Abstract
Podocytes form the visceral layer of a renal glomerulus and express a characteristic octopus—like cellular architecture specialized for the ultrafiltration of blood. The cytoskeletal dynamics and structural elasticity of podocytes rely on the self-organization of highly interconnected actin bundles and the maintenance of these features is important for the intact glomerular filtration. Development of more differentiated podocytes in culture has dramatically increased our understanding of the molecular mechanisms regulating podocyte actin dynamics. Podocytes are damaged in a variety of kidney diseases and therapies targeting podocytes are being investigated with increasing efforts. Association between podocyte damage and disease severity—or between podocyte recovery and the performance of therapeutic molecules—have been the venues of research for years. In this perspective, more standardized high content screening (HCS) has emerged as a powerful tool for visualization and analysis of podocyte morphology. This high-throughput fluorescence microscopy technique is based on an automated image analysis with simultaneous detection of various phenotypes (multiplexing) across multiple phenotypic parameters (multiparametric). Here, we review the principles of HCS technology and summarize efforts to carry out small compound screen using podocytes.
Introduction
Urine formation starts with the filtration of blood through the glomerular filtration barrier, which is a three-layer interface formed by fenestrated endothelial cells1, glomerular basement membrane (GBM)2, and visceral epithelial cells, also known as podocytes3. Owing to its delicate structure and coordinated cell polarity, glomerular filtration barrier constitutes a size and charge selectivity that facilitates cation transport and filters all the small molecules (water, salt, glucose, amino acids, urea, etc.) out of the blood but retains cells, platelets and large proteins, such as albumin.
As a key player of the glomerular filtration, podocytes are aligned on the external surface of GBM and cover glomerular capillaries neatly with numerous cytoplasmic projections, called foot processes (FPs)4. These terminally differentiated epithelial cells form the final barrier to protein loss by interdigitating with the FPs of the adjacent podocytes and leaving between them the slit diaphragm (SD), narrow filtration slits that are bridged by modified adherens junction5. Podocyte’s functions, which are vital to glomerular filtration, depend on a highly regulated actin cytoskeletal network that is formed either by a central actin bundle along the long axis of FPs or by a relatively short cortical network aligning at the cell periphery and anchoring the components of SD6.
Podocytes are the major targets of several agents or molecules such as toxins, reactive oxygen species (ROS), complements, and antibodies7. Injury to podocytes may physically alter this elaborate structure causing the flattening and retraction of the FPs as well as the disappearance of the filtration slits, a process called FP effacement. Therefore, efforts to reverse the podocyte damage and rescue glomerular filtration generally focus on actin regulatory pathways8 and developing therapeutic agents that can ameliorate disruptions of actin organization9.
A successful disease-specific tailoring of therapeutics may be achieved by using an image-based screening, which enables to analyze a wide variety of phenotypes in cells. Such high content screening (HCS) platforms employ fully automated microscopes and image analysis software, making it possible to quantify changes in cellular and subcellular properties including cell area, morphology, actin fiber and focal adhesion intensity. We recently described a novel phenotype-based HCS using immortalized mouse podocyte cells and applied it to identify podocyte-protective small molecules10. This review aims to discuss the screening experiments and image analysis approaches, as this high-throughput technique is being used in the preclinical development of the drug discovery process.
PODOCYTE AS A DIRECT TARGET OF DRUGS
Kidneys have arguably the most complex membrane system and solute trafficking in the body, which attracted researchers with interests in kidney biology for many decades11. This is mostly due to the multicomponent nature of the glomerular filtration system, with endothelial cells, glomerular basement membrane (GBM), and visceral epithelial cells (podocytes) participating in the filtration process4. The function of this elegant filtration system is maintained by the interplay among these core constituents as well as the immaculate arrangement of the structural proteins within the membrane. The integrity12 and elasticity13 are other fundamental concepts since the capillary pressures far exceed those in other organs. The mechanical support required for glomerular capillaries are mainly provided by podocytes14 since GBM and its associated cells are not rigid, but rather flexible15. Furthermore, endothelial cells lack sufficient cytoskeletal structure (and contractile system) as demonstrated by the electron microscopy16. Hence, among the principal components of the glomerular filtration barrier, podocyte deserves a special attention. And it really has: during the past decade, podocyte research has remarkably expanded, with more than 3000 published papers directed toward delineating the mechanisms regulating podocyte structure and function.
Owing to its strategic location, podocyte is the major target of various agents soluble in the blood, including toxic and immunologic compounds, reactive oxygen species (ROS), complements, and antibodies to podocyte membrane antigens7. Podocyte is also injured by other means, such as genetic deletions or mutations impacting the proteins of podocyte itself, SD complex and GBM structure or charge distortion directly affecting its apical membrane domain7. Podocyte injury leads to reorganization of actin cytoskeleton from a dynamic state (characterized by parallel and contractile actin filaments) to a rigid state (represented by thicker stress fibers) and foot process (FP) effacement (fusion or retraction of podocyte terminal processes)17. Beyond these structural changes and phenotypic conversions, persistent injuries to podocyte might cause lethal alterations such as detachment from the underlying GBM18 (as a relevant note, podocytes disappearing from the glomerular tuft can be still alive and recovered from the urine19) and death20. The loss of podocytes is an irreversible event causing the loss of glomerular filtration function since podocytes are post-mitotic cells with a minimal capacity to replicate21. Once podocytes are lost, the remaining podocytes fail to completely cover the outer aspect of the GBM and become more vulnerable to any additional workload22. Potential mechanisms for podocyte replacement include the contribution of glomerular parietal epithelial cells (PECs) and cells of renin lineage (CoRL) as podocyte progenitors; however, uncertainties still remain regarding the routes of the migratory event that brought those progenitors to the glomerular tuft and the formation of complex cytoskeletal structure (i.e., FPs and SDs) in these candidate cells23.
Notably, there is abundant evidence indicating that any abnormality or change in podocyte cytoskeleton may contribute to proteinuria and nephrotic syndrome24, and more strikingly, disorders affecting glomerular filtration are responsible for 90% end-stage kidney diseases (ESKD) at a cost of $30 billion per year in the US alone25. Several pharmacological agents targeting podocytes are being evaluated such as corticosteroids, angiotensin I-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), peroxisome proliferator-activated receptors (PPAR) agonists, retinoids, and vitamin derivatives26–28. Despite tremendous research effort, there are still very limited therapeutic options to stop progressive decline in glomerular filtration rate9. Podocytes are therefore a promising target for investigating the pathogenetic mechanisms of renal protection and screening new treatment options.
CULTURED PODOCYTES ARE SUITABLE SOURCES FOR IMAGE ANALYSIS
Fundamental observations of biological processes can be consistently simplified in two dimensional cell culture conditions, where cells grow (and differentiate) in the presence of a defined medium and behave similar to the in vivo situation. Regardless of its limitation regarding the representation of the physiological microenvironment, in vitro assessment of cell viability, metabolism, and functionality is an important step to many aspects of biomedical research. Therefore, over the years, traditional in vitro cell culture helps researchers to predict the response of more complex organisms (e.g., tissues, organs) to potential pharmaceuticals.
Kidney diseases are mainly characterized by structural and functional changes in glomerulus that causes a gradual loss of kidney function (measured by glomerular filtration rate, GFR) and terminal renal failure, if not treated promptly. Physical properties of cells constituting glomerular filtration barrier have been shown to have a significant role in various kidney diseases, as in many instances podocyte cytoskeleton is disorganized and podocyte adhesion decreased when the cells are no longer healthy. In that sense, the availability of murine29 and human podocyte30 cell lines is crucial since the injury-induced cytoskeletal changes and injury-driven cell motility can be evaluated by well-defined protocols in cultured podocytes.
Using a temperature-sensitive transgene, conditionally immortalized podocytes are able to proliferate under permissive conditions (at 33°C and with the presence of interferon-γ □□ podocytes are of mouse origin) and display characteristic cobblestone shape, whereas under non-permissive temperature (37°C), the cells stop replicating and starts differentiating by developing a large arborized morphology containing well-developed processes31. Actually, this one-cell-thick podocyte monolayer cannot fully replicate the highly sophisticated in vivo kidney filtration barrier (i.e., neither podocyte-GBM interaction is represented with a great molecular detail by the interaction of podocyte and extracellular matrix (ECM) ligands in the tissue culture flask, nor the tight connections of podocytes with each other through specialized cell-adhesion molecules in vitro is really an accurate cellular representation of filtration slits) but still offers a quite successful research venue for mechanistic studies. This is mainly due to their ability to express virtually all podocyte-specific markers31 such as synaptopodin, which is an actin-binding protein and involved in cytoskeletal organization32. Synaptopodin is profoundly expressed in podocyte FPs in vivo and synaptopodin-deficient mice abrogates stress-fiber formation and has an impaired recovery from proteinuria33. Since most glomerular diseases are characterized by FP effacement leading to proteinuria8, loss of stress fibers or reduced expression of synaptopodin in cultured podocytes can serve as surrogate markers for these pathological events in vitro. Rearrangement of actin cytoskeleton also modulates cell-matrix adhesion and plays a direct causative role in development of migratory phenotype, which can be analyzed in vitro by a functional podocyte assay34. If performed on a large scale by HCS platforms, these well-designed assays and related phenotypic measurements prepare the stage for potential clinical translation.
HIGH THROUGHPUT PLATFORM FOR STUDYING PODOCYTES
Over the last 20 years following the establishment of immortalized mouse podocyte cell line, it became more obvious that podocytes—as targets of various pathogenic pathways—have a key function in kidney diseases, regardless of the initial etiology, and cultured podocytes have potential to provide a suitable informational content to explore the molecular mechanisms mediating podocyte function and to validate kidney disease models. However, our capability to evaluate effective therapies to kidney dysfunction has somehow been limited by the poor translation of phenotypic changes to disease outcomes. In preclinical studies, it is particularly important to track changes in phenotype over time to better characterize the cellular responses to various external factors. To this end, high-throughput platforms using animal- or human-derived cell models are integral to facilitating the efforts to investigate the function of cells in disease and to improve our ability to predict whether the compounds of interest would lead to clinically useful drugs, irrespective of the level of disease complexity.
High content screening (HCS) combines the power of automated fluorescence microscopy and image analysis software for quantitative and dynamic measurements of the biological changes in cells35,36. The algorithm includes assay design, image acquisition, image analysis, and data interpretation. The major advantage of this technology is its ability of multiplex profiling (i.e., detection of diverse cellular states simultaneously) and multiparametric analysis (i.e., measurement of multiple phenotypic parameters at the same time) in an automated and robust manner. Applied to drug discovery, HCS campaign allows us to discover new compounds that are capable of enhancing cell’s stress response, improving the phenotype, and preventing an additional damage37,38.
We recently developed a podocyte-based HCS assay10 to screen compounds for their antiproteinuric activity (see Figure 1 for the schematic overview of experimental workflow). We took advantage of using immortalized murine podocytes29, which are highly proliferative when cultured under permissive conditions and exhibit the characteristics of differentiated podocytes with elongated cellular processes protruding from the cell body and concomitant expression of synaptopodin and other podocyte marker proteins such as nephrin, podocin, CD2AP, TRPC6, α3βi integrin, and α-actinin-431. The initial effort started with standardizing the cell culture conditions (passage number, seeding density, plate type, media, ECM composition, etc.) to eliminate assay-to-assay variability and make historical performance comparisons easy.
Figure 1. Set up of the podocyte-based HCS platform.
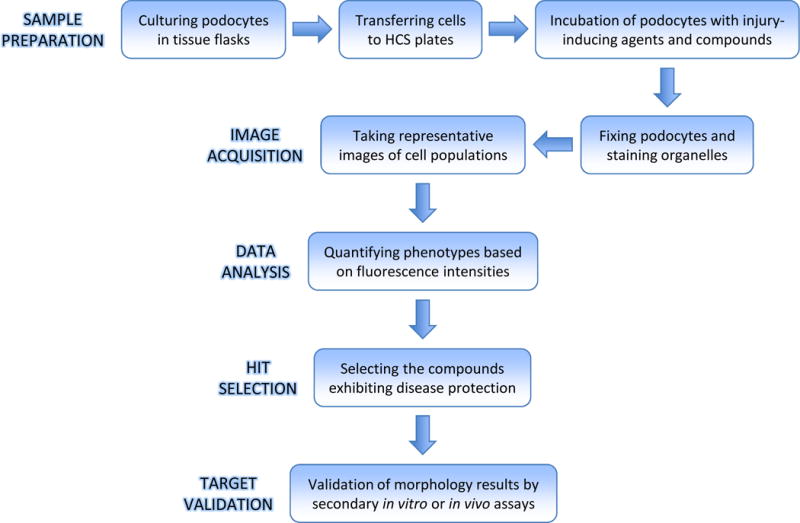
Immortalized mouse podocytes are proliferated at permissive temperature (33°C). When the culture reach 75 to 80% confluency, podocytes are harvested and cultured in tissue flasks at 37°C for differentiation. After 7 days, cells are detached and transferred to multiwell plates in which they are further cultured at 37°C for 4 days. Podocytes are treated with disease agent (PAN) and the library of compounds and fixed at the end of the treatment period. The nuclei, cytoplasmic boundaries, actin fibers, and focal adhesions are stained before acquiring images from each well of the plate on fully automated HCS system. Image analysis is performed using high content analysis software to examine (i.e., quantify) cell phenotypes. New targets (hits) are identified by screening compounds with desired mechanisms of action, i.e., alleviating adverse phenotypes. The biological activities of the most promising targets need to be further validated by secondary in vitro and in vivo assays before being introduced into the clinic.
We used puromycin aminonucleoside (PAN), a well-established rat model of proteinuria39 and a commonly used experimental model of podocyte injury in vitro34 to induce podocyte damage, which is manifested by the appearance of a relatively high proportion of rounded cells with reduced actin fibers and focal contacts and weak adhesion to ECM40,41. Following the treatment of cells with varying doses of PAN, we captured representative images of hundreds of cells simultaneously in an automated fashion. Then, we quantified the cellular phenotypes (i.e., cell size and morphology, intensity and spatial distribution of F-actin fibers and focal adhesions) in an unbiased manner by the computer-assisted image analysis (Figure 2), which is the key step in the protocol since it translates the assay readout into podocyte response and function using the immense amount of information stored in these microscope-generated images42. PAN-mediated podocyte injury can be ameliorated by several pharmacological agents, including dexamethasone, fluvastatin, darbepoetin, mizoribine, sialic acid, and nuclear factor kappa B (NF-κB) inhibitor dehydroxymethylepoxyquinomicin (DHMEQ)7. Among those, we chose mizoribine (MZR)43 and demonstrated that we were able to protect podocytes and rule out the adverse effects of PAN on podocyte cytoskeleton by MZR treatment. To validate our findings with a relevant protective agent, we used the glucocorticoid dexamethasone (DEX)44 and treated podocytes with varying doses of DEX to investigate its efficacy against PAN. We consistently obtained similar results toward the recovery from PAN-induced injury from each of the agents we employed, highlighted with the increased F-actin fibers and focal adhesions, higher synaptopodin levels and decreased cell roundness. This suggests that our HCS methodology is reliable across plates and experiments and not limited to a single model.
Figure 2. Image analysis of a podocyte phenotypic assay.
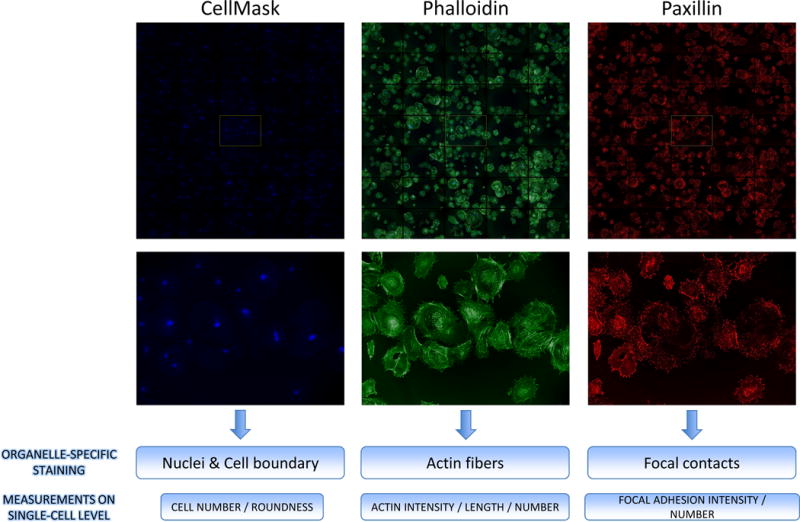
Prior to imaging, nuclei and cytoplasmic boundaries are stained with CellMask (blue), F-actin is stained with phalloidin (green), and focal adhesions are stained with paxillin antibody (red). Cellular phenotypes are calculated on a single-cell level by quantitative imaging platform of HCS system. Additional details regarding the staining and image analysis protocols can be found in Lee et al.10.
We further verified the assay performance by testing the reproducibility and robustness of our HCS algorithm. In particular, we determined Z′ factor across multiple replicates on an analysis plate45. It is a statistical test used to compare the conditions (e.g., positive and negative controls) and indicates a better separation (with a low standard deviation) between these groups when it approaches 1.045. It is suited to most HCS readouts to assess the quality of screening assays46. Repeating the multiparametric analysis for untreated and PAN-treated podocytes resulted in Z′ values of ≥0.46 and 0.44 for changes in cell roundness and F-actin fibers, respectively, indicating the robustness and reproducibility of our assay.
Based on the aggregated data (from individual cells to treatment wells) and the dynamics of the injury and recovery process, we chose cell roundness as the major feature to use at our primary HCS assay employing the library of 2121 pharmacologically active compounds. Approximation to one-dimensional parametric analysis improved the efficiency of our HCS campaign by speeding the screening and reducing the data storage. This single parameter approach is also applied to perform phenotypic screening to detect drug candidates47 or RNAi libraries48 in cancer pharmacology, where cell viability represents the reliable readout. Here, the ultimate goal was to screen those compounds on PAN-treated podocytes and identify hits (compounds exhibiting the protective properties) for additional testing. Our analysis resulted in a primary hit list of 24 compounds (with approximately 1% hit rate), which were re-analyzed using independently obtained powder forms. Our strategy here was to cluster the top primary hits in functional groups and start with the ones having potential protective roles for significant podocyte-related proteins. Among those, the small molecule β1-agonist pyrintegrin drew our attention due to a recent study reporting that pyrintegrin increased cell-ECM adhesion-mediated integrin activity in human embryonic stem cells (hESCs), in which β1 integrin is regarded as a major integrin49. Almost a decade ago, we had shown that PAN-treated mouse podocytes demonstrated a marked down-regulation of α3β1 integrin34, which is the anchoring unit of podocyte FPs to GBM50. Podocyte-specific deletion of β1 in mice leads to severe proteinuria as early as 3 weeks of age51 and death within 15 weeks52 indicating the critical role of α3β1 integrin in maintaining the structural integrity of the glomerulus. In the light of these findings, we were able to identify β1-agonist pyrintegrin as a podocyte-protective agent by HCS and validated it in vivo (in the setting of PAN-induced nephropathy in rats).
Overall, our HCS platform had the unique ability to quantify multiple phenotypic properties of podocytes with high sensitivity and identify the repertoire of small molecules preventing PAN-mediated podocyte disease. This fully automated and computer-aided analysis platform will accelerate the progress exploring therapies for preserving glomerular filtration function.
Conclusion
Podocytes are highly differentiated epithelial cells covering glomerular capillaries of the kidneys. They are well decorated with a dense array of stress fibers containing F-actin and hence have elasticity and contractile properties to bear the excess capillary pressure exerted by blood (filtrate) on the glomerular capsule. Several molecular pathways directly or indirectly contribute to podocyte injury, which is a common denominator of various glomerular diseases associated with proteinuria. Unfortunately, unlike other self-renewing epithelial cells, podocytes have a very limited ability to regenerate. Therefore, once podocyte cytoskeleton is altered or podocyte loss starts, these pathologic states operating in conjunction with each other further aggravate podocyte injury. This is clinically significant because of their critical role in keeping the glomerular filtration functioning properly. Knowing that podocytes are sensitive to many pathological conditions that lead to kidney damage, we aimed to develop a high-throughput phenotypic assay to study the ameliorative effect of a library of compounds on cultured podocytes in the context of a well-characterized glomerular disease model. We presented a fully automatic and quantitative image analysis approach that exploits multiple morphological details simultaneously at a single cell level. To our knowledge, this is the first HCS algorithm applied to podocytes and it is applicable to other physiologically relevant systems (i.e., systems designed to promote podocyte repair from other injury-inducing factors or to protect other glomerular cells from similar proteinuric agents) after an appropriate modification and agent-or cell-specific testing. Indeed, in pursuit of this methodology, HCS technology was used to screen compounds that reduced podocyte migration for the purpose of identification of potential therapeutic targets in a recent study53,54. These multi-parametric screening approaches, together with supporting studies, will enhance our systematic understanding of the regulation of podocyte cytoskeleton, function, and survival as well as basic mechanisms of glomerular diseases and drug actions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
J.R. and V.G. are inventors on pending patent applications related to this study and have the potential for future financial benefit from them.
References
- 1.Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009;296(5):F947–F956. doi: 10.1152/ajprenal.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318(9):973–978. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192(5):385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 4.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83(1):253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 5.Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11(1):1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 6.Ichimura K, Kurihara H, Sakai T. Actin filament organization of foot processes in rat podocytes. J Histochem Cytochem. 2003;51(12):1589–1600. doi: 10.1177/002215540305101203. [DOI] [PubMed] [Google Scholar]
- 7.Reiser J, Altintas MM. Podocytes. F1000 Res. 2016;5(114) doi: 10.12688/f1000research.7255.1. 1000research.7255.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17(9):428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kidney Int. 2010;77(8):662–668. doi: 10.1038/ki.2009.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HW, Khan SQ, Faridi MH, et al. A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol. 2015;26(11):2741–2752. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farquhar MG. Editorial: the primary glomerular filtration barrier—basement membrane or epithelial slits? Kidney Int. 1975;8(4):197–211. doi: 10.1038/ki.1975.103. [DOI] [PubMed] [Google Scholar]
- 12.Kriz W, Elger M, Mundel P, Lemley KV. Structure-stabilizing forces in the glomerular tuft. J Am Soc Nephrol. 1995;5(10):1731–1739. doi: 10.1681/ASN.V5101731. [DOI] [PubMed] [Google Scholar]
- 13.Embry AE, Mohammadi H, Niu X, et al. Biochemical and cellular determinants of renal glomerular elasticity. PLoS One. 2016;11(12):e0167924. doi: 10.1371/journal.pone.0167924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endlich N, Endlich K. The challenge and response of podocytes to glomerular hypertension. Semin Nephrol. 2012;32(4):327–341. doi: 10.1016/j.semnephrol.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Takami H, Naramoto A, Shigematsu H, Ohno S. Ultrastructure of glomerular basement membrane by quick-freeze and deep-etch methods. Kidney Int. 1991;39(4):659–664. doi: 10.1038/ki.1991.79. [DOI] [PubMed] [Google Scholar]
- 16.Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59(5):673–682. [PubMed] [Google Scholar]
- 17.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in masugi nephritis. Am J Pathol. 1996;148(4):1283–1296. [PMC free article] [PubMed] [Google Scholar]
- 18.Hara M, Yamamoto T, Yanagihara T, et al. Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron. 1995;69(4):397–403. doi: 10.1159/000188509. [DOI] [PubMed] [Google Scholar]
- 19.Petermann AT, Krofft R, Blonski M, et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64(4):1222–1231. doi: 10.1046/j.1523-1755.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 20.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13(12):3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 21.Kriz W. Progressive renal failure-inability of podocytes to replicate and the consequences for development of glomerulosclerosis. Nephrol Dial Transplant. 1996;11(9):1738–1742. [PubMed] [Google Scholar]
- 22.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67(2):404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- 23.Altintas MM, Reiser J. Bridges to cross, burn, and mend: cells of renin lineage as podocyte progenitors. Am J Physiol Renal Physiol. 2015;309(6):F499–F500. doi: 10.1152/ajprenal.00301.2015. [DOI] [PubMed] [Google Scholar]
- 24.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71(12):1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD: 2014. [Google Scholar]
- 26.Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15(1):1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 27.Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62(14):1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Mathieson PW. The podocyte as a target for therapies — new and old. Nat Rev Nephrol. 2011;8(1):52–56. doi: 10.1038/nrneph.2011.171. [DOI] [PubMed] [Google Scholar]
- 29.Mundel P, Reiser J, Zúñiga Mejía Borja A, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236(1):248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 30.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 31.Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72(1):26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 32.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139(1):193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asanuma K, Kim K, Oh J, et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115(5):1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279(33):34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 35.Taylor DL. Past, present, and future of high content screening and the field of cellomics. Methods Mol Biol. 2007;356:3–18. doi: 10.1385/1-59745-217-3:3. [DOI] [PubMed] [Google Scholar]
- 36.Zanella F, Lorens JB, Link W. High content screening: seeing is believing. Trends Biotechnol. 2010;28(5):237–245. doi: 10.1016/j.tibtech.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Perlman ZE, Slack MD, Feng Y, Mitchison TJ, Wu LF, Altschuler SJ. Multidimensional drug profiling by automated microscopy. Science. 2004;306(5699):1194–1198. doi: 10.1126/science.1100709. [DOI] [PubMed] [Google Scholar]
- 38.Boutros M, Heigwer F, Laufer C. Microscopy-based high-content screening. Cell. 2015;163(6):1314–1325. doi: 10.1016/j.cell.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Whiteside CI, Cameron R, Munk S, Levy J. Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis. Am J Pathol. 1993;142(5):1641–1653. [PMC free article] [PubMed] [Google Scholar]
- 40.Fishman JA, Karnovsky MJ. Effects of the aminonucleoside of puromycin on glomerular epithelial cells in vitro. Am J Pathol. 1985;118(3):398–407. [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall CB, Pippin JW, Krofft RD, Shankland SJ. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 2006;70(11):1962–1973. doi: 10.1038/sj.ki.5001965. [DOI] [PubMed] [Google Scholar]
- 42.Buchser W, Collins M, Garyantes T, et al. Assay development guidelines for image-based high content screening, high content analysis and high content imaging. In: Sittampalam GS, Coussens NP, Nelson H, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Lemmon V, Li Z, McGee J, McManus O, Minor L, Napper A, Riss T, Trask OJ, Weidner J, editors. Assay Guidance Manual. Bethesda, MD: 2004. [Google Scholar]
- 43.Takeuchi S, Hiromura K, Tomioka M, et al. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron Exp Nephrol. 2010;116(1):e3–e10. doi: 10.1159/000314668. [DOI] [PubMed] [Google Scholar]
- 44.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16(9):2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 46.Singh S, Carpenter AE, Genovesio A. Increasing the content of high-content screening: An overview. J Biomol Screen. 2014;19(5):640–650. doi: 10.1177/1087057114528537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez CN, Antczak C, Djaballah H. Cell viability assessment: toward content-rich platforms. Expert Opin Drug Discov. 2010;5(3):223–233. doi: 10.1517/17460441003596685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antczak C, Takagi T, Ramirez CN, Radu C, Djaballah H. Live-cell imaging of caspase activation for high-content screening. J Biomol Screen. 2009;14(8):956–969. doi: 10.1177/1087057109343207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y, Zhu X, Hahm HS, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci USA. 2010;107(18):8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adler S. Characterization of glomerular epithelial cell matrix receptors. Am J Pathol. 1992;141(3):571–578. [PMC free article] [PubMed] [Google Scholar]
- 51.Pozzi A, Jarad G, Moeckel GW, et al. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008;316(2):288–301. doi: 10.1016/j.ydbio.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanasaki K, Kanda Y, Palmsten K, et al. Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol. 2008;313(2):584–593. doi: 10.1016/j.ydbio.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Widmeier E, Tan W, Airik M, Hildebrandt F. A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol. 2017;312(1):F157–F171. doi: 10.1152/ajprenal.00386.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta V, Reiser J. Stop that podocyte! Am J Physiol Renal Physiol. 2017;312(2):F373–F374. doi: 10.1152/ajprenal.00499.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


