Summary
Plasma membrane (PM) free cholesterol (FC) is emerging as an important modulator of signal transduction. Here, we show that hepatocyte-specific knockout (HSKO) of the cellular FC exporter, ATP binding cassette transporter A1 (ABCA1), leads to decreased PM FC content and defective trafficking of lysosomal FC to the PM. Chow-fed HSKO mice had reduced hepatic: 1) insulin-stimulated Akt phosphorylation, 2) activation of the lipogenic transcription factor Sterol Regulatory Element Binding Protein (SREBP)-1c, and 3) lipogenic gene expression, versus controls. Consequently, Western-type diet-fed HSKO mice were protected from steatosis. Surprisingly, HSKO mice had intact glucose metabolism; they showed normal gluconeogenic gene suppression in response to re-feeding and normal glucose and insulin tolerance. We conclude that: 1) ABCA1 maintains optimal hepatocyte PM FC, through intracellular FC trafficking, for efficient insulin signaling; and 2) hepatocyte ABCA1 deletion produces a form of selective insulin resistance, such that lipogenesis is suppressed but glucose metabolism remains normal.
Graphical abstract

Introduction
On binding to its receptor, insulin stimulates signaling events that ultimately profoundly change metabolism (Biddinger and Kahn, 2006). One such change is stimulation of hepatic lipogenesis (Horton et al., 1998). Defective insulin signaling occurs in obesity, diabetes, and metabolic syndrome (Leavens and Birnbaum, 2011; Saltiel and Kahn, 2001). Changes in plasma membrane (PM) free cholesterol (FC) content and phospholipid species (i.e., fatty acyl and head group) modulate signaling of multiple receptors (Simons and Toomre, 2000), suggesting that PM FC content could also regulate insulin signaling. In support of this notion, excess PM FC in Niemann-Pick C1 knockout hepatocytes, or chemical depletion of PM FC (i.e., too little PM FC) in wild-type hepatocytes, blunted insulin receptor autophosphorylation, hinting that optimal PM FC is necessary for efficient insulin receptor activation (Vainio et al., 2005; Vainio et al., 2002).
ATP binding cassette transporter A1 (ABCA1) effluxes FC and phospholipid (PL) across the PM to combine with apolipoproteins (i.e. apoA-I), forming nascent high density lipoproteins (HDLs) (Oram and Vaughan, 2006). While a role for ABCA1 in bulk cellular FC export is well-recognized (Oram and Vaughan, 2006), recent studies suggest ABCA1 modifies PM lipid composition, which results in altered signaling via PM receptors. For example, myeloid-specific deletion of Abca1 results in macrophages with increased PM FC and lipid raft content (Zhu et al., 2008; Zhu et al., 2010). These macrophages are hyper-responsive to pathogen-associated molecular pattern molecules due to increased localization of Toll-like receptors and adaptor proteins in lipid rafts (Ito et al., 2015; Zhu et al., 2012), which increases macrophage secretion of proinflammatory cytokines (Zhu et al., 2008), chemotaxis in vitro and in vivo, and clearance of live bacteria from circulation (Zhu et al., 2012). Increased PM FC in hematopoietic stem and multipotential progenitor cells augments signaling through the IL-3 receptor and cell proliferation (Murphy et al., 2011; Yvan-Charvet et al., 2010). Recently, ABCA1 was implicated in modifying membrane lipid composition and multiple signaling activities in response to cell crowding (Frechin et al., 2015).
Several lines of indirect evidence suggest that ABCA1 may be involved in insulin signaling. Chow-fed Abca1 global KO mice develop hyperglycemia by four months of age and pancreatic β cell-specific deletion of Abca1 results in islet cell FC accumulation and defective insulin release, leading to hyperglycemia (Brunham et al., 2007). Chow-fed hepatocyte-specific Abca1 knockout (HSKO) mice show phenotypic lipid changes similar to those in type 2 diabetes and metabolic syndrome (Adiels et al., 2005), including elevated non-fasting plasma TG levels from hepatic overproduction of large TG-enriched VLDL1 particles and low plasma HDL concentrations (Chung et al., 2010). Chow-fed HSKO mice also have diminished phosphoinositide 3-kinase (PI3K) and Akt activation with fasting-refeeding or acute insulin injection (Chung et al., 2010). Chow-fed female HSKO mice have impaired glucose tolerance but unchanged insulin sensitivity (de Haan et al., 2014a). Mounting evidence suggests that ABCA1 single nucleotide polymorphisms are associated with type 2 diabetes and metabolic syndrome in humans (Daimon et al., 2005; Porchay et al., 2006; Villarreal-Molina et al., 2007; Villarreal-Molina et al., 2008). Nonetheless, a direct role of ABCA1 in insulin receptor signaling has not been reported.
Here, we sought to determine whether hepatocyte ABCA1 expression affects PM lipid composition, insulin signaling, and lipogenesis. Although liver FC and CE content are similar in chow-fed HSKO and control mice (Chung et al., 2010), we hypothesized that hepatocyte PM cholesterol is elevated in HSKO mice, resulting in blunted insulin signaling in liver of chow-fed HSKO vs. control mice. This hypothesis was based on reports that myeloid-specific deletion of ABCA1 results in macrophages with increased PM FC and lipid raft content (Zhu et al. 2008 and 2010), and optimal PM FC content is necessary for hepatocyte insulin signaling (Vainio et al., 2005; Vainio et al., 2002).
Results
Hepatocyte-specific Abca1 deletion modifies hepatic insulin and glucagon signaling in chow-fed mice
Chow-fed hepatocyte-specific Abca1 knockout (HSKO) mice had total plasma cholesterol concentrations 72–78% lower (p<0.0001) than in Abca1floxed/floxed (fl/fl) control mice (Fig. 1A), as previously reported (Chung et al., 2010; Timmins et al., 2005). Although body weight and food intake decreased and blood glucose and plasma insulin increased in chow-fed HSKO mice, the systemic metabolic phenotype (Fig. S1A–Q) was not statistically different from fl/fl mice.
Figure 1. Hepatic insulin and glucagon signaling and transcriptome profile. See also Figure S1 and S2.
(A) Mice (n=4 per genotype) were fasted and then fed chow for 2 h. Blood samples were obtained and isolated plasma used to measure total cholesterol (TC) concentration. (B) Western blot of liver Akt activation. (C) Western blot of hepatocyte Akt activation. (D–E) Glucagon signaling in primary hepatocytes. (D) Cyclic AMP (cAMP) levels measured with ELISA. ***P<0.001 (E) PKA phosphorylation substrates (pPKA Substrates) were determined by Western blotting. (A–E) Results are representative of 2–3 separate experiments. (F–G) Chow-fed mice were fasted for 4 hr and livers harvested for RNA sequencing (n=3 per genotype). (F) Heatmap and clustering of 3,000 most variably expressed genes in the normalized RNA-seq data set. (G) PCA analysis. (H) Pathway analysis of RNA-seq data set using the Reactome 2016 database.
We reported earlier that chow-fed HSKO mice had reduced hepatic phosphorylation of Akt serine residue 473 (S473) and PI3K p85 compared to controls, suggesting hepatic insulin resistance in HSKO mice (Chung et al., 2010). Complete Akt activation requires phosphorylation at S473 and threonine residue 308 (T308) (Cheng and White, 2012; Wu and Williams, 2012). Here, we observed that both phosphorylated sites were decreased in liver (Fig. 1B) and hepatocytes (Fig. 1C) of chow-fed HSKO vs. control mice.
We also investigated whether hepatocyte ABCA1 deletion also affected glucagon signaling, which activates adenylate cyclase resulting in increased cyclic adenosine monophosphate (cAMP) concentration and cAMP-induced protein kinase A (PKA) activity (Campbell and Drucker, 2015). Incubation of hepatocytes from chow-fed HSKO mice with glucagon led to a doubling of cAMP levels (Fig. 1D; p<0.001) and substrates of PKA phosphorylation, compared to hepatocytes from fl/fl control mice (Fig. 1E).
Hepatocyte-specific ABCA1 deletion impairs hepatic de novo lipogenesis
We applied an unbiased RNA-sequencing (RNA-seq) approach to identify differential gene signatures in livers of chow-fed HSKO vs. fl/fl control mice. We obtained an average of ~100 million paired-end reads per sample, ~85% of which mapped uniquely to the mouse genome. Clustering analysis using the 3,000 most variably expressed genes correctly distinguished the fl/fl and HSKO mice (Fig. 1F). This clustering was confirmed by analyzing the 3,000 most highly expressed genes (Fig. S2A) and the 10,000 most variable or most highly expressed genes (data not shown), and by principal components analysis (PCA) (Fig. 1G). The mRNA expression levels of 906 genes were significantly altered in livers of HSKO versus fl/fl control mice, including 226 down-regulated (<2 fold) and 680 up-regulated (>2 fold) genes (both unadjusted p=0.05; Tables S1, S2). As expected, Abca1 mRNA was among the most significantly down-regulated (~90% loss, unadj. p=3.2E-05; Table S1). Using the Reactome 2016 database, the 226 down-regulated genes were most significantly overrepresented in pathways related to lipogenesis, including fatty acyl-coA synthesis (p=0.0006) and TG biosynthesis (p=0.0009) (Fig. 1H; Table S3). Moreover, using the BioCarta 2016 resource, Sterol Regulatory Element Binding Protein (SREBP) control of lipid synthesis is one of only two significantly enriched pathways (p=0.01) among down-regulated genes (Table S3). We verified these findings by real-time RT-qPCR for genes that encode key enzymes in lipogenesis (Fig. S2B).
Primary hepatocytes were isolated from chow-fed mice and rates of triglyceride (TG), free cholesterol (FC), phospholipid (PL), and cholesteryl ester (CE) synthesis were determined by measuring incorporation of [14C]-acetic acid (i.e., de novo lipogenesis) and [3H]-oleic acid (TG formation by fatty acid [FA] esterification) into hepatocyte lipids. TG (Fig. 2A), FC (Fig. 2B), PL (Fig. 2C), and CE (Fig. 2D) synthesis from [14C]-acetic acid was significantly decreased in hepatocytes from HSKO versus fl/fl control mice, supporting the RNA-seq results. TG (Fig. 2E), but not PL (Fig. 2F) and CE (Fig. 2G), formation by [3H]-oleic acid esterification was significantly reduced in HSKO hepatocytes, suggesting the primary effect of hepatocyte ABCA1 deletion was on de novo lipogenesis and somewhat on FA uptake and esterification into TG.
Figure 2. Lipid metabolism in hepatocytes from chow-fed mice. See also Figure S2.
(A–G) Primary hepatocytes were incubated with the indicated radiolabels and newly synthesized triglyceride (TG) (A, E), free cholesterol (FC) (B), phospholipid (PL) (C, F), and cholesteryl ester (CE) (D, G) measured (n= 3 per time point). (H–I) Mice (n= 3 per genotype) underwent fasting and refeeding; nuclear and total liver protein (H) and total RNA (I) from mouse livers was subjected to Western blotting and real-time PCR quantification, respectively. (J) Primary hepatocytes were treated with saline (−) or insulin (+) for 2 min to examine pTSC2, TSC2, pPRAS40, and PRAS40 or 5 min to examine pS6K1, S6K1, p4E–BP1, and 4E–BP1 protein expression. (K) Real-time PCR quantification of glucose metabolism genes. Same experimental conditions and animals as panel I. Results are representative of 2–3 separate experiments.
Since insulin signaling was blunted and lipogenesis was decreased in HSKO hepatocytes compared to fl/fl hepatocytes, we examined expression and proteolytic processing of SREBP1c, the master regulator of de novo lipogenesis (Goldstein and Brown, 2015). Mice were subjected to a 24 h fast followed by 8 h refeeding of a high-carbohydrate chow diet, which results in robust expression and proteolytic processing of SREBP1c as insulin levels rise during the refeeding period (Engelking et al., 2004; Haas et al., 2012; Horton et al., 1998). After a 24 h fast, minimal full-length or processed SREBP1c was observed (Fig. 2H) due to low insulin levels. However, after 8 h of refeeding when insulin is high, full-length SREBP1c and nuclear processed SREBP1c were markedly reduced in HSKO versus fl/fl liver (Fig. 2H). As anticipated (Yabe et al., 2003), after 8 h of refeeding, Srebp1c (25-fold, p<0.0001), fatty acid synthase (Fasn; 8-fold, p<0.01), acetyl-coenzyme A carboxylase 1 (Acc1; 2-fold, p<0.01), Srebp2 (2-fold, p<0.05), and Insig1 (11-fold, p<0.01) hepatic mRNA increased and Insig2a and Lipin1a decreased (Fig. 2I) in both genotypes. However, HSKO mouse liver had significantly lower mRNA for Srebp1c after the 8 h refeeding period and a trend towards less Fasn and Insig1 mRNA versus refed fl/fl livers (Fig. 2I).
We also examined signaling proximal and distal to the mechanistic target of rapamycin C1 (mTORC1), a bifurcation point between insulin-stimulated lipogenesis and gluconeogenesis (Li et al., 2010), using insulin-stimulated primary hepatocytes isolated from chow-fed mice. We observed significantly decreased phosphorylation of Tuberous Sclerosis Complex 2 (TSC2), Ribosomal Protein S6 Kinase 1 (S6K1), Eukaryotic Initiation Factor 4E–Binding Protein 1 (4E–BP1) and Glycogen Synthase 3β (GSK3β), but not Proline-rich Akt Substrate of 40 kDa (PRAS40), in hepatocytes from chow-fed HSKO vs. fl/fl mice (Fig. 2J; Fig. S2C), supporting decreased mTORC activation and de novo lipogenesis in HSKO hepatocytes.
Hepatocyte-specific ABCA1 deletion does not impair hepatic glucose metabolism
As anticipated (Yabe et al., 2003), 8 h of refeeding after a 24 h fast decreased mRNA abundance for the gluconeogenic genes phosphoenolpyruvate carboxykinase (Pepck; p<0.01), glucose-6-phosphatase (G6pc; p=0.2), insulin receptor substrate 2 (Irs2; p=0.1), and insulin-like growth factor binding protein 1 (Igfbp1; p<0.01) and increased expression of the glycolysis gene glucokinase (Gck; p=0.001) in livers of fl/fl control mice (Fig. 2K). HSKO mouse liver had higher mRNA abundance for G6pc (p<0.05) and Igfbp1 (p<0.0001) after the 24 h fast and lower Pepck, G6pc, Irs2, and Igfbp1 mRNA versus refed fl/fl livers (Fig. 2K). In agreement with results in Fig. 2K, we observed increased nuclear FoxO1; on refeeding, nuclear FoxO1 appeared slightly lower in HSKO livers versus fl/fl livers (Fig. 2H). Overall, these results show that FoxO1 signaling is unimpaired in fasted-refed HSKO versus fl/fl mice.
Hepatocyte-specific ABCA1 deletion stimulates hepatic FA oxidation
Since our RNA-seq results suggested that FA oxidation pathways were downregulated in HSKO liver (Table S3), we measured rates of FA uptake and oxidation of [14C]-palmitic acid into [14C]-CO2 and [14C]-acid soluble metabolites (ASM). FA oxidation was increased in hepatocytes from chow-fed HSKO versus fl/fl mice (Fig. S2D, S2E), whereas FA uptake was similar (Fig. S2F).
Hepatocyte-specific ABCA1 deletion improves metabolic phenotype of WTD-fed mice
We challenged chow-fed HSKO mice with a Western-type diet (WTD), which decreased their weight versus fl/fl mice (Fig. 3A). WTD-fed HSKO mice had significantly increased oxygen consumption (Fig. 3B) and energy expenditure (Fig. 3C), without changes in food intake, physical activity, or respiratory exchange ratio (Fig. S3A–C). Similar to our previous reports (Chung et al., 2010; Timmins et al., 2005), HSKO mice had reduced plasma LDL cholesterol and HDL cholesterol concentrations versus fl/fl mice on both chow and WTD (Fig. S3D). Other measures of a systemic metabolic phenotype between WTD-fed HSKO and fl/fl mice were similar (Fig. S3E–L). However, liver TG (Fig. 3D, 3E) and FC (Fig. 3F) levels were decreased 70% (p<0.0001) and 20% (p<0.01), respectively, in WTD-fed HSKO vs. fl/fl control mice. TG (Fig. 3G) and FC (Fig. 3H) levels were also decreased 70% (p<0.0001) and 30% (p<0.01), respectively, in hepatocytes from WTD-fed HSKO versus fl/fl control mice.
Figure 3. Metabolic phenotype of Western-type diet (WTD)-fed mice. See also Figure S3.
Male mice were fed a WTD for 16–24 weeks. (A) Body weight (BW); n=9. (B) Oxygen consumption rates (VO2; ml/kg/h), and (C) energy expenditure (EE; kcal/kg/h). (D) Oil Red O staining of representative liver sections from 16 week WTD-fed mice. Liver TG (E) and FC (F) and hepatocyte TG (G) and FC (H) from WTD-fed mice. Data are representative of 2–3 separate experiments.
To determine the mechanism for reduced hepatosteatosis in WTD-fed HSKO mice, we examined several pathways that influence hepatic TG content. Incorporation of [14C]-acetic acid into TG (Fig. 4A) and PL (Fig. 4B) was significantly decreased in primary hepatocytes isolated from WTD-fed HSKO vs. fl/fl control mice, demonstrating decreased hepatic de novo lipogenesis. Newly synthesized FC (Fig. 4C) from [14C]-acetic acid was similar between genotypes, likely due to feedback inhibition by cholesterol in the WTD. [3H]-oleic acid esterification into hepatocyte TG (Fig. 4D) and PL (Fig. 4E) was also reduced in hepatocytes from WTD-fed HSKO mice, similar to results with [14C]-acetic acid. WTD-fed HSKO vs. fl/fl control mice also had decreased hepatocyte [14C]-palmitic acid uptake (Fig. 4F), decreased liver CD36/FA translocase mRNA levels (Fig. 4G; 50%, p<0.001), and reduced liver PM and cytosolic CD36/FA translocase protein expression (Fig. 4H); however, hepatocyte FA oxidation was similar (Fig. S4A, S4B), as was hepatic gene expression of peroxisome proliferator-activated receptor α (Pparα), carnitine palmitoyltransferase 1 (Cpt1), and Acc2 (Fig. S4C). Lastly, in vivo secretion of new synthesized VLDL-TG from [3H]-oleic acid (Fig. S4D, S4E) was similar for WTD-fed WT and HSKO mice. Thus, attenuated diet-induced hepatosteatosis in HSKO mice was most likely due to decreased hepatic de novo lipogenesis and FA uptake.
Figure 4. Lipid metabolism in primary hepatocytes from Western-type diet (WTD)-fed mice. See also Figure S4.
(A–E) Primary hepatocytes from WTD-fed mice (n=2/ genotype) were treated as in Figure 2 (n= 3/time point). (F) Fatty acid uptake by primary hepatocytes was measured as in Figure 2 (n= 3 per time point); Two-way ANOVA with Bonferroni’s multiple comparison test. (G), Cd36 expression by real-time PCR. (H) Hepatic CD36, Na+/P+ ATPase, and GAPDH protein expression. Results are representative of 2–3 separate experiments.
Impaired hepatic insulin signaling in HSKO liver is associated with decreased PM FC and increased hepatic lysophospholipid levels
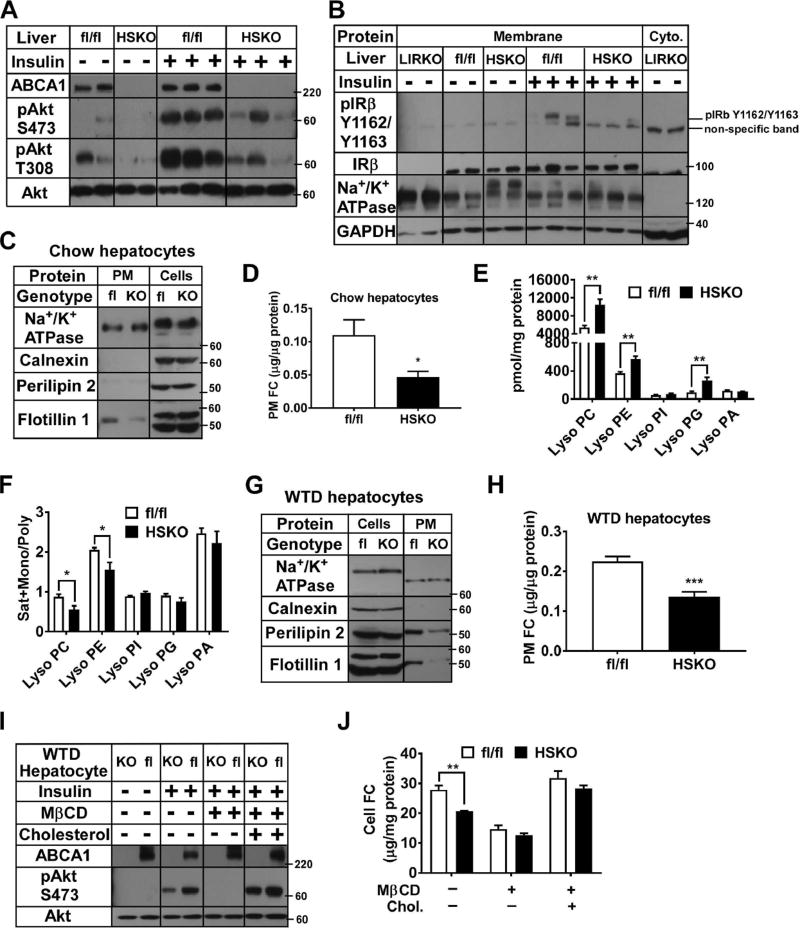
Portal vein insulin injection in WTD-fed HSKO mice resulted in decreased hepatic insulin-stimulated Akt S473 and T308 phosphorylation versus controls (Fig. 5A). However, white adipose tissue (Fig. S5A) and skeletal muscle (Fig. S5B) insulin signaling was similar for both genotypes, suggesting that insulin signaling was selectively impaired in hepatocytes lacking ABCA1 expression. We also isolated liver PM from mice with portal vein insulin injection. WTD-fed HSKO liver had decreased PM insulin receptor phosphorylation, but no change in insulin receptor b protein expression vs. fl/fl control mice, suggesting decreased activation of hepatic insulin receptor in WTD-fed HSKO mice (Fig. 5B).
Figure 5. Primary hepatocyte plasma membrane (PM) free cholesterol (FC) content modulates insulin signaling. See also Figure S5.
Mice consuming a WTD for 16–24 weeks received portal vein saline (n=2 per genotype) or insulin (0.5 U/kg body weight; n=3 per genotype) injection; 5 min later, livers were harvested. (A) Liver Western blots. (B) Western blots of hepatic membrane-associated and cytosolic proteins. LIRKO, liver-specific insulin receptor knockout. (C–D) Primary hepatocytes from chow-fed mice (n=2 per genotype) were used to purify PM fractions (n=3 per treatment) for Western blot (C) and FC measures (D). (E–F) Liver lipids were extracted from chow-fed mice (n=4 per genotype) for lipidomic analyses. (G–H) Primary hepatocytes from WTD-fed mice (n=2 per genotype) were used to purify PM fractions (n=6 per treatment). Western blotting and lipid analyses: as in panels C–D. (I) Primary hepatocytes from WTD-fed mice (n=2 per genotype) were FC depleted (+MβCD) and repleted (+cholesterol) before insulin stimulation (n=3 per treatment) and Western blotting. (J) Cells from panel I were lipid extracted and FC measured. Results are representative of 2–3 separate experiments.
We hypothesized that hepatocyte-specific ABCA1 deletion increased PM FC content, leading to impaired insulin signaling. Primary hepatocytes from chow- or WTD-fed mice were used to isolate PM fractions, which were then lipid extracted to measure FC. The PM fraction (enriched in Na+/K+ ATPase) isolated from chow-fed mouse hepatocytes was relatively free of calnexin and perilipin 2 (Fig. 5C). We also examined the abundance of PM flotillin 1, a protein associated with caveolar lipid rafts (Bickel, 2002; Bickel et al., 1997). Hepatocyte PM flotillin 1 (Fig. 5C) and FC (Fig. 5D; ~60%, p<0.05) content were significantly decreased in chow-fed HSKO versus control mice. As with our chemical measurement of PM FC, filipin staining was decreased in the PM region of chow HSKO versus fl/fl hepatocytes (Fig. S5C).
HSKO hepatocyte PM flotillin 1 and FC content were normalized after addition of 10% fetal bovine serum (FBS; 300 µg cholesterol/ml) to control hepatocyte levels (not shown), demonstrating acute repletion of HSKO hepatocyte PM cholesterol was possible with FBS-containing lipoproteins.
We performed a lipidomic analysis of PL species in chow-fed fl/fl and HSKO mouse liver. Hepatic content of phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidic acid (PA) were unaffected by ABCA1 expression (Fig. S5D). However, lysoPC, lysoPE, and lysophatidylglycerol (lysoPG) were significantly increased (1.9, 1.5, and 2.8-fold, respectively, p<0.01) in HSKO vs. fl/fl liver (Fig. 5E). HSKO liver lysoPC and lysoPE species also contained less saturated and monounsaturated fatty acyl species versus polyunsaturated species (Fig. 5F, Sat+Mono/Poly ratio) compared to fl/fl liver (p<0.05), suggesting a selective remodeling of hepatic lysoPL without ABCA1.
We also measured hepatocyte PM cholesterol and flotillin 1 content from WTD-fed mice. Compared to chow-fed mice (Fig. 5C), PM fractions from WTD-fed mice were likely contaminated with lipid droplets based on perilipin 2 content (Fig. 5G). Regardless, PM flotillin 1 (Fig. 5G) and FC content (Fig. 5H) were decreased by ~30% (p<0.001) in primary hepatocytes isolated from WTD-fed HSKO mice versus controls.
To directly test effects of acute cholesterol depletion and repletion on insulin signaling, we treated hepatocytes from WTD-fed mice with methyl-β-cyclodextrin (MβCD) or cholesterol-loaded MβCD, and then stimulated them acutely with insulin and measured Akt phosphorylation. MβCD-treated hepatocytes had no detectable Akt phosphorylation for both genotypes (Fig. 5I) and reduced cellular FC content (Fig. 5J). Incubation of MβCD-treated hepatocytes with cholesterol-loaded MβCD increased cellular FC and restored insulin-stimulated Akt phosphorylation, both above basal levels for both genotypes (Fig. 5I, 5J), demonstrating that acute FC depletion and repletion resulted in decreased and increased insulin-stimulated signaling, respectively.
Hepatic ABCA1 deletion impairs antegrade FC trafficking from lysosomes to the PM
Since we observed a significant decrease in HSKO hepatocyte PM FC content (Fig. 5D), but not total cellular FC level (Fig. S1I), compared to fl/fl controls, we hypothesized that intracellular cholesterol trafficking is defective in ABCA1-deficient hepatocytes. Because LDL trafficking from endosomes to lysosomes is blocked at 18°C (Jones, 1997; Sugii et al., 2003), we incubated primary hepatocytes isolated from chow-fed mice with [3H]-cholesteryl oleate-containing LDL at 18 °C for 5 h to pulse label the endosomal compartment. Radiolabel was chased for 90 min at 37°C to allow LDL trafficking to the lysosome and hydrolysis of LDL [3H]-cholesteryl oleate to [3H]-FC and oleic acid. Cell-associated (bound + internalized) [3H] total (CE + FC) cholesterol trended higher (p=0.06) in chow-fed HSKO vs. fl/fl hepatocytes (Fig. 6A) after the pulse period, possibly due to increased hepatic LDL receptor protein expression in HSKO liver (Chung et al., 2010). During the pulse period, some hydrolysis of LDL [3H]-cholesteryl oleate occurred, detected as higher cell-associated FC radiolabel compared to the LDL tracer (5% FC; 95% CE) (Fig. 6B); increased FC radiolabel was significantly higher in HSKO (28% FC) vs. fl/fl (17% FC) hepatocytes (Fig. 6B), likely reflecting the higher total cell-associated radiolabel in HSKO hepatocytes (Fig. 6A). To monitor arrival of [3H]-FC at the PM during the chase period, hepatocytes were incubated with MβCD for 5 min at 37°C, followed by lipid extraction of cells and media and isolation of [3H]-FC by thin layer chromatography (Sugii et al., 2003). Despite more [3H]-FC radiolabel in HSKO cells (Fig. 6B), MβCD-extractable PM [3H]-FC was lower for HSKO hepatocytes at zero time (42%, p<0.001) and all three chase times (32% p<0.01, 31% p<0.01, 20% p<0.05, respectively) relative to fl/fl hepatocytes (Fig. 6C), suggesting impaired antegrade trafficking of lysosomal FC to the PM in HSKO hepatocytes.
Figure 6. Defective intracellular free cholesterol (FC) trafficking in ABCA1 deficient hepatocytes.
Hepatocytes (n=3 per treatment) were isolated from chow-fed, male mice and incubated with [3H]-cholesteryl oleate-radiolabeled low density lipoprotein (LDL tracer) for 5 h at 18°C. (A) After the 5 h pulse, cell-associated [3H]-total cholesterol (TC) and (B) %FC were quantified. (C) After incubation with LDL tracer, cells were chased for 0, 15, 30, and 90 min at 37°C and then treated with 3 mM methyl-β-cyclodextrin (MβCD) for 5 min at 37°C to extract plasma membrane (PM) [3H]-FC. Results are representative of 2 separate experiments.
Discussion
Prior studies suggest that ABCA1 may be important in regulating systemic glucose metabolism and hepatic insulin signaling (Brunham et al., 2007; Chung et al., 2010; de Haan et al., 2014b), but mechanisms were unclear. A summary of our findings appears in Figure 7A. Compared to control (fl/fl) mice, HSKO mice had decreased hepatic insulin receptor signaling due to decreased PM FC content that likely resulted from defective antegrade lysosomal FC trafficking to the PM and decreased cholesterol biosynthesis. Decreased PM FC content and impaired PM signaling led to decreased mTORC activation, a ~50% reduction in SREBP1c mRNA abundance, decreased unprocessed, full-length SREBP1c, and low levels of processed nuclear SREBP1c, a critical transcriptional activator of de novo lipogenesis. Feeding a WTD to HSKO mice compared to fl/fl controls markedly decreased hepatic TG content, associated with decreased de novo lipogenesis and FA uptake and a mild metabolic phenotype (reduced body weight and increased energy expenditure). Nonetheless, hepatic and systemic glucose metabolism remained intact. Our results support the concept that hepatocyte ABCA1 is critical for maintaining optimal PM FC content for efficient membrane (particularly insulin) signaling. Without hepatocyte ABCA1, a unique form of insulin resistance is unmasked, where de novo lipogenesis is suppressed, but gluconeogenesis remains responsive (Figure 7B).
Figure 7. Summary of experimental results.
A) Compared to chow-fed control (fl/fl) mice, hepatic insulin signaling is reduced in chow-fed hepatocyte-specific Abca1 knockout (HSKO) mice due to reduced plasma membrane (PM) free cholesterol (FC) content from decreased (a) trafficking of lysosomal FC to the PM and (b) cholesterol biosynthesis (c). Blunted insulin signaling leads to decreased activation of the Akt-mTORC pathway (d) and decreased expression and processing of SREBP1c (e), resulting in reduced de novo lipogenesis (f). Regulation of gluconeogenesis is unchanged in HSKO vs. fl/fl liver (g), but fatty acid (FA) oxidation is increased (h), perhaps due to activation of glucagon signaling (i). Western-type diet (WTD)-fed HSKO vs. control mice have a similar phenotype with decreased PM FC (a), decreased insulin signaling (d), and reduced lipogenesis (f), resulting in diminished hepatosteatosis. However, unlike chow-fed mice, WTD-fed HSKO mouse liver had decreased FA uptake (j; dashed arrow) and similar FA oxidation (k) versus WTD-fed control mice. B) Proposed sequence leading to unique selective insulin resistance in HSKO mouse liver. Downward arrows = decreased signal pathway/molecule activation relative to fl/fl (control) mice; horizontal arrows denote no change. The HSKO mouse liver shows a defect in the Akt2 and mTORC1 response to insulin, with a concomitant reduction in de novo lipogenesis. FoxO1 and gluconeogenesis remain suppressed by insulin.
A striking result of our study was decreased hepatic insulin signaling and de novo lipogenesis in HSKO mice. Li et al showed that insulin signaling bifurcates at the level of mTORC activation, with one pathway stimulating de novo lipogenesis and one inhibiting gluconeogenesis (Li et al., 2010). Akt-dependent lipogenesis requires mTORC activation (Porstmann et al., 2008). Our results show that components of the insulin signaling pathway proximal (PI3K (Chung et al., 2010) and Akt) and distal (S6K and 4E–BP1) to mTORC are attenuated in HSKO vs. fl/fl liver. Decreased de novo lipogenesis in HSKO liver is supported by reduced SREBP1c mRNA, protein expression, and protein processing and by biochemical measurements. Decreased SREBP1c processing and lipogenesis in HSKO livers were most likely due to impaired insulin receptor activation resulting from reduced PM FC, since SREBP1c mRNA and full-length, unprocessed protein decreased proportional to processed nuclear SREBP1c. However, increased ER FC content, which would result in increased ER retention of SCAP-SREBP1c by INSIG (Engelking et al., 2004; Yabe et al., 2002), may be partially responsible for decreased de novo lipogenesis in HSKO liver.
HSKO mice subjected to fasting-refeeding appropriately downregulated gluconeogenic gene expression and nuclear FoxO1, and upregulated Gck, similar to control mice, resulting in no overt systemic abnormality in glucose regulation. In agreement with our results, other studies have shown that hepatic insulin signaling is dispensable for normal postprandial response of gluconeogenic genes, but required for inducing lipogenesis through SREBP1c activation (Titchenell et al., 2015; Titchenell et al., 2016).
Although ABCA1 is critical for generating plasma HDL (Timmins et al., 2005), it is also involved in endocytosis (Zha et al., 2001), exocytosis (Kruit et al., 2011), intracellular vesicular trafficking (Robenek and Schmitz, 1991; Schmitz et al., 1985; Zha et al., 2003) and reducing membrane lipid rafts (Landry et al., 2006; Zarubica et al., 2009; Zhu et al., 2008; Zhu et al., 2010). More recently, ABCA1 was implicated in retrograde transport of FC from PM to ER (Yamauchi et al., 2015) to regulate ER cholesterol content, a key step in controlling sterol biosynthesis (Radhakrishnan et al., 2008), and autonomous regulation of cellular membrane composition in response to cell crowding (Frechin et al., 2015). In the latter study, cultured cells with low crowding responded by down-regulating ABCA1, resulting in higher cellular cholesterol content, more lipid droplets, and more saturated cell membrane PL species. A more rigid membrane structure results that affects membrane receptor signaling, such as Akt phosphorylation, which critically depends on membrane lipid raft domains for optimal activation (Lasserre et al., 2008). Frechin et al (Frechin et al., 2015) postulate that the PM may act as a capacitor that converts signals to the correct timescale and is tuned by enzymes that alter membrane lipid composition and order. Our results with hepatocytes, and those of Frechin et al using non-hepatic cells, demonstrate a generalizable and key role for ABCA1 in determining PM composition and order, which modulates cellular signaling (i.e., insulin and glucagon).
Using mouse embryonic fibroblasts from whole body Abca1 knockout mice, endocytic retrograde FC movement from the PM to the ER was impaired compared to WT mouse embryonic fibroblasts, which increased activation of the SREBP2 pathway and cholesterol biosynthesis (Yamauchi et al., 2015). Furthermore, these authors documented increased PM FC and lipid raft content (i.e., flotillin-1 and caveolin-1), the opposite of our findings in HSKO hepatocytes. We propose that ABCA1 is more dominant in antegrade FC transport than retrograde transport in hepatocytes, which are programmed to be secretory cells. Defective vesicular trafficking and antegrade movement of lipid occurs in cells lacking ABCA1 (Robenek and Schmitz, 1991; Schmitz et al., 1985; Zha et al., 2003). Despite increased cell-associated [3H]-FC in HSKO hepatocytes, movement of lysosomal [3H]-FC cholesterol, liberated by hydrolysis of LDL [3H]-CE, to the PM, is impaired in ABCA1-deficient hepatocytes. With reduced intracellular FC trafficking to the PM, ER FC may increase, leading to down-regulation of FC and TG synthesis, by decreasing SREBP1c processing and nuclear SREBP1c content (Nohturfft et al., 2000; Wang et al., 1994), as supported by our results (Fig. 2H). Thus, the role of ABCA1 in antegrade and retrograde FC transport may be cell-specific and dictated by the overall need to take up or secrete lipid.
Mice completely lacking hepatocyte insulin receptors (i.e., LIRKO) are hyperinsulinemic, with mildly elevated blood glucose, but plasma TG concentrations are lower than controls (Biddinger et al., 2008). The pure insulin resistance in LIRKO mice results in decreased de novo lipogenesis due to decreased SREBP1c activation (Haas et al., 2012), unlike the selective insulin resistance observed in mouse models of obesity/type 2 diabetes (i.e., ob/ob) or mice fed a WTD, both of which have increased de novo lipogenesis, hepatosteatosis, and hypertriglyceridemia (Brown and Goldstein, 2008). Chow-fed HSKO mice have some phenotypic features of LIRKO mice (Biddinger et al., 2008; Haas et al., 2012), including elevated plasma glucose concentrations (Fig. S1J) and lower fasting plasma TG concentrations (Chung et al., 2010). When challenged with a HFD, both LIRKO (Haas et al., 2012) and HSKO mice (Figure 3) had decreased body weight gain and were relatively protected from hepatosteatosis versus control mice. However, unlike LIRKO mice, HSKO mice were not hyperinsulinemic (Fig. S1K), likely due to normal plasma insulin clearance by hepatocyte insulin receptors and normal pancreatic insulin secretion without significantly elevated plasma glucose levels. Insulin signaling strongly activates lipogenesis by increasing SREBP1c mRNA abundance and protein processing (Jeon and Osborne, 2012). Chow-fed LIRKO and HSKO mice have reduced SREBP1c expression and processing, compared to their respective controls, when refed with a high-carbohydrate chow diet after a prolonged fast. These similarities suggest that HSKO mice exhibit a phenotype more similar to pure insulin resistance than selective insulin resistance (Brown and Goldstein, 2008). If defective lysosomal FC trafficking to the PM reroutes FC trafficking to the ER, as suggested by our RNA-seq and in vitro cholesterol biogenesis data, then SREBP1c processing may be further attenuated by ER INSIG-mediated retention of the SCAP-SREBP1c complex (Engelking et al., 2004; Yabe et al., 2003).
Experimental procedures
Mice
Hepatocyte-specific Abca1 knockout mice (HSKO) were generated by crossing Abca1flox/flox (fl/fl) mice (Timmins et al., 2005) (backcrossed into the >99% C57BL/6 background) with albumin Cre recombinase transgenic mice (Jackson Laboratories). Age-matched (8–24 weeks of age) male mice on chow (LabDiet 5P00 Prolab RMH 3000) or a WTD were used. Eight-week-old chow-fed male mice were switched to WTD (42% fat calories, 0.2% cholesterol; Harlan Laboratories TD88137) for 16–24 weeks to induce obesity, insulin resistance, and hepatic steatosis. All mice were maintained in a specific pathogen-free environment on a 12:12 h light:dark cycle (dark from 6 p.m. to 6 a.m.) and allowed free access to food and water. All experiments were performed using a protocol approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine, in facilities approved by American Association for Accreditation of Laboratory Animal Care.
For the fasting/refeeding study, chow-fed mice (n=3 per genotype) were fasted for 24 h (6 a.m. to 6 a.m.), and another group was fasted for 24 h (10 p.m. to 10 p.m.) followed by refeeding a high carbohydrate chow (LabDiet 5P00 Prolab RMH 3000) for 8 h (10 p.m. to 6 a.m.). All mice were terminated between 6 a.m. and 7 a.m. Fresh liver (~200 mg) was used to isolate nuclear protein to examine SREBP1 expression (Bennett et al., 2008). The remainder was immediately frozen in liquid nitrogen and stored at −80°C until proteins were isolated and RNA was extracted for Western blotting and real-time PCR, respectively.
Indirect calorimetry
Indirect calorimetry was conducted as described previously (Thomas et al., 2013). Food intake was independently verified over 14 days, using mice housed in individual wire bottom cages (Thomas et al., 2013).
Metabolic measurements
Metabolic measurements were conducted as described previously (Chung et al., 2010; Thomas et al., 2013) with slight modifications as detailed in Supplemental Experimental Procedures.
In vivo triglyceride (TG) secretion rate
In vivo TG secretion was measured by a detergent lipolysis block procedure with radiolabeled oleic acid (Bi et al., 2013; Chung et al., 2010).
In vivo insulin signaling analysis
Liver, adipose tissue and muscle insulin signaling was determined as previously described (Chung et al., 2010) with slight modifications; see Supplemental Experimental Procedures.
RNA sequencing and real-time PCR
RNA sequencing and real-time PCR were performed as described in Supplemental Experimental Procedures.
Western blotting
Western blots were performed after protein fractionation using SDS polyacrylamide gel electrophoresis (Chung et al., 2010). Antibodies used for Western blots are listed in Supplemental Experimental Procedures.
In vitro metabolic analyses
Primary hepatocytes were isolated as described in Supplemental Experimental Procedures (Kreamer et al., 1986). Hepatic insulin signaling (Zhu et al., 2010), glucagon signaling (Miller et al., 2013), lipogenesis (Thomas et al., 2013), FA uptake (Li et al., 2013), and FA oxidation (Huynh et al., 2014) were analyzed using methods described in Supplemental Experimental Procedures.
Lipid analysis of plasma membrane (PM) fraction isolated from primary hepatocytes
Primary hepatocyte PM isolation was performed as previously described (Das et al., 2014; Das et al., 2013); see Supplemental Experimental Procedures.
Intracellular cholesterol trafficking
Trafficking of lysosomal FC to the PM was measured as described in Supplemental Experimental Procedures (Sugii et al., 2003).
Lipidomic analyses
Liver lipids were extracted from chow-fed mice (Folch et al., 1957) for mass spectrometry quantification of lysophospholipid (Bollinger et al., 2010) and phospholipid head group and fatty acyl species (Sorci-Thomas et al., 2012); see Supplemental Experimental Procedures.
Immunofluorescence
Immunofluorescent staining was performed as described in Supplemental Experimental Procedures (Tamura and Yui, 2014).
Statistics
Results are presented as mean ± the standard error of the mean (SEM). Data were analyzed using an unpaired two-tailed Student’s t-test, one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test, or two-way ANOVA with Bonferroni’s multiple comparison test. When unequal variance among groups was present, data were log transformed before performing ANOVA. All analyses were performed using GraphPad Prism 7 software. Significant differences are denoted as follows: *p<0.05, **p<0.01, ***p<0.001, ***p<0.0001; for multiple comparisons, different lowercase letters denote significant differences (p<0.05)
Supplementary Material
Table S1- Significantly down-regulated genes based on RNA-seq in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S2- Significantly up-regulated genes based on RNA-seq in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S3- Pathway enrichment analysis using Enrichr for genes down-regulated in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S4- Pathway enrichment analysis using Enrichr for genes up-regulated in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Highlights.
Hepatocyte ABCA1 regulates lysosome free cholesterol to plasma membrane trafficking
Plasma membrane free cholesterol is decreased in the absence of hepatocyte ABCA1
Glucose metabolism is responsive to insulin in the absence of hepatocyte ABCA1
Hepatocyte-specific Abca1 deletion protects against high fat diet-induced steatosis
In Brief.
Key et al. find that deletion of hepatocyte ABCA1 leads to decreased lysosomal free cholesterol trafficking to the plasma membrane and attenuated cholesterol biosynthesis, resulting in decreased insulin signaling to mTORC1 and attenuated lipogenesis. However, gluconeogenesis remains responsive. Mice lacking hepatocyte ABCA1 are resistant to high fat diet-induced hepatosteatosis.
Acknowledgments
This work was supported by NIH grants R01 HL119962 (J.S.P.), R01DK105965 (P.S.), HL48044 (T.O.), and HL109650 (SB). We gratefully acknowledge services provided by: 1) the Lipid, Lipoprotein and Atherosclerosis Analysis Laboratory of Internal Medicine Department/Section on Molecular Medicine, and 2) the Proteomics and Metabolomics Shared Resource, and 3) the Cell and Viral Vector Core Laboratory (supported in part by NCI P30 CA121291-37) of the Comprehensive Cancer Center at Wake Forest School of Medicine. We also acknowledge the editorial assistance of Karen Klein, MA, in the Wake Forest Clinical and Translational Science Institute (UL1 TR001420; PI: McClain).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS- RNA sequencing files are deposited in the GEO repository (GSE96093).
SUPPLEMENTAL INFORMATION Supplemental information including Supplemental Experimental Procedures, five figures, and four tables can be found with this article online.
AUTHOR CONTRIBUTIONS
Chia-Chi C. Key: C.C.K. designed and performed all the experiments and analysis except RNA-sequencing study. C.C.K. wrote the manuscript.
Mingxia Liu: M.L. assisted with the experiments of indirect calorimetry, in vivo insulin signaling analysis, and primary hepatocyte isolation, and conducted the experiment of in vivo determination of triglyceride secretion. M.L. generated preliminary data.
C. Lisa Kurtz: C.L.K. performed real time-PCR experiments and analysis and assisted with RNA-sequencing analysis
Soonkyu Chung: S.C. initiated the project and generated preliminary data.
Elena Boudyguina: E.B. assisted with real-time PCR analysis.
Timothy A. Dinh: T.A.D. assisted with RNA-sequencing analysis.
Alexander Bashore: A.B. prepared [3H]-cholesteryl oleate radiolabeled low density lipoproteins.
Peter E. Phelan: P.E.P. assisted in studying SREBP processing.
Timothy F. Osborne: T.F.O. provided SREBP1 antibody and assisted in studying SREBP processing.
Sudha B. Biddinger: S.B.B. provided liver samples of liver-specific insulin receptor knockout mice, assisted in studying insulin signaling, and edited the manuscript.
Xuewei Zhu: X.Z. assisted in measuring cholesterol and lipid raft content.
Praveen Sethupathy: P.S. designed RNA-sequencing experiments and performed analysis.
Barry I. Freedman: B.I.F, assisted in immunofluorescence experiments and manuscript editing.
Lijun Ma: L.M. assisted in immunofluorescence experiments.
John S. Parks: J.S.P. designed all the experiments except RNA-sequencing study.
J.S.P. wrote the manuscript.
Competing financial interests- none
References
- Adiels M, Boren J, Caslake MJ, Stewart P, Soro A, Westerbacka J, Wennberg B, Olofsson SO, Packard C, Taskinen MR. Overproduction of VLDL1 driven by hyperglycemia is a dominant feature of diabetic dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2005;25:1697–1703. doi: 10.1161/01.ATV.0000172689.53992.25. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Seo YK, Datta S, Shin DJ, Osborne TF. Selective binding of sterol regulatory element-binding protein isoforms and co-regulatory proteins to promoters for lipid metabolic genes in liver. J. Biol. Chem. 2008;283:15628–15637. doi: 10.1074/jbc.M800391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Zhu X, Duong M, Boudyguina EY, Wilson MD, Gebre AK, Parks JS. Liver ABCA1 deletion in LDLrKO mice does not impair macrophage reverse cholesterol transport or exacerbate atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2013;33:2288–2296. doi: 10.1161/ATVBAHA.112.301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel PE. Lipid rafts and insulin signaling. American Journal of Physiology -Endocrinology and Metabolism. 2002;282:E1–E10. doi: 10.1152/ajpendo.2002.282.1.E1. [DOI] [PubMed] [Google Scholar]
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Bollinger JG, Ii H, Sadilek M, Gelb MH. Improved method for the quantification of lysophospholipids including enol ether species by liquid chromatography-tandem mass spectrometry. J. Lipid Res. 2010;51:440–447. doi: 10.1194/jlr.D000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus Total Insulin Resistance: A Pathogenic Paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Islet alpha cells and glucagon--critical regulators of energy homeostasis. Nat. Rev. Endocrinol. 2015;11:329–338. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- Cheng Z, White MF. kNOXing on the door of selective insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2012;32:1063–1065. doi: 10.1161/ATVBAHA.112.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Timmins JM, Duong M, Degirolamo C, Rong S, Sawyer JK, Singaraja RR, Hayden MR, Maeda N, Rudel LL, et al. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem. 2010;285:12197–12209. doi: 10.1074/jbc.M109.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimon M, Kido T, Baba M, Oizumi T, Jimbu Y, Kameda W, Yamaguchi H, Ohnuma H, Tominaga M, Muramatsu M, et al. Association of the ABCA1 gene polymorphisms with type 2 DM in a Japanese population. Biochem. Biophys. Res. Commun. 2005;329:205–210. doi: 10.1016/j.bbrc.2005.01.119. [DOI] [PubMed] [Google Scholar]
- Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. In eLife. 2014 doi: 10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Goldstein JL, Anderson DD, Brown MS, Radhakrishnan A. Use of mutant 125I-perfringolysin O to probe transport and organization of cholesterol in membranes of animal cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10580–10585. doi: 10.1073/pnas.1309273110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J. Lipid Res. 2014a;55:516–523. doi: 10.1194/jlr.M045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W, Karasinska JM, Ruddle P, Hayden MR. Hepatic ABCA1 Expression Improves β-Cell Function and Glucose Tolerance. Diabetes. 2014b;63:4076–4082. doi: 10.2337/db14-0548. [DOI] [PubMed] [Google Scholar]
- Engelking LJ, Kuriyama H, Hammer RE, Horton JD, Brown MS, Goldstein JL, Liang G. Overexpression of Insig-1 in the livers of transgenic mice inhibits SREBP processing and reduces insulin-stimulated lipogenesis. J. Clin. Invest. 2004;113:1168–1175. doi: 10.1172/JCI20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Frechin M, Stoeger T, Daetwyler S, Gehin C, Battich N, Damm EM, Stergiou L, Riezman H, Pelkmans L. Cell-intrinsic adaptation of lipid composition to local crowding drives social behaviour. Nature. 2015;523:88–91. doi: 10.1038/nature14429. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas Joel T, Miao J, Chanda D, Wang Y, Zhao E, Haas Mary E, Hirschey M, Vaitheesvaran B, Farese Robert V, Jr, Kurland Irwin J, et al. Hepatic Insulin Signaling Is Required for Obesity-Dependent Expression of SREBP-1c mRNA but Not for Feeding-Dependent Expression. Cell Metab. 2012;15:873–884. doi: 10.1016/j.cmet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh FK, Green MF, Koves TR, Hirschey MD. Measurement of fatty acid oxidation rates in animal tissues and cell lines. Methods Enzymol. 2014;542:391–405. doi: 10.1016/B978-0-12-416618-9.00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. In eLife. 2015:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 2012;23:65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL. Simultaneous labeling of lipoprotein intracellular trafficking in pigeon monocyte-derived macrophages. Am. J. Pathol. 1997;150:1113–1124. [PMC free article] [PubMed] [Google Scholar]
- Kreamer BL, Staecker JL, Sawada N, Sattler GL, Hsia MT, Pitot HC. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986;22:201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- Kruit JK, Wijesekara N, Fox JE, Dai XQ, Brunham LR, Searle GJ, Morgan GP, Costin AJ, Tang R, Bhattacharjee A, et al. Islet cholesterol accumulation due to loss of ABCA1 leads to impaired exocytosis of insulin granules. Diabetes. 2011;60:3186–3196. doi: 10.2337/db11-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 2006;281:36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- Lasserre R, Guo XJ, Conchonaud F, Hamon Y, Hawchar O, Bernard AM, Soudja SM, Lenne PF, Rigneault H, Olive D, et al. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat. Chem. Biol. 2008;4:538–547. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- Leavens KF, Birnbaum MJ. Insulin signaling to hepatic lipid metabolism in health and disease. Crit. Rev. Biochem. Mol. Biol. 2011;46:200–215. doi: 10.3109/10409238.2011.562481. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Dong J, Ding T, Kuo MS, Cao G, Jiang XC, Li Z. Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor gamma2 suppression. Arterioscler Thromb Vasc Biol. 2013;33:1513–1520. doi: 10.1161/ATVBAHA.113.301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J. Clin. Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohturfft A, Yabe D, Goldstein JL, Brown MS, Espenshade PJ. Regulated step in cholesterol feedback localized to budding of SCAP from ER membranes. Cell. 2000;102:315–323. doi: 10.1016/s0092-8674(00)00037-4. [DOI] [PubMed] [Google Scholar]
- Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- Porchay I, Pean F, Bellili N, Royer B, Cogneau J, Chesnier MC, Caradec A, Tichet J, Balkau B, Marre M, et al. ABCA1 single nucleotide polymorphisms on high-density lipoprotein-cholesterol and overweight: the D.E.S.I.R. study. Obesity (Silver Spring, Md.) 2006;14:1874–1879. doi: 10.1038/oby.2006.217. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like Control of SREBP-2 Transport Triggered by Small Changes in ER Cholesterol: A Delicate Balance. Cell Metab. 2008;8:512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robenek H, Schmitz G. Abnormal processing of Golgi elements and lysosomes in Tangier disease. Arterioscler. Thromb. 1991;11:1007–1020. doi: 10.1161/01.atv.11.4.1007. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Assmann G, Robenek H, Brennhausen B. Tangier disease: a disorder of intracellular membrane traffic. Proc. Natl. Acad. Sci. U. S. A. 1985;82:6305–6309. doi: 10.1073/pnas.82.18.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Sorci-Thomas MG, Owen JS, Fulp B, Bhat S, Zhu X, Parks JS, Shah D, Jerome WG, Gerelus M, Zabalawi M, et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugii S, Reid PC, Ohgami N, Du H, Chang TY. Distinct endosomal compartments in early trafficking of low density lipoprotein-derived cholesterol. J. Biol. Chem. 2003;278:27180–27189. doi: 10.1074/jbc.M300542200. [DOI] [PubMed] [Google Scholar]
- Tamura A, Yui N. Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating Niemann-Pick type C disease. Sci. Rep. 2014;4:4356. doi: 10.1038/srep04356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Betters JL, Lord CC, Brown AL, Marshall S, Ferguson D, Sawyer J, Davis MA, Melchior JT, Blume LC, et al. The serine hydrolase ABHD6 Is a critical regulator of the metabolic syndrome. Cell reports. 2013;5:508–520. doi: 10.1016/j.celrep.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchenell PM, Chu Q, Monks BR, Birnbaum MJ. Hepatic insulin signalling is dispensable for suppression of glucose output by insulin in vivo. Nature communications. 2015;6:7078. doi: 10.1038/ncomms8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, Chen H, Monks BR, Chen J, Rabinowitz JD, et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016;23:1154–1166. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Bykov I, Hermansson M, Jokitalo E, Somerharju P, Ikonen E. Defective insulin receptor activation and altered lipid rafts in Niemann-Pick type C disease hepatocytes. Biochem. J. 2005;391:465–472. doi: 10.1042/BJ20050460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heino S, Mansson JE, Fredman P, Kuismanen E, Vaarala O, Ikonen E. Dynamic association of human insulin receptor with lipid rafts in cells lacking caveolae. EMBO Rep. 2002;3:95–100. doi: 10.1093/embo-reports/kvf010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Molina MT, Aguilar-Salinas CA, Rodriguez-Cruz M, Riano D, Villalobos-Comparan M, Coral-Vazquez R, Menjivar M, Yescas-Gomez P, Konigsoerg-Fainstein M, Romero-Hidalgo S, et al. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes. 2007;56:1881–1887. doi: 10.2337/db06-0905. [DOI] [PubMed] [Google Scholar]
- Villarreal-Molina MT, Flores-Dorantes MT, Arellano-Campos O, Villalobos-Comparan M, Rodriguez-Cruz M, Miliar-Garcia A, Huertas-Vazquez A, Menjivar M, Romero-Hidalgo S, Wacher NH, et al. Association of the ATP-binding cassette transporter A1 R230C variant with early-onset type 2 diabetes in a Mexican population. Diabetes. 2008;57:509–513. doi: 10.2337/db07-0484. [DOI] [PubMed] [Google Scholar]
- Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77:53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Wu X, Williams KJ. NOX4 pathway as a source of selective insulin resistance and responsiveness. Arterioscler. Thromb. Vasc. Biol. 2012;32:1236–1245. doi: 10.1161/ATVBAHA.111.244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Brown MS, Goldstein JL. Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12753–12758. doi: 10.1073/pnas.162488899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Iwamoto N, Rogers MA, Abe-Dohmae S, Fujimoto T, Chang CCY, Ishigami M, Kishimoto T, Kobayashi T, Ueda K, et al. Deficiency in the Lipid Exporter ABCA1 Impairs Retrograde Sterol Movement and Disrupts Sterol Sensing at the Endoplasmic Reticulum. J. Biol. Chem. 2015;290:23464–23477. doi: 10.1074/jbc.M115.662668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubica A, Plazzo AP, Stockl M, Trombik T, Hamon Y, Muller P, Pomorski T, Herrmann A, Chimini G. Functional implications of the influence of ABCA1 on lipid microenvironment at the plasma membrane: a biophysical study. FASEB J. 2009;23:1775–1785. doi: 10.1096/fj.08-122192. [DOI] [PubMed] [Google Scholar]
- Zha X, Gauthier A, Genest J, McPherson R. Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J. Biol. Chem. 2003;278:10002–10005. doi: 10.1074/jbc.C300024200. [DOI] [PubMed] [Google Scholar]
- Zha X, Genest J, Jr, McPherson R. Endocytosis is enhanced in Tangier fibroblasts: possible role of ATP-binding cassette protein A1 in endosomal vesicular transport. J. Biol. Chem. 2001;276:39476–39483. doi: 10.1074/jbc.M105067200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Westcott MM, Bi X, Liu M, Gowdy KM, Seo J, Cao Q, Gebre AK, Fessler MB, Hiltbold EM, et al. Myeloid cell-specific ABCA1 deletion protects mice from bacterial infection. Circ. Res. 2012;111:1398–1409. doi: 10.1161/CIRCRESAHA.112.269043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1- Significantly down-regulated genes based on RNA-seq in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S2- Significantly up-regulated genes based on RNA-seq in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S3- Pathway enrichment analysis using Enrichr for genes down-regulated in livers of HSKO mice relative to fl/fl mice, related to Figure 1.
Table S4- Pathway enrichment analysis using Enrichr for genes up-regulated in livers of HSKO mice relative to fl/fl mice, related to Figure 1.