anxiety-like behavior in formalin-induced pain: involvement of ERK activation in the anterior cingulate cortex. Psychopharmacology (Berl) 2015;232:4433–44. doi: 10.1007/s00213-015-4071-2. https://doi.org/10.1007/s00213-015-4071-2. [DOI] [PubMed] [Google Scholar]
- 30.Vafaei AA, Rashidy-Pour A, Taherian AA. Peripheral injection of dexamethasone modulates anxiety related behaviors in mice: an interaction with opioidergic neurons. Pak J Pharm Sci. 2008;21:285–9. [PubMed] [Google Scholar]
- 31.Baud O, Verney C, Evrard P, Gressens P. Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res. 2005;57:149–56. doi: 10.1203/01.PDR.0000148069.03855.C4. https://doi.org/10.1203/01.PDR.0000148069.03855.C4. [DOI] [PubMed] [Google Scholar]


