Abstract
Resilience is the ability to adequately adapt and respond to homeostatic perturbations. Although resilience has been associated with positive health outcomes, the neuro-biological basis of resilience is poorly understood. The aim of the study was to identify associations between regional brain morphology and trait resilience with a focus on resilience-related morphological differences in brain regions involved in cortico-limbic inhibition. The relationship between resilience and measures of affect were also investigated. Forty-eight healthy subjects completed structural MRI scans. Self-reported resilience was measured using the Connor and Davidson Resilience Scale. Segmentation and regional parcellation of images was performed to yield a total of 165 regions. Gray matter volume (GMV), cortical thickness, surface area, and mean curvature were calculated for each region. Regression models were used to identify associations between morphology of regions belonging to executive control and emotional arousal brain networks and trait resilience (total and subscales) while controlling for age, sex, and total GMV. Correlations were also conducted between resilience scores and affect scores. Significant associations were found between GM changes in hypothesized brain regions (subparietal sulcus, intraparietal sulcus, amygdala, anterior mid cingulate cortex, and subgenual cingulate cortex) and resilience scores. There were significant positive correlations between resilience and positive affect and negative correlations with negative affect. Resilience was associated with brain morphology of regions involved in cognitive and affective processes related to cortico-limbic inhibition. Brain signatures associated with resilience may be a biomarker of vulnerability to disease.
Keywords: resilience, brain morphology, executive control network, emotional arousal network, corto-limbic inhibition, cognitive-affective processes
Graphical abstract
The relationship between resilience measures and morphology of regions involved in cognitive and affective processes are demonstrated, consistent with previous suggestions that individuals with low resilience may have compromised cortico-limbic inhibition, increasing their vulnerability to stress related morbidity. Higher resilient individuals have a better ability to bounce back from adverse events, have greater emotional and cognitive control, and are more persistent.
INTRODUCTION
Resilience is the process of adapting and coping with stress and adversity (Russo et al. 2012; Southwick and Charney 2012). Compromised adaptive responses to adversity (low resilience) can increase vulnerability to illness (Karatsoreos and McEwen 2013b). Exposure to trauma can lead to stress-related pathologies such as anxiety, depression, post-traumatic stress disorders (PTSD) (Sexton et al. 2015), and physical illnesses (Gupta et al. 2014), but not everyone exposed to adversity develops these disorders (Iacoviello and Charney 2014; Yehuda and LeDoux 2007). Multiple factors contribute towards the development of disease after exposure to adversity, including timing of exposure relative to critical periods of neurodevelopment such as prenatal and adolescence, developmental history, severity and number of traumatic events, social support, cognitive flexibility, locus of control, and environment (Kalisch et al. 2014; Karatsoreos and McEwen 2013a; Wu et al. 2013). However, the definition of resilience as a lack of disease following adversity limits the scope of resilience to an observable phenomenon after some acute or chronic adverse event, and focuses on an active process or mechanism (Kalisch et al. 2015a).
A recent paradigm shift in operationalizing resilience has moved away from the focus on the non-emergence of pathology or symptoms after exposure to adversity, to include “resilient-conductive” factors such as personality traits, confidence, flexibility, optimism, or emotional lability, which can help promote positive subjective appraisal, negotiation, adaptation, or management of adverse situations with increased coping (Kalisch et al. 2015b; Windle et al. 2011). Psychosocial and spiritual factors play an important role in enhancing resilience (Haase et al. 2014b; Johnson et al. 2014; Pietrzak et al. 2010; Southwick et al. 2014; Southwick and Charney 2012). Individual differences, beyond personality traits such as subjective well-being (both hedonic or eudaimonic) could also be protective factors against adversity (Di Fabio and Palazzeschi 2015). Hedonic well-being refers to cognitive evaluation of life satisfaction and positive affect, whereas eudaimonic well-being is related to the determination of life-meaning and self-actualization (Di Fabio and Palazzeschi 2015); resilience is related to both types of well-being (He et al. 2013; Smith and Hollinger-Smith 2015). Positive affect is thought to facilitate resilience by broadening one’s attention and coping abilities (Fredrickson and Branigan 2005), and by decreasing susceptibility to disease through increased vagal control (Oveis et al. 2009). The overlap between measures of positive affect and resilience has also been observed in various conditions such as chronic pain (Montpetit et al. 2010; Strand et al. 2006; Xing and Sun 2013; Zautra et al. 2005). Positive (or negative) affect is typically conceptualized as a state variable that can both promote resilience to an incoming stressor as well as index resilience in terms of ability to respond to a stressor (Lu et al. 2014). Resilience as a trait measure can be differentiated from current affect levels in that it should be predictive of affective and biological responses to a range of stressors (Lu et al. 2014; Robinson et al. 2014; Schilling and Diehl 2014). In this context, although an individual’s resilience can be evidenced by examining the physical or mental state following specific stressors, it can also be assessed directly as a general trait based on self-report responses to common stressors (McEwen 2016; Osorio et al. 2016). Moreover, the use of measures that tap into these resilience-related conductive traits, offer the opportunity to gain insights into broader aspects of resilience not captured by the traditional model focused on the degree of psychopathology following adversity (Windle et al. 2011).
The brain is continuously adapting to perturbations in bodily homeostasis. To date, little information exists regarding the neurobiology of resilience in the absence of disease or traumatic adversity (van der Werff et al. 2013c; Wu et al. 2013). Resilience is likely determined by adaptive responses in brain systems that regulate behaviors associated with coping, fear, attention, cognitive flexibility, and emotional regulation (Baratta et al. 2013; Feder et al. 2009; Fleshner et al. 2011; Russo et al. 2012; van der Werff et al. 2013a). High resilience individuals display more effective modulation of brain circuits involved in emotion and fear (Southwick and Charney 2012).
A few neuroimaging studies have investigated the response to adversity as a “proxy” of resilience, and have reported resilience-related differences in brain structure (DeYoung et al. 2010), responses to acute experimental paradigms (Daniels et al. 2012; Peres et al. 2011; Reynaud et al. 2013; Vythilingam et al. 2009; Waugh et al. 2008), and in resting-state brain activity (Kunisato et al. 2011). These studies demonstrated impaired cortico-limbic inhibition in response to trauma, suggesting that executive control and emotional arousal networks may play a critical role in the mediation of low resilience or vulnerability to disease. Identification of neurobiological endophenotypes associated with resilience may be a critical first step in the identification of individuals with increased vulnerability to develop diseases.
In order to avoid confounding effects of trauma and illness, the main aim of this study was to identify self-reported resilience-related brain morphological signatures. The hypotheses of the study were: 1) Resilience-related structural differences will be observed in brain regions belonging to the executive control and emotional arousal networks, and in regions involved in cortico-limbic inhibition. 2) Regional brain morphology will show differential associations with specific sub-dimensions of resilience. As a secondary aim, the relationship between resilience and positive affect was investigated in order to discover how resilience-related structural differences differ from those related to current positive/negative affect. The hypothesis for this aim is that some areas will overlap between resilience and positive affect but that resilience specific alterations will also be evident.
MATERIALS AND METHODS
Subjects
A total of 48 male and female healthy subjects were recruited from the community through advertisements. Subjects were screened by medical examination for absence of significant health conditions. Exclusionary criteria for all subjects included pregnancy or lactation, substance abuse, tobacco dependence (smoked half a package of cigarettes or more daily), current or past psychiatric illness, extreme strenuous exercise (exercise more than one hour per day), and major medical or neurological conditions. In addition, subjects with current use of analgesic drugs (including narcotics, opioids, and α2-δ ligands) were excluded. Subjects did not meet any criteria for current anxiety or depressive symptoms as measured by the Hospital Anxiety and Depression (HAD) scale, a 14-item self-report instrument (Zigmond and Snaith 1983). All subjects were right-handed and female subjects were premenopausal as confirmed by self-report. Due to the modulation of brain activity, structure, and function by gonadal steroid hormones (Comasco and Sundstrom-Poromaa 2015), females were scanned during the follicular phase of their menstrual cycle as determined by the number of days since last menstrual period. All procedures were performed after approval from the University Institutional Review Board and all subjects provided written informed consent in accordance with the Declaration of Helsinki.
Behavioral Measures
Questionnaires were administered before MRI scanning
The most widely used measure of trait resilience is the Connor-Davidson Resilience (CD-RISC), a 25-item instrument (Connor and Davidson 2003d) which measures resilience as the ability to cope with stress and adversity. The total CD-RISC score ranges from 0 to 100 and also yields individual factor subscale scores (both 4- and 5-factor solutions have been described and used in various studies) (Connor and Davidson 2003a; Lamond et al. 2008). Subscales for the 5factor solution represent the following: self-efficacy, high standards, and tenacity (Factor 1); emotional and cognitive control under pressure, trust in one’s intuition (Factor 2); adaptability/ability to bounce back (Factor 3); sense of control of one’s life (Factor 4); and faith (Factor 5) (Connor and Davidson 2003a). Specific items on the Factor 1 subscale (8 items) measures an individual’s approach to challenges, ability to not give up when things seem hopeless, ability to achieve goals, and pride in achievements. Specific items on the Factor 2 subscale (7 items) measure an individual’s ability to think clearly and focus under pressure, ability to manage unpleasant feelings, and the ability to see the good in bad situations. Specific items on the Factor 3 subscale (5 items) include the ability to deal with life as it comes, the ability to adapt to change, and ability to recover after illness or hardship. Specific items on the Factor 4 subscale (3 items) measure the presence of a support system, control in life, and a sense of confidence. Specific items on the Factor 5 subscale (2 items) measure whether an individual believes that things happen for a reason, and that fate or God plays a role in their life (Connor and Davidson 2003a). For this analysis, we used a 4-factor structure, consisting of the original Factors 1–3 (persistence, emotional and cognitive control under pressure, and the ability to bounce back), and combining Factors 4 and 5 (control of one’s life and faith) due to their similarity in previous factor analyses (Bitsika et al. 2010; Lamond et al. 2008; Singh and Yu 2010). The CD-RISC has been successfully used in the general healthy population, in clinical trial studies, in psychiatric outpatients, and in patients with medical conditions, showing good psychometric properties such as internal consistency, test-retest reliability, and convergent and divergent validity (http://www.cd-risc.com/). Studies have also shown that resilience scores on the CD-RISC are moderately negatively associated with early adverse life events and with current psychiatric symptoms (Campbell-Sills et al. 2006). Treatment effects have also been shown in PTSD patients with improved resilience scores on the CD-RISC (Davidson et al. 2005).
The Positive Affect Negative Affect (PANAS) was used to measure both positive (attentive, interested, alert, excited, enthusiastic, inspired, proud, determined, strong, and active) and negative affect (distressed, upset-distressed, hostile, irritable-angry, scared, afraid-fearful, ashamed, guilty, nervous, and jittery) (Crawford and Henry 2004; Watson et al. 1988).
History of childhood traumatic events was measured using the Early Trauma Inventory Self Report (ETI-SR), a 27-item questionnaire that investigated four areas of traumatic and adverse life events that occur before the age of 18 years: general trauma (11 items), physical punishment (5 items), emotional abuse (5 items), and sexual abuse (6 items) (Bremner et al. 2007b). In addition to calculating subscale scores, the number of items receiving a positive response was calculated for each subject, resulting in a total ETI-SR score (range 0–27). The ETI-SR has been found to have good internal consistency (Cronbach α=.70) (Bremner et al. 2007a).
Structural MRI Acquisition
High-resolution T1-weighted brain images were acquired using a Siemens 3 Tesla Trio with a magnetization-prepared rapid gradient echo (MP-RAGE) sequence with the following scanning parameters: TR = 2200 ms, TE = 3.26 ms, flip angle = 9°, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 1.0 × 1.0 × 1.0 mm voxel size.
Structural MRI Preprocessing
Segmentation and regional parcellation of the T1-image was performed using Freesurfer according to the nomenclature described in Destrieux et al. (Destrieux et al. 2010). Based on the Destrieux and Harvard-Oxford atlases, 74 bilateral cortical structures, 7 subcortical structures, the cerebellum and the brainstem (a midline structure) were processed and parcelled out for a complete set of 165 regions for the entire brain. Four representative morphological measures were computed for each cortical parcellation: grey matter volume (GMV), surface area (SA), cortical thickness (CT), and mean curvature (MC). Using Freesurfer Freeview (http://surfer.nmr.mgh.harvard.edu/) each brain reconstruction was visually inspected and assessed based on various quality control measures including correct segmentation of gray and white matter and cerebral spinal fluid, and the absence of artifacts and anomalies such as motion, clipping, and atrophy. Scans with such errors were omitted from further analyses.
Region of Interest Analysis
We selected regions of interest (ROI) based on previous studies examining resilience to psychological trauma (Baratta et al. 2013; Franklin et al. 2012; Ganzel et al. 2008; Haase et al. 2014a; Johnson et al. 2014; Kasai et al. 2008; Thom et al. 2014; Wu et al. 2013). These ROIs were also selected based on review articles regarding models of resilience (Southwick et al. 2014; Southwick and Charney 2012). These ROIs included regions in the executive control network: dorsal lateral prefrontal cortex (dlPFC), dorsal medial prefrontal cortex (dmPFC), and posterior parietal cortex (PPC), and the emotional arousal network: cingulate subregions (anterior cingulate cortex [ACC], anterior mid cingulate cortex [aMCC], subgenual anterior cingulate cortex [sgACC]), amygdala, hippocampus (Figure 1, Table 1).
Figure 1. Regions in the Salience, Executive Control and Emotional Arousal Networks.
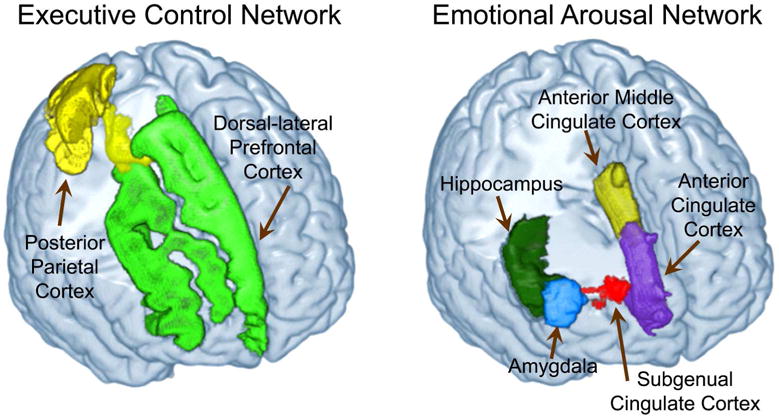
Executive Control Network: Dorsal-lateral Prefrontal Cortex (dlPFC), Posterior Parietal Cortex (PPC)
Emotional Arousal Network: Anterior Cingulate Cortex (ACC), Anterior Mid-Cingulate Cortex (aMCC), Subgenual Anterior Cingulate Cortex (sgACC), Amygdala (AMYG), Hippocampus (Hipp)
Table 1.
List of brain regions of interest and their representative Destrieux regions
| Region | Full Destrieux Name | Destrieux Label |
|---|---|---|
| Emotional Arousal Network | ||
| Anterior Cingulate Cortex (ACC) | Anterior part of the cingulate gyrus and sulcus | ACgG |
| Subgenual Anterior Cingulate (sgACC) | Subcallosal area, subcallosal gyrus | SbCag |
| Middle Anterior Cingulate (aMCC) | Middle-anterior part of the cingulate gyrus and sulcus | MACgG |
| Amygdala (AMYG) | Amygdala | Amg |
| Hippocampus (Hipp) | Hippocampus | Hip |
| Executive Control Network | ||
| Dorsal Lateral Prefrontal Cortex (dlPFC) | Middle frontal gyrus (F2) | MFG |
| Inferior Frontal Sulcus | InfFS | |
| Posterior Parietal Cortex (PPC) | Superior Parietal Lobule | SupPL |
| Parieto-occipital sulcus (or fissure) | Pocs | |
| Subparietal Sulcus | SbPS | |
| Intraparietal sulcus (interparietal sulcus) and transverse parietal sulci | IntPS/TrPS | |
Executive Control Network: Dorsal-lateral Prefrontal Cortex (dlPFC), Posterior Parietal Cortex (PPC)
Emotional Arousal Network: Anterior Cingulate Cortex (ACC), Anterior Mid-Cingulate Cortex (aMCC), Subgenual Anterior Cingulate Cortex (sgACC), Amygdala (AMYG), Hippocampus (Hipp)
Statistical Analysis
Subject Characteristics
A summary of the various demographic and behavioral measures (age, Positive Affect and Negative Affect (PANAS-current scores) and CD-RISC total and subscale scores were evaluated. For descriptive purposes, the percentage of subjects scoring in the high resilience vs. low resilience range (cut off score ≥ 80 on the total score) was also calculated, based on the average score for the general United States population (http://www.cd-risc.com/).
Regional Anatomical Changes Using ROI Analysis
1. Resilience-related differences in the four structural metrics of each ROI (grey matter volume, cortical thickness, mean curvature, and surface area) were evaluated using a linear regression model against the resilience total score and each resilience subscale score (Factor 1: persistence, Factor 2: emotional and cognitive control under pressure, Factor 3: ability to bounce back, and Factor 4: control of one’s life and faith), for a total of 5 models. Age, sex, and total gray matter volume (TGMV) were included as covariates.
If there was a significant main effect for a ROI within the executive control network (Table 3) or the emotional arousal network (Table 4) for any of the resilience measures, then main effects for “age” or “sex” were also tested. If a significant sex main effect was found, it would suggest differences in brain structures between males and females. In order to assess if age and/or sex moderated the relationship between resilience and brain structure, secondary analyses were run to test for interaction effects of resilience with age and sex. If a significant sex*resilience score interaction effect was found, it would suggest that the association between resilience and the ROI brain structure differed between males and females. If a significant age*resilience score interaction effect was found, it would suggest that the association between resilience and the ROI brain structure differed by age. In order to clarify the effect of age on brain regions, the dataset was stratified into two groups, those individuals below 25 years old (N=26) and those individuals above 25 years old (N=22) and linear regressions were rerun for only the aMCC and the personal/spiritual meaning resilience subscale, which showed significant age*resilience-related differences in the previous analysis. In addition, we investigated the effect of positive affect as measured by the PANAS on morphological measurements.
TABLE 3.
Significant Effects of Resilience in the Executive Control Network
| EXECUTIVE CONTROL NETWORK
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Hemisphere | CD-RISC (DF = 43)
|
Factor 1 (DF = 43)
|
Factor 2 (DF = 41) |
Factor 3 (DF = 43)
|
Factor 4 (DF = 42)
|
||||||||||||||||||||
| B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | ||
| SbPS_V | Left | 29.120 | 12.040 | 0.293 | 2.419 | 0.002 | ||||||||||||||||||||
| Age | −0.369 | 7.375 | −0.006 | −0.050 | 0.960 | |||||||||||||||||||||
| Sex | 7.899 | 109.500 | 0.009 | 0.072 | 0.943 | |||||||||||||||||||||
| TGMV | 0.004 | 0.001 | 0.567 | 4.676 | 0.00003 | |||||||||||||||||||||
| Right | 28.580 | 12.610 | 0.303 | 2.266 | 0.003 | 39.340 | 19.030 | 0.279 | 2.067 | 0.005 | ||||||||||||||||
| Age | −5.678 | 7.727 | −0.099 | −0.735 | 0.466 | −7.513 | 7.790 | −0.131 | −0.964 | 0.341 | ||||||||||||||||
| Sex | 12.120 | 114.700 | 0.014 | 0.106 | 0.916 | 6.146 | 115.600 | 0.007 | 0.053 | 0.958 | ||||||||||||||||
| TGMV | 0.003 | 0.001 | 0.424 | 3.178 | 0.003 | 0.003 | 0.001 | 0.388 | 2.871 | 0.006 | ||||||||||||||||
| SbPS_SA | Left | 10.230 | 4.796 | 0.262 | 2.132 | 0.004 | ||||||||||||||||||||
| Age | −2.181 | 2.938 | −0.092 | −0.742 | 0.462 | |||||||||||||||||||||
| Sex | 21.620 | 43.620 | 0.061 | 0.496 | 0.623 | |||||||||||||||||||||
| TGMV | 0.002 | 0.0003 | 0.570 | 4.650 | 0.00003 | |||||||||||||||||||||
| Right | 4.174 | 1.926 | 0.294 | 2.167 | 0.006 | 13.680 | 5.173 | 0.348 | 2.645 | 0.002 | 17.390 | 7.910 | 0.296 | 2.198 | 0.002 | |||||||||||
| Age | −2.580 | 3.259 | −0.108 | −0.792 | 0.433 | −2.880 | 3.169 | −0.121 | −0.909 | 0.369 | −3.726 | 3.240 | −0.156 | −1.150 | 0.257 | |||||||||||
| Sex | 9.819 | 48.420 | 0.028 | 0.203 | 0.840 | 9.867 | 47.050 | 0.028 | 0.210 | 0.835 | 5.715 | 48.020 | 0.016 | 0.119 | 0.906 | |||||||||||
| TGMV | 0.001 | 0.0004 | 0.402 | 2.988 | 0.005 | 0.001 | 0.0004 | 0.416 | 3.167 | 0.003 | 0.001 | 0.0004 | 0.376 | 2.792 | 0.008 | |||||||||||
| IntPS/TrPS_SA | Left | −27.120 | 13.310 | −0.251 | −2.038 | 0.005 | ||||||||||||||||||||
| Age | −0.769 | 5.711 | −0.016 | −0.135 | 0.894 | |||||||||||||||||||||
| Sex | 214.100 | 84.800 | 0.304 | 2.524 | 0.015 | |||||||||||||||||||||
| TGMV | 0.002 | 0.001 | 0.452 | 3.771 | 0.001 | |||||||||||||||||||||
| IntPS/TrPS_V | Left | −74.050 | 30.410 | −0.279 | −2.435 | 0.004 | ||||||||||||||||||||
| Age | 6.306 | 13.050 | 0.055 | 0.483 | 0.632 | |||||||||||||||||||||
| Sex | 331.600 | 193.800 | 0.192 | 1.711 | 0.095 | |||||||||||||||||||||
| TGMV | 0.007 | 0.001 | 0.545 | 4.873 | 0.00002 | |||||||||||||||||||||
Represents regions in the Executive Control Network that show significant main effects with CD-RISC total score and subscales as well as main effects due to age, sex and TGMV
Abbreviations: ROI, region of interest; TGMV, total gray matter volume; SA, surface area; CT, cortical thickness; MC, mean curvature; V, volume; B, unstandardized beta weight; β, standardized beta weight; DF, degrees of freedom; CD-RISC, Connor-Davidson Resilience Scale; Factor 1: Persistence; Factor 2: Intuition-Trust; Factor 3: Bounce Back; Factor 4: Personal/Spiritual Meaning
Regions: SbPS = Subparietal Sulcus; IntPS/TrPS = Intraparietal sulcus(interparietal sulcus) and transverse parietal sulci
Significance: Significance was considered at p < .05
TABLE 4.
Significant Effects of Resilience in the Emotional Arousal Network
| EXECUTIVE CONTROL NETWORK
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Hemisphere | CD-RISC (DF = 43) |
Factor 1 (DF = 43)
|
Factor 2 (DF = 41) |
Factor 3 (DF = 43)
|
Factor 4 (DF = 42)
|
||||||||||||||||||||
| B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | B | se | β | t | p | ||
| ACgC_CT | Right | 0.0241 | 0.011 | 0.243 | 0.701 | 0.009 | ||||||||||||||||||||
| Age | 7.320 | 3.401 | 0.241 | 2.152 | 0.037 | |||||||||||||||||||||
| Sex | 21.040 | 50.500 | 0.046 | 0.417 | 0.679 | |||||||||||||||||||||
| TGMV | 0.002 | 0.0004 | 0.703 | 6.638 | 0.0000002 | |||||||||||||||||||||
| SbCag_SA | Left | −9.820 | 4.713 | −0.281 | −2.083 | 0.004 | ||||||||||||||||||||
| Age | 4.621 | 1.931 | 0.325 | 2.393 | 0.021 | |||||||||||||||||||||
| Sex | −7.442 | 28.620 | −0.035 | −0.260 | 0.796 | |||||||||||||||||||||
| TGMV | 0.0004 | 0.0002 | 0.246 | 1.827 | 0.075 | |||||||||||||||||||||
| MACgG_CT | Right | −0.018 | 0.007 | −0.357 | −2.529 | 0.005 | ||||||||||||||||||||
| Age | 0.003 | 0.003 | 0.153 | 1.092 | 0.281 | |||||||||||||||||||||
| Sex | −0.090 | 0.046 | −0.271 | −1.958 | 0.057 | |||||||||||||||||||||
| TGMV | 0.0000006 | 0.0000004 | −0.229 | −1.662 | 0.104 | |||||||||||||||||||||
| Amg_V | Right | 12.490 | 6.092 | 0.235 | 2.050 | 0.004 | ||||||||||||||||||||
| Age | −4.331 | 3.732 | −0.134 | −1.160 | 0.252 | |||||||||||||||||||||
| Sex | 106.100 | 55.410 | 0.221 | 1.914 | 0.062 | |||||||||||||||||||||
| TGMV | 0.002 | 0.0004 | 0.612 | 5.346 | 0.000003 | |||||||||||||||||||||
Represents regions in the Emotional Arousal Network that show significant main effects with CD-RISC total score and subscales as well as main effects due to age, sex and TGMV
Abbreviations: ROI, region of interest; TGMV, total gray matter volume; SA, surface area; CT, cortical thickness; MC, mean curvature; V, volume; B, unstandardized beta weight; β, standardized beta weight; DF, degrees of freedom; CD-RISC, Connor-Davidson Resilience Scale; Factor 1: Persistence; Factor 2: Intuition-Trust; Factor 3: Bounce Back; Factor 4: Personal/Spiritual Meaning
Regions: ACgC = Anterior part of the cingulate gyrus and sulcus (ACC); SbCag = Subcallosial Area (sgACC); MACgG = Middle-anterior part of the cingulate gyrus and sulcus (aMCC); Amg = Amygdala
Significance: Significance was considered at p < .05
Bivariate correlations were conducted between resilience total and subscale scores and measures of affect (Positive Affect and Negative Affect (PANAS-current scores). Significance was considered at p < .05, uncorrected, but we emphasized effect size in this sample, where r = .30 (r2 = .09) is considered a moderate effect and r = .50 (r2 = .25) is considered a large effect. In addition, standardized betas values were reported in order to aid with interpretation of the effect sizes.
All analyses were done in SPSS v22 (IBM Corp., Armonk, NY). In total, 5 emotional arousal and 2 executive control (6 subdivisions) regions were tested. Permuted probability values were corrected using an FDR adjusted p value, where a FDR q <0.05 was considered significant (Benjamini and Hochberg 2000; Benjamini et al. 2006). This correction was performed within each network (emotional, executive control), for each morphological metric (volume, cortical thickness, surface area, mean curvature), and by laterality.
RESULTS
Subject Characteristics
A summary of subjects’ clinical and behavioral data is presented in Table 2. The mean age of all subjects was 26.3 years old (SD=7.0, range=18–46). Based on the total score of the CD-RISC and using a cut off of 80, there were 27 low resilience subjects (mean total score=69.89, SD=7.55; 7 males and 20 females) and 21 high resilience subjects (mean total scores=88.48, SD=6.95; 8 males and 13 females). There were no significant differences in CD-RISC total scores between males and females. The mean resilience total score for all subjects was 78.02 (SD=11.69, range=53–100), and mean subscale scores included the following: Factor 1 (Persistence)=26.19 (SD=4.23, range=17–32), Factor 2 (Emotional Cognitive Control)=20.78 (SD=3.67, range=12–28), Factor 3 (Bounce Back)=16.63 (SD=2.83, range=9–20), and Factor 4 (Personal and Spiritual Meaning=14.55 (SD=3.01, range=8–20). There were no statistically significant resilience-group differences in the early trauma total or subscale scores and there was very little spread in scores.
Table 2.
Study Demographics and Clinical/Behavioral Measures
| Mean | SD | Range | N | ||
|---|---|---|---|---|---|
| Sex | 15 Males and 33 Females | 48 | |||
| %High Resilience | 44% | 48 | |||
| Age (yrs) | 26.31 | 6.96 | 18–46 | 48 | |
| Early Traumatic Inventory (ETI) | |||||
| General | 1.38 | 1.23 | 0–6 | 48 | |
| Physical | 1.26 | 1.64 | 0–5 | 47 | |
| Emotional | .40 | .99 | 0–5 | 47 | |
| Sexual | .30 | .93 | 0–4 | 47 | |
| Total | 3.39 | 3.29 | 0–15 | 47 | |
| Positive Affect Negative Affect (PANAS) | |||||
| Positive Affect (Current) | 33.17 | 8.70 | 15–50 | 40 | |
| Negative Affect (Current) | 12.21 | 4.23 | 10–29 | 40 | |
| Resilience Measure | |||||
| Connor & Davidson Resilience Scale (CD RISC) | |||||
| Persistence (Factor 1) | 26.19 | 4.23 | 17–32 | 48 | |
| Emotional Cognitive Control (Factor 2) | 20.78 | 3.67 | 12–28 | 46 | |
| Bounce Back (Factor 3) | 16.63 | 2.83 | 9–20 | 48 | |
| Personal and Spiritual Meaning (Factor 4) | 14.55 | 3.01 | 8–20 | 47 | |
| Total CD RISC Score | 78.02 | 11.69 | 53–100 | 48 | |
Abbreviations: Subject Number (N), Standard Deviation (SD)
Questionnaires: Early Traumatic Inventory (ETI); Positive Affect Negative Affect Scale (PANAS Current); Connor & Davidson Resilience Scale (CD RISC)
Groups: Low resilience, High resilience (A score ≥ 80 was used as a cutoff to determine percentage of high vs. low resilience)
Resilience total scores had large positive correlations with positive affect scores (r=.62, p=.001), but no significant correlations between resilience total scores and negative affect scores were found. Considering the resilience subscale scores, there were moderate to high correlations with positive affect scores (Factor 1/Persistence: r=.47, p=.021; Factor 2/Emotional and cognitive control under pressure: r=.77, p=.001; Factor 4/Control of one’s life and faith: r=.62, p=.002). On the other hand, current negative affect had large negative correlations with resilience Factor 2/Emotional and cognitive control under pressure (r=−.49, p=.019) and with Factor 3/Ability to bounce back (r=−.69, p=.009).
Morphological changes in regions of the executive control network associated with resilience total and subscale scores
Significant unstandardized beta weights (B) and standardized beta weights (β), with corresponding p-values for the ROIs in the executive control network with the resilience total score and each resilience subscale score are reported in Table 3 and represented in Figure 2.
Figure 2. Morphological Differences in Executive Control Network are Associated with Resilience.
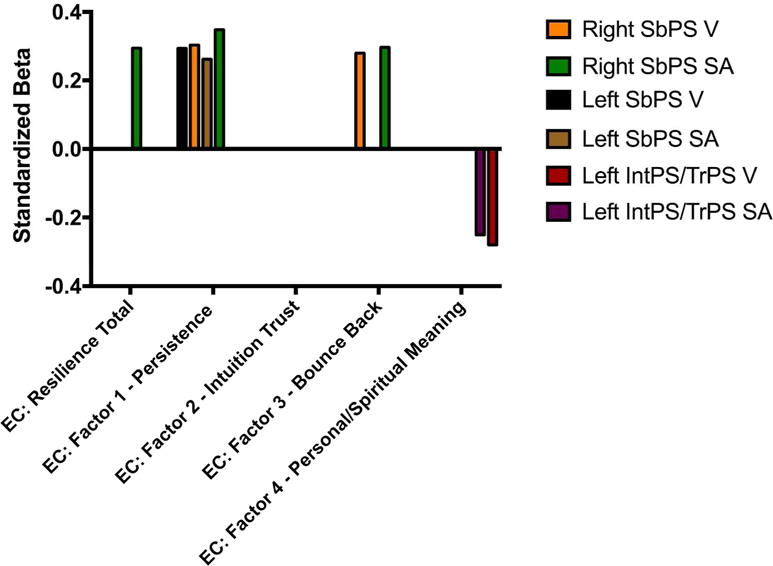
Findings showing significant morphological association with regions in the executive control network have been represented (standardized bets)
Abbreviations: EC, executive control; V, volume; SA, surface area
Resilience Scale Factors: CD-RISC, Connor-Davidson Resilience Scale; Factor 1: Persistence; Factor 2: Intuition-Trust; Factor 3: Bounce Back; Factor 4: Personal/Spiritual Meaning
Regions: SbPS, subparietal sulcus; IntPS/TrPS, intraparietal sulcus (interparietal sulcus) and transverse parietal sulci
Resilience Total Score
Greater resilience scores were associated with observed increased morphological differences in the right subparietal sulcus (a subregion of the PPC) (SA: B(4,43)=4.17, β=.29 se=1.93, n=48, q=.04).
Sex Effects
There were no significant main sex or sex*resilience total score interaction effects for the SA of the right subparietal sulcus.
Age Effects
There were no significant main age or age*resilience total score interaction effects for the SA of the right subparietal sulcus.
Persistence resilience (Factor 1 subscale)
Greater persistence scores were associated with observed morphological differences in the subparietal sulcus: Increased GMV bilaterally (Left (B(4,43)=29.12, β=.29 se=12.04, n=48, q=.04), Right (B(4,43)=28.58, β=.30, se=12.61, n=48, q=.04), and increased SA bilaterally (Left (B(4,43)=10.23, β=.26, se=4.79, n=48, q=.03; Right (B(4,43)=13.68, β=.35, se=5.17, n=47, q=.04).
Sex Effects
There were no significant main sex effects for the GMV or the SA of the bilateral subparietal sulcus. However, there was a significant sex*persistence score interaction effect for the SA of the left subparietal sulcus (p=.02).
Age Effects
There were no significant main age or age*persistence score interaction effects for the GMV or the SA of the left subparietal sulcus.
Trust in One’s Intuition (Factor 2 subscale)
No significant associations were found between brain morphology and the trust in one’s intuition subscale scores.
Bounce Back (Factor 3 subscale)
Greater bounce back scores were associated with increased GMV and SA in the right subparietal sulcus (GMV: B(4,43)=39.36, β=.28, se=19.03, n=48, q= .04; SA: B(4,43)=17.39, β=.30, se=7.91, n=48, q= .03).
Sex Effects
There were no significant main sex or sex*bounce back score interaction effects for the GMV or the SA of the right subparietal sulcus.
Age Effects
There were no significant main age or age*bounce back score interaction effects for the GMV or the SA of the right subparietal sulcus.
Personal/Spiritual Meaning (Factor 4 subscale)
Greater personal/spiritual meaning scores were associated with decreased GMV and SA of the left intraparietal sulcus, a subregion of the PPC (GMV: B(4,42)=−74.05, β=−.25, se=30.41, n=47, q=.029; SA: B(4,42)=−27.12, β=−.30, se=13.31, n=47, q=.04).
Sex Effects
There were no significant sex*personal/spiritual meaning score interaction effects for the GMV or the SA of the left intraparietal sulcus. However there was a significant main effect of sex on the left intraperietal sulcus SA (p=.02).
Age Effects
There were no significant main age or age*personal/spiritual meaning score interaction effects for the GMV or the SA of the left intraparietal sulcus.
Morphological changes in regions of the emotional arousal network are associated with resilience total and subscale scores
Significant unstandardized beta weights (B) and standardized beta weights (β), with corresponding p-values for the ROIs in the emotional arousal network with the resilience total score and each resilience subscale score are reported in Table 4 and represented in Figure 3.
Figure 3. Morphological Differences in Emotional Arousal Network are Associated with Resilience.
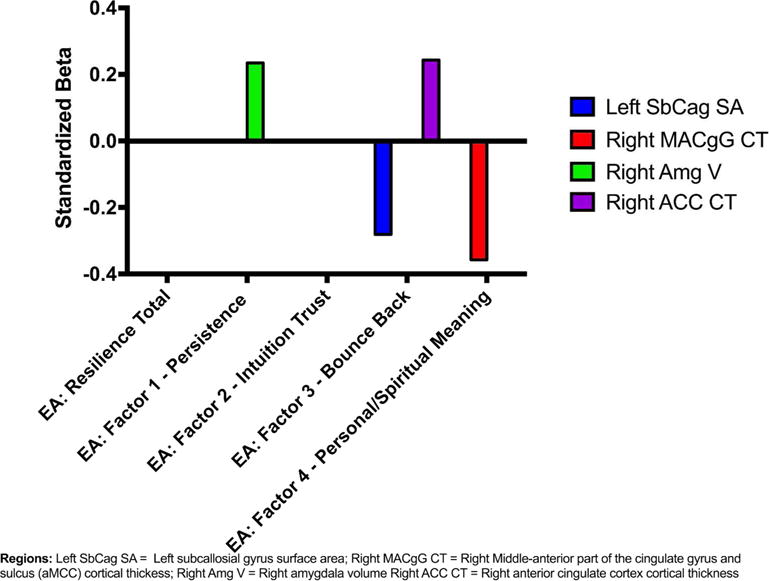
Findings showing significant morphological association with regions in the emotional arousal network have been represented (standardized bets)
Abbreviations: EA, emotional arousal; V, volume; SA, surface area; CT, cortical thickness
Resilience Scale Factors: Resilience CD-RISC Total scores; Factor 1: Persistence; Factor 2: Intuition-Trust; Factor 3: Bounce Back; Factor 4: Personal/Spiritual Meaning
Regions: SbCag, subcallosial gyrus; MACgG, middle-anterior part of the cingulate gyrus and sulcus; amg, amygdala; ACC, anterior cingulate cortex
Resilience Total Score
No significant associations were found between brain morphology and the resilience total scores.
Persistence resilience (Factor 1 subscale)
Increased persistence scores were associated with greater GMV in the right amygdala (B(4,43)=13.98, β=.24, se=6.87, n=48, q= .04).
Sex Effects
There were no significant main sex or sex*persistence score interaction effects for the GMV of the right amygdala.
Age Effects
There were no significant main age or age*persistence score interaction effects for the GMV of the right amygdala.
Trust in One’s Intuition (Factor 2 subscale)
No significant associations were found between brain morphology and the trust in one’s intuition scores.
Bounce Back (Factor 3 subscale)
Higher scores on the bounce back scores were positively associated with increased CT in the right ACC (B(4,43)=0.02, β=.24, se=0.01, n=48, q=.03), and decreased SA in the left sgACC (B(4,43)=−9.82, β=−.28, se=4.71, n=48, q=.04).
Sex Effects
There were no significant main sex or sex*bounce back score interaction effects for the CT of the right ACC and the SA of the left sgACC.
Age Effects
There were significant main age effects for the CT of the right ACC (p=.04) and the SA of the left sgACC (p=.02). However, there were no significant age*bounce back score interaction effects for the CT of the right ACC or the SA of the left sgACC.
Personal/Spiritual Meaning (Factor 4 subscale)
Higher personal/spiritual meaning scores were associated with decreased CT in the right aMCC (B(4,42)=−0.02, β=−.36, se=0.01, n=47, q= .03).
Sex Effects
There were no significant main sex or sex*personal/spiritual meaning score interaction effects for the CT of the right aMCC.
Age Effects
There was not a significant main age effect for the CT of the right aMCC, but there was a significant age*personal/spiritual meaning interaction effect for the CT of the right aMCC (p=.03).
Above 25 years old group: No significant associations were found for personal/spiritual meaning resilience subscale scores with the CT of the right aMCC.
Below 25 years old group: A significant association was found for personal/spiritual meaning resilience subscale scores and decreased CT of the right aMCC (p=.01).
Effects of Positive Affect on Brain Morphology
When investigating the association between positive affect (PANAS) and brain morphology, we found that in the executive control network, there was an association with increases in the MC of the right intraparietal sulcus, a subregion of the PPC (B(4,44)=.0013, β=.75, se=.0004, n=48, p=.001). In the emotional arousal network, there was an association between positive affect and decreased CT of the left aMCC (B(4,44)=−.01, β=−.57, se=.004, n=48, p=.01).
DISCUSSION
Resilience helps to promote health by protecting against stress or trauma, and by improving the recovery from such adverse events. In this study, we aimed to identify regional brain morphological differences associated with increased or decreased trait resilience in individuals without any previous or current major psychiatric or medical disease. The main findings were: 1. Subjective measures of resilience were significantly associated with morphological changes in subregions of the parietal cortex, cingulate subregions and in the amygdala. 2. Significant differential associations were observed between subdimensions of resilience and these brain regions. 3. Self-report measures of resilience were positively associated with positive affect. To our knowledge, this is the first study to use parcellation techniques to examine hypotheses regarding regional morphological alterations associated with resilience in healthy subjects.
Resilience-related morphological differences in the parietal cortex
Higher levels of resilience total scores were related to greater GMV and SA of the subparietal sulcus, and the same brain regions showed positive correlations with persistence resilience subscale scores. We also showed evidence for a negative association between GMV and SA of the left inferior parietal cortex and personal/spiritual meaning resilience subscale scores.
Several human studies have shown an association between experiences of trauma with decreased function of the parietal cortex (Bremner et al. 1999; Rauch et al. 1996; Shin et al. 1997; Shin et al. 1999). Retrieval of emotionally valenced words in females with histories of early abuse has been linked to decreased blood flow in the inferior parietal cortex (Bremner et al. 2001; Bremner et al. 2003). In an emotional Stroop task, there was decreased parietal cortex activity in females with histories of PTSD and abuse (Bremner et al. 2004). Since resilience has rarely been studied in non-trauma exposed healthy subjects, disease populations such as those with PTSD and depression are often used as a proxy for the interpretation of resilience. Although, these functional studies measured alterations in individuals with PTSD or abuse/trauma histories, they provide indirect support for the observed resilience related structural alterations within the parietal cortex.
The parietal cortex is a key region of the executive control network, and is associated with inhibitory control, attention, working memory, planning, and response (Uddin et al. 2011). Therefore, the findings are consistent with the hypothesis that high resilient individuals may be better able to engage the executive control network, including its role in inhibitory functions in relation to real or perceived threats in homeostasis.
Resilience-related morphological differences in subregions of the cingulate and the amygdala
We found greater GMV of the right amygdala was associated with increased persistence resilience subscale scores.
This is consistent with findings from several neuroimaging studies, which show that amygdala volume is reduced in individuals who have been exposed to early adverse life events or maltreatment. For example, smaller amygdalae have been observed in individuals exposed to childhood poverty (Luby et al. 2013) and in adolescents having histories of childhood maltreatment (Edmiston et al. 2011). Another study also found smaller amygdala volumes in individuals exposed to childhood adversities such as physical abuse, neglect, or being raised in poor households (Hanson et al. 2015). Consistent findings of reduced amygdala volumes have been found in PTSD populations compared to healthy controls (Depue et al. 2014; Lanius et al. 2001; Rauch et al. 2003; Shin et al. 2004). The findings of reduced amygdala volume in these studies are opposite to some other studies that have found increased amygdala volume (Mitra et al. 2005; Padival et al. 2013; Vyas et al. 2006; Vyas et al. 2002), or where there was functional hyperactivity of the amygdala (Padival et al. 2013; Rosenkranz et al. 2010) in populations exposed to adversity. However, it is possible that these morphological and activity alterations found in the amygdala could be secondary changes related to trauma or adversity. For example, several studies have demonstrated that hyperactivity of the amygdala early in life can result in apoptosis of amygdala cells later in life following repeated exposure to stress or trauma (Ding et al. 2010; Hodel et al. 2015; McEwen 2003; Sheline et al. 1998). This suggests that the time since the trauma could be important in accessing morphological changes.
The amygdala plays a key role in emotional processing and arousal, and fear conditioning, and increased amygdala responses are associated with reduced inhibitory control and decreased regulation of learned responses to fearful conditions (Klenowski et al. 2015; Rauch et al. 2003; Shin et al. 2004). Results from these earlier animal studies are consistent with the smaller amygdala volumes found with lower levels of resilience in our study. One may speculate that the reduced volumes associated with low resilience may develop in individuals with compromised inhibitory control.
Previous studies have found that reduced volumes (Woodward et al. 2006; Yamasue et al. 2003) and abnormal shape (Corbo et al. 2005) of the ACC were seen in patients with PTSD (Sherin and Nemeroff 2011), and that these morphological measures were correlated with PTSD symptom severity scores. A study investigating 40 pairs of identical twins (Vietnam veterans exposed to trauma versus their twins who were not exposed to trauma) found that the loss of GMV in the ACC was only evident in the veterans who were exposed, and was absent in the twins who were not exposed (Kasai et al. 2008). This observed reduced volume of the ACC could be an acquired feature in response to trauma exposure versus a predisposing risk factor. It is also possible that alterations and dysfunction in the ACC in PTSD patients may be related to secondary neuronal loss (Bremner et al. 1999; Lanius et al. 2001; Shin et al. 2001).
In our study we found that the thickness of the right ACC was positively associated with bounce-back resilience subscale scores. The findings from our study related to resilience are interesting in that the ACC is involved in emotion regulation (Devinsky et al. 1995), attention (Cohen et al. 2000), and extinction of fear responses (Sherin and Nemeroff 2011). When viewed together with the observed structural alterations in the parietal cortex, these findings of increased CT of the ACC suggest that a common feature of more resilient individuals may be a greater ability to engage feedback inhibition of the amygdala, limiting the extent and the duration of stress circuit activations.
We found reduced SA of the left sgACC with increasing bounce back resilience subscale scores, and reduced CT of the right aMCC with increasing personal/spiritual meaning subscale resilience scores. The mPFC (which includes the sgACC/subcallosal gyrus) can modulate emotional responses to stimuli by attenuating amygdala responses (Bremner 2006). Various neuroimaging studies, which have shown that PTSD patients usually do not activate the sgACC when exposed to traumatic stimuli that remind them of their experienced trauma (Bremner et al. 1999; Lanius et al. 2001; Lanius et al. 2003; Liberzon et al. 1999; Shin et al. 2004). In fact, individuals with PTSD or abuse histories displayed decreased activity in these regions during attention tasks (Semple et al. 2000) and during an emotional Stroop task (Bremner et al. 2001; Bremner et al. 2004; Shin et al. 2001), which measures cognitive flexibility during exposure to emotionally valenced words. Taken together with our results these studies suggest that high resilient individuals demonstrate morphological alterations that may be responsible for the modulation of emotional or fearful responses.
Associations between resilience and affect
As hypothesized, self-reported resilience was positively associated with current positive affect and negatively associated with negative affect from the PANAS. Positive affect was also associated with increased morphology of the intraparietal sulcus and the aMCC similar to that of several resilience scales. However, morphological changes in the subparietal sulcus, anterior cingulate cortex, subgenual anterior cingulate cortex, and amygdala were found for higher resilience, but were not found for greater positive affect or less negative affect. This is consistent with some but not complete overlap of brain networks associated with current affect and general resilience to stress. Other psychological mechanisms involved in resilience such as life satisfaction, coping, and hope may be related to these other brain networks. Since resilience is associated with adaptive responses in cortico-limbic inhibition to environmental adversity, would suggest that it plays an important role in decreasing the vulnerability to illness (Baratta et al. 2013; Feder et al. 2009; Fleshner et al. 2011; Karatsoreos and McEwen 2013b; Russo et al. 2012; van der Werff et al. 2013a). It appears that more effective modulation of brain circuits involved in emotion and fear, which are characteristic of highly resilient individuals (Southwick and Charney 2012), could lead to adaptive changes in the brain that are moderated by affect.
Study Limitations
Larger longitudinal studies will be important for both replication and determination of the extent these morphological changes are protective against disease or are associated with resilience operationalized as recovery from a specific trauma or challenge. Network analyses in larger datasets would help to identify if the observed regional changes are associated with more extensive alterations in network architecture and function, and provide a better understanding of how functional and anatomical connections relate to morphological changes. Although few interactions were found between participant’s sex and the resilience measures, the current sample was unbalanced with fewer males than females. Future studies with larger samples are needed to further investigate sex differences related to resilience. Although there was a wide range in age in the current sample, there was only one significant interaction finding between participant’s age and personal/spiritual meaning subscale resilience measures on a region of the emotional arousal network. Future studies with large stratified samples based on age will be needed to more specifically test for possible age-related changes in brain circuits involved in resilience. Additionally, future investigations with more diverse samples could further test for the influence of various covariates (e.g. adult adversity and stress, early life adversity and stress, etc.) on these associations. The assessment of resilience was based on a validated self-report questionnaire, and could therefore be influenced by reporting bias. However, previous studies have demonstrated that the CD-RISC resilience questionnaire used in this study has acceptable psychometric properties and it has been validated against more extensive interview measures of resilience (Campbell-Sills and Stein 2007; Connor and Davidson 2003d; Gonzalez et al. 2015; Rodriguez-Rey et al. 2016). The current data explored common and differential brain morphology for general trait resilience and a single measure of current positive/negative affect. Further studies using other measures of resilience and affect will be important to examine more specifically the overlap of brain mechanisms involved. Finally, it needs to be determined if the observed structural differences related to resilience are a consequence of learned behaviors or skills, or represent a trait influenced by genetic/epigenetic factors, which would have to be confirmed using longitudinal studies.
Conclusions and Clinical Implications
This study demonstrates that higher levels of resilience are related to distinct morphological alterations in brain regions involved in executive control and emotional arousal networks, suggesting individuals with low resilience may have compromised cortico-limbic inhibition, making them more vulnerable to stress related morbidity. Higher resilient individuals have a better ability to bounce back from adverse events, have greater emotional and cognitive control, and are more persistent. Our results also indicate that resilience scores varied with regard to a sense of control over personal and spiritual life. Brain signatures of low resilience in healthy individuals have the potential to serve as biomarker of vulnerability to stress-related diseases. Prophylactic interventions in the form of training in more effective coping styles and stress management may decrease the risk of future morbidity in such individuals.
Significance Statement.
This manuscript demonstrates the relationship between resilience measures and morphology of regions involved in cognitive and affective processes, consistent with previous suggestions that individuals with low resilience may have compromised cortico-limbic inhibition, increasing vulnerability to stress-related morbidity. Higher-resilient individuals have a better ability to bounce back from adverse events, have greater emotional and cognitive control, and are more persistent. The findings from this study have implications for using brain signatures of resilience as a biomarker of vulnerability to stress-related diseases and have implications for the development of training interventions that increase effective coping and stress management.
Acknowledgments
Funding Support: Supported by NIH grants DK106528 (AG), DK048351 (EAM), DK064539 (EAM), DK041301, and pilot funds were provided for brain scanning by the Ahmanson-Lovelace Brain Mapping Center
Footnotes
Authors Roles:
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Below is listed the contributions made by each author.
AG: study conceptualization and design, data analyses, manuscript preparation
AL: data analysis
LAK: study conceptualization and design, manuscript preparation
JSL: study conceptualization and design, data analysis
RB: data analysis, revision of manuscript
LC: study conceptualization and design, manuscript preparation
KT: study conceptualization and design, provided study funding
BN: provided study funding, study conceptualization and design, manuscript preparation
EAM: provided study funding, study conceptualization and design, manuscript preparation
Data Accessibility
Data can be requested by sending an email to the UCLA G Oppenheimer Center for Neurobiology of Stress and Resilience (CNSR) at OCNSadmin@mednet.ucla.edu. CNSR may deny requests if they conflict with the Data Sharing plan outlined by the funding source(s) for which the data were obtained. Recipients of approved request must agree to a Data Sharing Agreement, which applies to both the raw data as well as to any new data derived solely or in part from the data received. Any resulting publications utilizing CNSR data should acknowledge the methods of CNSR data gathering using language recommended by CNSR as well as their funding sources.
Associate Editor: Dr. Larry Cahill
Disclosures: The authors’ report that no conflicts of interest exist
References
- Baratta MV, Rozeske RR, Maier SF. Understanding stress resilience. Front Behav Neurosci. 2013;7:158. doi: 10.3389/fnbeh.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery fate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25(1):60–83. [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. [Google Scholar]
- Bitsika V, Sharpley C, Peters K. How is resilience associated with anxiety and depression? Analysis of factor score interactions within a homogenous sample. German Journal of Psychiatry. 2010;13(1):9–16. [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues in clinical neuroscience. 2006;8(4):445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007a;195(3):211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007b;195(3):211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Soufer R, McCarthy G, Delaney R, Staib LH, Duncan JS, Charney DS. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmacol Bull. 2001;35(3):55–78. [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biological psychiatry. 2004;55(6):612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological psychiatry. 2003;53(10):879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther. 2006;44(4):585–599. doi: 10.1016/j.brat.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): Validation of a 10-item measure of resilience. Journal of Traumatic Stress. 2007;20(6):1019–1028. doi: 10.1002/jts.20271. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Botvinick M, Carter CS. Anterior cingulate and prefrontal cortex: who’s in control? Nature neuroscience. 2000;3(5):421–423. doi: 10.1038/74783. [DOI] [PubMed] [Google Scholar]
- Comasco E, Sundstrom-Poromaa I. Neuroimaging the Menstrual Cycle and Premenstrual Dysphoric Disorder. Curr Psychiatry Rep. 2015;17(10):619. doi: 10.1007/s11920-015-0619-4. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depression and anxiety. 2003a;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT. Development of a new resilience scale: The Connor-Davidson Resilience scale (CD-RISC) Depress Anxiety. 2003d;18(2):76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biological psychiatry. 2005;58(2):119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Hegadoren KM, Coupland NJ, Rowe BH, Densmore M, Neufeld RW, Lanius RA. Neural correlates and predictive power of trait resilience in an acutely traumatized sample: a pilot investigation. The Journal of clinical psychiatry. 2012;73(3):327–332. doi: 10.4088/JCP.10m06293. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Payne VM, Connor KM, Foa EB, Rothbaum BO, Hertzberg MA, Weisler RH. Trauma, resilience and saliostasis: effects of treatment in post-traumatic stress disorder. Int Clin Psychopharmacol. 2005;20(1):43–48. doi: 10.1097/00004850-200501000-00009. [DOI] [PubMed] [Google Scholar]
- Depue BE, Olson-Madden JH, Smolker HR, Rajamani M, Brenner LA, Banich MT. Reduced amygdala volume is associated with deficits in inhibitory control: a voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. BioMed research international. 2014;2014:691505. doi: 10.1155/2014/691505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci. 2010;21(6):820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fabio A, Palazzeschi L. Hedonic and eudaimonic well-being: the role of resilience beyond fluid intelligence and personality traits. Front Psychol. 2015;6:1367. doi: 10.3389/fpsyg.2015.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Han F, Shi Y. Single-prolonged stress induces apoptosis in the amygdala in a rat model of post-traumatic stress disorder. Journal of psychiatric research. 2010;44(1):48–55. doi: 10.1016/j.jpsychires.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10(6):446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Maier SF, Lyons DM, Raskind MA. The neurobiology of the stress-resistant brain. Stress. 2011;14(5):498–502. doi: 10.3109/10253890.2011.596865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75(5):747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cogn Emot. 2005;19(3):313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Glover GH, Temple E. Resilience after 9/11: multimodal neuroimaging evidence for stress-related change in the healthy adult brain. NeuroImage. 2008;40(2):788–795. doi: 10.1016/j.neuroimage.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez VBA, Sierra MTC, Martinez BA, Martinez-Molina A, Ponce FP. An in-depth psychometric analysis of the Connor-Davidson Resilience Scale: calibration with Rasch-Andrich model. Health Qual Life Out. 2015;13 doi: 10.1186/s12955-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Kilpatrick L, Labus J, Tillisch K, Braun A, Hong JY, Ashe-McNalley C, Naliboff B, Mayer EA. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. 2014;76(6):404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Paulus MP, Johnson DC. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Social cognitive and affective neuroscience. 2014a doi: 10.1093/scan/nsu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase L, Thom NJ, Shukla A, Davenport PW, Simmons AN, Stanley EA, Paulus MP, Johnson DC. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Social cognitive and affective neuroscience. 2014b doi: 10.1093/scan/nsu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Cao R, Feng Z, Guan H, Peng J. The impacts of dispositional optimism and psychological resilience on the subjective well-being of burn patients: a structural equation modelling analysis. Plos One. 2013;8(12):e82939. doi: 10.1371/journal.pone.0082939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, Thomas KM. Duration of early adversity and structural brain development in post-institutionalized adolescents. NeuroImage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoviello BM, Charney DS. Psychosocial facets of resilience: implications for preventing posttrauma psychopathology, treating trauma survivors, and enhancing community resilience. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Thom NJ, Stanley EA, Haase L, Simmons AN, Shih PA, Thompson WK, Potterat EG, Minor TR, Paulus MP. Modifying resilience mechanisms in at-risk individuals: a controlled study of mindfulness training in Marines preparing for deployment. Am J Psychiatry. 2014;171(8):844–853. doi: 10.1176/appi.ajp.2014.13040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Muller MB, Tuscher O. A conceptual framework for the neurobiological study of resilience. Behav Brain Sci. 2014:1–49. doi: 10.1017/S0140525X1400082X. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Muller MB, Tuscher O. Advancing empirical resilience research. Behav Brain Sci. 2015a;38:e128. doi: 10.1017/S0140525X15000023. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Muller MB, Tuscher O. A conceptual framework for the neurobiological study of resilience. Behav Brain Sci. 2015b;38:e92. doi: 10.1017/S0140525X1400082X. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Annual Research Review: The neurobiology and physiology of resilience and adaptation across the life course. Journal of child psychology and psychiatry, and allied disciplines. 2013a;54(4):337–347. doi: 10.1111/jcpp.12054. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Resilience and vulnerability: a neurobiological perspective. F1000prime reports. 2013b;5:13. doi: 10.12703/P5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological psychiatry. 2008;63(6):550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM, Fogarty MJ, Belmer A, Noakes PG, Bellingham MC, Bartlett SE. Structural and functional characterization of dendritic arbors and GABAergic synaptic inputs on interneurons and principal cells in the rat basolateral amygdala. J Neurophysiol. 2015;jn008240:2014. doi: 10.1152/jn.00824.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Wang X, Hu S, Liu J. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. NeuroImage. 2015;123:165–172. doi: 10.1016/j.neuroimage.2015.08.020. [DOI] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Okada G, Aoyama S, Nishiyama Y, Onoda K, Yamawaki S. Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neurosci Lett. 2011;492(2):109–113. doi: 10.1016/j.neulet.2011.01.067. [DOI] [PubMed] [Google Scholar]
- Lamond AJ, Depp CA, Allison M, Langer R, Reichstadt J, Moore DJ, Golshan S, Ganiats TG, Jeste DV. Measurement and predictors of resilience among community-dwelling older women. Journal of psychiatric research. 2008;43(2):148–154. doi: 10.1016/j.jpsychires.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biological psychiatry. 2003;53(3):204–210. doi: 10.1016/s0006-3223(02)01466-x. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biological psychiatry. 1999;45(7):817–826. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- Lu W, Wang ZH, Liu Y, Zhang H. Resilience as a mediator between extraversion, neuroticism and happiness, PA and NA. Pers Indiv Differ. 2014;63:128–133. [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. Jama Pediatr. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.13020. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpetit MA, Bergeman CS, Deboeck PR, Tiberio SS, Boker SM. Resilience-as-process: negative affect, stress, and coupled dynamical systems. Psychol Aging. 2010;25(3):631–640. doi: 10.1037/a0019268. [DOI] [PubMed] [Google Scholar]
- Osorio C, Probert T, Jones E, Young AH, Robbins I. Adapting to stress: understanding the neurobiology of resilience. Behav Med. 2016;0 doi: 10.1080/08964289.2016.1170661. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9(2):265–270. doi: 10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres JF, Foerster B, Santana LG, Fereira MD, Nasello AG, Savoia M, Moreira-Almeida A, Lederman H. Police officers under attack: resilience implications of an fMRI study. J Psychiatr Res. 2011;45(6):727–734. doi: 10.1016/j.jpsychires.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Johnson DC, Goldstein MB, Malley JC, Rivers AJ, Morgan CA, Southwick SM. Psychosocial buffers of traumatic stress, depressive symptoms, and psychosocial difficulties in veterans of Operations Enduring Freedom and Iraqi Freedom: the role of resilience, unit support, and postdeployment social support. J Affect Disord. 2010;120(1–3):188–192. doi: 10.1016/j.jad.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14(7):913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of general psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Reynaud E, Guedj E, Souville M, Trousselard M, Zendjidjian X, El Khoury-Malhame M, Fakra E, Nazarian B, Blin O, Canini F, Khalfa S. Relationship between emotional experience and resilience: an fMRI study in fire-fighters. Neuropsychologia. 2013;51(5):845–849. doi: 10.1016/j.neuropsychologia.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Larson CL, Cahill SP. Relations Between Resilience, Positive and Negative Emotionality, and Symptoms of Anxiety and Depression. Psychol Trauma-Us. 2014;6:S92–S98. doi: 10.1037/a0033733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rey R, Alonso-Tapia J, Hernansaiz-Garrido H. Reliability and Validity of the Brief Resilience Scale (BRS) Spanish Version. Psychol Assessment. 2016;28(5):E101–E110. doi: 10.1037/pas0000191. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biological psychiatry. 2010;67(12):1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling OK, Diehl M. Reactivity to Stressor Pile-Up in Adulthood: Effects on Daily Negative and Positive Affect. Psychol Aging. 2014;29(1):72–83. doi: 10.1037/a0035500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63(1):65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- Sexton MB, Hamilton L, McGinnis EW, Rosenblum KL, Muzik M. The roles of resilience and childhood trauma history: main and moderating effects on postpartum maternal mental health and functioning. J Affect Disord. 2015;174:562–568. doi: 10.1016/j.jad.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9(9):2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Nemeroff CB. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues in clinical neuroscience. 2011;13(3):263–278. doi: 10.31887/DCNS.2011.13.2/jsherin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Archives of general psychiatry. 1997;54(3):233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological psychiatry. 2001;50(12):932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Singh K, Yu X. Psychometric evaluation of the Connor-Davidson Resilience Scale (CD-RISC) in a sample of Indian students. Psychology. 2010:23–30. [Google Scholar]
- Smith JL, Hollinger-Smith L. Savoring, resilience, and psychological well-being in older adults. Aging Ment Health. 2015;19(3):192–200. doi: 10.1080/13607863.2014.986647. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bonanno GA, Masten AS, Panter-Brick C, Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol. 2014;5 doi: 10.3402/ejpt.v5.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338(6103):79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Strand EB, Zautra AJ, Thoresen M, Odegard S, Uhlig T, Finset A. Positive affect as a factor of resilience in the pain-negative affect relationship in patients with rheumatoid arthritis. Journal of psychosomatic research. 2006;60(5):477–484. doi: 10.1016/j.jpsychores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Thom NJ, Johnson DC, Flagan T, Simmons AN, Kotturi SA, Van Orden KF, Potterat EG, Swain JL, Paulus MP. Detecting emotion in others: increased insula and decreased medial prefrontal cortex activation during emotion processing in elite adventure racers. Social cognitive and affective neuroscience. 2014;9(2):225–231. doi: 10.1093/scan/nss127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werff SJ, Pannekoek JN, Stein DJ, van der Wee NJ. Neuroimaging of resilience to stress: current state of affairs. Hum Psychopharmacol. 2013a;28(5):529–532. doi: 10.1002/hup.2336. [DOI] [PubMed] [Google Scholar]
- van der Werff SJ, van den Berg SM, Pannekoek JN, Elzinga BM, van der Wee NJ. Neuroimaging resilience to stress: a review. Front Behav Neurosci. 2013c;7:39. doi: 10.3389/fnbeh.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Nelson EE, Scaramozza M, Waldeck T, Hazlett G, Southwick SM, Pine DS, Drevets W, Charney DS, Ernst M. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry research. 2009;172(1):75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social cognitive and affective neuroscience. 2008;3(4):322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. 2011;9:8. doi: 10.1186/1477-7525-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biological psychiatry. 2006;59(7):582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, Mathe AA. Understanding resilience. Front Behav Neurosci. 2013;7:10. doi: 10.3389/fnbeh.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Sun JM. The role of psychological resilience and positive affect in risky decision-making. Int J Psychol. 2013;48(5):935–943. doi: 10.1080/00207594.2012.729840. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(15):9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56(1):19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73(2):212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]


