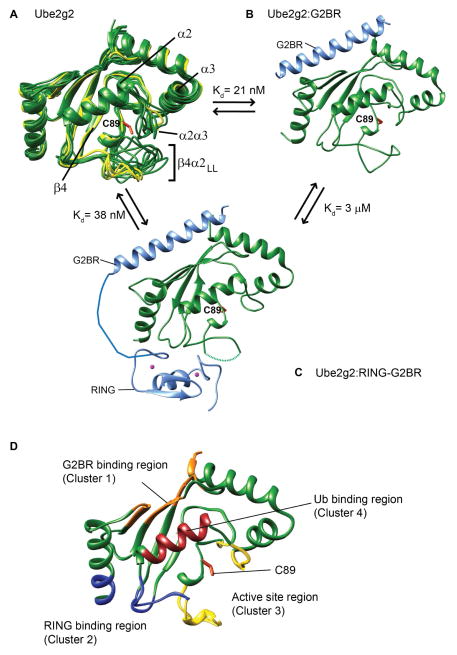
Figure 1. Conformational changes and binding sites in Ube2g2 upon interaction with gp78.
(A) Ube2g2 NMR structures (20 conformers in green, PDB: 2KLY) are superimposed with three structures from the crystal asymmetrical unit of Ube2g2 (yellow, PDB: 2CYX (Arai et al., 2006)). Binary Ube2g2:G2BR complex (B) (PDB: 3H8K) and ternary Ube2g2:RING-G2BR complex (C) (PDB: 4LAD), where Ube2g2 is green and G2BR and RING are light blue. The missing electron density of the loop in (C) is indicated by a dashed line. (D) The regions critical for binding and activity in Ube2g2 are depicted as: ‘backside’ G2BR binding site in orange, RING binding site in blue, and Ub binding in dark red. Regions around the active site are colored in yellow. The active site cysteine side-chain is shown in orange in (A–D).