Abstract
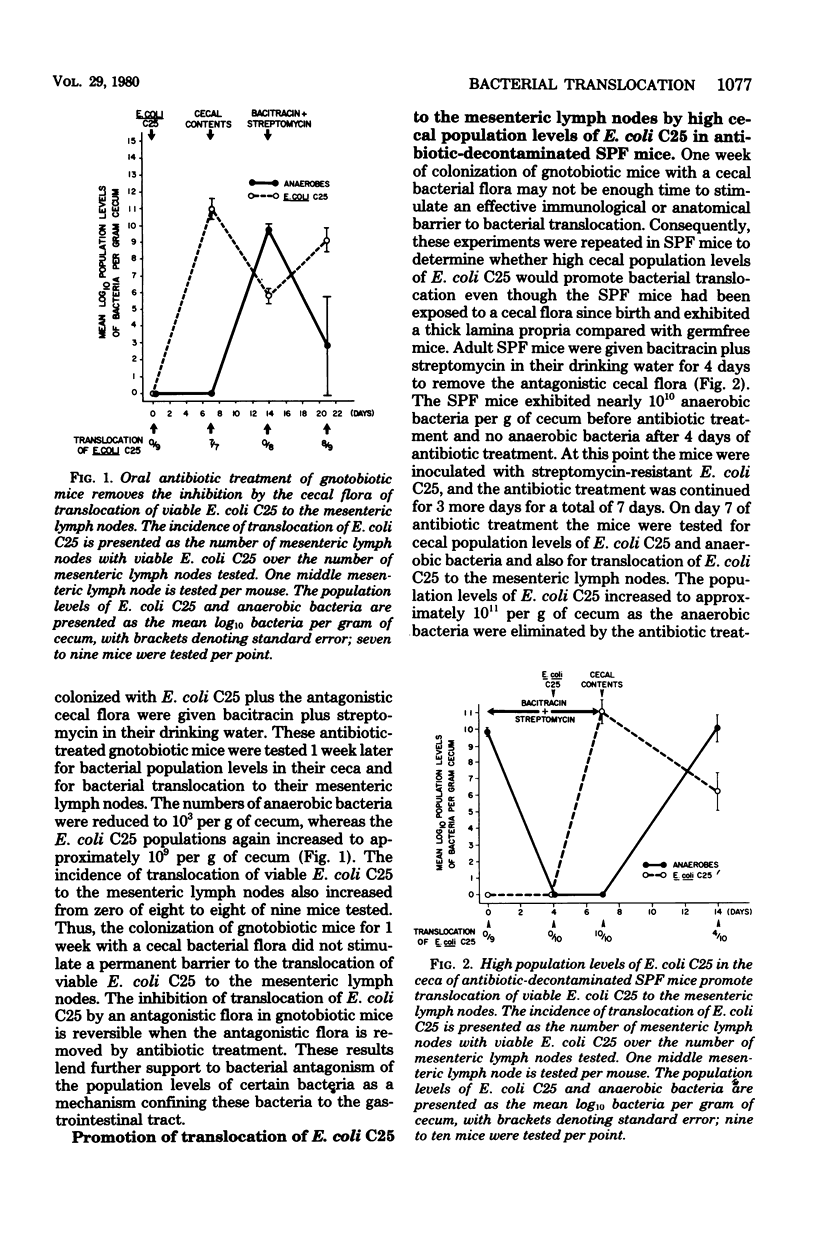
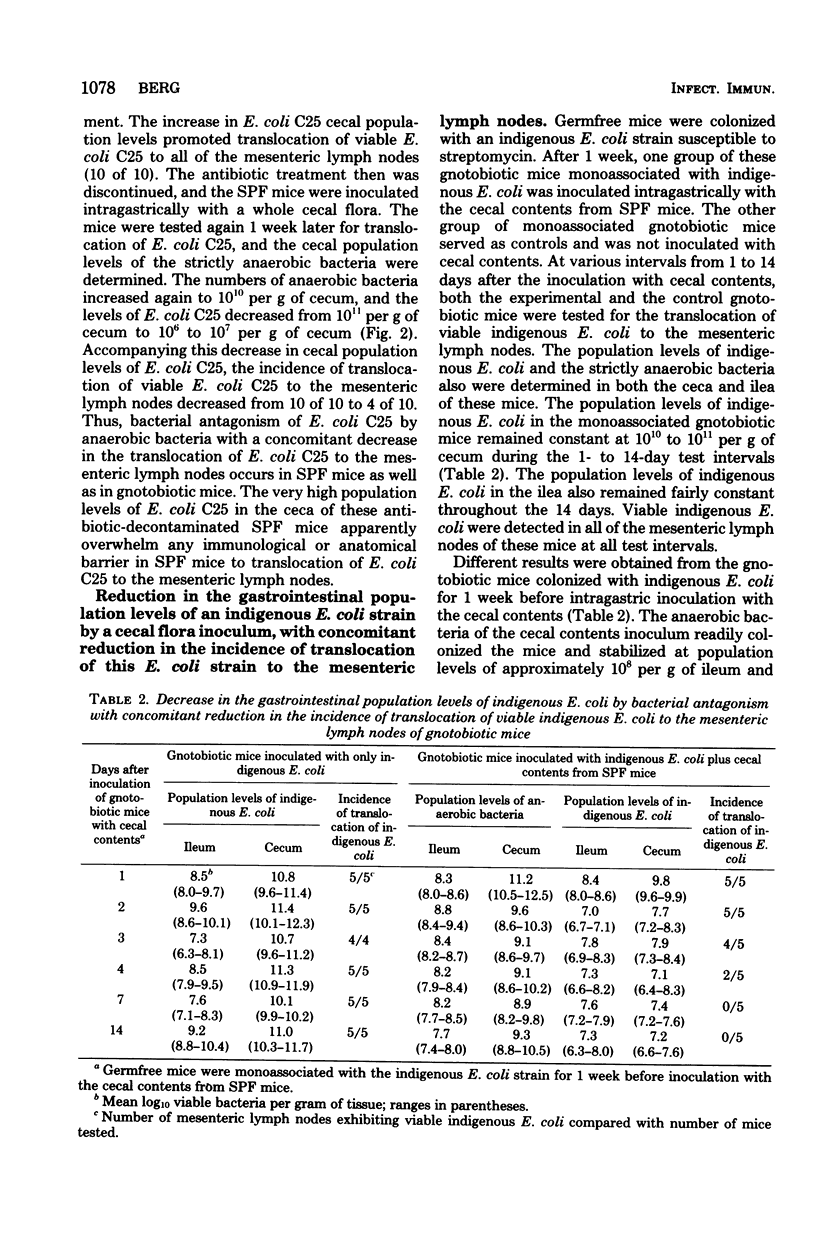
Escherichia coli C25 maintained population levels of 109 to 1010 per g of cecum and translocated to 100% of the middle mesenteric lymph nodes in gnotobiotic mice monoassociated with E. coli C25. Intragastric inoculation of these mice with the cecal contents from specific-pathogen-free mice reduced the population levels of E. coli C25 to 106 per g of cecum and completely inhibited translocation to the mesenteric lymph nodes. Intragastric inoculation with heat-treated, Formalintreated, or filtered cecal contents did not reduce the population levels of E. coli C25 or reduce the incidence of translocation of E. coli C25 to the mesenteric lymph nodes. Thus, viable bacteria apparently are required in the cecal contents inocula to reduce the population levels and the incidence of translocation of E. coli C25. Treatment with streptomycin plus bacitracin decreased the anaerobic bacterial levels in these gnotobiotic mice, allowing increased population levels of E. coli C25 and increased translocation to the mesenteric lymph nodes. E. coli C25 also translocated to the mesenteric lymph nodes of specific-pathogen-free mice treated with streptomycin and bacitracin before colonization with E. coli C25. The high cecal population levels of E. coli C25 in these antibiotic-decontaminated specific-pathogen-free mice apparently overwhelm any barrier to translocation exerted by the immunologically developed lamina propria of the specific-pathogen-free mice. Inoculation of gnotobiotic mice with a cecal flora also reduced the population levels of an indigenous strain of E. coli with a concomitant inhibition of translocation of the indigenous E. coli to the mesenteric lymph nodes. Thus, bacterial antagonism of the gastrointestinal population levels of certain indigenous bacteria, such as E. coli, by other members of the normal bacterial flora appears to be an important defense mechanism confining bacteria to the gastrointestinal tract.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Bammann L. L., Clark W. B., Gibbons R. J. Impaired colonization of gnotobiotic and conventional rats by streptomycin-resistant strains of Streptococcus mutans. Infect Immun. 1978 Dec;22(3):721–726. doi: 10.1128/iai.22.3.721-726.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Antagonism among the normal anaerobic bacteria of the mouse gastrointestinal tract determined by immunofluorescence. Appl Environ Microbiol. 1978 Jun;35(6):1066–1073. doi: 10.1128/aem.35.6.1066-1073.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Owens W. E. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun. 1979 Sep;25(3):820–827. doi: 10.1128/iai.25.3.820-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Savage D. C. Immune responses of specific pathogen-free and gnotobiotic mice to antigens of indigenous and nonindigenous microorganisms. Infect Immun. 1975 Feb;11(2):320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Savage D. C. Immunological responses and microorganisms indigenous to the gastrointestinal tract. Am J Clin Nutr. 1972 Dec;25(12):1364–1371. doi: 10.1093/ajcn/25.12.1364. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Davis C. P., McAllister J. S., Savage D. C. Microbial colonization of the intestinal epithelium in suckling mice. Infect Immun. 1973 Apr;7(4):666–672. doi: 10.1128/iai.7.4.666-672.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Ecologic factors in dental caries. The fate of antibiotic-resistant cariogenic streptococci in hamsters. Am J Pathol. 1963 Jun;42:759–772. [PMC free article] [PubMed] [Google Scholar]
- FRETER R. In vivo and in vitro antagonism of intestinal bacteria against Shigellaflexneri. II. The inhibitory mechanism. J Infect Dis. 1962 Jan-Feb;110:38–46. doi: 10.1093/infdis/110.1.38. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Interactions between mechanisms controlling the intestinal microflora. Am J Clin Nutr. 1974 Dec;27(12):1409–1416. doi: 10.1093/ajcn/27.12.1409. [DOI] [PubMed] [Google Scholar]
- Lee A., Gemmell E. Changes in the mouse intestinal microflora during weaning: role of volatile fatty acids. Infect Immun. 1972 Jan;5(1):1–7. doi: 10.1128/iai.5.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAWA A., FRETER R. ECOLOGICAL MECHANISM CONTROLLING GROWTH OF ESCHERICHIA COLI IN CONTINUOUS FLOW CULTURES AND IN THE MOUSE INTESTINE. J Infect Dis. 1964 Jun;114:235–242. doi: 10.1093/infdis/114.3.235. [DOI] [PubMed] [Google Scholar]
- Owens W. E., Berg R. D. Bacterial translocation from the gastrointestinal tract of athymic (nu/nu) mice. Infect Immun. 1980 Feb;27(2):461–467. doi: 10.1128/iai.27.2.461-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Abrams G. D., Freter R. Efficiency of various intestinal bacteria in assuming normal functions of enteric flora after association with germ-free mice. Infect Immun. 1970 Oct;2(4):376–386. doi: 10.1128/iai.2.4.376-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



