Abstract
Background
African Americans (AA) develop chronic kidney disease (CKD) and pulmonary hypertension (PH) at disproportionately high rates. Little is known whether PH heightens the risk of heart failure (HF) admission or mortality among CKD patients, including non-end stage renal disease.
Methods and Results
We analyzed AA participants with CKD (eGFR<60 ml/min/1.73m2 or urine albumin/creatinine >30 mg/g) and available echocardiogram-derived pulmonary artery systolic pressure (PASP) from the Jackson Heart Study (N=408). We used Cox models to assess whether PH (PASP>35 mmHg) was associated with higher rates of HF hospitalization and mortality. In a secondary, cross-sectional analysis, we examined the relationship between cystatin C (a marker of renal function) and PASP and potential mediators including b-type natriuretic peptide [BNP] and endothelin-1. In our cohort, the mean age was 63±13 years, 70% were female, 78% had hypertension, and 22% had PH. 85% had an eGFR>30 ml/min/m2. During follow-up, 13% were hospitalized for HF and 27% died. After adjusting for potential confounders, including BNP, PH was associated with HF hospitalization (HR 2.37, 95% CI: 1.15–4.86) and the combined outcome of HF hospitalization or mortality (HR 1.84, CI: 1.09–3.10). Log cystatin C was directly associated with PASP (adjusted β=2.5 [95% CI 0.8–4.1] per standard deviation change in cystatin C). Mediation analysis showed that BNP and endothelin-1 explained 56% and 40%, respectively, of the indirect effects between cystatin C and PASP.
Conclusions
Among AA with CKD, PH, which is likely pulmonary venous hypertension, was associated with a higher risk of HF admission and mortality.
Keywords: Pulmonary hypertension, chronic kidney disease, heart failure, echocardiography
INTRODUCTION
Chronic kidney disease (CKD) affects more than 10% of all United States adults and is associated with high morbidity and mortality.1 The role of pulmonary hypertension (PH) as a potential contributor to adverse events in CKD remains unclear. Furthermore, African Americans develop both CKD and PH independently at disproportionate rates compared to other ethnicities.2, 3 However, data evaluating the relationship between CKD and PH, particularly in African American populations, are limited. The associations between CKD, PH, and adverse cardiovascular events have been explored mostly in patients with end-stage renal disease (ESRD) or post-renal transplant, and limited data are available in earlier stages of CKD.4, 5 These studies in ESRD and post-transplantation are small, show significant variability in the definition of PH, or were performed after surgical fistula procedures for hemodialysis.6–8 Understanding the link between earlier stages of renal disease and PH may allow for timely targeted therapy and prevention of disease progression. Furthermore, whether CKD patients with PH are at higher risk of heart failure (HF) hospitalization or mortality merits further attention given limited published data.4, 5
We sought to assess the association of CKD with estimated pulmonary artery systolic pressure (PASP) and subsequent morbidity and mortality in African Americans. We hypothesized that in patients with baseline CKD, PH would be associated with higher HF hospitalization and mortality rates. We also hypothesized that measures of glomerular filtration rate (GFR) would be inversely associated with PASP.
METHODS
Study Population
The Jackson Heart Study is a prospective, population-based cohort study of 5,306 self-identified African American participants recruited from 2000–2004 in Jackson, MS. The methodology of the study has been previously reported.9 In brief, participants answered predefined questionnaires and underwent comprehensive echocardiography during the first examination period from 2000–2004. Participants have been followed for two subsequent examinations, with the last follow-up occurring in 2012. All Jackson Heart Study participants gave written informed consent, and the Jackson Heart Study was approved by the University of Mississippi Medical Center review board. The current analysis was also approved by the Partners Healthcare institutional review board. For the present analysis, study participants with CKD as defined below and with measurable PASP were included.
Clinical Characteristics
Demographic, clinical, physical exam, and laboratory data were collected during the initial JHS visit. At baseline, information on age, sex, body-mass index (BMI), heart rate, systolic blood pressure, diastolic blood pressure, comorbidities, and cardiovascular medications was collected. Diabetes mellitus was defined by a history of diabetes mellitus, use of diabetes mellitus medications, or a fasting blood glucose ≥126 mg/dL. Presence of systemic hypertension was defined by a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Atrial fibrillation was based upon direct clinical examination, while myocardial infarction, chronic lung disease (including asthma or chronic obstructive pulmonary disease), alcohol use, and smoking were obtained by self-report. History of HF was considered present if the participant answered in the affirmative to the following question: “Has a doctor ever said you had HF or congestive HF?”.
Laboratory markers include serum creatinine, blood urea nitrogen, b-type natriuretic peptide (BNP), plasma endothelin-1, low density lipoprotein, cystatin C, 25-hydroxy vitamin D3, spot urine albumin, spot urine creatinine as well as 24-hour collection of albumin and creatinine.
Chronic kidney disease was defined using the Kidney Disease Improving Global Outcomes (KDIGO) criteria, which included either a reduced estimated GFR (eGFR) ≤ 60 ml/min/1.73m2 or presence of albuminuria [urine albumin to creatinine ratio (UACR) ≥ 30 mg/g]. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. Albuminuria measurements were derived from either 24 hour collection or spot collection; previous analysis in the JHS has shown high correlation between the two tests in participants who underwent collection of both tests.10 Pulmonary function testing includes forced expiratory volume in 1 second and forced vital capacity.
Echocardiography
Echocardiography (including 2D, M-mode, and Doppler imaging) was acquired on all study participants by certified ultrasonographers and interpreted by cardiologists at the University of Mississippi Medical Center.11, 12 Windows from the parasternal, apical, and subcostal views were recorded. The reading cardiologist was blinded to the clinical characteristics of the study participants. PASP was calculated by adding 5 mmHg (assumed right atrial pressure) to the tricuspid regurgitant jet peak. Assessment and categorization of left ventricular (LV) ejection fraction, LV hypertrophy, valvular disease, and other measurements can be found elsewhere.13 Diastolic function was assessed using early diastolic (E) and late/atrial diastolic (A) transmitral velocities, E/A ratio, and isovolumic relaxation time.
Outcome variables
The primary outcome of the study is a composite of HF hospitalization and all-cause mortality. Secondary outcomes include HF hospitalization as well as all-cause mortality. Follow-up telephone interviews were conducted to obtain incident information on hospitalizations. All HF hospitalizations were adjudicated starting on January 1st, 2005, using history, physical exam, laboratory analysis, and medication use data by a trained abstractor. HF hospitalizations defined as either probable or definite were included. Data on the HF outcome are missing in 106 participants who self-reported HF hospitalization or had an uncertain HF hospitalization status before incidence assessment start time point. Hence, analytical sample for HF hospitalization was 302. Death was determined by family member interviews, physician short questionnaires, and coroner records.14
Statistical Analysis
Clinical, laboratory, and echocardiographic data are stratified by the presence or absence of PH. Continuous data are presented as mean ± standard deviation. Categorical variables are presented as a count and percentage. Skewed data are presented as median and 25th–75th percentile and log-transformed for regression analyses. We compared groups using t-tests for continuous variables (or non-parametric equivalent) and Chi-squared (or Fisher’s) tests for categorical variables.
We investigated the association of PH with HF hospitalization and mortality using cumulative incidence curves and Cox proportional hazard models. We estimated cumulative incidence curves as (1 - the Kaplan-Meier estimator). For Cox regression, we constructed sequential models: after the crude model, Model 1 adjusted for age, sex, diabetes mellitus, eGFR, and LV ejection fraction. Model 2 included all variables used in Model 1 with addition of b-type natriuretic peptide (BNP). Candidate covariates were chosen based on clinical relevance (pre-specified based on face validity) or association with HF or mortality (either in prior studies or in our study).15, 16 We used the partial likelihood ratio test within nested models to support that we did not miss important confounding variables for covariate selection. Person-time of follow up was computed from baseline to the first occurrence of the primary outcome, loss to follow-up, end of study period, or death. In sensitivity analysis, we excluded patients with prevalent HF or use of hemodialysis. Because incident ESRD is not documented in a consistent fashion in the JHS, there was no explicit exclusion of these patients in the present analysis. We explored interactions by sex and higher eGFR value (defined as >30 ml/min/1.73m2). A p-value <0.05 was significant for further exploration.
For cross-sectional analysis, we performed multivariable-adjusted regression models to assess whether cystatin C was associated with PASP. We used cystatin C as the surrogate marker of GFR given that it is more strongly correlated to true GFR than creatinine and possesses superior test characteristics.17 Using a model that established risk factors for PH in the Jackson Heart Study, we adjusted for age, sex, BMI, hypertension, diabetes, coronary heart disease, severe mitral/aortic valvular heart disease, chronic lung disease, spirometry profile (normal, obstructive, and restrictive), and a LV ejection fraction < 50% (pulse pressure was initially omitted since it was later entered as a mediating factor).15 Beta-coefficients are reported per standard deviation increase in the parameter.
To examine the extent to which the relationship between PASP and renal function is explained by potential intermediate factors, we estimated the beta-coefficient after addition of four covariates. Based upon putative mechanisms, potential mediators included pulse pressure (marker of arterial stiffness), 25-hydroxy vitamin D level (associated with vascular hyper-proliferation), BNP (marker of volume overload), and plasma endothelin-1 level (marker of endothelial dysfunction).4, 18, 19 We calculated the proportion explained by the intermediate factors as follows: 100% × [Beta-coefficientmodel – Beta-coefficientmodel+intermediate factor]/[Beta-coefficientmodel].20
We performed multiple imputation analyses for missing model covariates using the Markov Chain Monte Carlo method in PROC MI and PROC MIANALYZE in SAS. We imputed all missing data using 10 sets of values using non-missing predictors and ultimately pooled using Rubin’s combination rules. A two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS v.9.4.
RESULTS
Characteristics of Study Participants
Out of the 728 participants with CKD, 408 had available PASP data and were included in the study population (Table 1). Nearly a quarter of the study participants had PH at baseline (88/408, 22%). The mean age was 63±13 years and 70% were female, 78% had hypertension, and 38% had diabetes mellitus. Only 9% had prevalent HF, while 3% were dialysis-dependent. Mean systolic blood pressure was 132±19/mmHg, and the mean BMI was 31.8±7.2 kg/m2. Median BNP was 16 [25th–75th percentile: 6–42] pg/ml, and most participants had an eGFR >30 ml/min/1.73m2 (85%). Individuals with PH were older, more often female, more often had hypertension and atrial fibrillation, had higher systolic blood pressure and lower diastolic blood pressure, and had lower eGFR, higher UACR, and higher BNP.
Table 1.
Characteristics of the Chronic Kidney Disease Cohort According to Presence of Pulmonary Hypertension
| Characteristic | All Participants (N=408) |
Pulmonary Hypertension (N=88) |
No Pulmonary Hypertension (N=320) |
P-value |
|---|---|---|---|---|
| Age, y | 63±13 | 69±10 | 61±13 | <0.001 |
| Female, n (%) | 284 (70) | 69 (78) | 215 (67) | 0.043 |
| Comorbidities, n (%) | ||||
| Hypertension | 317 (78) | 77 (87) | 240 (75) | 0.013 |
| Diabetes mellitus | 153 (39) | 34 (41) | 119 (39) | 0.75 |
| Coronary heart disease | 75 (18) | 19 (22) | 56 (18) | 0.38 |
| Atrial fibrillation | 7 (2) | 4 (5) | 3 (1) | 0.042 |
| Chronic lung disease | 17 (6) | 3 (5) | 14 (6) | 0.99 |
| Heart failure | 35 (9) | 8 (9) | 27 (8) | 0.85 |
| Dialysis | 14 (3) | 4 (5) | 10 (3) | 0.51 |
| Alcohol history | 0.003 | |||
| • Never drinker | 133 (33) | 42 (48) | 91 (28) | |
| • Former drinker | 142 (34) | 24 (27) | 118 (37) | |
| • Current drinker | 133 (33) | 22 (25) | 111 (35) | |
| Smoking history | 0.55 | |||
| • Never smoker | 275 (68) | 63 (72) | 212 (67) | |
| • Former smoker | 86 (21) | 15 (17) | 71 (22) | |
| • Current smoker | 44 (11) | 10 (11) | 34 (11) | |
| Medications, n (%) | ||||
| Anti-hypertensive medication | 210 (51) | 50 (57) | 160 (50) | 0.26 |
| ACE-inhibitor | 84 (21) | 21 (24) | 63 (20) | 0.39 |
| Angiotensin receptor blocker | 39 (10) | 8 (9) | 31 (10) | 0.87 |
| Beta-blocker | 81 (20) | 19 (22) | 62 (19) | 0.64 |
| Diuretic | 202 (50) | 47 (53) | 155 (48) | 0.47 |
| Physical examination | ||||
| Systolic blood pressure, mm Hg | 132±19 | 137±21 | 131±19 | 0.006 |
| Diastolic blood pressure, mm Hg | 75±10 | 72±9 | 75±10 | 0.005 |
| Heart rate, beats per minute | 68±11 | 67±12 | 68±11 | 0.59 |
| Body-mass index, kg/m2 | 31.8±7.2 | 32.8±7.2 | 31.5±6.7 | 0.22 |
| Laboratory data | ||||
| eGFR, ml/min/1.73m2 | 68±32 | 60±30 | 70±32 | 0.012 |
| eGFR classification, n (%) | 0.23 | |||
| • eGFR>60 ml/min/1.73m2 | 180 (44) | 32 (36) | 148 (46) | |
| • 45<eGFR≤60 ml/min/1.73m2 | 120 (30) | 27 (31) | 93 (29) | |
| • 30<eGFR≤45 ml/min/1.73m2 | 45 (11) | 12 (14) | 33 (10) | |
| • 15<eGFR≤ ml/min/1.73m2 | 21 (5) | 8 (9) | 13 (4) | |
| • eGFR≤15 ml/min/1.73m2 | 42 (10) | 9 (10) | 33 (10) | |
| Urine albumin-creatinine ratio, mg/g* | 48 (18, 108) | 61 (17, 218) | 45 (18, 94) | 0.14 |
| Cystatin C, mg/L* | 0.93 (0.74, 1.21) | 1.04 (0.83, 1.41) | 0.90 (0.72, 1.62) | 0.003 |
| 25-hydroxy vitamin D, ng/ml | 13±6 | 13±5 | 13±6 | 0.49 |
| Low density lipoprotein, mg/dl | 128±41 | 129±44 | 128±40 | 0.81 |
| Plasma endothelin-1, pg/ml* | 1.4 (1.1, 1.8) | 1.7 (1.1, 2.3) | 1.4 (1.1, 1.8) | 0.007 |
| B-type natriuretic peptide, pg/ml* | 16 (6, 42) | 39 (11, 83) | 13 (5, 28) | <0.001 |
| Spirometry | ||||
| FEV1/FVC | 0.79±0.09 | 0.78±0.08 | 0.80±0.10 | 0.07 |
| Echocardiographic parameter | ||||
| Pulmonary artery systolic pressure, mmHg | 30±8 | 42±7 | 27±5 | <0.001 |
| Tricuspid regurgitation | <0.001 | |||
| • None | 8 (2) | 0 (0) | 8 (3) | |
| • Mild | 315 (77) | 51 (59) | 264 (83) | |
| • Moderate | 63 (15) | 24 (28) | 39 (12) | |
| • Severe | 21 (5) | 12 (14) | 9 (3) | |
| LV end-diastolic diameter, mm | 50±5 | 51±6 | 50±5 | 0.20 |
| LV end-systolic diameter, mm | 31±6 | 30±6 | 31±6 | 0.78 |
| LV mass index, g/m2 | 41.9±13.1 | 48.6±16.4 | 40.0±11.5 | 0.001 |
| LV hypertrophy, n (%) | 49 (20) | 22 (41) | 27 (14) | <0.001 |
| Left atrial diameter, cm | 37±5 | 39±6 | 36±5 | 0.002 |
| LV ejection fraction, % | 61±9 | 61±12 | 62±8 | 0.94 |
| E/A ratio | 1.00±0.38 | 1.07±0.50 | 0.99±0.34 | 0.14 |
| Isovolumic relaxation time, ms | 98±24 | 94±24 | 99±24 | 0.07 |
ACE, angiotensin-converting enzyme inhibitor; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 second; FVC; forced vital capacity; LV, left ventricular.
Data presented as median (25th–75th percentile).
The mean pulmonary artery pressure was 30±8 (minimum to maximum: 11–66) mmHg. The average LV dimensions fell within normal limits (LV end-systolic diameter 31±6 mm; LV end-diastolic diameter 50±5 mm; LV mass index 41.9±13.1 kg/m2), though one-fifth had evidence of LV hypertrophy. The ejection fraction was 61±9%. Individuals with PH had more significant tricuspid regurgitation, higher LV mass index and larger left atrial diameter, but no difference was observed in LV dimensions, ejection fraction, E/A ratio, and isovolumic relaxation time. Very few participants had any evidence of right atrial or right ventricular enlargement (4 and 1 participants, respectively).
Association of PH with HF Hospitalization and Mortality
The mean follow-up was 6.71±2.18 years for HF hospitalization and 8.35±3.26 years for death. 27% and 10% of the participants with and without PH were hospitalized for HF, while 44% and 22% died during follow-up, respectively. After adjusting for covariates in model 1, PH was still associated with the composite outcome (HR 2.18, 95% CI 1.20, 3.70) (Table 2). Additional adjustment for BNP in model 2 did not eliminate the association. Exclusion of participants with prevalent HF or hemodialysis yielded similar results. For secondary outcomes, PH was associated with higher cumulative incidence for heart failure and mortality (p<0.001, Fig 1). Table 3 shows that PH was associated with higher risk for HF hospitalization (HR 2.90, 95% CI 1.45, 5.81) and mortality (HR 1.83, 95% CI 1.21, 2.76) after adjustment for variables in model 1. Additional adjustment for BNP in model 2 did not alter the statistical significance for HF hospitalization (p=0.019), though it did for all-cause mortality (p=0.16). Table S1 shows no interaction by sex or eGFR (p>0.05 for all outcomes).
Table 2.
HR (95% CI) for Heart Failure Hospitalization or Death by Pulmonary Hypertension Status in the Jackson Heart Study
| Outcome | Events N (%) |
Crude | Model 1* | Model 2† | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | ||
| Heart failure hospitalization or death‡ | |||||||
| • No pulmonary hypertension | 52 (21) | 1.0 (ref) | — | 1.0 (ref) | — | 1.0 (ref) | — |
| • Pulmonary hypertension present | 28 (47) | 2.19 (1.36, 3.53) | 0.001 | 2.18 (1.20, 3.70) | 0.003 | 1.84 (1.09, 3.10) | 0.02 |
Model 1 adjusted for age, sex, diabetes mellitus, estimated glomerular filtration rate, and ejection fraction.
Model 2 adjusted for b-type natriuretic peptide in addition to covariates in model 1.
Because of missing data on heart failure hospitalization (N=106), the total N for this analysis is 302 (242 in the no PH group and 60 in the PH group). Please see method sections for more details.
HR, hazard ratio; CI, confidence interval.
Figure 1. Cumulative Incidence of Heart Failure Hospitalization and All-Cause Mortality Stratified by the Presence of Pulmonary Hypertension in Patients with Chronic Kidney Disease.
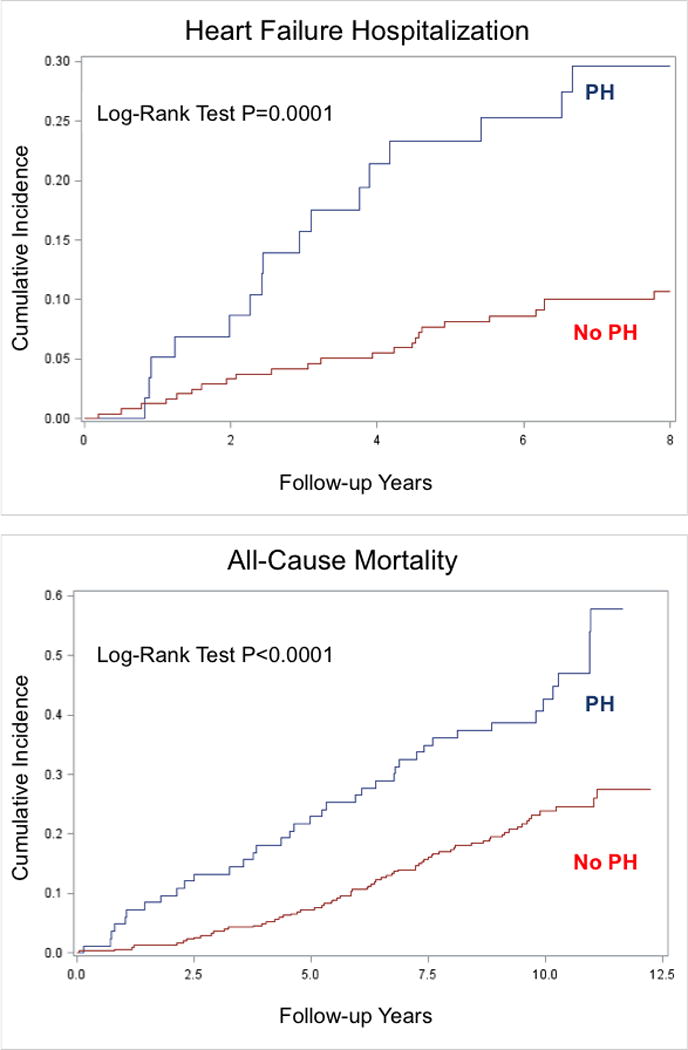
Pulmonary hypertension (PH) is associated with greater risk for heart failure hospitalization and all-cause mortality in participants with pre-existing chronic kidney disease. P-values are shown for the log-rank test.
Table 3.
HR (95% CI) for Heart Failure Hospitalization and Death by Pulmonary Hypertension Status in the Jackson Heart Study
| Outcome | Events N (%) |
Crude | Model 1* | Model 2† | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | ||
| Heart failure hospitalization‡ | |||||||
| • No pulmonary hypertension | 24 (10) | 1.0 (ref) | — | 1.0 (ref) | — | 1.0 (ref) | — |
| • Pulmonary hypertension present | 16 (27) | 3.19 (1.70, 6.02) | <0.001 | 2.90 (1.45, 5.81) | 0.003 | 2.37 (1.15, 4.86) | 0.0189 |
| Death | |||||||
| • No pulmonary hypertension | 70 (22) | 1.0 (ref) | — | 1.0 (ref) | — | 1.0 (ref) | — |
| • Pulmonary hypertension present | 39 (44) | 2.39 (1.62, 3.54) | <0.001 | 1.83 (1.21, 2.76) | 0.004 | 1.37 (0.89, 2.13) | 0.16 |
Model 1 adjusted for age, sex, diabetes mellitus, estimated glomerular filtration rate, and ejection fraction.
Model 2 adjusted for b-type natriuretic peptide in addition to covariates in model 1.
Because of missing data on heart failure hospitalization (N=106), the total N for this analysis is 302 (242 in the no PH group and 60 in the PH group). Please see method sections for more details.
HR, hazard ratio; CI, confidence interval.
Association of Cystatin C with Pulmonary Artery Systolic Pressure on Mediation Analysis
After adjusting for age, sex, BMI, hypertension, diabetes, coronary heart disease, severe valvular heart disease, chronic lung disease, spirometry profile, and reduced ejection fraction, log cystatin C was associated with PASP (beta-coefficient per standard deviation change 2.5, 95% CI 0.8, 4.1), as shown in Table 4. Mediation analysis was performed to evaluate the contribution of several potential intermediate factors. LogBNP and plasma endothelin-1 levels explained 56% and 40%, respectively, of the indirect effects of the relationship between cystatin C and higher PASP; together, they explained 88% of the indirect effects.
Table 4.
Association of Cystatin C with Pulmonary Artery Systolic Pressure on Mediation Analysis in the Jackson Heart Study
| Dependent variable | Multivariable Adjustment* | Proportion of PASP Explained by Mediator |
|
|---|---|---|---|
|
| |||
| β-Coefficient (95% CI) |
P-value | ||
| Log Cystatin C (mg/L) | 2.5 (0.8, 4.1) | 0.0029 | - |
| Log Cystatin C + logBNP (pg/dl) | 1.1 (−0.6, 2.8) | 0.22 | 56% |
| Log Cystatin C + pulse pressure (mmHg) | 2.3 (0.7, 4.0) | 0.0047 | 8% |
| Log Cystatin C+ 25-hydroxy vitamin D level (ng/ml) | 2.5 (0.8, 4.1) | 0.0035 | 0% |
| Log Cystatin C+ log endothelin-1 level (pg/ml) | 1.5 (−0.3, 3.2) | 0.10 | 40% |
| Log Cystatin C+ log BNP + log endothelin-1 level | 0.3 (−1.5 2.1) | 0.74 | 88% |
| Log Cystatin C + all intermediary factors | 0.2 (−1.53, 1.97) | 0.81 | 92% |
PASP, pulmonary artery systolic pressure.
All models adjusted for age, sex, body-mass index, hypertension, diabetes, chronic lung disease, spirometry profile, coronary heart disease, severe mitral/aortic valvular heart disease, and reduced ejection fraction.
DISCUSSION
In an analysis of 408 African American participants from the Jackson Heart Study with long-term follow-up, we found that PH was common in an unselected cohort of CKD patients (22%) and was associated with a significantly higher risk for HF hospitalization and all-cause mortality. These associations persisted after adjusting for a number of covariates, including BNP (with the exception of all-cause mortality). In addition, cystatin C, a surrogate marker of GFR, was significantly associated with PASP on multivariable analysis. These data show that echocardiographic PH identifies a high-risk cohort of CKD patients beyond that predicted by BNP, and may offer insight into the relationship between CKD and adverse cardiovascular events.
Several studies7, 8, 18 have shown a relationship between CKD and PH in late stage renal disease, but very few studies have examined this relationship in earlier stage CKD,4 which is much more common. Only a very small percentage of patients in our study were dialysis-dependent, and the vast majority of participants had an eGFR>45 ml/min/1.73m2. Thus, the association between PH and adverse events found in this cohort demonstrates the adverse association with PH despite largely mild renal insufficiency. Notably, pulmonary pressures in our PH participants were only mildly elevated in most patients with PH (average PASP 42 mmHg), which demonstrates that even such pressures are valuable in identifying a high-risk phenotype. In two recent analyses of CKD patients, echocardiographic PH was present in a similar percentage of patients and also predicted adverse events, including HF.5, 21 These studies, however, were limited by a potential referral bias5, failed to adjust for BNP to show the additional benefits of PASP5, 21, and lacked additional laboratory data to understand the relationship between renal function and PASP5.
Elevated PASP in CKD patients may indicate a pre-clinical HF with preserved ejection fraction state. Interestingly, the median BNP in our cohort was 16 pg/ml (25th–75th percentile: 6–42), while those with echocardiographic PH had a median BNP of 39 pg/ml (25th–75th percentile: 11–83). Thus, the majority of patients in our study had a BNP<40 pg/ml even with echocardiographic PH and there was significant overlap in BNP values. This underscores two important points. First, the range of “normal” BNP is truly narrow. For instance, while some practitioners consider values less than 100 pg/ml to rule out HF, this cutoff only applies to acutely decompensated patients.22 In a study of elderly patients with stable heart failure, the average BNP level for those with diastolic HF was 56 pg/ml, while control patients had an average BNP of 3 pg/ml.23 Indeed, in another study of stable HF with preserved ejection fraction patients, BNP levels were less than 100 pg/mL in nearly 30%.24 Thus, BNP levels even greater than 40 pg/ml (or likely less) should at least raise concern for the progression to clinical signs and symptoms of heart failure. Secondly, because the range of BNP values in our study is narrow with significant overlap between those with and without echocardiographic PH, we have demonstrated that PASP is a useful adjunct to risk stratification for HF hospitalization and mortality in CKD patients.
The pathophysiological correlates of CKD with PH are numerous and complex. CKD is associated with volume overload, endothelial dysfunction, vascular calcification, and arterial stiffening.8, 18 These processes are most apparent in late stage renal disease. We attempted to understand the contribution of these components on mediation analysis, which suggested a role for both BNP (a measure of volume overload) as well as endothelin-1 (a measure of endothelial dysfunction) on elevated pulmonary pressures. Endothelin-1 is a potent vasopressor and is disproportionately elevated in African Americans.25 Endothelin levels are associated with pulmonary vascular remodeling and are increased in both systemic and pulmonary circulations in PH.26
Strengths of the study include detailed echocardiographic analysis, long duration of follow-up, and adjudication of events. Additionally, our results show the utility of measuring PASP beyond BNP. There are some limitations. PH was defined by echocardiography, though the gold standard is right heart catheterization.27 Right heart catheterization is also useful in distinguishing pulmonary arterial hypertension and pulmonary venous hypertension. However, echocardiography is non-invasive, less costly, and more widely available. Thus, it is more conducive to larger epidemiologic studies of PH. Tissue Doppler measurements as well as left atrial volume index were not available in JHS, which would be helpful to better evaluate LV diastolic function in this population as a cause of the elevated pulmonary pressures. However, given the larger left atrial dimensions and LV mass index (without much right ventricular or right atrial remodeling), it is likely that the majority of patients had pulmonary venous hypertension. Another limitation is the lack of serial echocardiograms to assess changes in cardiac structure and function over time in relation to renal disease. Additionally, ejection fraction was not collected at the time of HF hospitalization. Finally, an assumed right atrial pressure was used for all patients given lack of inferior vena cava measurements.
In summary, we found that PH is associated with elevated risk for HF hospitalization and all-cause mortality in African Americans with CKD. The relationships, with the exception of all-cause mortality, remained significant after adjustment for BNP. In addition, cystatin C was directly associated with higher PASP. Based upon the clinical and echocardiographic phenotype of these participants, PH is likely due to increased venous pressures. Whether screening echocardiography may be useful in patients with CKD to identify high-risk groups in need of further testing and therapies and to reduce morbidity and mortality should be further evaluated.
Supplementary Material
Acknowledgments
The authors thank the participants and data collection staff of the Jackson Heart Study.
FUNDING
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Footnotes
DISCLOSURES
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS. Risks for end-stage renal disease, cardiovascular events, and death in hispanic versus non-hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17:2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 3.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance--united states, 1980–2002. MMWR Surveill Summ. 2005;54:1–28. [PubMed] [Google Scholar]
- 4.Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C. Pulmonary hypertension in ckd. Am J Kidney Dis. 2013;61:612–622. doi: 10.1053/j.ajkd.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Navaneethan SD, Roy J, Tao K, Brecklin CS, Chen J, Deo R, Flack JM, Ojo AO, Plappert TJ, Raj DS, Saydain G, Sondheimer JH, Sood R, Steigerwalt SP, Townsend RR, Dweik RA, Rahman M, Chronic Renal Insufficiency Cohort I Prevalence, predictors, and outcomes of pulmonary hypertension in ckd. J Am Soc Nephrol. 2016;27:877–886. doi: 10.1681/ASN.2014111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation. 2008;86:1384–1388. doi: 10.1097/TP.0b013e318188d640. [DOI] [PubMed] [Google Scholar]
- 7.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, Reisner SA. Pulmonary hypertension in patients with end-stage renal disease. Chest. 2003;123:1577–1582. doi: 10.1378/chest.123.5.1577. [DOI] [PubMed] [Google Scholar]
- 8.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohe C, Nickenig G, Woitas R, Skowasch D. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: Results of the pepper-study. PLoS One. 2012;7:e35310. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor HA, Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in african americans: Design and methods of the jackson heart study. Ethn Dis. 2005;15 S6-4-17. [PubMed] [Google Scholar]
- 10.Bansal N, Katz R, Himmelfarb J, Afkarian M, Kestenbaum B, de Boer IH, Young B. Markers of kidney disease and risk of subclinical and clinical heart failure in african americans: The jackson heart study. Nephrol Dial Transplant. 2016;31:2057–2064. doi: 10.1093/ndt/gfw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmieri V, Dahlof B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: The preserve study. Prospective randomized study evaluating regression of ventricular enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]
- 12.Devereux RB, Roman MJ, de Simone G, O'Grady MJ, Paranicas M, Yeh JL, Fabsitz RR, Howard BV. Relations of left ventricular mass to demographic and hemodynamic variables in american indians: The strong heart study. Circulation. 1997;96:1416–1423. doi: 10.1161/01.cir.96.5.1416. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed September 2016];Jackson heart study protocol: Manual 6: Echocardiography. [Google Scholar]
- 14.Keku E, Rosamond W, Taylor HA, Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L, Sarpong D. Cardiovascular disease event classification in the jackson heart study: Methods and procedures. Ethn Dis. 2005;15 S6-62-70. [PubMed] [Google Scholar]
- 15.Choudhary G, Jankowich M, Wu WC. Prevalence and clinical characteristics associated with pulmonary hypertension in african-americans. PLoS One. 2013;8:e84264. doi: 10.1371/journal.pone.0084264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in african americans: Jackson heart study. Circ Heart Fail. 2014;7:558–564. doi: 10.1161/CIRCHEARTFAILURE.114.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin c is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 18.Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: Epidemiology, potential mechanisms and implications. Am J Nephrol. 2013;37:281–290. doi: 10.1159/000348804. [DOI] [PubMed] [Google Scholar]
- 19.Jankowich MD, Wu WC, Choudhary G. Association of elevated plasma endothelin-1 levels with pulmonary hypertension, mortality, and heart failure in african american individuals: The jackson heart study. JAMA Cardiol. 2016;1:461–469. doi: 10.1001/jamacardio.2016.0962. [DOI] [PubMed] [Google Scholar]
- 20.Djousse L, Lee IM, Buring JE, Gaziano JM. Alcohol consumption and risk of cardiovascular disease and death in women: Potential mediating mechanisms. Circulation. 2009;120:237–244. doi: 10.1161/CIRCULATIONAHA.108.832360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reque J, Garcia-Prieto A, Linares T, Vega A, Abad S, Panizo N, Quiroga B, Collado Boira EJ, Lopez-Gomez JM. Pulmonary hypertension is associated with mortality and cardiovascular events in chronic kidney disease patients. Am J Nephrol. 2016;45:107–114. doi: 10.1159/000453047. [DOI] [PubMed] [Google Scholar]
- 22.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of b-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. doi: 10.1056/NEJMoa031681. [DOI] [PubMed] [Google Scholar]
- 23.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 24.Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal b-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ergul S, Parish DC, Puett D, Ergul A. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension. 1996;28:652–655. doi: 10.1161/01.hyp.28.4.652. [DOI] [PubMed] [Google Scholar]
- 26.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 27.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


