Abstract
Background and Objectives:
Oral submucous fibrosis (OSMF) is a potentially malignant disorder of oral mucosa affecting mainly population in South and Southeast Asia. The aim of this study is to compare the effectiveness of oral colchicine with intralesional injection of hyaluronidase or injection triamcinolone acetonide in patients with Grade II OSMF.
Materials and Methods:
The study included thirty patients of clinically diagnosed Grade II OSMF. Patients were divided randomly into two groups: Group A patients were treated by administrating tablet colchicine 0.5 mg twice daily with an intralesional injection of hyaluronidase 1500 IU with 0.5 ml of lignocaine hydrochloride at weekly interval for 12 weeks. Group B patients were treated by administering tablet colchicine 0.5 mg twice daily with an intralesional injection of triamcinolone acetonide 10 mg/ml at weekly interval for 12 weeks. Clinical diagnosis was based on burning sensation in mouth, blanching of mucosa, presence of vesicles or ulceration in oral cavity, and reduced mouth opening. Outcome assessment was done at intervals of 3 weeks, 6 weeks, 3 months, and 6 months.
Results:
Improvement in mouth opening and reduction in burning sensation was seen more in Group A patients. Improvement in blanching of mucosa was seen in both the groups.
Conclusion:
In conclusion, use of injection hyaluronidase with oral colchicine gave better results in terms of increase in mouth opening and improvement in burning sensation without notable side effects. However, for a definite conclusion, further study with large sample size and long follow-up is required.
Keywords: Colchicine, hyaluronidase, triamcinolone acetonide
INTRODUCTION
Oral submucous fibrosis (OSMF), reported in 1952 by Schwartz as “atrophia idiopathica mucosae oris”[1] is a potentially malignant disorder of oral mucosa affecting mainly population in South and Southeast Asia.[2] There is an increased prevalence of squamous cell carcinoma in patients with OSMF. The highest malignant transformation rate of OSMF of about 7.6% was reported from India as documented in International Agency for Research on Cancer monograph on OSMF.[3]
The etiology of OSMF is multifactorial. Areca nut chewing is considered the most important etiologic factor,[4] but other factors such as nutritional deficiency, altered salivary constituents, collagen disorders, and genetic susceptibility are also involved in the etiopathogenesis of OSMF.[5] The constituents of areca nut including arecoline and tanin interfere with normal collagen metabolism by increasing the synthesis of collagen and decreasing its breakdown. This results in increased collagen deposition and thus resulting in fibrosis of oral tissues.[4]
Various treatment modalities including drug therapy, surgical therapy, and physiotherapy have been proposed till date for the management of OSMF. Various drugs with antifibrotic, anti-inflammatory, and antioxidant activity have been used in the management of OSMF but with unpredictable results and incomplete remission. No single drug has been reported to be effective in treatment of OSMF. Hence, a combination of drugs has been used in the treatment of OSMF.
Colchicine is an alkaloid chemically known as colchicinum-N-(5,5,79-Tetrahydro-1,2,310-tetramethoxy-9- oxobenzo[alpha]heptalen-7-yl)acetamide.[6] Various studies have established the role of colchicine as an antifibrotic agent by inhibiting collagen synthesis and increasing collagenolytic activity.[7] It has been used in reducing fibrosis in liver and kidney diseases.[8] Besides, it also has some anti-inflammatory properties. This anti-inflammatory property is related to drug's effect on polymorphonuclear leukocytes and monocyte chemotaxis, leukocyte adhesiveness, and also its effect on prostaglandin E, which suppresses the leukocyte function.[9] Very few studies have reported the use of colchicine in OSMF.
The aim of this study is to compare the effectiveness of oral colchicine with intralesional injection of hyaluronidase or injection triamcinolone acetonide in patients with Grade II OSMF.
MATERIALS AND METHODS
This study included thirty patients of clinically diagnosed Grade II OSMF who attended the OPD of Department of Oral and Maxillofacial Surgery. Patients were enrolled after obtaining informed consent. Approval from Institute's Research Ethical Committee was taken.
A detailed case history including their habits was taken, and clinical examination was done. Clinical diagnosis was based on reduced mouth opening, burning sensation in mouth, blanching of mucosa, presence of vesicles or ulceration in oral cavity.
Burning sensation
Burning sensation in buccal mucosa was recorded before the start of treatment as a baseline. It was recorded then at intervals of 3 weeks, 6 weeks, 3 months, and 6 months during treatment using a visual analog scale. Scoring was done from 0 to 10 based on patient's response (Score 0: No pain; Score 10: Severe pain).
Mouth opening
The distance between the mesioincisal edge of right upper central incisor till the mesioincisal edge of right lower central incisor was measured for the assessment of mouth opening. In case of missing teeth, left central incisor was taken for measurement. Baseline measurement was taken before the start of treatment, and then measurements were taken subsequently at intervals of 3 weeks, 6 weeks, 3 months, and 6 months.
Clinical examination of oral mucosa for blanching of the mucosa and presence of ulceration was done.
Inclusion criteria
Patients with clinically diagnosed Grade II OSMF[10] were included in the study:
Grade 1 (mild): Any features of the disease triad for OSMF (burning, depapillation, blanching, or leathery mucosa) may be reported and interincisal opening >35 mm
Grade 2 (moderate): Above features of OSMF + interincisal limitation of opening 20–35 mm
Grade 3 (severe): Above features of OSMF + interincisal opening <20 mm
Grade 4a: OSMF + other potentially malignant disorder on clinical examination
Grade 4b: OSMF with any grade of oral epithelial dysplasia on biopsy
Grade 5: OSMF + oral squamous cell carcinoma.
Exclusion criteria
Medically compromised patients
Those who had received previous treatment were excluded from the study
Other accompanying mucosal disorders, if present
Persons with a history of drug allergy to hyaluronidase, triamcinolone, lignocaine, or colchicine
Pregnant females.
Patients were divided randomly into two groups:
Group A patients were treated by administrating tablet colchicine 0.5 mg twice daily with an intralesional injection of hyaluronidase 1500 IU with 0.5 ml of lignocaine hydrochloride at weekly interval for 12 weeks
Group B patients were treated by administering tablet colchicine 0.5 mg twice daily with an intralesional injection of triamcinolone acetonide 10 mg/ml at weekly interval for 12 weeks.
Patients were asked to discontinue their habits.
Outcome assessment was done at intervals of 3 weeks, 6 weeks, 3 months, and 6 months.
RESULTS
In a total of thirty patients, 15 patients were included in Group A and 15 patients were included in Group B. Among thirty patients, 90% were male and young adults.
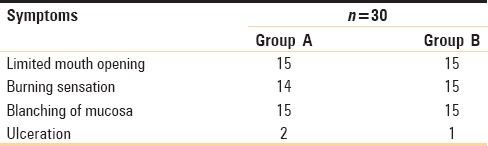
In both the groups, almost all patients had limited mouth opening, blanching of oral mucosa, and burning sensation in oral cavity on consumption of hot or spicy food. Ulceration was present in only two cases in Group A and in only one case in Group B [Table 1].
Table 1.
Comparison of symptoms between Group A and Group B
There was an improvement in mouth opening in both the groups with a maximum increase of about 8 mm seen in Group A and an increase of about 5 mm seen in Group B at 6-month follow-up [Figure 1].
Figure 1.
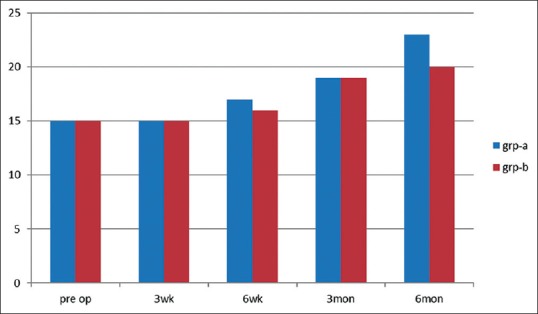
Comparison of mouth opening in Group A and Group B
Burning sensation in oral cavity was reduced in both the groups except in two patients in Group A. Burning sensation persisted in six patients in Group B with partial remission [Figure 2].
Figure 2.
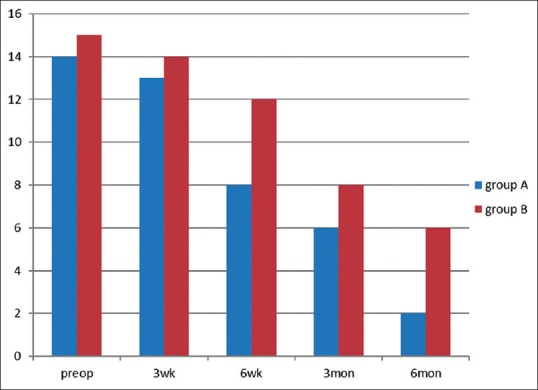
Comparison of decrease in burning sensation in Group A and Group B
Blanching of mucosa was seen in both the groups preoperatively which reduced after treatment in both the groups. Blanching of mucosa was present in six patients in Group A, whereas in four patients in Group B at 6-month follow-up [Figure 3]. After completion of treatment, a noticeable change in color was seen more in Group B patients than in Group A.
Figure 3.
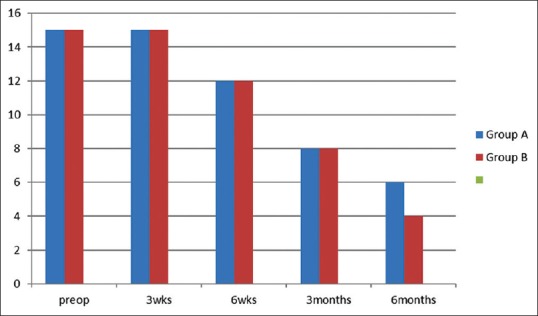
Comparison of decrease in blanching of mucosa in Group A and Group B
Ulceration persisted in both the groups even after 6-month follow-up.
DISCUSSION
OSMF is an insidious, chronic disease with multifactorial etiology. Various treatment modalities had been proposed for OSMF but with unpredictable results.
In this study, male preponderance was seen (90% males). This is in accordance with previous studies.[11] A maximum number of patients in our study (25 out of 30) were young adults. This is similar to a study by Maher et al., in which 70% were below 30 years of age.[12] All the patients had a habit of gutka chewing which brings to light the lack of awareness and easy availability of these products.
The specific etiology of OSMF is still not clear, but various mechanisms for explaining the etiopathogenesis of OSMF have been suggested.[2,13] There is excessive secretion of collagen and increased proliferation of fibroblasts along with inhibition of collagenase and fibrinolytic system. The cellular macromolecule damage caused by an excessive amount of reactive oxygen species (ROS) and other reactive metabolic intermediates from betel quid also play a crucial role in the etiopathogenesis of OSMF. Besides, effects of fibrogenic cytokines and inflammatory factors secreted by activated macrophages and T lymphocytes are also important in the etiopathogenesis of OSMF. Upregulation of various cytokines such as transforming growth factor-beta and connective tissue growth factor which are main triggers for increased collagen production and downregulation of bone morphogenic protein 7 which is a negative modulator of fibrosis have been reported in OSMF.[14] In later stages, collagen deposits increase in the submucous tissue involving blood vessels, salivary glands, and muscle leading to vascular occlusion and thus the symptoms.
Colchicine has been shown to inhibit collagen synthesis. It has been reported that colchicine inhibited procollagen secretion and its conversion to collagen and thus specifically inhibited collagen synthesis.[15] It disrupts the microtubule formation and inhibits microtubule polymerization by binding to tubulin.[16]
Colchicine also exerts an anti-inflammatory effect by its destabilizing action on microtubules. It blunts the tumor necrosis factor alpha (TNF-α)-induced activation of macrophages and diminishes the number of TNF-α receptors on the surface of macrophages and endothelial cells. It also interrupts the mast cell degranulation process, thus preventing the release of inflammatory mediators.[17] The correlation of TNF-α levels with the severity of OSMF has been recognized.[18]
There is an excessive amount of ROS production in OSMF which leads to cellular macromolecule damage. Colchicine has been shown to inhibit ROS production which is necessary for inflammasome activation by its microtubule disrupting effect.[17]
Among the various treatment methods, use of steroids and use of hyaluronidase have shown to improve the symptoms in OSMF. Steroids act as immunosuppressive agents by opposing the action of soluble factors released by sensitized lymphocytes. Steroids have also been used in OSMF due to their anti-inflammatory action.[19] They prevent fibrosis by decreasing the proliferation of fibroblasts and collagen deposition.[20]
Triamcinolone is preferred due to its high potency, duration of action, and decreased systemic absorption.[21,22]
Hyaluronidase breaks down hyaluronic acid and lowers the viscosity of intercellular cement substances. It also decreases collagen formation.[23]
In a study by Kakar et al., patients receiving hyaluronidase alone showed a quicker improvement in symptoms, whereas combination with dexamethasone gave better long-term results.[24] In a study by Borle and Borle,[25] improvement in burning sensation of about 89.9% was seen with a combination of injection triamcinolone and injection hyaluronidase.
In this study also, amelioration of symptoms was seen in patients in both the groups. On 6-month follow–up, less number of patients in Group B, in which triamcinolone injection with oral colchicine was used, showed a decrease in burning sensation as compared to patients in Group A, in which oral colchicine was used along with injection hyaluronidase.
Blanching of mucosa was decreased more in patients in Group B than in Group A.
On comparison of increase in mouth opening between both the groups, more increase in mouth opening was seen in patients in Group A than in Group B patients. This is in accordance with the randomized, double-blind trial conducted by Alora Veedu et al.[26] The rapid breakdown of collagen by hyaluronidase and decreased collagen formation due to its action on hyaluronic acid gives a better result in improvement in mouth opening.[27]
The improvement in mouth opening achieved more in Group A, i.e., in patients with injection hyaluronidase with oral colchicine is in accordance with the study by Krishnamoorthy and Khan.[28] A significant improvement in burning sensation and mouth opening was seen in patients on oral colchicine 0.5 mg twice daily along with intralesional hyaluronidase 1500 IU was also seen in their study similar to this study.
Colchicine, with its anti-inflammatory and antifibrotic action, has been used in reducing fibrosis in liver and renal diseases. The use of colchicine in OSMF has been reported by very few studies till date. At a minimal dose of colchicine of 0.5 mg orally, no side effects were noticed. The combination of colchicine with hyaluronidase is beneficial due to the different mechanisms of action of both drugs which leads to additive effect. Besides, addition of colchicine with other drug gives the advantage of low dose of colchicine used and thus decreased toxicity.
The study had certain limitations. The first being that only patients with Grade II OSMF were evaluated. Most patients in Stage I are reluctant for treatment since they are mostly asymptomatic and thus unwilling to bear the cost and pain of injections. Patients in Grade III have mostly associated ulcerations and other mucosal diseases. Second, histological assessment of change could not be done in this study as patients with Grade II OSMF were unwilling to undergo surgical procedure of biopsy.
CONCLUSION
Use of injection hyaluronidase with oral colchicine gave better results in terms of increase in mouth opening and improvement in burning sensation without notable side effects. However, for a definite conclusion, further study with large sample size and long follow-up is required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schwartz J. Atrophia Idiopathica Mucosae Oris. London: Demonstrated at the 11th International Dent Congress; 1952. [Google Scholar]
- 2.Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Wang YY, Tail YH, Wang WC, Chen CY, Kao YH, Chen YK, et al. Malignant transformation in 5071 southern Taiwanese patients with potentially malignant oral mucosal disorders. BMC Oral Health. 2014;14:99. doi: 10.1186/1472-6831-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 5.Yadav S, Verma A, Sachdeva A, Virdi M. Etiopathogenesis and management of oral submucus fibrosis. Internet J Bioeng. 2010;5:1. [Google Scholar]
- 6.Malkinson FD. Colchicine. New uses of an old, old drug. Arch Dermatol. 1982;118:453–7. doi: 10.1001/archderm.118.7.453. [DOI] [PubMed] [Google Scholar]
- 7.Diegelmann RF, Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972;69:892–6. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solak Y, Siriopol D, Yildiz A, Yilmaz MI, Ortiz A, Covic A, et al. Colchicine in renal medicine: New virtues of an ancient friend. Blood Purif. 2016;43:125–35. doi: 10.1159/000454669. [DOI] [PubMed] [Google Scholar]
- 9.Wallace SI, Bernstein D, Diamond H. Diagnostic value of the colchicine therapeutic trial. JAMA. 1967;199:525–8. [PubMed] [Google Scholar]
- 10.Kerr AR, Warnakulasuriya S, Mighell AJ, Dietrich T, Nasser M, Rimal J, et al. A systematic review of medical interventions for oral submucous fibrosis and future research opportunities. Oral Dis. 2011;17(Suppl 1):42–57. doi: 10.1111/j.1601-0825.2011.01791.x. [DOI] [PubMed] [Google Scholar]
- 11.Ranganathan K, Devi MU, Joshua E, Kirankumar K, Saraswathi TR. Oral submucous fibrosis: A case-control study in Chennai, South India. J Oral Pathol Med. 2004;33:274–7. doi: 10.1111/j.0904-2512.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 12.Maher R, Lee AJ, Warnakulasuriya KA, Lewis JA, Johnson NW. Role of areca nut in the causation of oral submucous fibrosis: A case-control study in Pakistan. J Oral Pathol Med. 1994;23:65–9. doi: 10.1111/j.1600-0714.1994.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Reddy MV, Harinath BC. Role of oxidative stress and antioxidants in aetiopathogenesis and management of oral submucous fibrosis. Indian J Clin Biochem. 2004;19:138–41. doi: 10.1007/BF02872409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan I, Agarwal P, Thangjam GS, Radhesh R, Rao SG, Kondaiah P. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119–27. doi: 10.3109/08977194.2011.582839. [DOI] [PubMed] [Google Scholar]
- 15.Allison AC. Effects of agents interacting with microtubules on collagen synthesis and breakdown. Ann Rheum Dis. 1977;36(Suppl 2):65–6. [Google Scholar]
- 16.Andreu JM, Timasheff SN. Tubulin bound to colchicine forms polymers different from microtubules. Proc Natl Acad Sci U S A. 1982;79:6753–6. doi: 10.1073/pnas.79.22.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin Ther. 2014;36:1465–79. doi: 10.1016/j.clinthera.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Sodhi S, Sodhi JS, Khambete N, Kumar R, Marthala M, Sodhi NK. Expression of tumor necrosis factor a and its correlation with severity of oral submucous fibrosis: A case-control study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:704–8. doi: 10.1016/j.oooo.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Rao PK. Efficacy of alpha lipoic acid in adjunct with intralesional steroids and hyaluronidase in the management of oral submucous fibrosis. J Cancer Res Ther. 2010;6:508–10. doi: 10.4103/0973-1482.77087. [DOI] [PubMed] [Google Scholar]
- 20.Gupta D, Sharma SC. Oral submucous fibrosis – A new treatment regimen. J Oral Maxillofac Surg. 1988;46:830–3. doi: 10.1016/0278-2391(88)90043-2. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Niranjan HS, Mehrotra R, Sharma D, Gupta SC. Efficacy of hydrocortisone acetate/hyaluronidase vs. triamcinolone acetonide/hyaluronidase in the treatment of oral submucous fibrosis. Indian J Med Res. 2010;131:665–9. [PubMed] [Google Scholar]
- 22.Jiang XW, Zhang Y, Yang SK, Zhang H, Lu K, Sun GL. Efficacy of salvianolic acid B combined with triamcinolone acetonide in the treatment of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:339–44. doi: 10.1016/j.oooo.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Laxmipathi G. Hyalase in Submucous Fibrosis in Oral Cavity. Jaipur, India, 32nd Annual Conference of the Association of Otolaryngologists of India.1980. [Google Scholar]
- 24.Kakar PK, Puri RK, Venkatachalam VP. Oral submucous fibrosis – Treatment with hyalase. J Laryngol Otol. 1985;99:57–9. doi: 10.1017/s0022215100096286. [DOI] [PubMed] [Google Scholar]
- 25.Borle RM, Borle SR. Management of oral submucous fibrosis: A conservative approach. J Oral Maxillofac Surg. 1991;49:788–91. doi: 10.1016/0278-2391(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 26.Alora Veedu R, Balan A, Sankar SP. A randomized double-blind, multiple-arm trial comparing the efficacy of submucosal injections of hyaluronidase, dexamethasone, and combination of dexamethasone and hyaluronidase in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:588–93.e1. doi: 10.1016/j.oooo.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Sirsat SM, Khanolkar VR. Submucous fibrosis of the palate in diet-preconditioned Wistar rats. Induction by local painting of capsaicin – An optical and electron microscopic study. Arch Pathol. 1960;70:171–9. [PubMed] [Google Scholar]
- 28.Krishnamoorthy B, Khan M. Management of oral submucous fibrosis by two different drug regimens: A comparative study. Dent Res J (Isfahan) 2013;10:527–32. [PMC free article] [PubMed] [Google Scholar]


