Abstract
Microglia regulate brain development through many processes, such as promoting neurogenesis, supporting cell survival, and phagocytizing progenitor, newly-born, and dying cells. Many of these same developmental processes show robust sex differences, yet very few studies have assessed sex differences in microglia function during development. Hormonally-induced sexual differentiation of the brain occurs during the perinatal period, thus we examined sex differences in microglial morphology, phagocytosis, and proliferation in the hippocampus during the early postnatal period. We found that the neonatal female hippocampus had significantly more microglia with phagocytic cups than the male hippocampus. We subsequently found that female microglia phagocytized more neural progenitor cells and healthy cells compared to males, but there were no sex differences in the number of newly-born or dying cells targeted by microglial phagocytosis. We found that the number of phagocytic microglia in females was reduced to male-typical levels by treatment with estradiol, the hormone responsible for masculinizing the rodent brain. Females also had higher expression of several phagocytic pathway genes in the hippocampus compared to males. In contrast to robust sex differences in phagocytic microglia, we found no sex differences in the number of microglia with amoeboid, transitioning, or ramified morphologies or differences in three-dimensional reconstructions of microglial morphology. While we did not find a baseline sex difference in microglial proliferation during or following the prenatal gonadal hormone surge in males, we found that estradiol treatment increased microglia proliferation in females. Overall, these data show that there are important sex differences in microglia function in the hippocampus during the early neonatal period.
1. Introduction
Sexual differentiation of the rodent brain occurs during the perinatal period. The male testes produce a surge in testosterone that begins at embryonic day (E) 18, peaks at E20, and concludes on the day of birth (Rhoda et al., 1984; Weisz et al., 1980). Testosterone is converted to estradiol in the developing brain by the enzyme, p450 aromatase, and estradiol in turn acts on estrogen receptors to masculinize and defeminize the brain (Forger et al., 2016; McCarthy et al., 2008). Rodents are sensitive to the masculinizing and defeminizing effects of estradiol throughout the first postnatal week, thus females can be masculinized during the early postnatal period via exogenous treatment with estradiol in the early postnatal period (Bowers et al., 2010; Nugent et al., 2015). During brain development, estradiol drives sex differences in cell proliferation, dendritic spine patterning, cell death, and neural and glial cell complexity in several brain areas, including the hippocampus, amygdala, hypothalamus and preoptic area (Ahern et al., 2013; Amateau and McCarthy, 2004; Bowers et al., 2010; Krebs-Kraft et al., 2010; Lenz et al., 2013). Interestingly, some of these same developmental processes are known to be regulated by microglia, the primary innate immune cells of the brain. Yet, little is known regarding the extent to which microglia are sexually dimorphic in the immature brain and whether they regulate the process of sexual differentiation.
Microglia colonize the rodent brain beginning at approximately E8 (Alliot et al., 1999; Ginhoux et al., 2010). In the perinatal brain, microglia secrete a variety of diffusible factors that support cell survival and proliferation (Shigemoto-Mogami et al., 2014; Ueno et al., 2013). Microglia also remove synapses and dying cells via a process of engulfment and degradation, called phagocytosis (Cunningham et al., 2013; Schafer et al., 2012). Microglia target healthy progenitor cells as well as recently divided cells in the cerebral cortex after the peak in progenitor proliferation, and induce neuronal cell death during the peak period for apoptosis (Cunningham et al., 2013; Eyo et al., 2016; Wakselman et al., 2008). There are previous reports of sex differences in microglial morphology in the hippocampus, amygdala and preoptic area (Lenz et al., 2013; Schwarz et al., 2012). In the case of the preoptic area, microglial sex differences are driven by the masculinizing action of estrogens and in turn contribute to the masculinization of dendritic spine patterning (Lenz et al., 2013). It has not been determined if sex differences in microglia in other brain areas are hormonally programmed and contribute mechanistically to sex differences in brain development.
During the early postnatal period, cell proliferation peaks in the hippocampus (Altman and Bayer, 1990; Khalaf-Nazzal and Francis, 2013). Interestingly, males have higher levels of cell proliferation in the hippocampus compared to females, and females treated postnatally with estradiol have male-typical levels of cell proliferation (Bowers et al., 2010). Microgl ia could potentially contribute to this developmental sex difference in hippocampal neurogenesis, either by secreting pro-neurogenic mediators, such as growth factors or cytokines (Shigemoto-Mogami et al., 2014), or by phagocytizing progenitors and thereby limiting the number of progenitors that can produce new cells (Cunningham et al., 2013). We sought to determine whether there are sex differences in microglia properties that could be related to the sex differences in cell proliferation in the neonatal hippocampus.
In the current studies, we performed an in-depth analysis of microglia in the neonatal hippocampus based on the location of hippocampal progenitor populations. We found that females had more microglia with phagocytic cups, and that treating neonatal females with estradiol reduced the number of phagocytic cups to male levels within days. Additionally, we found that microglia in the hippocampus engulf newly-born, dying, and neural progenitor cells. Microglia in the female hippocampus phagocytize more Sox2+ neural progenitor cells than males, but not recently-divided or pyknotic cells. We found that females also had higher expression of several phagocytic pathway genes. We did not find other major sex differences in microglia morphology or any effects of estradiol treatment of females on microglial morphology. Interestingly, while there was no baseline sex difference in microglial proliferation in the neonatal hippocampus, estradiol increased microglia proliferation in females. Overall, our results indicate that there are sex differences in microglial phagocytosis in the neonatal hippocampus and that progenitor cells are phagocytized by microglia in females more than males. These sex differences in microglial function may be responsible for the lower level of cell genesis in the neonatal female hippocampus, and may have implications for lifelong sex differences in hippocampal function.
2. Methods
2.1 Animals
All procedures were conducted in accordance with The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by The Ohio State University Institutional Animal Care and Use Committee. Adult Sprague Dawley rats were mated in our facilities, or timed pregnant animals were ordered to deliver within a week of arrival at the animal facility (breeders and timed pregnant dams obtained from Envigo Inc., Indianapolis, IN). Animals were housed in a temperature and humidity controlled room with ad libitum food and water, and the room was maintained on a 12h/12h light/dark cycle (lights on at 20 hrs). Pregnant females were allowed to deliver naturally. On the day of birth, designated postnatal day (PN) 0, pups were sexed via measurement of anogenital distance. A separate cohort of animals was used for each experiment.
2.2 Experiment 1: Hormonal programming of sex differences in hippocampal microglia morphology
2.2.1 In vivo manipulations
Subcutaneous (sc) hormone injections were performed at a volume of 0.1 mL on the dorsal surface of the pup and the injection site was sealed with VetBond (3M) to prevent drug leakage. To masculinize females, females were injected for two days starting on PN1 with 100 μg of estradiol in sterile sesame seed oil (E2; 100 μg/0.1 mL) or sterile sesame seed oil vehicle as previously reported (Bowers et al., 2010; Zhang et al., 2008). This dosage in females mimics levels of E2 in males and is known to masculinize females’ brain morphology and behavior (Amateau et al., 2004). Male and female controls were injected for two days with 100 μL sterile sesame seed oil starting on PN1. All animals were also injected intraperitoneally (ip) with the thymidine analog bromodeoxyuridine (BrdU) (200 mg/kg) on PN2, 18 hr before brain collection, in order to label cells undergoing mitosis within approximately 4 hr of injection. For all injections, the separation of pups from the dam was limited to 15 min or less. For Experiment 1, a total of nine males, ten females, and eight estradiol-treated females were used.
2.2.2 Brain collection and immunohistochemistry
Animals were deeply anesthetized on PN3 with FatalPlus (Vortech Pharmaceuticals), transcardially perfused with 0.1M PBS followed by 4% paraformaldehyde (PFA), brains removed and postfixed overnight in 4% PFA, and cryoprotected with 30% sucrose in PBS. Brains were coronally-sectioned on a cryostat into two alternate series at a thickness of 45 μm and mounted on charged slides at the time of sectioning. Slide-mounted sections from one alternate series underwent immunohistochemical staining for the microglial/macrophage specific marker, ionized calcium binding adapter molecule 1 (Iba1; Wako Chemicals). Sections were extensively rinsed with PBS, non-specific staining blocked via 1 hr incubation with 5% bovine serum albumin (BSA) in 0.1M PBS + 0.4% Triton-X, and endogenous peroxidase activity quenched by incubation in 0.3% H2O2 in 50% methanol for 1 hr. Sections were then rinsed and incubated overnight at 4°C in antiserum to Iba1 (1:1000) in 0.1M PBS + 2.5% BSA + 0.4% Triton-X. On day 2, sections were rinsed and incubated for 1 h at room temperature with biotinylated anti-rabbit secondary antibody (1:500; Vector Laboratories) in 0.1M PBS + 2.5% BSA + 0.4% Triton-X. Sections were then rinsed in 0.1M PBS + 0.4% Triton-X followed by 1 h in ABC complex (solution A and solution B, 1:500 each; Vector Laboratories) in 0.1M PBS + 0.4% Triton-X. Sections were rinsed in 0.1M PBS + 0.4% Triton-X followed by rinses in 0.175M sodium acetate. A 10 min incubation with Ni-DAB chromogen visualized the reaction product (2.5% Ni, 0.05% 3,3 diaminobenzidine tetrachloride, 0.005% H2O2 in 0.175M sodium acetate). Sections were thoroughly rinsed in PBS, cleared with ascending alcohol, defatted with xylenes, and coverslipped with Permount mounting medium.
2.2.3 Stereological cell counts
Stereological cell counts were performed for the dorsal hippocampus using computer-based stereology software (StereoInvestigator, MBF Bioscience Inc) interfaced with a Zeiss AxioImager.M2 microscope and a Zeiss MRc digital camera. Microglia in the dorsal hippocampus were counted in one of two series using the optical fractionator method. The hippocampus was divided into three parts: the stratum oriens (SO), stratum radium/molecular lucidium (SR/ML), and the molecular layer of the dentate gyrus (DG) (see Fig. 1a). The pyramidal layer, and the dentate granular and subgranular layers were omitted from analysis as there were very few to no microglia located in those layers. Although microglial cells are sufficiently small that it is unlikely that a single cell would be visible in consecutive sections in an alternate series, cells that did not have a complete, darkly-labeled cell body were not counted. Microglia were first assessed to determine whether or not they were phagocytic, based on the presence of at least one phagocytic cup, which often are found at the end of extended processes (Fig. 1b). Microglial morphology can also be qualitatively characterized on the basis of morphology (Schafer et al., 2012; Schwarz et al., 2012), thus microglia were additionally characterized morphologically at the time of counting to determine whether there were group or regional differences in the microglial activation profile. Quiescent or ramified micro glia show long, thin, highly-branched processes (Fig. 1c); transitioning microglia show short, thick, processes (Fig. 1d); amoeboid microglia show enlarged cell bodies and display a characteristic round morphology devoid of processes (Fig. 1e) (Kloss et al., 2001; Stence et al., 2001; Schafer et al., 2012; Schwarz et al., 2012). The optical fractionator estimates the total cell population number by using the area counted, thickness of the tissue, as well as distance between sections. Cell counts are expressed in total estimated microglia population by combining total microglial counts for the left and right halves of the hippocampus.
Figure 1. Hippocampal subdivisions and microglia morphologies.
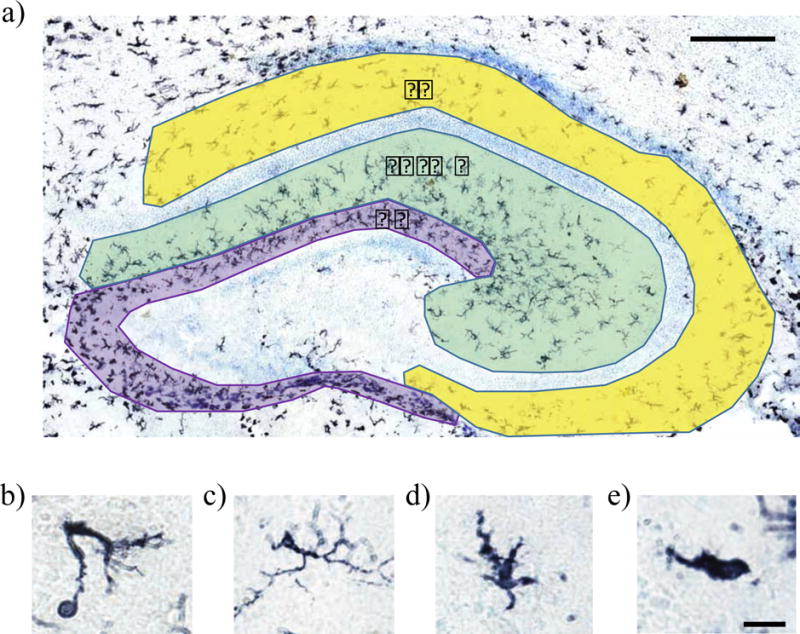
Hippocampal subdivisions and representative images of microglial morphological categories used in cell counting. The hippocampus was divided into the dentate gyrus (DG; purple), the molecular layer/stratum radiatum (SR/ML; green), and the stratum oriens (SO; yellow). Microglia morphology was assessed by classifying microglia as phagocytic (b), ramified (c), transitioning (d), or amoeboid (e) following immunohistochemical staining for the pan-microglial marker, Iba1. Scale bar = 200 μm for panel (a), and 12.5 μm for panels (b)–(e).
2.2.4 Microglia morphology analysis
To confirm that our qualitative analysis of microglial morphology was accurately capturing group differences in microglial morphology, we also performed three-dimensional reconstructions of microglia using Neurolucida software (MBF Bioscience Inc). This analysis has been successfully used to confirm sex differences in microglial morphology and validate qualitative morphological counts of microglia (Lenz et al., 2013). Microglia were chosen for analysis if they had a complete, darkly-stained cell body and multiple processes extending from the cell body. Thus our analysis focused on the SR/ML area, as there were very few microglia in the SO and DG with multiple processes. A total of five ramified microglia per animal were analyzed from 5 males, 5 females and 5 estradiol-treated females. Animals used for analysis were chosen randomly from the larger cohort of subjects.
2.3 Experiment 2: Sex differences in hippocampal microglia proliferation and phagocytosis
2.3.1 In vivo manipulations
To determine if the masculinizing effects of hormones on microglial properties was due to the actions of estrogens or androgens, female rats were injected for two days starting on PN0 with 100μg of either estradiol (E2; 100 μg/0.1 mL) or the non-aromatizable androgen, dihydrotestosterone (DHT; 100 μg/0.1 mL), in sterile sesame seed oil (Bowers et al., 2010; Waddell et al., 2013; Zhang et al., 2008). Sc hormone injections were performed as described in Experiment 1. Male and female controls were injected for two days with sterile sesame seed oil. Rats treated postnatally were also injected ip with BrdU (200 mg/kg) on PN1, 18 hr before brain collection. Eleven males, eight females, eight estradiol-treated females, and eight DHT-treated females were used. An additional cohort of unmanipulated rats was used to assess microglia proliferation and phagocytosis during the height of the prenatal testosterone surge in males, E20. Five males and four females were used. On E20, a timed pregnant dam was euthanized via overdose with Fatal Plus and transcardial perfusion, and sex of the fetuses was confirmed by measuring anogenital distance before fetal brains were collected and fixed in 4% PFA overnight.
2.4 Experiment 3: Sex differences in microglia phagocytosis of pyknotic and progenitor cells
2.4.1 In vivo manipulations
Another cohort of animals was used to replicate and verify estimated counts of phagocytic microglia obtained in Experiments 1–2 using a specific stain for phagocytic cells and to further determine the cellular targets of microglial phagocytosis, including newly-born cells, dying cells, and neural progenitor cells. Untreated males and females were euthanized on PN2, brains removed, postfixed, and cryoprotected as described above, and bisected into left and right hemispheres to increase the number of markers that could be assessed in each animal. A total of three males and three females were used for Experiment 3.
2.5 Immunofluorescence staining for Experiments 2–3
All brains were coronally-sectioned on a cryostat into two alternate series at a thickness of 45 μm and mounted on charged slides at the time of sectioning. Slide mounted sections from one alternate series underwent immunofluorescent staining. The antibodies and dilutions used for staining were: Iba1, a pan-microglial marker (1:1000; Wako Chemicals); BrdU, a marker of recently divided cells (1:100; BD Biosciences, B44); Ki67, a marker of mitotic cells (1:100; eBiosciences, 41-5698-80); CD68, a lysosomal marker that allows for the identification of phagocytic microglia (1:500; Bio-rad; MCA341R); and Sox2, a marker for neural stem/early progenitor cells (1:1500; Abcam; ab97959). For BrdU and Iba1 double staining, sections were first incubated in a heated 0.1M citric acid solution, pH = 6. Citric acid was brought to a boil for 30 s in a microwave and sections were added to the solution and microwaved for 5 min at power level 2. Slides were then left to incubate in the heated 0.1M citric acid solution for 10 min. Slides were then rinsed in PBS and then incubated in 2N HCl for 30 min in a water bath heated to 37°C. For all other staining protocols, the citric acid and HCl steps were omitted and processed as follows. Sections were extensively rinsed with PBS, then incubated in 50% methanol for 20 minutes. Sections were rinsed in PBS then blocked for 1 hr with 5% NDS in PBS + 0.4% Triton-X. Sections were incubated overnight at 4°C in antiserum to the respective primary antibodies in PBS + 2.5% NDS + 0.4% Triton-X. On day 2, sections were rinsed in PBS and incubated in the dark at room temperature for 2 hr in appropriate donkey secondary antibodies conjugated to Alexa Fluor 555 (1:200), 657 (1:200), or 488 (1:333) in PBS + 2.5% NDS + 0.4% Triton-X. Sections were then rinsed and coverslipped with Vectashield Hard Set with DAPI.
2.6 Immunofluorescence imaging and cell counts for Experiments 2–3
For Experiment 2, cell counts were performed using computer-based stereology software. In one of two alternate series the first four sections containing the whole dorsal hippocampus were analyzed using an exhaustive counting method. Image stacks for the left and right hippocampus were acquired at 10×, and analyzed at 20×. Microglia were classified as newly-divided (Iba1+/BrdU+ cell body), phagocytic (showing a phagocytic cup), as well as classifying whether the phagocytic cup was BrdU+, to determine if a microglial cell was phagocytizing a newly-divided cell. BrdU injections are not easily feasible in animals prenatally, thus for the E20 tissue, microglia were classified as newly-divided (Iba1+Ki67+ cell body), phagocytic (showing a phagocytic cup), as well as classifying if the phagocytic cup was Ki67+, to determine if a microglial cell was phagocytizing a newly-divided cell.
For Experiment 3, two sets of image stacks were acquired at 20× on three channels for Iba1, CD68 and DAPI, or Iba1, Sox2 and DAPI for first four sections of the left or right hippocampus. For phagocytosis analysis, microglia were classified as phagocytic if they expressed CD68 and had a phagocytic cup that was DAPI+. We also classified whether each phagocytic cup surrounded a pyknotic cell, to determine if a microglial cell was phagocytizing a cell undergoing apoptosis. Pyknotic cells were identified by the characteristic morphology of a darkly-stained, condensed or fragmented DAPI-positive nucleus (Fig. 5a). For the second set of images, we determined whether each phagocytic microglia was phagocytizing a Sox2+ progenitor cell based on similar criteria to that used by Cunningham, et al. (2013). Briefly, to be counted as a phagocytized progenitor, the Sox2+ cell had to be fully engulfed by the microglial cup.
Figure 5. Representative images of phagocytic microglia.
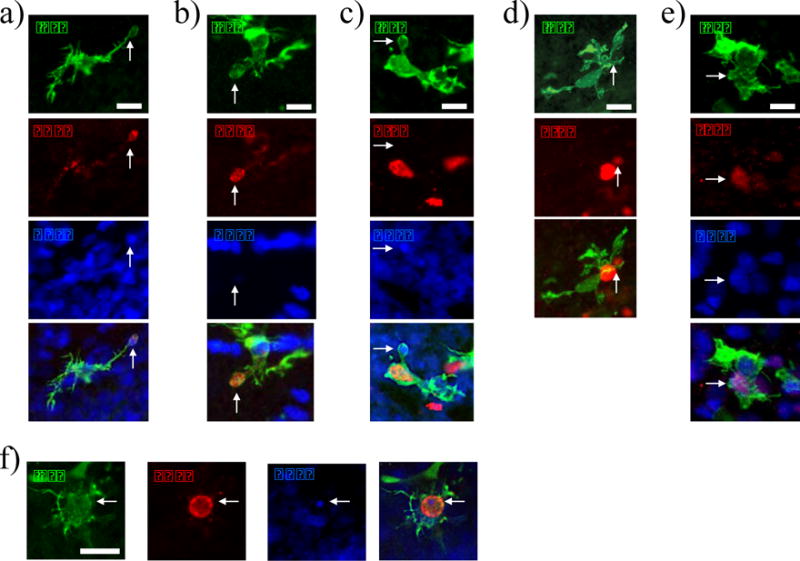
Microglia with a CD68+/DAPI+ phagocytic cup that contains a pyknotic nucleus (a), Microglia with a CD68+ phagocytic cup that is DAPI− (b), recently divided (BrdU+) phagocytic microglia (c), microglia with a BrdU+ phagocytic cup (d), microglia with a Sox2+ phagocytic cup (e), and representative image of the smallest DAPI+ particle that was quantified as being phagocytized (f). Scale bars = 12.5 μm.
2.7 Experiment 4: Gene expression analysis of phagocytic receptors and associated proteins
To further determine whether there are sex differences in microglial markers and phagocytic function in the developing hippocampus, we performed gene expression analyses of key microglial genes and genes related to phagocytosis using qPCR. There are a wide variety of receptors and phagocytic molecules that have been identified, we chose to focus on the most well-established and studied phagocytic genes, including C1q, C3, Itgam (part of CD11b), Tyrobp, Cybb (Nox2), CD68, Mfge8, and GPR34 (Bennett et al., 2016; Brown and Neher, 2014; Lelli et al., 2013; Preissler et al., 2014; Spittau et al., 2015; Wakselman et al., 2008; Zhang et al., 2014). All the genes chosen are highly expressed by microglia, except Mfge8, which is highly expressed by astrocytes and could show non-microglial control of phagocytosis (Bennett et al., 2016; Zhang et al., 2014). We also chose CX3CR1 and P2ry12 as they are common microglial markers. A total of seven females and seven males were used. Animals were deeply anesthetized on PN2 with FatalPlus (Vortech Pharmaceuticals), transcardially perfused with 0.1M PBS to remove potential contamination by peripheral immune cells, brains removed and the dorsal hippocampus rapidly microdissected. RNA was extracted using Qiazol and the Qiagen RNeasy Lipid Tissue Mini Kit. cDNA was synthesized from mRNA using the 1st strand cDNA Synthesis System (Origene). qPCR was run using Bio-Rad Sso Advanced Universal Sybr Green Supermix on a Bio-Rad CFX-96 thermocycler. Samples were run in triplicate and the average Ct (threshold cycle) was used along with a calibrator sample, not included in experimental data, to normalize and convert all qPCR data to fold change using the Pfaffl method. Hprt1 was used as a housekeeping gene. The primer sequences used are included in Table S1. All primers had efficiencies between 95% and 105%, and had single amplification product in melt curve analyses.
2.8 Statistics
For Experiment 1, data was analyzed using a repeated measures ANOVA with hippocampal sub-region as a within-subjects repeated measure factor and sex as a between-subjects factor. Each microglia morphological category was analyzed as a separate repeated-measures ANOVA. All p-values were adjusted using Bonferroni corrections for multiple comparisons. For microglia morphologies that had significant effects of sex, multiple t-tests were used to determine sex differences in each specific sub-region of the hippocampus for a total of 9 comparisons. All t-test p-values were corrected using the Bonferroni method. 3D reconstruction data was analyzed using one-way ANOVA. For Experiments 2 and 3, data were analyzed using a one-way ANOVA for data with 3 or more treatment groups or a t-test when only male and females were compared. Raw cell counts for Experiment 3 were doubled prior to statistical analysis and consistency with other experiments, as only one hemisphere was counted. For Experiment 4, t-tests were used to analyze sex differences in gene expression. Differences between genes were not analyzed and fold change values do not represent differences in expression between genes. Several outliers for the qPCR data were found using the ROUT method on Graphpad Prism, and were removed from the analysis for the qPCR experiments. For Trem2, two males and two females were removed; for Cybb, one male was removed; for Mfge8, one female was removed; for C3, one female was removed. There was no difference in housekeeping gene expression or quality of mRNA for outlier samples. All data are expressed as means + standard error of the mean (SEM). Statistical analyses were conducted in SPSS v24 and GraphPad Prism v7 for Mac/PC.
3. Results
3.1 Microglia morphology and distribution
For phagocytic microglia in the hippocampus, there was a significant effect of group (Fig. 2c; F(2,24) = 9.74, p = 0.006), and a significant within-subjects effect of area (Fig. 3a; F(2,48) = 15.6, p < 0.0001), but no sex by area interaction (F(4,48) = 1.11, ns). Post-hoc comparisons showed that females had more phagocytic microglia compared to males and estradiol-treated females. The dentate gyrus (DG) had significantly fewer phagocytic microglia compared to the stratum radiatum/molecular layer (SR/ML) and the stratum oriens (SO) (Fig. 3a). To further investigate the sex difference in phagocytic microglia, we assessed group differences in specific sub-regions of the hippocampus. Females had significantly more phagocytic microglia compared to males in the DG and SR/ML areas, and females had significantly more phagocytic microglia compared to estradiol-treated females in the DG (Fig. 3a). 2.88% of microglia were phagocytic in males, 5.72% in females, and 3.33% in estradiol-treated females. For ramified microglia, there were no significant differences between groups (Fig 2d; F(2,24) = 3.29, ns). Within-subjects comparisons showed that there was a significant effect of area (Fig. 3b; F(2,48) = 177, p < 0.0001) and a significant sex by area interaction (F(4,48) = 3.64, p = 0.011). Pairwise comparisons for area showed there were significantly more ramified microglia in the SR/ML area compared to the DG and SO. To further investigate the sex by area interaction, we compared sex differences within each region, however there were no significant post-hoc comparisons (Fig. 3b). 21.9% of microglia were ramified in males, 24.5% in females, and 36.5% in estradiol-treated females. For transitioning microglia, there was no effect of group (Fig. 2e; F(2,24) = 0.312, ns). Within-subjects tests showed that there was a significant effect of area (Fig. 3c; F(2,48) = 18.5, p < 0.0001), but no sex by area interaction (F(4,48) = 1.83, ns). Pairwise comparisons showed there were significantly more transitioning microglia in the DG and SO areas compared to the SR/ML area. 35.4% of microglia were transitioning in males, 35.0% in females, and 29.1% in estradiol-treated females. For amoeboid microglia, there was no effect of sex/hormonal treatment group (Fig. 2f; F(2,24) = 1.53, ns). Within-subjects tests on amoeboid microglia showed that there was a significant effect of area (Fig. 3d; F(2,48) = 93.9, p < 0.0001), but no sex by area interaction. (F(4,48) = 1.54, ns). Post-hoc comparisons showed there were significantly more amoeboid microglia in the SR/ML area compared to the DG and SO. 42.7% of microglia were amoeboid in males, 40.5% in females, and 34.4% in estradiol-treated females.
Figure 2. Sex differences in microglial morphology.
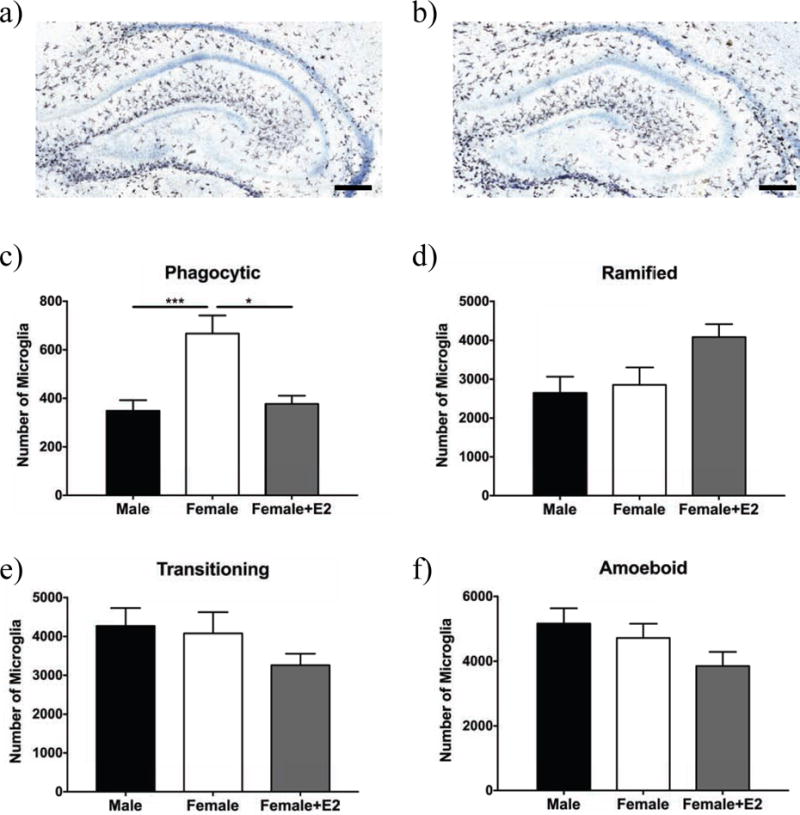
Representative images of Iba1 immunostaining in the hippocampus of males (a) and females (b) on PN3. Microglia morphology was assessed across the hippocampus independent of region for phagocytic (c), ramified (d), transitioning (e), and amoeboid (f). Females had more phagocytic microglia than ma l es and estradiol (E2)-treated females (c). There were no effects of sex or hormones on the number of ramified (d), transitioning (e), or amoeboid microglia (f). * p < 0.05, and *** p < 0.001. Scale bar = 200 μm.
Figure 3. Differences in microglial number across hippocampal sub-region and sex differences within sub-regions.
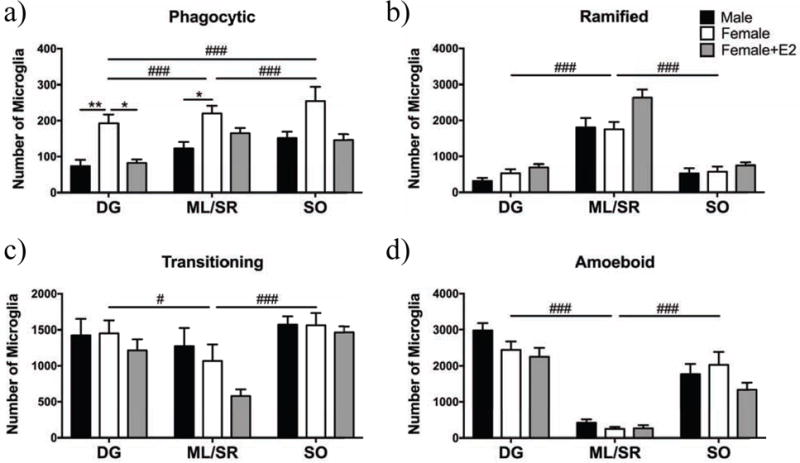
Microglia morphology was classified as phagocytic and/or transitioning, ramified or amoeboid across three sub-regions of the hippocampus in males (black), females (white), and females treated with E2 (gray). There were more phagocytic microglia in the ML/SR and SO compared to the DG, and more phagocytic microglia in the SO compared to the ML/SR (a). Females had significantly more phagocytic microglia compared to males in the DG and ML/SR (a). Females had more phagocytic microglia in the DG compared to estradiol-treated females (a). There were more ramified microglia in the ML/SR compared to the SO and DG (b). There were more transitioning microglia in the DG and SO compared to the ML/SR (c). There were more amoeboid microglia in the DG and SO compared to the ML/SR (d). DG = dentate gyrus, ML/SR = molecular layer/stratum radiatum, and SO = statrum oriens. * p < 0.05, ** p < 0.01 for pairwise comparisons within areas/morphologies; # p < 0.01, ### p < 0.001 for comparisons between areas independent of sex.
There was no sex difference in the total number of microglia in the hippocampus (Fig. S1a; F(2,24) = 1.06, ns). We also analyzed differences in total microglial number between areas. We found that there was no significant effect of group (Fig. S1b; F(2,24) = 0.904, ns), and a significant within-subjects effect of area (Fig. S1b; F(2,24) = 31.4, p < 0.0001), but no sex by area interaction (F(4,48) = 1.06, ns). Pairwise comparisons showed there were more microglia in the DG compared to the SR/ML and OR area, and more microglia in the SR/ML compared to the OR area.
Microglia were also reconstructed in three-dimensions to determine if there were any differences in morphology that were not captured in microglia counts using qualitative criteria (e.g., amoeboid vs. transitioning vs. ramified). There were no sex differences in microglia cell body area (Fig. S1c; F(2,12) = 0.389, ns), number of main processes (Fig. S1d; F(2,12) = 0.563, ns), number of nodes (Fig. S1e; F(2,12) = 0.483, ns), or total length of processes (Fig. S1f; F(2,12) = 0.811, ns) between males, females, and estradiol-treated females.
3.2 Microglial Proliferation
To determine whether there was a sex difference in microglia proliferation during the critical period for sexual differentiation of the brain, we used BrdU to identify recently-divided microglia (for representative images, see Fig. 4a–d). There was a significant effect of sex on the number of BrdU+/Iba1+ double-labeled cells at PN2 (Fig. 4e; F(4,31) = 4.09, p = 0.0147), with estradiol-treated females having significantly more double-labeled cells than vehicle males. We again found no sex difference in the total number of microglia (Fig. S2a; F(4,31) = 0.494, ns). 9.36% of microglia were BrdU+ in males, 11.2% in females, 13.6% in estradiol-treated females, and 11.8% in DHT-treated females. Given that we found no basal sex differences in microglia proliferation during the early postnatal period (following the completion of the perinatal male androgen surge), we quantified proliferating microglia in the hippocampus on embryonic day (E) 20 using Ki67, to determine if there was a sex difference in microglial proliferation and phagocytosis during the peak of the testosterone surge. However, we found no difference in the number of Ki67+/Iba1+ cells in the male versus female hippocampus at E20 (Fig. 4f; t(7) = 0.799, ns).
Fig. 4. Proliferating microglia in the hippocampus.
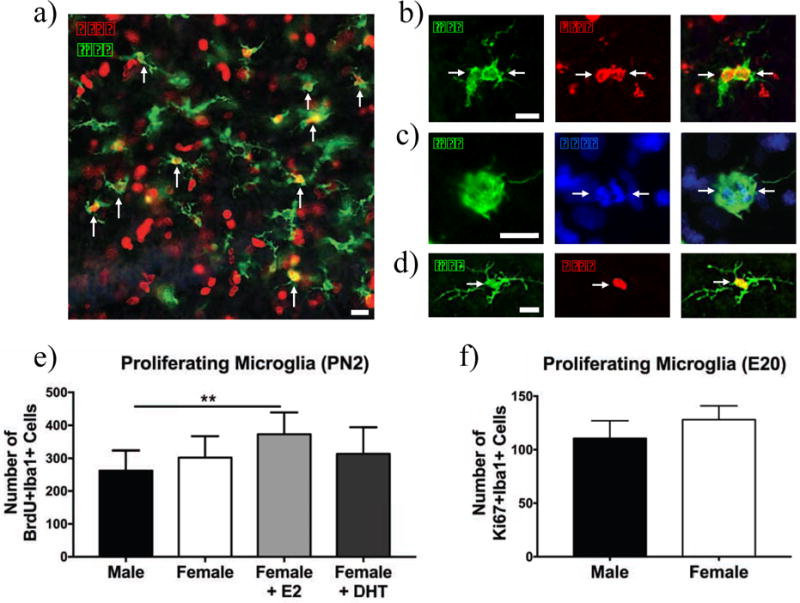
10× image of BrdU+/Iba+ cells in the hippocampus at PN2 (a), 20× image of a BrdU+Iba1+ cell (b), dividing microglia seen by parallel chromosomes in DAPI staining (c), recently divided microglia with ramified morphology (d), proliferating microglia counts on PN2 (e) and proliferating microglia counts on E20 (f). There were no basal sex differences in the number of proliferating microglia at PN2 (e) or E20 (f), however estradiol significantly increased microglial proliferation in females relative to vehicle males on PN2 (e). **p < 0.01. Scale bar = 100 μm for panel (a) and 12.5 μm for panels (b)–(d).
3.3 Microglia phagocytosis
After finding a sex difference in phagocytic microglia in Experiment 1, we sought to better characterize phagocytic microglia and determine what cells microglia are phagocytizing. Figure 5 shows representative pictures of phagocytic microglia and their targets in the hippocampus. We replicated our initial finding on phagocytosis in Experiment 2, observing a significant effect of sex on the number of microglia with phagocytic cups (Fig. 6a; F(4,31) = 6.68, p = 0.0013), with females having more microglia with phagocytic cups compared to males and estradiol-treated females, however we found no effect of DHT. 3.46% of microglia were phagocytic in males, 5.53% in females, 3.32% in estradiol-treated females, and 4.29% in DHT-treated females. To confirm that we correctly classified Iba1+ microglia as phagocytic using morphological criteria in Experiment 1, we stained hippocampal tissue for CD68, a lysosomal marker used to biochemically identify phagocytic microglia (Schafer et al., 2012) in males and females on PN2. We classified a microglial cell as phagocytic if it was Iba1+, showed a phagocytic enlargement surrounding a DAPI+ nucleus and the cell or phagocytic cup or microglia was also CD68+ (Fig. 5a and f). CD68+/DAPI− phagocytic microglia were separately quantified as phagocytizing an unidentified target. We found that females had more CD68+ phagocytic microglia that were phagocytizing DAPI+ nuclei compared to males (Fig. 6b; t(4) = 3.32, p = 0.0293). We found no sex difference in the total number of microglia for Experiment 3 (Fig. S2b; t(4) = 0.7107, ns). 3.41% of microglia were phagocytic in males, and 4.79% in females. We also found no sex difference in the % area stained by Iba1 (Fig. S2c and d; t(4) = 0.688, ns) or CD68 (Fig. S2e and f; t(4) = 0.332, ns).
Fig. 6. Sex differences in microglial phagocytosis.
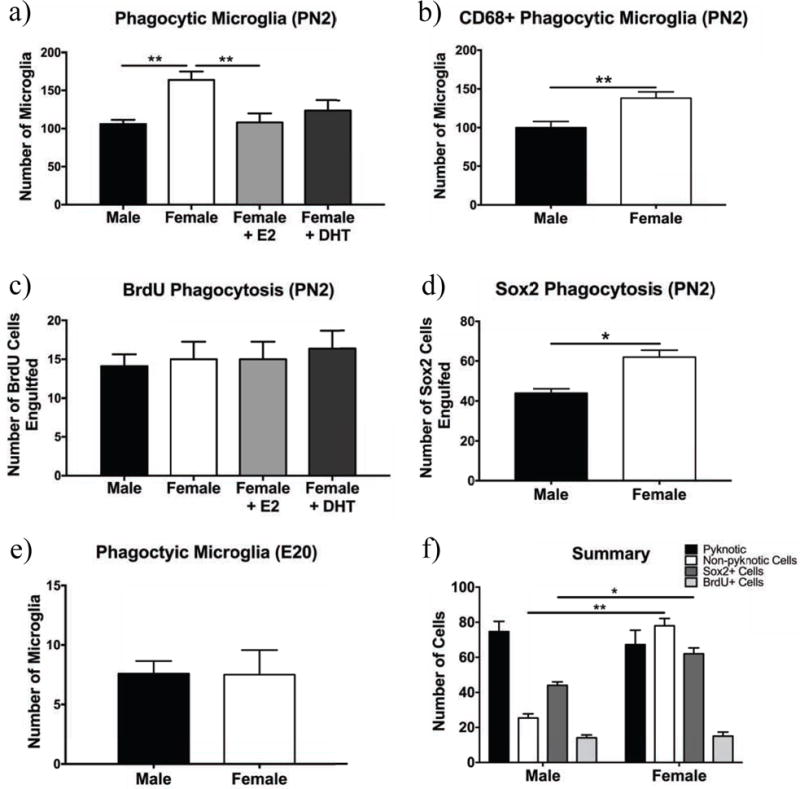
The number of Iba1+ phagocytic cells at PN2 (a), Iba1+ cells with CD68+/DAPI+ phagocytic cups (b), the number of Iba1+ cells phagocytizing BrdU+ cells (c), the number of Iba1+ cells phagocytizing Sox2+ progenitor cells (d), the total number of Iba1+ phagocytic microglia at E20 (e), and summary of phagocytic targets (f). There were significantly more phagocytic microglia in females compared to males and females treated with estradiol (E2) (a). There were significantly more CD68+ phagocytic microglia in females compared to males (b). There were no differences in the number of BrdU+ cells phagocytized across groups (c). There were more microglia phagocytizing Sox2+ cells in females than in males (d). There was no sex difference in phagocytic microglia at E20 (e). * p < 0.05, ** p < 0.01.
We found no sex differences in phagocytosis of cells with pyknotic nuclei (Fig. S3a, t(4) = 0.466, ns), however we did find a sex difference in the number of non-pyknotic cells that were being phagocytized (Fig. S3b; t(4) = 4.97, p = 0.0077). 56.5% of cups in females and 74.7% of cups in males contained pyknotic nuclei. 14.9% of total pyknotic nuclei in females and 16.1% of total pyknotic nuclei in males were being phagocytized by a microglial cell. There was no overall sex difference in number of pyknotic cells (Fig. S3c; t(4) = 0.531, ns).
There was no significant effect of sex on the number of microglia phagocytizing BrdU+ cells (Fig. 6c, and Fig. S4a for a representative image of BrdU distribution in the hippocampus; F(3,31) = 0.219, ns). Additionally, fewer than 15% of microglia with phagocytic cups had cups that were BrdU+ for all groups. To determine whether microglia were phagocytizing progenitor cells, we stained for Sox2, a neural stem cell marker that is also expressed in early progenitor cells and some intermediate progenitor cells (Hodge et al., 2013). We found that female microglia phagocytized more Sox2+ cells than males (Fig. 6d, and Fig. S4b for a representative image of Sox2 distribution in the hippocampus; t(4) = 4.5, p = 0.011). 1.46% of microglia in males were phagocytizing Sox2+ cells, and 2.19% in females. Additionally, to determine if there was a sex difference in microglial phagocytosis closer to the prenatal testosterone surge we analyzed hippocampi from E20 rats. There was no sex difference in the number of phagocytic microglia at E20 (Fig. 6e; t(7) = 0.0464, ns). For summary of findings see Fig. 6f.
3.4 Phagocytic receptor gene expression
To determine the potential molecular mechanisms that may regulate sex differences in microglial phagocytosis, we analyzed the gene expression of several phagocytic genes specifically expressed by microglia, and one expressed by astroglia (Mfge8) in PN2 whole hippocampal tissue (Fig. 7). We found a significant sex difference in several phagocytic genes, including Trem2 (t(8) = 4.78, p = 0.0014) Tyrobp (t(12) = 3.03, p = 0.0104), Cybb (t(11) = 0.0199, p = 0.0199), and CD68 (t(12) = 2.236, p = 0.0451). We found no sex difference in phagocytic molecules C1q (t(12) = 1.19, ns), C3 (t(11) = 0.319, ns), or Mfge8 (t(11) = 0.273, ns). We also found no sex difference in phagocytic receptors Gpr34 (t(12) = 0.864, ns), or Itgam (t(12) = 0.219, ns). We also assessed gene expression of markers used to identify microglia to further assess overall sex differences in hippocampal microglia. Further supporting the lack of sex differences in microglial number or staining density assessed in our previous experiments, we found no sex difference in expression of P2ry12 (t(12) = 1.24, ns), and CX3CR1 (t(12) = 0.911, ns) in the hippocampus.
Fig. 7. Hippocampal expression of phagocytic genes.
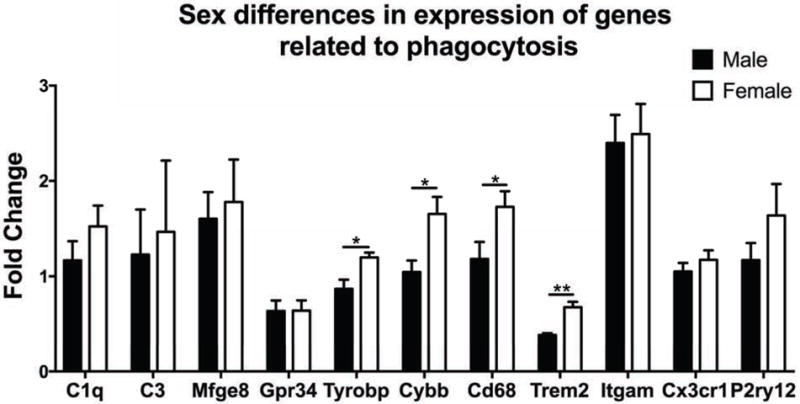
All genes are highly express e d by microglia except for Mfge8, which is related to phagocytosis but expressed by astrocytes. Females had significantly higher expression of the phagocytic pathway genes, Tyrobp, Cybb, Cd68, and Trem2 than males. There were no sex differences in three common microglial markers (Cx3cr1, Itgam, and P2ry12). All genes are normalized to fold expression relative to a calibrator sample. * p < 0.05, ** p < 0.01.
4. Discussion
In the current studies we assessed sex differences in microglial morphology in the developing hippocampus and whether sex steroid hormones influence microglial morphology or function during the critical period for sexual differentiation. We found that there is a major sex difference in phagocytic microglia in the developing hippocampus. Females had more phagocytic microglia than males, and treatment of females with masculinizing hormones reduced microglial phagocytosis to male-typical levels. We further found that females phagocytize more Sox2+ neural progenitor cells compared to males, but not newly-born or dying cells. We found a sex difference in several genes that are highly expressed by microglia and involved in the phagocytic pathway. We found no major sex differences in microglial morphology or microglial proliferation in the neonatal hippocampus, but we observed that estradiol treatment increased microglial proliferation in females. Overall these data suggest that microglial phagocytic function in the hippocampus is different in males and females during the critical period for sexual differentiation of the brain, which may have implications for lifelong sex differences in hippocampal function.
4.1 Sex differences in hippocampal microglia morphology and gene expression
We observed no sex differences in the number of amoeboid, transitioning and ramified microglia, or total number of microglia in the hippocampus. A previous survey study of sex differences in microglial morphology across development found that males have more amoeboid microglia at PN4 compared to females in the hippocampus (Schwarz et al., 2012). Several reasons could explain the discrepancy between the two studies. First, we subdivided the hippocampus for analysis based on areas of high progenitor proliferation and neurogenesis rather than cornu ammonis areas as in Schwarz et al (2012). Additionally, we used thicker tissue sections to facilitate optimal stereological cell counts (45 μm vs 14 μm). Thinner sections could potentially allow for misidentification of ramified microglia as stout or amoeboid, as thin sections could remove ramified microglia processes, which often extend considerably farther than 14 μm in the z-dimension. While we did not find sex differences in amoeboid versus ramified microglial morphology in the hippocampus, this does not preclude functional sex differences in the microglial support of neurogenesis via secreted factors or phagocytosis of synapses. Ramified microglia in the developing brain stain positive for traditional markers of both “activated” and “resting” cells and pro-inflammatory cytokine release by microglia can precede changes in microglial morphology (Lenz et al., 2013; Norden et al., 2016). Thus, morphological criteria alone are not the best indicators of microglial signaling or function, which is why we assessed microglial phagocytosis and phagocytic gene expression. We also did not find a sex difference in gene expression of common microglial markers (Itgam, CX3CR1, and P2ry12) in the hippocampus, again suggesting that there is no large sex difference in microglial number. There are also known sex differences in the expression of immune-related molecules, such as cytokines, chemokines and their receptors, that could derive from or act on microglia and thereby impact brain development differentially in males and females (Schwarz et al., 2012). Analyses of these sex differences in immune signaling in the developing hippocampus warrant future mechanistic inquiry.
4.2 Sub-region differences in hippocampal microglia morphology
It was previously shown that hippocampal microglia do not occupy the proliferative zones in the hippocampal neuroepithelium and dentate gyrus prenatally or in the early postnatal period (Dalmau et al., 1998, 1997). We similarly observed that few to no microglia are present within the granular zone of the dentate gyrus, where large amounts of cellular proliferation occur postnatally, or in the pyramidal layer (see Fig. 1 for representative image). However, there were many microglia adjacent to the dentate gyrus and hippocampal progenitor areas as well as in the dorsal migratory stream. Hippocampal neurogenesis peaks just before birth, whereas proliferation in the dentate gyrus peaks in the first week of life, thus different proportions of progenitors could be present throughout the hippocampus and explain the differential distribution of microglia within the hippocampus at this developmental time point (Altman and Bayer, 1990). Microglia invade the dentate gyrus by the end of the first postnatal week, which would correlate with the peak in cell proliferation that occurs in that region (Dalmau et al., 1998). Progenitor cells can modulate microglia function and colonization of the brain by releasing CXCL12 and VEGF (Arnò et al., 2014; Mosher et al., 2012). Additionally, amoeboid microglia preferentially occupy the neurogenic zones in the developing cortex (Cunningham et al., 2013; Shigemoto-Mogami et al., 2014). We found very few amoeboid microglia in the SR/ML, and many amoeboid microglia in the DG and SO areas near the progenitor and proliferative zones, which suggests that the progenitor cells may influence the function and/or colonization of microglia.
4.3 The role of microglial phagocytosis in brain development
Our results extend previously published work on microglia phagocytosis during development with several novel findings. We found more microglia phagocytosis occurs in the SO and SR/ML areas where cellular proliferation is at peak levels during the early postnatal period, as compared to the dentate gyrus, where cellular proliferation is just beginning to peak (Altman and Bayer, 1990). Additionally, we found that females had more phagocytic microglia and that sex steroid hormones program this sex difference, which is a potentially important sex difference for hippocampal development that has not previously been documented. Microglial phagocytosis in the subventricular and ventricular zones (SVZ/VZ) that supply new cells to the developing cortex has been shown to increase during development as the period for robust proliferation ends and the progenitor pool size is reduced (Cunningham et al., 2013). Interestingly, this previous work also showed that microglia primarily target healthy progenitor cells in the SVZ at the close of this period of high proliferation (Cunningham et al., 2013). Proliferation peaks in the dentate gyrus postnatally, while hippocampal proliferation is decreasing (Altman and Bayer, 1990). Thus, we may have observed greater numbers of phagocytic microglia in the SO and SR/ML areas because microglia are targeting progenitor cells at the close of this proliferative period. Higher levels of phagocytosis may be present in the dentate gyrus at the end of the first postnatal week when microglia have colonized the entire dentate gyrus (Dalmau et al., 1998).
Microglia can phagocytize healthy cells, progenitor cells, recently divided cells, and apoptotic cells during development (Cunningham et al., 2013; McArthur et al., 2010; Wakselman et al., 2008). We found that female microglia phagocytize more Sox2+ neural progenitor cells compared to males. However, as there were few microglia present in the subgranular zone of the dentate gyrus where the adult proliferative zone forms, it is unclear whether microglial phagocytosis could be important for regulating the size of the adult progenitor pool or resultant adult neurogenesis. Future research could assess later time points (e.g. PN7 or PN12) when microglia fully colonize the dentate gyrus to determine whether microglia phagocytize progenitor cells in the subgranular zone of the dentate gyrus at this time. It is likely that the phagocytized Sox2+ cells we identified are contributing to pyramidal neuron, interneuron, or glia population in the hippocampus proper. Future fate-mapping studies coupled with manipulations of phagocytic function of microglia would be required to determine the long-term impacts of sex differences in microglia phagocytosis in the developing hippocampus.
We did not find a sex difference in phagocytosis of pyknotic cells. There is no sex difference in the number of apoptotic cells, and hippocampal apoptosis quickly decreases after the first postnatal day (Mosley et al., 2016; Wakselman et al., 2008). Research in the cerebellum has also found that microglia did not preferentially target dying cells during the first three weeks of life (Perez-Pouchoulen et al., 2015). Additionally, microglia can induce death in healthy cells before phagocytizing them (Guadagno et al., 2015; Neher et al., 2014; Wakselman et al., 2008). Microglia also preferentially target healthy progenitor cells for phagocytosis during development (Cunningham et al., 2013). We did not find a sex difference in the number of phagocytic microglia during the late embryonic period, and analysis of microglial phagocytosis across the full extent of the neonatal period could determine the full duration of the sex difference in phagocytosis.
We hypothesize that the sex differences we observed may have occurred because microglia phagocytosis is coupled to the rate of proliferation during the neonatal period. Previous studies have shown that males have more cellular proliferation in the hippocampus than females, and estradiol treated females have male typical levels of cellular proliferation (Bowers et al., 2010). We found that females had more phagocytic microglia and microglia phagocytosis could be decreased by treatment with estradiol. Future research will need to determine what factors may be regulating microglial phagocytosis and whether microglia phagocytosis is tied to the rate of cellular proliferation.
Interestingly, we found that females had higher expression of several genes involved in the phagocytic pathway in the hippocampus that corroborate our observed sex difference in the number of phagocytic microglia. We specifically found that females had higher expression of CD68, Cybb, Trem2, and Tyrobp, all phagocytic pathway genes that are highly expressed in microglia compared to other cell types (Zhang et al., 2014). Cybb (Nox2) is part of the NADPH oxidase complex, which is involved in initial oxidative degradation of phagocytized material and is thought to be expressed on a forming phagocytic cup (Russell et al., 2009). Interestingly, Cybb is an X-linked gene, thus its higher expression in females may result from incomplete x-inactivation (Russell et al., 2009). Furthermore, Cybb has been shown to be important for infiltration of microglia into the postnatal subventricular zone and knockout animals for the gene show a transient decrease in microglial density at PN3, but not at PN0 or PN10 (Lelli et al., 2013). A past study in the hippocampus found that cell death of neurons in the CA3 and subiculum was dependent on microglial DAP12/Tyrobp and generation of reactive oxygen species, although it is unknown through what mechanism reactive oxygen species were synthesized (Wakselman et al., 2008). Future research will need to determine whether female microglia are inherently more phagocytic during development or whether there is a sex difference in the expression of a phagocytic ligand to which female microglia are responding. One study found that peritoneal macrophages in females had higher baseline phagocytic and NADPH activity compared to males (Scotland et al., 2011). How might microglia identify cells to phagocytize? Trem2 detects a specific subset of lipids that are present on neurons and astrocytomas (Cannon et al.; 2012; Hsieg, et al., 2013; Wang et al., 2015). Additionally, Trem2 is highly expressed in microglia around the subventricular zones and white matter in the neonatal mouse brain, and is co-expressed in a small subset of CD68+ microglia (Chertoff et al., 2013). Since Trem2 is expressed throughout the brain during development, this suggests that there may be more widespread sex differences in phagocytic microglia beyond the hippocampus that warrant future investigation.
4.4 Hormonal effects on microglia
We found that estradiol treatment of females decreased the number of phagocytic microglia to male-typical levels. Thus, estradiol, in decreasing phagocytosis of neural progenitor cells, may create a permissive environment for higher levels of cellular proliferation seen in males (Bowers et al., 2010; Zhang et al., 2008). We also found that estradiol increased microglial proliferation in females. Interestingly, we found no effect of the androgen, DHT, on microglial phagocytosis or proliferation even though androgens are important for higher levels of cell proliferation in the neonatal male hippocampus (Waddell et al., 2013). Future work will need to focus on how estradiol is driving sex differences in microglial phagocytosis and proliferation. Several studies have measured estradiol content or estrogen receptor (ER) expression during development. Males do not have higher levels of estradiol in the hippocampus during development, even though aromatase expression has been reported to be higher in males (Amateau et al., 2004; Ivanova and Beyer, 2000; Konkle and McCarthy, 2011). Both ER-alpha and ER-beta have been detected in the early postnatal hippocampus, however expression seems to be localized primarily to pyramidal neurons (Ivanova and Beyer, 2000; Solum and Handa, 2001; Zuloaga et al., 2014). Notably, none of these studies have found a sex difference in ER expression during the first postnatal week, suggesting that differential ER expression may not be the means through which hormones influence sex differences in microglial phagocytosis.
How might microglia be affected by estradiol? If progenitor cells express estrogen receptors, they could release diffusible factors or hormones could influence ‘tags’ expressed on the cellular membrane that regulate microglia homing and phagocytic activity (Brown and Neher, 2014). Progenitor cells have been shown to regulate microglia function in adult animals by releasing diffusible factors such as CXCL12 and VEGF (Arnò et al., 2014; Mosher et al., 2012). Alternatively, hippocampal microglia could be directly affected by estradiol signaling if they express estrogen receptors. However, ERs are preferentially expressed in pyramidal cells or neurons and there are few to no microglia in the pyramidal layer during the early neonatal period, suggesting that microglia do not express estrogen receptors in the hippocampus during this early neonatal period. Similarly, microglia in the preoptic area of the hippocampus do not express ER-alpha (Lenz et al., 2013). Although ER-alpha has been detected in whole brain microglia at PN3 (Crain et al., 2013), this may only be a specific subset of microglia outside the hippocampal neurogenic zone. It is possible that microglia may also express the G-protein coupled estrogen receptor (GPER), however this has not been tested. Future work will need to determine how estrogens are affecting microglial function during development.
4.5 Conclusions
Microglia are involved in many developmental functions such as secreting factors that promote neurogenesis and synapse formation, and phagocytizing progenitor cells and synapses. However, very few of these studies have analyzed for sex differences. Our data contributes novel and important information on sex differences in microglia, showing that there are sex differences in microglia phagocytosis during development. These sex differences in microglial phagocytosis may impact the development and lifelong function of the hippocampus in males and females, both at baseline and in response to early life perturbations, such as early life stress, inflammation, or injury, that are known to negatively impact hippocampal function throughout the lifespan.
Supplementary Material
Highlights.
We quantified sex differences and hormonal programming of microglial morphology and phagocytosis in the neonatal rat hippocampus.
Females had more phagocytic microglia compared to males, and male-typical hormones are responsible for this sex difference.
More progenitor and non-pyknotic cells were targeted by microglial phagocytosis in females.
Estradiol increased microglia proliferation in neonatal females.
We found that females had higher expression of several phagocytic pathway genes.
Acknowledgments
We would like to thank Brenden Bishop for his help with the statistical analysis. This work was supported by OSU Fellowship to LHN and OSU Startup Funds to KML.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern TH, Krug S, Carr AV, Murray EK, Fitzpatrick E, Bengston L, McCutcheon J, De Vries GJ, Forger NG. Cell death atlas of the postnatal mouse ventral forebrain and hypothalamus: Effects of age and sex: Cell Death Atlas, Hypothalamus and Forebrain. J Comp Neurol. 2013;521:2551–2569. doi: 10.1002/cne.23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain Estradiol Content in Newborn Rats: Sex Differences, Regional Heterogeneity, and Possible de No vo Synthesis by the Female Telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Arnò B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B, Martino G, Muzio L. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:5611. doi: 10.1038/ncomms6611. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1:10–1186. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- Cannon JP, O’Driscoll M, Litman GW. Specific lipid recognition is a general feature of CD300 and TREM molecules. Immunogenetics. 2012;64:39–47. doi: 10.1007/s00251-011-0562-4. [DOI] [PubMed] [Google Scholar]
- Chertoff M, Shrivastava K, Gonzalez B, Acarin L, Giménez-Llort L. Differential Modulation of TREM2 Protein during Postnatal Brain Development in Mice. PLoS ONE. 2013;8:e72083. doi: 10.1371/journal.pone.0072083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice: Microglial Gene Expression in Healthy Brain. J Neurosci Res. 2013;91:1143–1151. doi: 10.1002/jnr.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Tønder N, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the prenatal rat hippocampus. J Comp Neurol. 1997;377:70–84. [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, González B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Miner SA, Weiner JA, Dailey ME. Developmental changes in microglial mobilization are independent of apoptosis in the neonatal mouse hippocampus. Brain Behav Immun. 2016;55:49–59. doi: 10.1016/j.bbi.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Strahan JA, Castillo-Ruiz A. Cellular and molecular mechanisms of sexual differentiation in the mammalian nervous system. Front Neuroendocrinol. 2016;40:67–86. doi: 10.1016/j.yfrne.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno J, Swan P, Shaikh R, Cregan SP. Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis. 2015;6:e1779. doi: 10.1038/cddis.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, Garcia AJ, Elsen GE, Nelson BR, Mussar KE, Reiner SL, Ramirez JM, Hevner RF. Tbr2 Expression in Cajal-Retzius Cells and Intermediate Neuronal Progenitors Is Required for Morphogenesis of the Dentate Gyrus. J Neurosci. 2013;33:4165–4180. doi: 10.1523/JNEUROSCI.4185-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, Seaman WE. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. Journal of Neurochemistry. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor- αβ; mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. doi: 10.1007/s004410000199. [DOI] [PubMed] [Google Scholar]
- Khalaf-Nazzal R, Francis F. Hippocampal development — Old and new findings. Neuroscience. 2013;248:225–242. doi: 10.1016/j.neuroscience.2013.05.061. [DOI] [PubMed] [Google Scholar]
- Konkle ATM, McCarthy MM. Developmental Time Course of Estradiol, Testosterone, and Dihydrotestosterone Levels in Discrete Regions of Male and Female Rat Brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Gervais A, Colin C, Chéret C, de Almodovar CR, Carmeliet P, Krause KH, Boillée S, Mallat M. The NADPH oxidase Nox2 regulates VEGFR1/CSF-1R-mediated microglial chemotaxis and promotes early postnatal infiltration of phagocytes in the subventricular zone of the mouse cerebral cortex: Microglial Nox2 in Development. Glia. 2013;61:1542–1555. doi: 10.1002/glia.22540. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia Are Essential to Masculinization of Brain and Behavior. J Neurosci. 2013;33:2761–2772. doi: 10.1523/JNEUROSCI.1268-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur S, Cristante E, Paterno M, Christian H, Roncaroli F, Gillies GE, Solito E. Annexin A1: A Central Player in the Anti-Inflammatory and Neuroprotective Role of Microglia. J Immunol. 2010;185:6317–6328. doi: 10.4049/jimmunol.1001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Schwarz JM, Wright CL, Dean SL. Mechanisms Mediating Oestradiol Modulation of the Developing Brain. J Neuroendocrinol. 2008;20:777–783. doi: 10.1111/j.1365-2826.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T. Neural progenitor cells regulate microglia functions and activity. Nat Neurosci. 2012;15:1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley M, Shah C, Morse KA, Miloro SA, Holmes MM, Ahern TH, Forger NG. Patterns of cell death in the perinatal mouse forebrain: Cell death in the perinatal mouse brain. J Comp Neurol. 2016 doi: 10.1002/cne.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Neniskyte U, Hornik T, Brown GC. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo: P2Y6 Purinergic Signaling in Phagoptosis. Glia. 2014;62:1463–1475. doi: 10.1002/glia.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge: Iba1 and GFAP Are Unreliable Activation Markers. Glia. 2016;64:300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pouchoulen M, VanRyzin JW, McCarthy MM. Morphological and Phagocytic Profile of Microglia in the Developing Rat Cerebellum. eNeuro. 2015;2 doi: 10.1523/ENEURO.0036-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler J, Grosche A, Lede V, Le Duc D, Krügel K, Matyash V, Szulzewsky F, Kallendrusch S, Immig K, Kettenmann H, Bechmann I, Schöneberg T, Schulz A. Altered microglial phagocytosis in GPR34-deficient mice: Microglial Phagocytosis in GPR34−/− Mice. Glia. 2015;63:206–215. doi: 10.1002/glia.22744. [DOI] [PubMed] [Google Scholar]
- Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 beta-estradiol. Endocrinology. 1984;114:1754–1760. doi: 10.1210/endo-114-5-1754. [DOI] [PubMed] [Google Scholar]
- Russell DG, VanderVen BC, Glennie S, Mwandumba H, Heyderman RS. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nature Reviews Immunology. 2009;9:594–600. doi: 10.1038/nri2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain: Sex differences in microglial colonization. 2012 doi: 10.1111/j.1471-4159.2011.07630.x. no–no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia Enhance Neurogenesis and Oligodendrogenesis in the Early Postnatal Subventricular Zone. J Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ERa) in pyramidal neurons of the developing rat hippocampus. Dev Brain Res. 2001:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Spittau B, Rilka J, Steinfath E, Zöller T, Krieglstein K. TGFβ1 increases microglia-mediated engulfment of apoptotic cells via upregulation of the milk fat globule-EGF factor 8: TGFβ1 Upregulates Mfge8 Expression. Glia. 2015;63:142–153. doi: 10.1002/glia.22740. [DOI] [PubMed] [Google Scholar]
- Surace MJ, Block ML. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cellular and Molecular Life Sciences. 2012;69:2409–2427. doi: 10.1007/s00018-012-1015-4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Waddell J, Bowers JM, Edwards NS, Jordan CL, McCarthy MM. Dysregulation of neonatal hippocampal cell genesis in the androgen insensitive Tfm rat. Horm Behav. 2013;64:144–152. doi: 10.1016/j.yhbeh.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental Neuronal Death in Hippocampus Requires the Microglial CD11b Integrin and DAP12 Immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. Journal of Neuroscience. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor β expression in the mouse forebrain: Age and sex differences: Estrogen receptor β forebrain distribution. J Comp Neurol. 2014;522:358–371. doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


