Abstract
Lipid-based nanoparticle technology has developed from chemical drug carrier into an efficient multifunctional siRNA tumor targeting delivery system. In this review, we start with an overview of the lipid-based nanomedicine history and the two classes of lipidic vectors for DNA or siRNA delivery. Then we discuss the features of lipid-based nanomedicine that lead to effective tumor targeting and the principles behind. We also discuss nanoparticle surface modification, classes of tumor targeting ligands, and other state-of-the-art strategies for enhancing endosome release primarily focused on lipid-based systems. At the end, we show that multifunctional self-assembled lipid-based nanoparticles could also be versatile delivery vehicles for cancer molecular imaging probes.
Keywords: Self-Assembly, Lipid, Liposome, Nanoparticles, Nanomedicine, siRNA, Tumor Targeting, Endosome Escape
1. INTRODUCTION
Drugs formulated in liposomes are considered the very first class of nanomedicine used in clinics. Liposomes are artificial cell-like vesicles that have an aqueous compartment inside the surrounding one or multiple lipid bilayers. The bilayer usually consists of a lipid component (usually a cationic lipid and/or a fusogenic lipid) and cholesterol. Some may further contain a polyethylene glycol-lipid conjugate for surface protection.1 The aqueous compartment and the lipid bilayer have both been used to carry drugs. By formulating doxorubicin into liposomal dosage form, it can increase the tumor uptake and reduce the cardio-toxicity.2,3 For the drugs with very poor solubility such as paclitaxel, loading the drug into the bilayer compartment can increase the delivering dose.4,5 For some drugs, such as camptothecins that are not stable under physiological pH, formulation into liposomes can also protect them from degradation.6,7 Furthermore, it can improve the pharmacokinetic profile primarily by increasing the circulation time of the drug.8
Lipid-based systems have also been developed for poly- or oligo-nucleotide delivery for decades. In 1987, Felgner et al.9 showed that lipofection, i.e., cationic lipid mediated transfection, is more efficient for delivering DNA into cells than calcium phosphate10,11 or DEAE-dextran.12 Later, various cationic lipid formulations such as the popular Lipofectamine13,14 or cardiolipin analogs15,16 have been developed and used extensively for in vitro DNA or siRNA delivery.
An important milestone for lipid-based nanomedicines is the clinical trial for liposome-mediated gene therapy conducted in 1992. This clinical trial used a liposome formulation consisting of a cationic derivative of cholesterol, 3-β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol and dioleoylphosphatidylethanolamine (DC-Chol/DOPE) to transfer a xenogenic MHC class I antigen gene to the cutaneous melanoma lesions.17 Although the transfection efficiency, the duration of expression, and the overall therapeutic effect was not as promising as anticipated, no adverse clinical effects were observed.18 There were other clinical trials for cystic fibrosis using other cationic lipids.19,20
The discovery of RNA interference has brought a new category of therapeutics that can be used for genetic diseases,21–25 viral infections,26–31 or cancers by inhibiting various pathways. Compared to delivering plasmid DNA for expressing short-hairpin RNA (shRNA) in cells, delivering synthetic siRNA can silence protein expression and is more favorable in terms of drug delivery. The great advantage of siRNA therapy is that the site of action is the cytoplasm, not the nucleus. Lipid-based systems and other non-viral vectors are excellent vehicles for siRNA delivery.
2. CLASSES OF LIPIDIC VECTORS
DNA or siRNA delivery systems can be divided into viral and non-viral vector systems. Based on the type of the target disease, local or systemic administration is a factor of consideration. Viral vectors have been used for both systemic and local administrations. The strategy is, by genetic engineering, replacing pathogenic viral genes with desired genes or shRNA expression cassette. One great advantage of using viral vectors is that some viral vectors such as lentiviral and retroviral vectors can achieve stable long term expression due to their host genome-insertion nature.32 In addition, viral vectors are generally more efficient in terms of expression level. However, immunogenicity and other safety issues are always the major concerns of using viral based systems, especially in humans. For the field of tumor targeting siRNA therapy, since stable long term expression is not needed and the site of action for siRNA is only the cytoplasm, non-viral vectors are more favorable.
There are different types of non-viral vectors for siRNA or DNA delivery such as polymers, block co-polymers, proteins, or peptides.33–36 Various designs have been established based on either chemical conjugation or self-assembly processes. Self-assembled nanomedicines are more desirable due to their easy preparation and potential for scale-up manufacturing. Desimone et al.37–40 established an imprint lithographic technique called PRINT™ (Particle Replication In Non-wetting Templates) for nanoparticle production. A variety of materials including synthetic polymers, hydrogels, active pharmaceutical ingredients, and proteins41 could be made into shape-specific, monodisperse, and surface modifiable nanoparticles.37 Bioactive agents including proteins, DNA, and small-molecule therapeutics42 have also been encapsulated into PRINT™ nanoparticles. There are also other non-viral physical methods such as hydrodynamic injection,43–45 electroporation,46–48 and particle bombardment49 that could be used for local DNA or siRNA delivery. In this review, we will primarily focus on lipid-based self-assembled nanoparticles with tumor as the target disease.
2.1. Lipoplex
There are two main types of self-assembled lipid nanomedicines, one is the traditional type that formed simply by mixing positively charged liposome with negatively charged DNA or siRNA to make a complex. These types of reagents have been extensively used for in vitro gene transfection or silencing. The other type is a more sophisticated lipopolyplex nanoparticle such as the LPD (liposome-polycation-DNA) nanoparticles designed in our lab in the mid 90s.50
Verma et al. reported the first lipoplex mediated in vivo tumor siRNA delivery via intraperitoneal (i.p.) injection to a HCT116 colon cancer xenograft model with commercially available Oligofectamine (Invitrogen, Carlsbad, CA). They showed successful β-catenin expression reduction and HCT116 tumor growth inhibition.51 Sorensen et al. used DOTAP (N-[1-(2,3-dioleoyloxy)]-N-N-N trimethyl ammonium propane) liposomes to make lipoplex for systemic siRNA delivery.52 They showed inhibited exogenous green fluorescent protein expression in liver and spleen via systemic intravenous (i.v.) injection and endogenous tumor necrosis factor expression in macrophages via i.p. injection.
The problem for lipoplex is that the complex is not very stable. Especially when diluted in the blood circulation after injection. The lipoplex is usually made fresh immediately before use. Also, the works mentioned above did not really target siRNA to solid tumors via i.v. injection. To achieve siRNA solid tumor targeted delivery via systemic i.v. injection, a more sophisticated system that can produce a nanoparticle stable long enough before reaching the solid tumor is required.
2.2. LPD
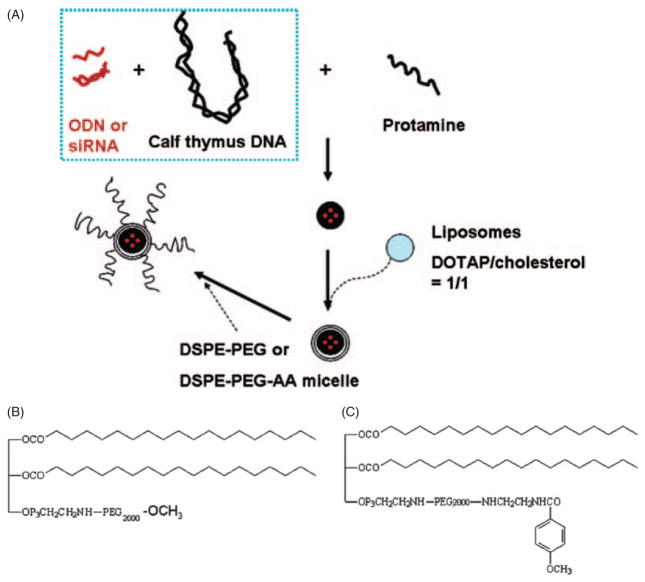
Unlike a liposome that has an aqueous phase inside the particle, LPD nanoparticles consist of a solid core inside of the lipid bilayer.53 The core formation is a self-assembly process driven by charge–charge interaction. In the LPD formulation designed in our lab, we use FDA approved protamine with the help of a high molecular weight calf thymus DNA to condense DNA or siRNA into a solid core.54,55 Protamine is a highly positive charged arginine-rich nuclear protein from salmon sperm. Its natural function is to replace histones in the haploid phase of spermatogenesis and stabilize the DNA. With slightly excess amounts of negatively charged DNA or siRNA to positively charged protamine, the solid core remains negatively charged and thus allows further coating with positively charged DOTAP/cholesterol cationic liposomes. The self-assembled LPD nanoparticles were further modified by post-inserting either DSPE-PEG for surface protection or DSPE-PEG-anisamide for targeting to the sigma receptor (Fig. 1).54–56
Fig. 1.
(A) Preparation of the PEG and PEG-anisamide (PEG-AA) modified LPD. (B) Chemical structures of DSPE-PEG2000 and (C) DSPE-PEG2000-anisamide. Reproduced with permission from [54], S. D. Li and L. Huang, Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol. Pharm. 3, 579 (2006). © 2006.
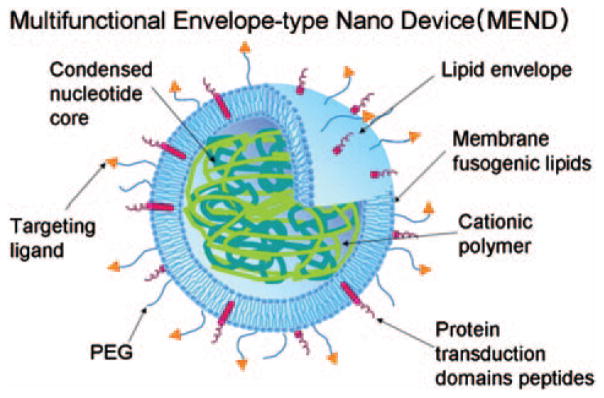
With a similar approach, Harashima group used poly-L-lysine to condense shRNA encoding plasmid DNA or siRNA into their octaarginine modified Multifunctional Envelope type Nano Device (MEND) (Fig. 2). The octaarginine function for cell penetration will be discussed later. In this work, over 80% of luciferase gene expression silencing in HeLa cells was reported.57,58
Fig. 2.
The multifunctional envelope-type nano device (MEND) has a condensed nucleotide core coated with lipid envelope. The lipid envelope contains membrane fusogenic lipids and is further modified with PEG, targeting ligand and protein transduction domain peptides. Reproduced width permission from [58], K. Kogure et al., Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv. Drug Deliv. Rev. 60, 559 (2008). © 2006.
Besides lipoplex and LPD formulation, Zimmermann et al. used a stable nucleic acid lipid particles (SNALP) formulation consisting of 3-N-[(ω-methoxypoly(ethyleneglycol)2000) carbamoyl]-1, 2-dimyristoyloxy-propylamine (PEG-C-DMA), 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane, (DLin DMA), 1,2-distearoyl-sn-glycerol-3-phosphocholine (DSPC) and cholesterol, in a 2:40:10:48 molar ratio to deliver siRNA against apolipoprotein B (apoB) in the liver. In this formulation, the siRNA was encapsulated within the liposomes. More than 80% silencing of apoB mRNA and apoB-100 protein could be achieved with a single 1 mg/kg dose in non-human primate.59
3. FEATURES THAT LEAD TO EFFECTIVE TUMOR TARGETING
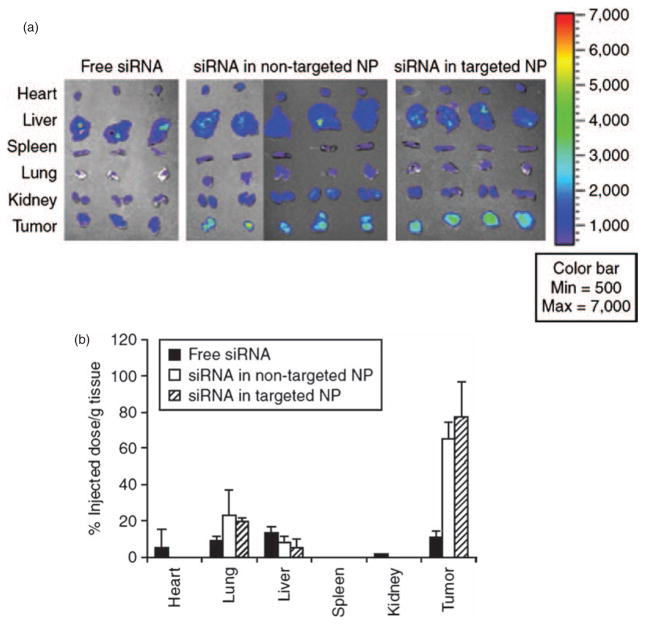
For targeting nanoparticles to solid tumors, there are several important barriers that have to be overcome. The pharmacokinetic and pharmacodynamic profiles of nanoparticles are completely different from conventional small chemical drugs or some protein drugs that are usually eliminated or metabolized by the kidneys, liver, or lungs.60 Nanoparticles are cleared from the blood circulation primarily by the reticuloendothelial system (RES), especially the Kupffer cell in the liver 61 and the macrophages in the spleen. After injecting the nanoparticles into the blood circulation, opsonins such as IgM, IgG, fibronectins, or complement C3 will absorb to their surface. Phagocytic cells will recognize the opsonins and will rapidly and effectively take up the opsonized nanoparticles. The uptake of the nanoparticles by the tumor is a slower and less efficient process. Thus, the RES uptake represents a major “kinetic barrier” for drug delivery to the tumor by nanoparticles. Once the nanoparticles arrive at the tumor, there are other “physical barriers” preventing the cargo drugs from entering the cytoplasm. With appropriate design, self-assembled lipid nanomedicines have been successfully used for siRNA tumor targeting delivery. Li et al.55,62 showed that by taking advantage of the enhanced permeability and retention (EPR) effect of the tumor (see below), PEGylated LPD could accumulate up to 60–80% injected dose per gram of tissue in the H460 lung cancer xenograft model (Fig. 3). With the help of a targeting ligand–anisamide, significant siRNA uptake and almost complete oncogene silencing and significant tumor growth inhibition in vivo were observed. By delivering MDM2, c-myc, and VEGF siRNA combination, significant pulmonary metastasis inhibition in a B16F10 murine melanoma model was also observed (Fig. 4). In the following sections, we will discuss in detail how self-assembled nanoparticles overcome these barriers.
Fig. 3.
Tissue distribution study of siRNA formulated in different LPD formulations. (A) FAM-labeled siRNA was formulated into LPD formulations and i.v. injected into nude mice through tail vein. After 4 hours, tumor and major organs were collected. FAM fluorescence signals were detected by Xenogen IVIS-100 imaging system. (B) Quantitative results of FAM-siRNA tissue distribution. Data =mean ±SD, n = 3–4. NP, nanoparticles. Reproduced with permission from, [55], S. D. Li et al., Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol. Ther. 16, 163 (2008). © 2008.
Fig. 4.
LPD nanoparticles (NP) mediated siRNA delivery for metastatic tumor growth inhibition. Lung–homing B16F10 melanoma cells were i.v. injected into mice. 10 days later, mice were i.v. injected with siRNA twice (0.45 mg/kg, MDM2/c-myc/VEGF = 1:1:1, weight ratio). After six days, the mice were sacrificed and observed for melanoma growth in the lung. Only the mice received siRNA in targeted NP showed significant tumor growth reduction. Reproduced with permission from [62], S. D. Li et al., Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol. Ther. 16, 942 (2008). © 2008.
3.1. EPR Effect, PEGylation, Optimal Size
Tumor cells are rapidly differentiating and growing cells. They require a large amount of nutrient supply. Angiogenesis as induced by growth factors, e.g., VEGF, is important for tumor growth.63 Neovessels in the tumor are usually leaky and not well organized. However, the degree of leakiness is highly tumor dependent and could vary significantly. Matsumura and Maeda64 discovered that due to the leakiness of the vasculature in the solid tumor, macromolecules and colloidal nanoparticles that are too big to penetrate normal blood vessels could penetrate these leaky vasculature and accumulate at the tumor site. This is so called Enhanced Permeability and Retention (EPR) effect. Lacking lymphatic drainage might also contribute to the enhanced retention effect.64–66 To take advantage of the EPR effect, nanoparticles must be within an optimal size range. The optimum diameter should be around 100 nm.67 However, it is dependent on the leakiness of the tumor vasculature.
Nanoparticles need to stay in the blood circulation long enough to overcome the kinetic barrier for extravasating the leaky tumor vasculature. As previously mentioned, the primary elimination mechanism for nanoparticles is the uptake by phagocytic cells after opsonization of the nanoparticles. Modifying the nanoparticle surface with carbohydrate or polyethylene glycol (PEG) is a common strategy for protecting and shielding the surface charge.68 Studies have shown that PEGylated colloids69,70 and stealth liposomes55 could stay in the blood circulation up to 6–10 h in mice and 40 h in humans.71
3.2. Targeting Ligands
EPR effect is important in guiding the nanoparticles to tumor tissue, but EPR effect is not enough for delivering siRNA into the cancer cells. There still remains two physical barriers, cell membrane and endosome membrane, that prohibit siRNA from entering the cytoplasm. Drugs or siRNA that stay outside of the cancer cells are not bio-available and will not show therapeutic effect. Nanoparticles with a structure too stable may stay in the tumor extracellular matrix without releasing payload drugs. For example, a stealth liposomal-cisplatin formulation (SPI-077) accumulated efficiently at the tumor site, but it showed minimum therapeutic effect compared to free cisplatin.72 In order to prompt cancer cells to take up nanoparticles, targeting ligands are needed for triggering receptor mediated endocytosis. There are various types of targeting ligands being used for tumor targeting, including peptides, proteins, antibodies (including Fab, scFv, etc.), aptamers, and small molecular weight ligands, etc.
3.2.1. Peptide Ligands
Binding motifs between ligands and receptors usually involves only several amino acids. Based on this concept, investigators have establish phage display libraries to select special amino acid sequences that show strong binding affinities to tissues, cells, or organs of interests.73 This method has been established and improved for decades. Increasing numbers of peptide ligands have been identified with high affinities against neo-vasculature, various kinds of cancer cells, proteins, receptors, organs and even lymphatic vessels.74 For example, the RGD (Arg-Gly-Asp) motif that shows great binding around 1 nM has been used for targeting various drugs or nanoparticles to either tumor neo-vasculature or cancer cells that express integrin αvβ3 cell surface receptor.75–77 NGR (Asn-Gly-Arg) peptide targeting aminopeptidase N (APN, CD13) is another example for peptide ligand.78 There are also other non-specific cell penetrating peptides for drug delivery systems. They include the famous HIV-1 Tat, Drosophila Antennapedia transcription factor, herpes simplex virus type-1 VP22 transcription factor, or even simple oligoarginine (R8, R9) peptides.79,80
3.2.2. Antibodies (Including Fab, scFv, etc.)
Antibodies (mostly IgG) have been extensively used in biological laboratories due to their high binding affinity to specific epitopes. Humanized antibodies can be used solely or combined with other chemotherapy agents for cancer therapy. Two great examples are the FDA approved anti-Her2/neu monoclonal antibody Herceptin® for breast cancer and the anti-VEGF monoclonal antibody Avastin® for metastatic colorectal cancer. Taking advantage of their binding activity to cancer cell membrane proteins, Herceptin has also been used in targeting liposomes to breast cancer xenografts.81–85
IgG antibodies usually have a molecular weight around 150 kDa. In order to make them smaller to either increase the biological production efficiency or reduce the chances to generate immune response, several smaller versions of antibodies have been adapted.86 For example, removal of the Fc region of the IgG to become the Fab fragment, or combination of the variable regions of both light and heavy chains into a single chain peptide antibody (scFv) is commonly used. Fab and scFv can further be engineered into dimer, trimer, or tetramer forms to provide stronger multivalent binding.
3.2.3. Transferrin
Transferrin is an iron transporting protein that can specifically react with its receptor (Tf receptor) that is expressed in various tissues. Due to the rapid growth of cancer cells, Tf receptors are over-expressed on various kinds of cancers. Anti-Tf receptor antibody87,88 and transferrin have both been used as ligands for targeting liposomes to tumors89,90 or even brain cells.91
3.2.4. Small Molecule Ligands (Folic Acid, Anisamide)
Small molecule ligands that have good binding affinity and specificity are also suitable for tumor targeting, although they are relatively rare. The advantages of using small molecule ligands compared to small peptides, proteins, or antibodies may include: easy synthesis, more tolerant to chemical modification/conjugation, low immunogenicity, and stable for long-term storage.
The vitamin, folic acid, is the high affinity natural ligand for the folate receptor which is over-expressed in a wide range of human cancers, including ovary, lung, breast, endometrium, kidney, and brain cancers. Drugs including protein toxins, chemotherapeutic agents, oligonucleotides, radioimaging/therapeutic agents, MRI contrast agents, liposomes,92 etc. have been modified and targeted with folic acid to various tumors.93–95 Anisamide96 and haloperidol97,98 are good small molecule ligands for cancer cells over-expressing the sigma receptor. They include melanoma, non-small cell lung carcinoma, breast tumors of neural origin, and prostate cancers.97,99–101 The LPD tumor targeting work done in our lab uses mostly anisamide as the targeting ligand.54–56,62
3.2.5. Aptamers
Aptamers are nucleic acid-based ligands ranging in size from 20 to 80 bases (6 to 26 kDa). They were mostly identified through a procedure called “systemic evolution of ligands by exponential enrichment” (SELEX). Due to their unique nucleotide sequences, aptamers fold into unique 3D structures and are able to recognize, with high affinity, various molecules including proteins, sugars, phospholipids, or even small chemicals. One aptamer recognizing VEGF (Macugen®) has been approved by FDA as a therapeutic drug for the treatment of age-related macular degeneration (AMD).102 The aptamer that recognizes the prostate-specific membrane antigen is so far the most successful tumor targeting aptamer. With this aptamer, poly(d,l-lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-b-PEG) nanoparticles,103 aptamer-siRNA chimera,104 and quantum dots105 have been delivered to prostate cancer xenografts.
There are additional ligands under development such as protein scaffolds (e.g., affibody and monobody, which are protein domain-based frameworks).106 The options for tumor targeting ligands will keep growing. Since some targeting ligands may have their biological functions after binding to the receptors, choosing them carefully is important. For example, if the ligand serves as an agonist, it may promote cancer cell growth. It might not be a good ligand for tumor targeting.
3.3. Endosome Escape, Proton Sponge Effect, HII Phase, Ion-Pairs
Getting the nanoparticles endocytosed is not a major issue. The challenge that remains for the siRNA delivery field is how to bring the siRNA out of the endosome. For lipid-based systems, the mechanism through which cationic lipoplex can trigger endosome release has been proposed by Xu and Szoka.107 They proposed that in the endosome, the cationic lipid of the lipoplex can form ion-pairs with the anionic endosomal membrane. By excluding the interfacial water molecules, the ion-pairs destabilize the endosomal membrane. Furthermore, binding of cationic lipid with anionic lipids can form the inverted hexagonal HII phase, proposed by Cullis et al.,108 and leads to membrane fusion and release of cargo. Generally speaking, cationic lipids with smaller and less charged head groups and more bulky acyl/alkyl chains favor the HII phase formation.109 This is probably the reason why DOTAP (containing two C18:1 acyl chains) is used quite often In liposome transfection formulation as a cationic lipid but DSTAP (1,2-distearyl-3-trimethylammonium propane, a close analog of DOTAP but with two C18:0 chains) is not.
Cationic lipid is not the only category of cationic molecules that can form ion-pairs with the endosomal membrane. Protein transduction domains such as HIV Tat, Drosophila Antennapedia transcription factor, herpes simplex virus type-1 VP22 transcription factor, or oligo-arginines (R8 or R9) also show similar activity. It is interesting to know that these peptides all have multiple arginines but not lysines in their sequences. Sakai and Matile110 showed that this is because the charged groups of both the cationic guanidinium group of the poly-arginine and the anionic phosphate group of the endosomal membrane phospholipids contain delocalized electrons. They form stronger charge-charge interaction and hydrogen bonding than the interaction between phospholipid and lysine which does not contain delocalized electrons. Also, protamine used in the LPD formulation mentioned earlier also contains many arginines but not lysine.
Unlike cationic lipids that possess an intrinsic fusogenic property, polyplex formed by polymeric cationic carriers such as polyethyleneimine (PEI)111–114 shows a “proton sponge effect” for endosome destabilization.115–118 The polyplex has many crowded 1°-, 2°- and 3°-amines. Due to the crowding effect, these amines show different pKa within the endosomal pH range and serve as a buffering “proton sponge.” After endocytosis, the pH inside the endosome should drop from physiological pH 7.4 to around pH 5 during the endosome-lysosome maturation process. Due to the presence of the “proton sponge,” the pH would not drop as expected. The ATP-dependent proton-pump on the endosomal membrane would transport extra protons and chloride ions (counter ions), resulting in an increase in the osmotic pressure. Eventually, the endosome would swell and burst due to the large amount of water influx and the polyplex could be released. Polymers containing crowded histidines (imidazoles) or morpholinos also show a similar buffering effect.119 The buffering effect may also play a role in protecting siRNA from degradation during the early endosome to late endosome transport process.
Verkman et al.118 did an elegant piece of work visually showing the accumulation of chloride ions in the endosomes and the release of chloride ions after endosomes burst. However, they did not show exactly that the cargo was efficiently released. The endosome burst and release of chloride ions does not necessary accompany the release of the cargo of the polyplex.
4. ENDOSOME ESCAPE (A PROGRESSING TECHNOLOGY)
Although some self-assembled lipid siRNA tumor targeting nanoparticles already show therapeutic effects in some xenograft mouse models, the endosome escape is still inefficient. Most of the siRNA delivered to tumor cells are still trapped inside the endosome compartment (Li et al. unpublished observation). There might be several reasons for the problem: PEG dilemma, lack of ion-pair formation, not small enough particle size, and insufficient de-assembly of the particles.
4.1. PEG Dilemma
As previous mentioned, PEGylation is the most commonly used method to protect the bare surface of nanoparticles. However, as PEG chains prevent the attachment of opsonins, they also impede the contact between nanoparticles and the target cells. Inside the endosome, PEG may also prohibit the interaction between the cationic lipids of the lipoplex and the anionic endosomal membrane. This is so called “PEG dilemma.”120 Sophisticated designs such as tunable stealth liposomes,121 cleavable PEG-lipid linker,120 or acid labile PEG molecule might help dealing with this dilemma.
4.2. Enhance Endosome Escape
Boeckle et al.122 have done an interesting study on the effect of free PEI in transfection. They compared the gene transfer efficiency between purified PEI-DNA polyplex and un-purified PEI-DNA polyplex mixture (containing unbound PEI molecules). The result shows that without the presence of unbound free PEI, the gene transfer efficiency decreased dramatically. By applying free PEI 4 h after purified PEI-DNA polyplex transfection, they could rescue the gene transfer efficiency, probably by helping the previously transfected purified PEI-DNA polyplex escape from the endosome. This shows that free unbound cationic polymers such as PEI or poly-arginine may play a critical role in disrupting endosomes by forming ion-pairs with the anionic endosomal membrane. Poly-arginine may have stronger activity than PEI due to their ion-pair formation activity described by Sakai and Matile.110 If a nanoparticle formulation could sufficiently release free cationic polymers inside the endosome, there would be a great chance that the siRNA delivery be significantly improved.
4.3. Particle de-Assembly and Smaller Particles
Besides endosome escape, de-assembly of nanoparticles is also essential for sufficient siRNA release. If the structure of the nanoparticles is so stable that they will not release the siRNA inside, the siRNA will not be bio-available. De-assembly may take place either in the endosome or in the cytoplasm after the endosome escapes. Ideally, it should take place in the endosome with the release of endosome disrupting cationic materials, because, even though the endosome is disrupted or burst, the “hole” opened on the endosomal membrane may not be large enough to allow intact nanoparticles to pass through. The LPD nanoparticles established by our lab have a particle size around 120 ~ 150 nm. If the particle size could be smaller (perhaps under 100 nm), not only could they escape from the endosome more efficiently, the required siRNA dose for tumor killing may also decrease. Furthermore, they may reach those tumors with less leaky vasculatures.
5. THERANOSTIC NANOMEDICINES
Tumor imaging is a very powerful clinical technique for tumor detection and therapeutic effect monitoring. With the development of multifunctional nanoparticles that can carry both drugs and imaging agents in the same formulation,123 monitoring cancer therapeutic effects while delivering the therapeutic agents at the same time has become possible. Self-assembled lipids-based nanoparticles could be one of these multifunctional delivery systems for both therapeutic siRNA and a diagnostic agent. This is the so called theranostic smart nanomedicines. Since tumor cells will receive therapeutic siRNA and the diagnostic agent at the same time, cell-specific real-time monitoring of the therapeutic event can be achieved. By monitoring whether the tumor is undergoing apoptosis in the early phase of a given treatment, physicians could decide to either continue the treatment or changing the treatment strategy.
Apoptosis is a complex mechanism that involves various pro-apoptotic and anti-apoptotic molecules inside the cell. There are several methods for detecting apoptosis in vitro now, such as staining for the appearance of phosphatidylserine (PS) using annexin V or detecting the activation of caspase-3 which is an early apoptosis event. Annexin V is a human placental anticoagulant protein with four repeats each containing a putative Ca2+ dependent binding site for PS. PS was originally distributed in the inner plasma membrane. During apoptosis, the asymmetry of the cell membrane is disrupted, which results in the flip-out of PS and can be stained with annexin V as a marker of apoptosis. Several reports have demonstrated that annexin V labeled with indium-111, technetium-99 m, iodine-123, iodine-124 or fluoride-18 can be used for in vivo study.124–128
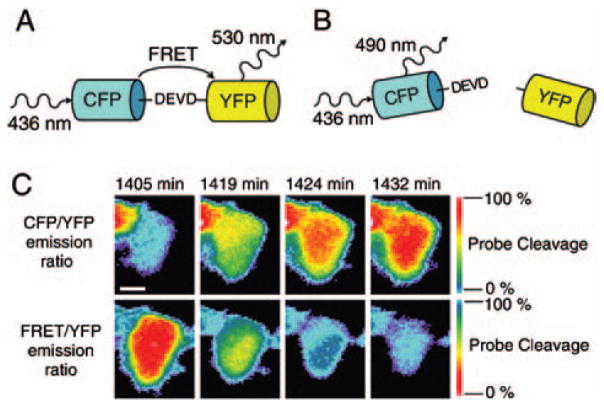
Monitoring caspase-3 activity is another widely applied in vitro apoptosis monitoring method. Several reports and commercially available kits have been designed based on the peptide sequence DEVD (asp-glu-val-asp) found in poly-ADP-ribose-polymerase (PARP), a natural substrate of caspase-3. Linking the DEVD sequence with two fluorescent proteins for fluorescence resonance energy transfer (FRET) (Fig. 5),129 a fluorophore with a quencher,130 or two subunits of luciferase131 as probes for apoptosis have been demonstrated in vitro or in vivo.
Fig. 5.
DEVD FRET probe containing DEVD as the specific cleavage site for caspase-3, Cyan Fluorescent Protein (CFP) as the FRET donor, and Yellow Fluorescent Protein (YFP) as the FRET acceptor. (A) Without the presence of caspase-3, CFP and YFP are linked by DEVD peptide. Upon CFP excitation, the energy was transferred to YFP by FRET. (B) With the presence of caspase-3, the DEVD linker was cleaved and the FRET between CFP and YFP was disappeared. (C) DLD-1 cell expressing DEVD FRET probe was treated with 1 μM staurosporine for inducing apoptosis. The changes of CFP/YFP and FRET/YFP emission ratio indicate the cleavage of the DEVD FRET probe. Reproduced with permission from [129], C. L. O’Connor et al., Intracellular signaling dynamics during apoptosis execution in the presence or absence of X-linked-inhibitor-of-apoptosis-protein. Biochim. Biophys. Acta. 1783, 1903 (2008). © 2008.
Many recent reports demonstrate that liposomes can be loaded with gadolinium (Gd)132–134 or Maghemite (γ-Fe2O3) nanocrystals135 for magnetic resonance imaging, quantum dots136 for optical imaging, or64 Cu for positron emission tomography (PET) imaging.137 Among all the imaging techniques, MRI can provide good resolution and PET is very sensitive with moderate resolution. Both of them have almost no tissue depth limitation, but, so far, they can only show the size and location of the tumor. Monitoring therapeutic efficacy based on the change in tumor size may not be early enough. On the other hand, taking advantage of the FRET with only fluorescence dyes or involving quantum dots as a donor,138 fluorescence imaging is capable of generating a signal that would change its profile. Thus, it has the potential for apoptosis monitoring.
Fluorescence with the wave length within visible range has been routinely used in fluorescence microscopy or intravital microscopy. But when it comes to in vivo imaging of the whole animal without any invasive procedure, high absorbance or scattering of the visible light by the tissues becomes a major issue. Inside the tissue, hemoglobin is the primary absorber for visible lights; water and lipids are the major absorbers of the infrared light. However, the absorbance coefficients of hemoglobin, water, and lipids are small within the near infrared (NIR) range (around 600 ~ 900 nm). Besides, imaging in the NIR range can also reduce the auto-fluorescence background from tissue and thus provide improved signal to noise ratio. Using advanced imaging methods such as fluorescence molecular tomography (FMT) with near infrared fluorophores can provide 7 to 14 cm penetration in tissue,139 which could be useful in clinical practice.
6. CONCLUDING REMARKS
Lipid-based nanomedicines have been known for their high biological compatibilities. Their pharmacokinetics and pharmacodynamics profiles are also well studied. This is a solid foundation for further development of advanced self-assembled lipid nanomedicines. The work done by our lab and other groups has shown that self-assembled lipid nanomedicines can specifically deliver siRNA to tumors in several xenograft and syngeneic models. Although the endosome escape of siRNA cargo still has room for improvement, the existing results are already promising. Finally, theranostic nanomedicine will be a new generation drug with high demand. Self-assembly nanoparticles are capable of carrying various cargos as long as these cargos meet the pre-requirement of the self-assembly process. Another advantage is that the manufacturing process of self-assembled lipid nanomedicines could be easily scaled-up. This also makes self-assembled lipid nanomedicines a versatile multifunctional delivery system for theranostic nanomedicine design.
Acknowledgments
The original work of this lab has been supported by NIH grant CA129825.
Biographies

Yu-Cheng Tseng received his bachelor’s degree in Pharmacy and master’s degree in Biochemistry and Molecular Biology from National Taiwan University. After finishing his military obligation, he worked as a research assistant for fifteen months at Institute of Biomedical Sciences, Academia Sinica in Taiwan before joining the Molecular Pharmaceutics Graduate Program in the School of Pharmacy, University of North Carolina at Chapel Hill. He is interested in molecular imaging and cancer targeted therapy with siRNA and peptide. Yu-Cheng Tseng is currently a Ph.D. graduate student in Dr. Leaf Huang’s lab.

Leaf Huang Ph.D. is the Fred N. Eshelman Distinguished Professor and Chair, Division of Molecular Pharmaceutics in the School of Pharmacy, University of North Carolina at Chapel Hill. Dr. Huang’s research has been in the area of gene therapy and targeted drug delivery. He has pioneered the liposome non-viral vector and has produced the vector for the first clinical trial in 1992. His current work centers on further improvement of liposome vectors for gene transfer in tumor, liver and lung. He also continues research in establishing a ligand targeted delivery system for siRNA and peptides for tumor growth inhibition and for peptide vaccines in treating cervical cancer. He has authored or co-authored more than 300 peer-reviewed papers and more than 120 reviews and book chapters. He is also the inventor or co-inventor of 16 US and foreign patents. In 2004, he received the Alec D. Bangham MD FRS Achievement Award, which is highest honor in the field of liposome research. Dr. Huang has also co-founded 4 biotech start-ups in the past.
References and Notes
- 1.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 2.Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): Reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 3.Lyass O, Uziely B, Ben-Yosef R, Tzemach D, Heshing NI, Lotem M, Brufman G, Gabizon A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer. 2000;89:1037. doi: 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Mayhew E, Bolcsak L, Cavanaugh C, Harmon P, Janoff A, Bernacki RJ. Activity of paclitaxel liposome formulations against human ovarian tumor xenografts. Int J Cancer. 1997;71:103. doi: 10.1002/(sici)1097-0215(19970328)71:1<103::aid-ijc17>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J Control Release. 2000;63:19. doi: 10.1016/s0168-3659(99)00166-2. [DOI] [PubMed] [Google Scholar]
- 6.Daoud SS, Fetouh MI, Giovanella BC, Fetouh MI, Giovanella BC. Antitumor effect of liposome-incorporated camptothecin in human malignant xenografts. Anticancer Drugs. 1995;6:83. doi: 10.1097/00001813-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Zamboni WC, Ramalingam S, Friedland DM, Edwards RP, Stoller RG, Strychor S, Maruca L, Zamboni BA, Belani CP, Ramanathan RK. Phase I and pharmacokinetic study of pegylated liposomal CKD-602 in patients with advanced malignancies. Clin Cancer Res. 2009;15:1466. doi: 10.1158/1078-0432.CCR-08-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litzinger DC, Buiting AM, van Rooijen N, Huang L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim Biophys Acta. 1994;1190:99. doi: 10.1016/0005-2736(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 9.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 11.Loyter, Scangos GA, Ruddle FH. Mechanisms of DNA uptake by mammalian cells: Fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci USA. 1982;79:422. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danielsen M, Northrop JP, Ringold GM. The mouse glucocorticoid receptor: Mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986;5:2513. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalby B, Cates S, Harris A, Ohki EC, Tilkins ML, Price PJ, Ciccarone VC. Advanced transfection with Lipofectamine 2000 reagent: Primary neurons, siRNA, and high-throughput applications. Methods. 2004;33:95. doi: 10.1016/j.ymeth.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Loffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gen Ther. 2006;13:1222. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 15.Chien PY, Wang J, Carbonaro D, Lei S, Miller B, Sheikh S, Ali SM, Ahmad MU, Ahmad I. Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gen Ther. 2005;12:321. doi: 10.1038/sj.cgt.7700793. [DOI] [PubMed] [Google Scholar]
- 16.Pal A, Ahmad A, Khan S, Sakabe I, Zhang C, Kasid UN, Ahmad I. Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int J Oncol. 2005;26:1087. [PubMed] [Google Scholar]
- 17.Gao X, Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991;179:280. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- 18.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, Durham SR, Jeffery PK, Hodson ME, Coutelle C, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 19.Porteous DJ, Dorin JR, McLachlan G, Davidson-Smith H, Davidson H, Stevenson BJ, Carothers AD, Wallace WA, Moralee S, Hoenes C, Kallmeyer G, Michaelis U, Naujoks K, Ho LP, Samways JM, Imrie M, Greening AP, Innes JA. Evidence for safety and efficacy of DOTAP cationic liposome mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Gen Ther. 1997;4:210. doi: 10.1038/sj.gt.3300390. [DOI] [PubMed] [Google Scholar]
- 20.Alton EW, Stern M, Farley R, Jaffe A, Chadwick SL, Phillips J, Davies J, Smith SN, Browning J, Davies MG, Hodson ME, Durham SR, Li D, Jeffery PK, Scallan M, Balfour R, Eastman SJ, Cheng SH, Smith AE, Meeker D, Geddes DM. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: A double-blind placebo-controlled trial. Lancet. 1999;353:947. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- 21.Miller VM, Gouvion CM, Davidson BL, Paulson HL. Targeting Alzheimer’s disease genes with RNA interference: An efficient strategy for silencing mutant alleles. Nucleic Acids Res. 2004;32:661. doi: 10.1093/nar/gkh208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller VM, Xia H, Marrs GL, Gouvion CM, Lee G, Davidson BL, Paulson HL. Allele-specific silencing of dominant disease genes. Proc Natl Acad Sci USA. 2003;100:7195. doi: 10.1073/pnas.1231012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding H, Schwarz DS, Keene A, Affarel B, Fenton L, Xia X, Shi Y, Zamore PD, Xu Z. Selective silencing by RNAi of a dominant allele that causes amyotrophic lateral sclerosis. Aging Cell. 2003;2:209. doi: 10.1046/j.1474-9728.2003.00054.x. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Burchard J, Linsley PS, Aronin N, Xu Z, Zamore PD. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2006;2:e140. doi: 10.1371/journal.pgen.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson BL, Paulson HL. Molecular medicine for the brain: Silencing of disease genes with RNA interference. Lancet Neurol. 2004;3:145. doi: 10.1016/S1474-4422(04)00678-7. [DOI] [PubMed] [Google Scholar]
- 26.McCaffrey AP, Nakai H, Pandey K, Huang Z, Salazar FH, Xu H, Wieland SF, Marion PL, Kay MA. Inhibition of hepatitis B virus in mice by RNA interference. Nat Biotechnol. 2003;21:639. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- 27.Randall G, Grakoui A, Rice CM. Clearance of replicating hepatitis C virus replicon RNAs in cell culture by small interfering RNAs. Proc Natl Acad Sci USA. 2003;100:235. doi: 10.1073/pnas.0235524100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JA, Jayasena S, Khvorova A, Sabatinos S, Rodrigue-Gervais IG, Arya S, Sarangi F, Harris-Brandts M, Beaulieu S, Richardson CD. RNA interference blocks gene expression and RNA synthesis from hepatitis C replicons propagated in human liver cells. Proc Natl Acad Sci USA. 2003;100:2783. doi: 10.1073/pnas.252758799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapadia SB, Brideau-Andersen A, Chisari FV. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc Natl Acad Sci USA. 2003;100:2014. doi: 10.1073/pnas.252783999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCaffrey AP, Meuse L, Pham TT, Conklin DS, Hannon GJ, Kay MA. RNA interference in adult mice. Nature. 2002;418:38. doi: 10.1038/418038a. [DOI] [PubMed] [Google Scholar]
- 31.Song E, Lee SK, Wang J, Ince N, Ouyang N, Min J, Chen J, Shankar P, Lieberman J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat Med. 2003;9:347. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- 32.Cattaneo R, Miest T, Shashkova EV, Barry MA. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat Rev Microbiol. 2008;6:529. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 34.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2008 doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjugate Chem. 2007;18:456. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, Jeong JH, Lee KY, Kim YH, Kim SW, Peipp M, Fey GH, Manjunath N, Shultz LD, Lee SK, Shankar P. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 38.Euliss LE, DuPont JA, Gratton S, DeSimone J. Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev. 2006;35:1095. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- 39.Gratton SE, Williams SS, Napier ME, Pohlhaus PD, Zhou Z, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, Desimone JM. The pursuit of a scalable nanofabrication platform for use in material and life science applications. Acc Chem Res. 2008;41:1685. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gratton SE, Napier ME, Ropp PA, Tian S, DeSimone JM. Microfabricated particles for engineered drug therapies: Elucidation into the mechanisms of cellular internalization of PRINT particles. Pharm Res. 2008;25:2845. doi: 10.1007/s11095-008-9654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly JY, DeSimone JM. Shape-specific, monodisperse nanomolding of protein particles. J Am Chem Soc. 2008;130:5438. doi: 10.1021/ja8014428. [DOI] [PubMed] [Google Scholar]
- 42.Petros RA, Ropp PA, DeSimone JM. Reductively labile PRINT particles for the delivery of doxorubicin to HeLa cells. J Am Chem Soc. 2008;130:5008. doi: 10.1021/ja801436j. [DOI] [PubMed] [Google Scholar]
- 43.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gen Ther. 1999;6:1258. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 44.Stoll SM, Sclimenti CR, Baba EJ, Meuse L, Kay MA, Calos MP. Epstein-Barr virus/human vector provides high-level, long-term expression of alpha1-antitrypsin in mice. Mol Ther. 2001;4:122. doi: 10.1006/mthe.2001.0429. [DOI] [PubMed] [Google Scholar]
- 45.Suda T, Suda K, Liu D. Computer-assisted hydrodynamic gene delivery. Mol Ther. 2008;16:1098. doi: 10.1038/mt.2008.66. [DOI] [PubMed] [Google Scholar]
- 46.Liu F, Huang L. A syringe electrode device for simultaneous injection of DNA and electrotransfer. Mol Ther. 2002;5:323. doi: 10.1006/mthe.2002.0540. [DOI] [PubMed] [Google Scholar]
- 47.Lee RC, River LP, Pan FS, Ji L, Wollmann RL. Surfactant-induced sealing of electropermeabilized skeletal muscle membranes in vivo. Proc Natl Acad Sci USA. 1992;89:4524. doi: 10.1073/pnas.89.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F, Huang L. Electric gene transfer to the liver following systemic administration of plasmid DNA. Gen Ther. 2002;9:1116. doi: 10.1038/sj.gt.3301733. [DOI] [PubMed] [Google Scholar]
- 49.Belyantseva IA. Helios Gene Gun-mediated transfection of the inner ear sensory epithelium. Methods Mol Biol. 2009;493:103. doi: 10.1007/978-1-59745-523-7_7. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gen Ther. 1997;4:891. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 51.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against beta-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291. [PubMed] [Google Scholar]
- 52.Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- 53.Tan Y, Whitmore M, Li S, Frederik P, Huang L. LPD nanoparticles–novel nonviral vector for efficient gene delivery. Methods Mol Med. 2002;69:73. doi: 10.1385/1-59259-141-8:073. [DOI] [PubMed] [Google Scholar]
- 54.Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3:579. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- 55.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16:163. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura Y, Kogure K, Futaki S, Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J Control Release. 2007;119:360. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Kogure K, Akita H, Yamada Y, Harashima H. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv Drug Deliv Rev. 2008;60:559. doi: 10.1016/j.addr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 60.Zamboni WC, Strychor S, Joseph E, Walsh DR, Zamboni BA, Parise RA, Tonda ME, Yu NY, Engbers C, Eiseman JL. Plasma, tumor, and tissue disposition of STEALTH liposomal CKD-602 (S-CKD602) and nonliposomal CKD-602 in mice bearing A375 human melanoma xenografts. Clin Cancer Res. 2007;13:7217. doi: 10.1158/1078-0432.CCR-07-1035. [DOI] [PubMed] [Google Scholar]
- 61.Popielarski SR, Hu-Lieskovan S, French SW, Triche TJ, Davis ME. A nanoparticle-based model delivery system to guide the rational design of gene delivery to the liver. 2. In vitro and in vivo uptake results. Bioconjug Chem. 2005;16:1071. doi: 10.1021/bc0501146. [DOI] [PubMed] [Google Scholar]
- 62.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 65.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 67.Nagayasu A, Uchiyama K, Kiwada H. The size of liposomes: A factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Adv Drug Deliv Rev. 1999;40:75. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 68.Maruyama K, Kennel SJ, Huang L. Lipid composition is important for highly efficient target binding and retention of immunoliposomes. Proc Natl Acad Sci USA. 1990;87:5744. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang G, Yang Z, Lu W, Zhang R, Huang Q, Tian M, Li L, Liang D, Li C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verbaan FJ, Oussoren C, Snel CJ, Crommelin DJ, Hennink WE, Storm G. Steric stabilization of poly(2-(dimethylamino)ethyl methacrylate)-based polyplexes mediates prolonged circulation and tumor targeting in mice. J Gene Med. 2004;6:64. doi: 10.1002/jgm.475. [DOI] [PubMed] [Google Scholar]
- 71.Woodle MC. Surface-modified liposomes: Assessment and characterization for increased stability and prolonged blood circulation. Chem Phys Lipids. 1993;64:1993. doi: 10.1016/0009-3084(93)90069-f. [DOI] [PubMed] [Google Scholar]
- 72.Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Zuhowski EG, Pluim D, Joseph E, Hamburger DR, Working PK, Colbern G, Tonda ME, Potter DM, Eiseman JL. Systemic and tumor disposition of platinum after administration of cisplatin or STEALTH liposomal-cisplatin formulations (SPI-077 and SPI-077 B103) in a preclinical tumor model of melanoma. Cancer Chemother Pharmacol. 2004;53:2004. doi: 10.1007/s00280-003-0719-4. [DOI] [PubMed] [Google Scholar]
- 73.Newton J, Deutscher SL. Phage peptide display. Handb Exp Pharmacol. 2008:145. doi: 10.1007/978-3-540-77496-9_7. [DOI] [PubMed] [Google Scholar]
- 74.Enback J, Laakkonen P. Tumour-homing peptides: Tools for targeting, imaging and destruction. Biochem Soc Trans. 2007;35:2007. doi: 10.1042/BST0350780. [DOI] [PubMed] [Google Scholar]
- 75.Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem. 2007;7:2007. doi: 10.2174/187152007781668706. [DOI] [PubMed] [Google Scholar]
- 76.Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des. 2006;12:2006. doi: 10.2174/138161206777947740. [DOI] [PubMed] [Google Scholar]
- 77.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA. 2008;105:2008. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Corti A, Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann N Y Acad Sci. 2004;1028:2004. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- 79.Stewart KM, Horton KL, Kelley SO. Cell-penetrating peptides as delivery vehicles for biology and medicine. Org Biomol Chem. 2008;6:2008. doi: 10.1039/b719950c. [DOI] [PubMed] [Google Scholar]
- 80.Vives E, Schmidt J, Pelegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta. 2008;1786:2008. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, Shao Y, Nielsen UB, Marks JD, Moore D, Papahadjopoulos D, Benz CC. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:2002. [PubMed] [Google Scholar]
- 82.Wei Q, Kullberg EB, Gedda L. Trastuzumab-conjugated boron-containing liposomes for tumor-cell targeting; development and cellular studies. Int J Oncol. 2003;23:2003. [PubMed] [Google Scholar]
- 83.Yang T, Choi MK, Cui FD, Kim JS, Chung SJ, Shim CK, Kim DD. Preparation and evaluation of paclitaxelloaded PEGylated immunoliposome. J Control Release. 2007;120:2007. doi: 10.1016/j.jconrel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Kikumori T, Kobayashi T, Sawaki M, Imai T. Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes. Breast Cancer Res Treat. 2009;113:2009. doi: 10.1007/s10549-008-9948-x. [DOI] [PubMed] [Google Scholar]
- 85.Kullberg M, Mann K, Owens JL. A two-component drug delivery system using Her-2-targeting thermosensitive liposomes. J Drug Target. 2009;17:2009. doi: 10.1080/10611860802471562. [DOI] [PubMed] [Google Scholar]
- 86.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:2005. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 87.Daniels TR, Ng PP, Delgado T, Lynch MR, Schiller G, Helguera G, Penichet ML. Conjugation of an anti transferrin receptor IgG3-avidin fusion protein with biotinylated saporin results in significant enhancement of its cytotoxicity against malignant hematopoietic cells. Mol Cancer Ther. 2007;6:2007. doi: 10.1158/1535-7163.MCT-07-0330. [DOI] [PubMed] [Google Scholar]
- 88.Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol Cancer Ther. 2002;1:2002. [PubMed] [Google Scholar]
- 89.Cardoso AL, Simoes S, de Almeida LP, Pelisek J, Culmsee C, Wagner E, Pedroso de Lima MC. siRNA delivery by a transferrin-associated lipid-based vector: A non-viral strategy to mediate gene silencing. J Gene Med. 2007;9:2007. doi: 10.1002/jgm.1006. [DOI] [PubMed] [Google Scholar]
- 90.Pirollo KF, Zon G, Rait A, Zhou Q, Yu W, Hogrefe R, Chang EH. Tumor-targeting nanoimmunoliposome complex for short interfering RNA delivery. Hum Gene Ther. 2006;17:2006. doi: 10.1089/hum.2006.17.117. [DOI] [PubMed] [Google Scholar]
- 91.Boado RJ. Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharm Res. 2007;24:1772. doi: 10.1007/s11095-007-9321-5. [DOI] [PubMed] [Google Scholar]
- 92.Yamada A, Taniguchi Y, Kawano K, Honda T, Hattori Y, Maitani Y. Design of folate-linked liposomal doxorubicin to its antitumor effect in mice. Clin Cancer Res. 2008;14:2008. doi: 10.1158/1078-0432.CCR-08-0159. [DOI] [PubMed] [Google Scholar]
- 93.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:2008. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 94.Reddy JA, Allagadda VM, Leamon CP. Targeting therapeutic and imaging agents to folate receptor positive tumors. Curr Pharm Biotechnol. 2005;6:2005. doi: 10.2174/1389201053642376. [DOI] [PubMed] [Google Scholar]
- 95.Hilgenbrink AR, Low PS. Folate receptor-mediated drug targeting: From therapeutics to diagnostics. J Pharm Sci. 2005;94:2005. doi: 10.1002/jps.20457. [DOI] [PubMed] [Google Scholar]
- 96.Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112:2004. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 97.John CS, Vilner BJ, Geyer BC, Moody T, Bowen WD. Targeting sigma receptor-binding benzamides as in vivo diagnostic and therapeutic agents for human prostate tumors. Cancer Res. 1999;59:1999. [PubMed] [Google Scholar]
- 98.Mukherjee A, Prasad TK, Rao NM, Banerjee R. Haloperidol-associated stealth liposomes: A potent carrier for delivering genes to human breast cancer cells. J Biol Chem. 2005;280:2005. doi: 10.1074/jbc.M409723200. [DOI] [PubMed] [Google Scholar]
- 99.Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995;55:1995. [PubMed] [Google Scholar]
- 100.John CS, Bowen WD, Saga T, Kinuya S, Vilner BJ, Baum-gold J, Paik CH, Reba RC, Neumann RD, Varma VM, et al. A malignant melanoma imaging agent: synthesis, characterization, in vitro binding and biodistribution of iodine-125-(2-piperidinylaminoethyl)4-iodobenzamide. J Nucl Med. 1993;34:1993. [PubMed] [Google Scholar]
- 101.John CS, Vilner BJ, Gulden ME, Efange SM, Langason RB, Moody TW, Bowen WD. Synthesis and pharmacological characterization of 4-[125I]-N-(N-benzylpiperidin-4-yl)-4-iodobenzamide: A high affinity sigma receptor ligand for potential imaging of breast cancer. Cancer Res. 1995;55:1995. [PubMed] [Google Scholar]
- 102.Lee JH, Canny MD, De Erkenez A, Krilleke D, Ng YS, Shima DT, Pardi A, Jucker F. A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF 165. Proc Natl Acad Sci USA. 2005;102:2005. doi: 10.1073/pnas.0509069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28:2007. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:2006. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 105.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett. 2007;7:2007. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 106.Nuttall SD, Walsh RB. Display scaffolds: Protein engineering for novel therapeutics. Curr Opin Pharmacol. 2008;8:2008. doi: 10.1016/j.coph.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 107.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:1996. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 108.Hafez IM, Cullis PR. Roles of lipid polymorphism in intra-cellular delivery. Adv Drug Deliv Rev. 2001;47:2001. doi: 10.1016/s0169-409x(01)00103-x. [DOI] [PubMed] [Google Scholar]
- 109.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:2001. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 110.Sakai N, Matile S. Anion-mediated transfer of polyarginine across liquid and bilayer membranes. J Am Chem Soc. 2003;125:2003. doi: 10.1021/ja037601l. [DOI] [PubMed] [Google Scholar]
- 111.Werth S, Urban-Klein B, Dai L, Hobel S, Grzelinski M, Bakowsky U, Czubayko F, Aigner A. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release. 2006;112:2006. doi: 10.1016/j.jconrel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 112.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:2005. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 113.Grayson AC, Doody AM, Putnam D. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm Res. 2006;23:2006. doi: 10.1007/s11095-006-9009-2. [DOI] [PubMed] [Google Scholar]
- 114.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA. 2005;102:2005. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc Natl Acad Sci USA. 1995;92:1995. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thomas M, Klibanov AM. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2002;99:2002. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:2005. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 118.Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:2003. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 119.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9:E18. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hatakeyama H, Akita H, Kogure K, Oishi M, Nagasaki Y, Kihira Y, Ueno M, Kobayashi H, Kikuchi H, Harashima H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14:68. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 121.Webb MS, Saxon D, Wong FM, Lim HJ, Wang Z, Bally MB, Choi LS, Cullis PR, Mayer LD. Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): Effects on the pharmacokinetics of liposomal vincristine. Biochim Biophys Acta. 1998;1372:1998. doi: 10.1016/s0005-2736(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 122.Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6:2004. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 123.Ferrari M. Cancer nanotechnology: Opportunities and challenges. Nat Rev Cancer. 2005;5:2005. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 124.Blankenberg FG. In vivo detection of apoptosis. J Nucl Med. 2008;49:81S. doi: 10.2967/jnumed.107.045898. [DOI] [PubMed] [Google Scholar]
- 125.Thimister PW, Hofstra L, Liem IH, Boersma HH, Kemerink G, Reutelingsperger CP, Heidendal GA. In vivo detection of cell death in the area at risk in acute myocardial infarction. J Nucl Med. 2003;44:2003. [PubMed] [Google Scholar]
- 126.Narula J, Acio ER, Narula N, Samuels LE, Fyfe B, Wood D, Fitzpatrick JM, Raghunath PN, Tomaszewski JE, Kelly C, Steinmetz N, Green A, Tait JF, Leppo J, Blankenberg FG, Jain D, Strauss HW. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7:2001. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]
- 127.van de Wiele C, Lahorte C, Vermeersch H, Loose D, Mervillie K, Steinmetz ND, Vanderheyden JL, Cuvelier CA, Slegers G, Dierck RA. Quantitative tumor apoptosis imaging using technetium-99m-HYNIC annexin V single photon emission computed tomography. J Clin Oncol. 2003;21:2003. doi: 10.1200/JCO.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 128.Wong E, Kumar V, Howman-Giles RB, Vanderheyden JL. Imaging of Therapy-Induced Apoptosis Using (99m) Tc-HYNIC-Annexin V in Thymoma Tumor-Bearing Mice. Cancer Biother Radiopharm. 2008 doi: 10.1089/cbr.2008.0504. [DOI] [PubMed] [Google Scholar]
- 129.O’Connor CL, Anguissola S, Huber HJ, Dussmann H, Prehn JH, Rehm M. Intracellular signaling dynamics during apoptosis execution in the presence or absence of X-linked-inhibitor-of-apoptosis-protein. Biochim Biophys Acta. 2008;1783:2008. doi: 10.1016/j.bbamcr.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 130.Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J Med Chem. 2005;48:2005. doi: 10.1021/jm050008p. [DOI] [PubMed] [Google Scholar]
- 131.Coppola JM, Ross BD, Rehemtulla A. Noninvasive imaging of apoptosis and its application in cancer therapeutics. Clin Cancer Res. 2008;14:2008. doi: 10.1158/1078-0432.CCR-07-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Esposito G, Crich SG, Aime S. Efficient cellular labeling by CD44 receptor-mediated uptake of cationic liposomes functionalized with hyaluronic acid and loaded with MRI contrast agents. Chem Med Chem. 2008;3:2008. doi: 10.1002/cmdc.200800234. [DOI] [PubMed] [Google Scholar]
- 133.Maiseyeu A, Mihai G, Kampfrath T, Simonetti OP, Sen CK, Roy S, Rajagopalan S, Parthasarathy S. Gadolinium containing phosphatidylserine liposomes for molecular imaging of atherosclerosis. J Lipid Res. 2008 doi: 10.1194/jlr.M800405-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Terreno E, Castelli DD, Cabella C, Dastru W, Sanino A, Stancanello J, Tei L, Aime S. Paramagnetic liposomes as innovative contrast agents for magnetic resonance (MR) molecular imaging applications. Chem Biodivers. 2008;5:2008. doi: 10.1002/cbdv.200890178. [DOI] [PubMed] [Google Scholar]
- 135.Martina MS, Fortin JP, Menager C, Clement O, Barratt G, Grabielle-Madelmont C, Gazeau F, Cabuil V, Lesieur S. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc. 2005;127:2005. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 136.Kostarelos K. Tumor Targeting of Functionalized Quantum Dot-Liposome Hybrids by Intravenous Administration. Mol Pharm. 2009 doi: 10.1021/mp800187d. [DOI] [PubMed] [Google Scholar]
- 137.Seo JW, Zhang H, Kukis DL, Meares CF, Ferrara KW. A novel method to label preformed liposomes with 64Cu for positron emission tomography (PET) imaging. Bioconjug Chem. 2008;19:2008. doi: 10.1021/bc8002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith AM, Duan H, Mohs AM, Nie S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev. 2008;60:2008. doi: 10.1016/j.addr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]