Abstract
Objective
The objective of this prospective cohort study was to determine if sleep disordered breathing during pregnancy is a risk factor for the development of hypertensive disorders of pregnancy and gestational diabetes mellitus.
Methods
Nulliparous women underwent in-home sleep disordered breathing assessments in early (6–15 weeks) and mid-pregnancy (22–31 weeks). Participants and providers were blinded to the sleep test results. An apnea-hypopnea index (AHI) of ≥5 was used to define sleep disordered breathing. Exposure-response relationships were examined grouping participants into four AHI groups: AHI=0, 0<AHI<5, 5≤AHI<15 and AHI≥15. The study was powered to test the primary hypothesis that sleep disordered breathing occurring in pregnancy is associated with an increased incidence of preeclampsia.. Secondary outcomes were rates of hypertensive disorders of pregnancy, defined as preeclampsia and antepartum gestational hypertension, and GDM. Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models.
Results
Three thousand seven hundred and five women were enrolled. AHI data were available for 3,132 (84.5%) and 2,474 (66.8%) women in early and mid-pregnancy, respectively. The corresponding prevalence of sleep disordered breathing was 3.6% and 8.3%. The prevalence of preeclampsia was 6.0%, hypertensive disorders of pregnancy 13.1% and GDM 4.1%. In early and mid-pregnancy the adjusted odds ratios for preeclampsia when sleep disordered breathing was present were 1.94 (95% CI 1.07–3.51) and 1.95 (95% CI 1.18–3.23), respectively; hypertensive disorders of pregnancy, 1.46 (95% CI 0.91–2.32) and 1.73 (95% CI 1.19–2.52); and GDM, 3.47 (95% CI 1.95–6.19) and 2.79 (95% CI 1.63–4.77). Increasing exposure-response relationships were observed between AHI and both hypertensive disorders and GDM.
Conclusions
There is an independent association between sleep disordered breathing and preeclampsia, hypertensive disorders of pregnancy, and GDM.
Introduction
Sleep-disordered breathing conditions are characterized by abnormal respiratory patterns and abnormal gas exchange during sleep.1 Obstructive sleep apnea is the most common type of sleep disordered breathing. In reproductive aged women, epidemiologic studies suggest a 2–13% prevalence of obstructive sleep apnea.2,3
Pregnancy is associated with changes that promote obstructive sleep apnea, such as increased body weight and upper airway edema.4 Frequent snoring, a cardinal symptom of obstructive sleep apnea, is endorsed by 15–25% of pregnant women.5,6 Health outcomes linked to obstructive sleep apnea in the non-pregnant population, such as hypertension and insulin-resistant diabetes, have correlates in pregnancy (preeclampsia, gestational diabetes).7–9 Obstructive sleep apnea has been linked to enhanced inflammatory and oxidative stress responses, endothelial damage and metabolic derangements.10,11 These same biological pathways have been associated with adverse pregnancy outcomes suggesting a mechanistic link between obstructive sleep apnea exposure in pregnancy and adverse outcomes.12
Several cross-sectional and retrospective studies suggest that sleep disordered breathing may increase the risk of developing hypertensive disorders and gestational diabetes (GDM) during pregnancy, but most of these studies relied on self-reported symptoms (e.g., snoring) as the exposure variable or sub-optimally controlled for body mass index (BMI).13–15 There are limited and conflicting data from small prospective observational cohorts.16–18 Addressing this knowledge gap is clinically relevant as hypertensive disorders of pregnancy and GDM are associated with maternal and perinatal morbidity and have long-term health consequences for both mothers and babies.17,19 The Sleep Disordered Breathing Substudy of the Nulliparous Pregnancy Outcomes Study was a multicenter, prospective cohort study. The objective was to determine if sleep-disordered breathing during pregnancy is a risk factor for the development of hypertensive disorders of pregnancy and GDM of pregnant women.
Materials and Methods
Details of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be parent and sleep disordered breathing substudy methods have been previously published.20,21 Briefly, the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be parent study was conducted at 8 clinical sites and managed by an independent Data Coordinating Center. Inclusion criteria for the parent study were nulliparity (no prior delivery ≥20 weeks’ gestation) and a viable singleton pregnancy at the time of screening (60–136 weeks’ gestation). Women were excluded from the sleep disordered breathing substudy if they were currently on continuous positive airway pressure (CPAP) treatment for sleep disordered breathing, had severe asthma requiring continuous oral steroid therapy for more than 14 days, or suffered from a condition requiring oxygen supplementation. The sleep disordered breathing substudy was designed and powered to test the primary hypothesis that sleep disordered breathing occurring early or appearing later in pregnancy is associated with an increased incidence of preeclampsia. Secondary aims were to examine the association between sleep disordered breathing and gestational hypertension and GDM.
For the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be-sleep disordered breathing substudy, Level 3 home sleep tests were performed using a 6-channel monitor that was self-applied by the participant twice during pregnancy, first between 60–150 weeks of pregnancy and then again between 220–310 weeks. Sleep study data were downloaded at the study site and electronically transmitted to a central Sleep Reading Center. The scoring and quality control protocol has been previously published.20 Sleep studies were scored using the following definitions:
Apnea: amplitude (peak to trough) of the nasal pressure signal flat for ≥ 10 seconds; if accompanied by effort on either respiratory band (obstructive apnea); if accompanied by complete absence of effort on both respiratory bands (central apnea).
Hypopnea: scored based on ≥ 30% reduction of amplitude in the nasal pressure signal or the respiratory sum channel (if no nasal pressure signal) for ≥ 10 seconds.
Apnea-Hypopnea Index (AHI): number of apneas and hypopneas per hour of estimated sleep, defined in this analysis as all apnea as regardless of oxygen desaturation and hypopneas accompanied by ≥ 3% oxygen desaturation
All apnea and hypopnea events were annotated and later linked with oxygen saturation values. Participants, investigators and care providers were blinded to the sleep test results unless urgent alert criteria were identified. Urgent alert studies included those with an AHI >50 events/hour or severe hypoxemia (oxygen saturation of <90% for ≥10% of sleep time). Criteria for urgent alerts were developed by expert consensus from members of the study team and approved by the Advisory and Safety Monitoring Board IRB approval was obtained at each site and informed consent was obtained from each participant.
For our primary analyses AHI was treated as a dichotomous variable with an AHI ≥ 5 defining the presence of sleep disordered breathing. To examine exposure-response relationships between increased AHI and pregnancy outcomes we also ran analyses grouping participants into four AHI groups: AHI=0, 0<AHI<5, 5≤AHI<15 and AHI ≥ 15 events per hour. The cutoff points of 5≤AHI<15 and AHI ≥ 15 represent mild and moderate- -severe sleep disordered breathing, respectively.
For any participant with hypertension, proteinuria, or a related condition documented in the chart, a detailed chart review was required by a site investigator or a staff member certified for abstraction of complicated charts. Appendix 2, available online at http://links.lww.com/xxx, outlines our study definitions of hypertensive disorders. Cases that presented atypically and were difficult to classify according to study criteria were adjudicated by the principal investigators and final classification was reached by consensus. As part of chart abstraction, the onset of the hypertensive disorder (gestational age; antepartum, intrapartum, postpartum) was ascertained. For analysis, preeclampsia was defined as all cases of mild, severe, or superimposed preeclampsia or eclampsia, regardless of the timing of onset. A hypertensive disorder related to pregnancy was defined as all cases of preeclampsia and antepartum gestational hypertension.
GDM was defined by one of the following glucose tolerance testing (GTT) criteria: 1) fasting 3-hour 100 gram GTT with two abnormal values: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL, 3-hour ≥140 mg/dL; 2) fasting 2-hour 75 gram GTT with one abnormal value: fasting ≥92 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥153 mg/dL; 3) non-fasting 50 gram GTT ≥200 mg/dL if no fasting 3-hour or 2-hour GTT was performed. In addition to GTT data, chart abstractors recorded if a diagnosis of GDM was made during the course of clinical care. If no GTT data were available, the information from chart abstraction was used for GDM classification. Women with pre-gestational diabetes were excluded from analysis of GDM.
A detailed description of our sample size calculation has been previously published.20 In summary, we aimed to enroll 3630 women in the SDB substudy anticipating that this would yield approximately 180 women (5%) with sleep disordered breathing in early pregnancy and 360 (10%) in late pregnancy. With these assumptions, and setting the Type I error at 2-sided α =0.05, the target sample size yields at least 80% power to detect a relative risk of 2.0 (1.8) for preeclampsia for women with sleep disordered breathing in early (late) pregnancy, assuming a 7% incidence of preeclampsia among the unexposed.22,23
Descriptive statistics were used to characterize the study population by AHI category. Chi-square tests assessed associations with characteristics that were categorical and analysis of variance F-tests were used for continuous measurements. Crude and adjusted odds ratios and 95% confidence intervals were calculated from univariate and multivariate logistic regression models to relate level of sleep disordered breathing in early and mid-pregnancy to hypertensive disorders of pregnancy and to GDM. Adjustment covariates included maternal age (≤21, 22–35, and >35 years), body mass index (BMI; <25, 25 to <30, ≥30 kg/m2), chronic hypertension (yes, no), and for mid-pregnancy, rate of weight gain per week between early and mid-pregnancy assessments, treated as a continuous variable. These covariates were selected prior to analysis of results based on data supporting an association between these variables and both sleep disordered breathing and the adverse pregnancy outcomes of interest.24,25 To avoid overfitting, other potential covariates were first evaluated for confounding on the relationship of sleep disordered breathing (AHI≥5) with hypertensive disorders of pregnancy and with GDM using a criteria of >10% modification in the sleep disordered breathing odds ratio by inclusion of the potential covariate. The interactions of BMI with hypertensive disorders of pregnancy on sleep disordered breathing and with GDM on sleep disordered breathing were investigated for early and mid-pregnancy assessments. Exposure-response relationships were assessed in post-hoc tests for linear and quadratic trends in the log-odds across the AHI categories using orthogonal contrasts. For all analyses, women with pregnancy losses prior to 200 weeks’ gestation were excluded.
All tests were performed at a nominal significance level of α=0.05. All single degree of freedom tests were 2-sided. No correction was made for multiple comparisons. Analyses were conducted using SAS 9.3/9.4 software.
Results
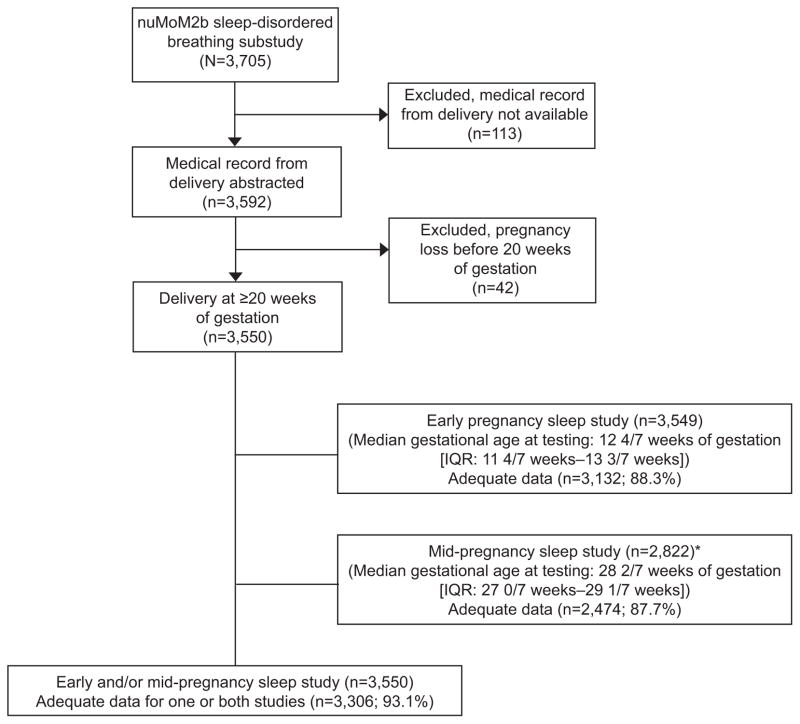
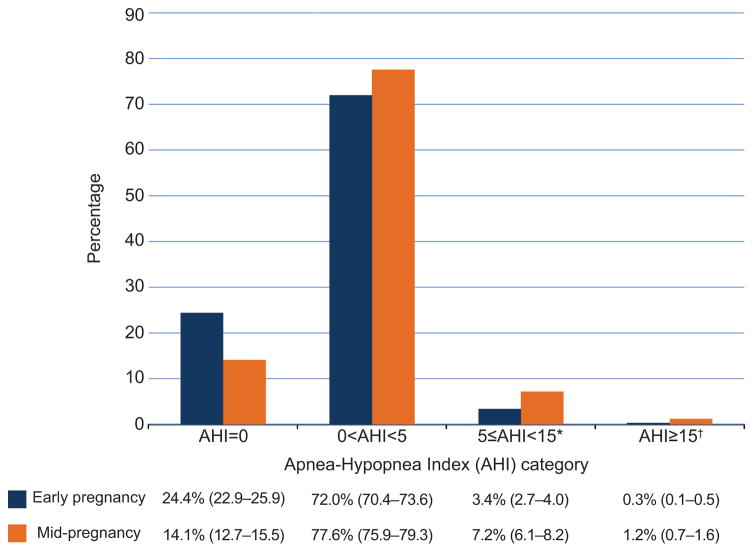
A total of 3705 women participating in the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be parent study were enrolled in the sleep disordered breathing substudy between March 2011 and September 2013 (Figure 1). Baseline characteristics were similar between Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be women who participated in the sleep disordered breathing substudy versus those who did not. (Appendix 3, available online at http://links.lww.com/xxx). AHI distributions in early and mid-pregnancy are presented in Figure 2. The prevalence of sleep disordered breathing (AHI ≥ 5) in early and mid-pregnancy was 3.6% and 8.3%, respectively. In both early and mid- pregnancy, the majority of sleep disordered breathing cases were mild (5 ≤ AHI<15). Urgent alerts for an AHI > 50 or severe hypoxemia occurred in only 6 cases (1 in early and 5 in mid-pregnancy). In women with sleep disordered breathing, the vast majority of apneaic events were obstructive (Appendix 4, available online at http://links.lww.com/xxx), indicating that almost all subjects identified as sleep disordered breathing positive had obstructive sleep apnea.
Figure 1.
Flowchart showing enrollment in nuMoM2b sleep-disordered breathing substudy and inclusion in analysis. *For the 728 women (3,550 minus 2,822) without sleep studies in mid-pregnancy, 30 had a preterm birth before the sleep study could be completed, 86 missed the study visit (including the sleep assessment), and 612 had the study visit but did not participate in the mid-pregnancy sleep study. In planning the study, it was assumed that 25% would refuse the second sleep study. The participation rate in the mid-pregnancy sleep study was higher than expected. nuMoM2b, Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be; IQR, interquartile range.
Figure 2.
The distributions of the women by apnea-hypopnea index (AHI) category in early and mid-pregnancy are presented, giving point estimate (95% confidence interval). *Mild sleep-disordered breathing. †Moderate or severe sleep-disordered breathing.
Characteristics of the participants according to the AHI at baseline are shown in Table 1. In early pregnancy, older age, higher BMI, larger neck circumference, non-Hispanic black race–ethnicity, smoking, and chronic hypertension were all associated with increased AHI. Similar findings were seen with the mid-pregnancy AHI (Appendix 5, available online at http://links.lww.com/xxx).
Table 1.
Baseline Characteristics of Participants Completing the Early Pregnancy Sleep Study According to the Apnea-Hypopnea Index (AHI) in Early Pregnancy
| Baseline Characteristics | Failed Study* (N = 417) | All Apneas and Hypopneas with ≥ 3% Oxygen Desaturation / Hour
|
p-value† | |||
|---|---|---|---|---|---|---|
| AHI=0 (N = 764) | 0<AHI<5 (N = 2,254) | 5 ≤AHI<15 (N = 105) | AHI≥15 (N = 9) | |||
|
| ||||||
| Maternal age, in years | ||||||
| Mean ± standard deviation | 25.8 ± 5.7 | 25.7 ± 5.5 | 27.1 ± 5.5 | 29.2 ± 5.8 | 34.0 ± 5.8 | <.001 |
| Category: n (%) | <.001 | |||||
| 13–21 | 120 (28.8) | 218 (28.5) | 417 (18.5) | 11 (10.5) | 0 (0.0) | |
| 22–35 | 275 (65.9) | 513 (67.1) | 1,686 (74.8) | 77 (73.3) | 5 (55.6) | |
| >35 | 22 (5.3) | 33 (4.3) | 151 (6.7) | 17 (16.2) | 4 (44.4) | |
| Maternal race: n (%) | 0.05 | |||||
| White Non-Hispanic | 227 (54.4) | 434 (56.8) | 1,406 (62.4) | 63 (60.0) | 3 (33.3) | |
| Black Non-Hispanic | 104 (24.9) | 106 (13.9) | 265 (11.8) | 18 (17.1) | 4 (44.4) | |
| Hispanic | 51 (12.2) | 156 (20.4) | 396 (17.6) | 13 (12.4) | 1 (11.1) | |
| Asian | 10 (2.4) | 28 (3.7) | 83 (3.7) | 6 (5.7) | 0 (0.0) | |
| Other | 25 (6.0) | 40 (5.2) | 104 (4.6) | 5 (4.8) | 1 (11.1) | |
| Body mass index, in kg/m2 | ||||||
| Mean ± standard deviation | 27.0 ± 6.3 | 24.4 ± 5.4 | 26.8 ± 6.5 | 36.3 ± 8.6 | 45.6 ± 11.3 | <.001 |
| Category: n (%) | <.001 | |||||
| <25 | 195 (47.4) | 496 (65.9) | 1,112 (49.8) | 8 (7.8) | 0 (0.0) | |
| 25 to <30 | 109 (26.5) | 182 (24.2) | 573 (25.7) | 12 (11.7) | 0 (0.0) | |
| ≥30 | 107 (26.0) | 75 (10.0) | 546 (24.5) | 83 (80.6) | 9 (100.0) | |
| Smoked during 3 months prior to pregnancy: n (%) | 102 (24.5) | 125 (16.4) | 383 (17.0) | 30 (28.6) | 0 (0.0) | 0.03 |
| Chronic hypertension: n (%) | 14 (3.4) | 11 (1.4) | 50 (2.2) | 7 (6.7) | 2 (22.2) | <.001 |
| Pre-gestational diabetes: n (%) | 6 (1.4) | 13 (1.7) | 33 (1.5) | 4 (3.8) | 0 (0.0) | 0.23 |
| Neck circumference, in cm | ||||||
| Mean ± standard deviation | 33.3 ± 3.1 | 32.3 ± 2.5 | 33.0 ± 2.8 | 36.5 ± 4.6 | 40.2 ± 3.2 | <.001 |
| Category: n (%) | <.001 | |||||
| <34 | 234 (61.6) | 563 (77.3) | 1,425 (65.8) | 23 (22.8) | 0 (0.0) | |
| 34 to 36.5 | 92 (24.2) | 127 (17.4) | 512 (23.6) | 32 (31.7) | 0 (0.0) | |
| >36.5 | 54 (14.2) | 38 (5.2) | 230 (10.6) | 46 (45.5) | 9 (100.0) | |
Abbreviations: AHI=apnea-hypopnea index; kg/m2=kilograms per square meters; cm=centimeters
Tests comparing baseline characteristics of women with failed studies versus those with adequate data found significant differences at p<0.05 for: age (continuous and categorical), race, smoking status, and neck circumference (continuous).
P-values are shown for chi-square tests for AHI and the categorical baseline characteristics and from ANOVA F-tests for AHI and continuous baseline characteristics. Due to the small number of women with AHI≥15, the 5≤AHI<15 and AHI≥15 categories were combined for these tests.
Among the 3,306 women included in the analysis (Figure 1), hypertensive disorders data were available on 3,304 participants; hypertensive disorders of pregnancy occurred in 433 (13.1%), specifically preeclampsia occurred in 199 (6.0%). The most common diagnosis was antepartum gestational hypertension (N=234, 7.1%), followed by severe preeclampsia (N=96, 2.9%), mild preeclampsia (N=86, 2.6%), superimposed preeclampsia (N=14, 0.4%), and eclampsia (N=3).
GDM occurred in 134 of 3245 women without pre-gestational diabetes (4.1%). The median gestational age in weeks at the time of GDM testing was 27 with an interquartile range of 25–28. Of the GDM cases, 55% (N=74)were diet controlled, 36% (N=48) required treatment with insulin and/or an oral hypoglycemic and in 9% of cases (N=12) information on therapy modality was not available.
Table 2 presents crude and adjusted odds ratios for preeclampsia and hypertensive disorders of pregnancy, according to AHI in early and mid-pregnancy. We found a statistically significant association between sleep disordered breathing (AHI ≥ 5) during pregnancy and preeclampsia. In early pregnancy analyses the adjusted OR (aOR) for preeclampsia when sleep disordered breathing was present versus absent was 1.94 (95% CI 1.07–3.51); and in mid-pregnancy was 1.95 (95% CI 1.18–3.23). Statistically significant linear trends were observed between AHI and the rate of preeclampsia in unadjusted analyses (early p=0.02 and mid p=0.001), but the trend did not remain statistically significant in the adjusted analyses.
Table 2.
Crude and Adjusted* Odds Ratios for Preeclampsia and Hypertensive Disorder of Pregnancy (Study Definition)† According to Apnea-Hypopnea Index (AHI) in Early and Mid- Pregnancy
| All Apneas and Hypopneas with 3% Oxygen Desaturation / hour | n/N (%) | Crude Odds Ratios | Adjusted Odds Ratios | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | ||
|
| |||||
| Preeclampsia | |||||
| Early Pregnancy (N=3,131) | |||||
| AHI<5 (referent) | 170/3017 (5.6) | 1.00 | <0.001 | 1.00 | 0.03 |
| AHI≥5 | 16/114 (14.0) | 2.73 (1.58–4.74) | 1.94 (1.07–3.51) | ||
| AHI=0 (referent) | 42/763 (5.5) | 1.00 | 0.004 | 1.00 | 0.16 |
| 0<AHI<5 | 128/2254 (5.7) | 1.03 (0.72–1.48) | Trend tests: | 0.96 (0.66–1.39) | |
| 5≤AHI<15 | 14/105 (13.3) | 2.64 (1.39–5.02) | 0.02 linear | 1.79 (0.89–3.61) | |
| AHI≥15 | 2/9 (22.2) | 4.90 (0.99–24.34) | 0.50 quadratic | 2.74 (0.51–14.73) | |
| Mid-pregnancy (N=2,472) | |||||
| AHI<5 (referent) | 114/2266 (5.0) | 1.00 | <0.001 | 1.00 | 0.01 |
| AHI≥5 | 26/206 (12.6) | 2.73 (1.73–4.29) | 1.95 (1.18–3.23) | ||
| AHI=0 (referent) | 15/347 (4.3) | 1.00 | <0.001 | 1.00 | 0.06 |
| 0<AHI<5 | 99/1919 (5.2) | 1.20 (0.69–2.10) | Trend tests: | 1.01 (0.57–1.78) | |
| 5≤ AHI<15 | 21/177 (11.9) | 2.98 (1.50–5.94) | 0.001 linear | 1.83 (0.86–3.89) | |
| AHI ≥15 | 5/29 (17.2) | 4.61 (1.54–13.76) | 0.68 quadratic | 2.95 (0.91–9.58) | |
| HTN Disorder of Pregnancy | |||||
| Early Pregnancy (N=3,131) | |||||
| AHI<5 (referent) | 378/3017 (12.5) | 1.00 | <0.001 | 1.00 | 0.11 |
| AHI≥5 | 28/114 (24.6) | 2.27 (1.46–3.53) | 1.46 (0.91–2.32) | ||
| AHI=0 (referent) | 79/763 (10.4) | 1.00 | <0.001 | 1.00 | 0.29 |
| 0<AHI<5 | 299/2254 (13.3) | 1.32 (1.02–1.72) | Trend tests: | 1.16 (0.88–1.53) | |
| 5≤ AHI<15 | 25/105 (23.8) | 2.71 (1.63–4.49) | 0.02 linear | 1.62 (0.94–2.79) | |
| AHI≥ 15 | 3/9 (33.3) | 4.33 (1.06–17.65) | 0.80 quadratic | 2.17 (0.52–9.14) | |
| Mid-pregnancy (N=2,472) | |||||
| AHI<5 (referent) | 266/2266 (11.7) | 1.00 | <0.001 | 1.00 | 0.004 |
| AHI≥5 | 51/206 (24.8) | 2.47 (1.76–3.48) | 1.73 (1.19–2.52) | ||
| AHI=0 (referent) | 28/347 (8.1) | 1.00 | <0.001 | 1.00 | 0.004 |
| 0<AHI<5 | 238/1919 (12.4) | 1.61 (1.07–2.43) | Trend tests: | 1.31 (0.86–1.99) | Trend tests: |
| 5≤ AHI<15 | 40/177 (22.6) | 3.33 (1.97–5.61) | <0.001 linear | 1.98 (1.12–3.48) | <0.001 linear |
| AHI≥ 15 | 11/29 (37.9) | 6.96 (2.99–16.19) | 0.58 quadratic | 4.27 (1.74–10.45) | 0.30 quadratic |
Abbreviations: AHI=apnea-hypopnea index; 95% CI=95% confidence interval; BMI=body mass index
Early and mid- pregnancy AHI results were adjusted for maternal age (≤21, 22–35, and >35), BMI (<25, 25 to <30, ≥30) and chronic hypertension (yes, no) as determined in early pregnancy. The mid-pregnancy results were also adjusted for rate of weight gain from early pregnancy to mid-pregnancy (as a continuous variable, linear on the log odds scale). The adequacy of a linear term for rate of weight gain was investigated using AHI (dichotomized: <5, ≥5), BMI categories, linear rate of change in weight, and quadratic rate of change in the model. The quadratic term was not significant and was not considered further. The adjusted analyses for early and mid- pregnancy included Ne=3,093 and Nm=2,413 observations, respectively. P-values from logistic regression are given for general association with AHI categories. For a significant test of general association with the 4-category AHI, post-hoc tests are given for linear and quadratic trends in the log odds across AHI categories using orthogonal contrasts.
Preeclampsia includes mild, severe, and superimposed preeclampsia and eclampsia. Hypertensive disorder of pregnancy includes mild, severe, and superimposed preeclampsia and eclampsia, plus antepartum gestational hypertension.
The adjusted OR for hypertensive disorders of pregnancy (preeclampsia and antepartum GHTN) when sleep disordered breathing was present versus absent in early-pregnancy did not reach statistical significance (aOR 1.46 (95% CI 0.91, 2.32)) but in mid-pregnancy the adjusted analysis was statistically significant (aOR 1.73 (95% CI 1.19–2.52)). Statistically significant linear trends were observed between AHI and the rate of all hypertensive disorders in unadjusted analyses (early and mid), and remained significant for mid-pregnancy AHI in the adjusted analyses (p<0.001). We examined the timing between the sleep study and the diagnosis of a hypertensive disorder. Hypertensive complications were diagnosed between 45 to 229 days after the early pregnancy sleep study, and from 44 days before to 113 days after the mid-pregnancy sleep study. Only 4.1% of hypertension diagnoses were made before the mid-pregnancy sleep disordered breathing assessment, while 91.7% of hypertension diagnoses were made more than two weeks after the mid-pregnancy assessment.
Table 3 presents the crude and adjusted odds ratios for GDM, according to AHI in early and mid-pregnancy. For sleep disordered breathing present (AHI≥5) versus absent, the adjusted OR for GDM was 3.47 (95% CI 1.95–6.19) and 2.79 (95% CI 1.63–4.77) in early and mid-pregnancy, respectively. In adjusted analyses for both early and mid-pregnancy, GDM rates increased with increasing AHI (p-values for linear trend 0.001, and <0.001, respectively). When we repeated all analyses excluding women whose GDM status was ascertained only through chart abstraction (without a confirmatory GTT), the direction, magnitude, and statistical significance of effects did not change (data not shown). We examined the timing of the sleep study relative to GTT testing. Only 93 women (3.2%) completed their GTT testing before the early pregnancy home sleep test, while 96.4% of women had their testing done greater than 1 week after the sleep test. With respect to mid-pregnancy, 61.7% of GTT testing was done prior to the mid-pregnancy home sleep test, which was expected given the gestational age window for the study visit (220–310 weeks).
Table 3.
Crude and Adjusted* Odds Ratios for Gestational Diabetes According to Apnea-Hypopnea Index (AHI) in Early and Mid- Pregnancy For Women without Pre-gestational Diabetes
| All Apneas and Hypopneas with 3% Oxygen Desaturation / hour | GDM n/N (%) | Crude Odds Ratios | Adjusted Odds Ratios | ||
|---|---|---|---|---|---|
|
| |||||
| Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | ||
|
| |||||
| Early Pregnancy (N=3,075) | |||||
| AHI<5 (referent) | 107/2965 (3.6) | 1.00 | <0.001 | 1.00 | <0.001 |
| AHI≥5 | 21/110 (19.1) | 6.30 (3.77–10.53) | 3.47 (1.95–6.19) | ||
| AHI=0 (referent) | 21/750 (2.8) | 1.00 | <0.001 | 1.00 | <0.001 |
| 0<AHI<5 | 86/2215 (3.9) | 1.40 (0.86–2.28) | Trend tests: | 1.13 (0.69–1.85) | Trend tests: |
| 5≤ AHI<15 | 17/101 (16.8) | 7.03 (3.57–13.84) | <0.001 linear | 3.50 (1.64–7.44) | 0.001 linear |
| AHI≥ 15 | 4/9 (44.4) | 27.77 (6.96–110.89) | 0.17 quadratic | 8.44 (1.90–37.60) | 0.34 quadratic |
| Mid-pregnancy (N=2,432) | |||||
| AHI<5 (referent) | 69/2231 (3.1) | 1.00 | <0.001 | 1.00 | <0.001 |
| AHI≥5 | 27/201 (13.4) | 4.86 (3.04–7.79) | 2.79 (1.63–4.77) | ||
| AHI=0 (referent) | 2/347 (0.6) | 1.00 | <0.001 | 1.00 | <0.001 |
| 0<AHI<5 | 67/1884 (3.6) | 6.36 (1.55–26.07) | Trend tests: | 4.96 (1.20–20.51) | Trend tests: |
| 5≤ AHI<15 | 22/173 (12.7) | 25.12 (5.84–108.17) | <0.001 linear | 12.62 (2.80–56.81) | <0.001 linear |
| AHI ≥15 | 5/28 (17.9) | 37.49 (6.90–203.79) | 0.11 quadratic | 15.64 (2.67–91.60) | 0.13 quadratic |
Abbreviations: AHI=apnea-hypopnea index; 95% CI=95% confidence interval; BMI=body mass index
Early and mid- pregnancy AHI results were adjusted for maternal age (≤21, 22–35, and >35), BMI (<25, 25 to <30, ≥30) and chronic hypertension (yes, no) as determined in early pregnancy. The mid-pregnancy results were also adjusted for rate of weight gain from early pregnancy to mid-pregnancy (as a continuous variable, linear on the log odds scale). The adequacy of a linear term for rate of weight gain was investigated using AHI (dichotomized: <5, ≥5), BMI categories, linear rate of change in weight, and quadratic rate of change in the model. The quadratic term was not significant and was not considered further. The adjusted analyses for early and mid- pregnancy included Ne=3,037 and Nm=2,374 observations, respectively. P-values from logistic regression are given for general association with AHI categories. For a significant test of general association with the 4-category AHI, post-hoc tests are given for linear and quadratic trends in the log odds across AHI categories using orthogonal contrasts.
Both race–ethnicity and smoking were considered for covariate adjustment to the analyses presented in Tables 2 and 3. Neither met the criteria for inclusion as a confounder. Specifically, adding either variable to a univariate model with sleep disordered breathing (AHI ≥ 5) for hypertensive disorder of pregnancy and for GDM modified the crude odd ratios by 0.0–2.6% and 0.4–4.1%, respectively. Adding either variable to the multivariate models shown in the tables modified the adjusted odds ratios by 0.0–1.4% and 0.3–1.1%, respectively.
Given the clear relationship between sleep disordered breathing and BMI, we also ran analyses with BMI as a continuous variable and considered the interaction between AHI and BMI. Adjusting for BMI as a continuous variable (linear and quadratic terms) did not alter the direction, magnitude, or statistical significance of effects (data not shown). For both hypertensive disorders and GDM the interaction of BMI and sleep disordered breathing was non-significant (early pregnancy: p=0.90 and p=0.31, respectively; and mid-pregnancy: p=0.61 and p=0.95, respectively).
Furthermore, we considered that weight gain from early to mid-pregnancy could be an intermediate variable in the causal pathway between AHI and pregnancy outcomes. However, the direction, magnitude, and significance of effects were similar to those in Tables 2 and 3 when we excluded weight gain as a covariate in the mid-pregnancy models (Appendixes 6 and 7, available online at http://links.lww.com/xxx).
Discussion
In this prospective analysis of objectively assessed sleep disordered breathing in pregnancy, the prevalence of sleep disordered breathing was 3.6% and 8.3% in early pregnancy and mid-pregnancy, respectively. Nearly all subjects in this cohort identified as sleep disordered breathing positive (AHI ≥ 5) had a nocturnal respiratory pattern consistent with obstructive sleep apnea. There was an independent association between sleep disordered breathing and preeclampsia, hypertensive disorders of pregnancy, and GDM after adjustment for age, BMI, chronic hypertension and pregnancy-related weight gain. Increasing exposure-response relationships were observed between AHI and pregnancy-related hypertension and GDM.
Prior to this report, the largest studies evaluating sleep disordered breathing and pregnancy-related hypertension have been retrospective or cross-sectional, and limited by the quality of sleep disordered breathing exposure and pregnancy outcome assessment.26 Data from smaller prospective cohorts, using objective assessments of sleep disordered breathing have yielded conflicting results.16–18 Louis et al reported on a cohort of 175 obese women and demonstrated that women with sleep disordered breathing (AHI ≥ 5) were more likely to develop preeclampsia (aOR 3.5, 95% CI 1.3, 9.9).16 However, two other small studies failed to demonstrate a positive association between sleep disordered breathing and pregnancy related hypertension.17,18 In our large prospective study, in which sleep disordered breathing was diagnosed using objective criteria, and confounding variables carefully considered, we found an association between sleep disordered breathing and preeclampsia and pregnancy-related hypertension. In adjusted analyses, an early pregnancy AHI≥5 was associated with preeclampsia. In mid-pregnancy, an AHI≥5 was associated with the development of preeclampsia and the composite of preeclampsia and antepartum gestational hypertension. In regards to our mid-pregnancy AHI data, because rapid weight gain and extra-vascular fluid retention may result in an increase in AHI, we additionally adjusted for weight gain between visits in the mid-pregnancy analyses. We also examined the timing of the mid-pregnancy sleep assessment in relation to hypertension diagnosis and found that 91.7% of diagnoses were made more than 2 weeks after the mid-pregnancy sleep test. In summary, the associations and dose response relationships observed in this study between AHI, preeclampsia and pregnancy-related hypertension could potentially signify a causal link between sleep disordered breathing exposure in pregnancy and the subsequent development of hypertensive disorders of pregnancy.
Our data regarding the association between sleep disordered breathing and GDM are robust. By excluding women with pre-gestational diabetes and defining GDM, we optimized case ascertainment. We observed increasing AHI with increasing incidence of GDM in both early and mid-pregnancy, independent of important covariates. The early pregnancy AHI data (6–15 weeks) predated the GTT testing by more than 1 week in over 96% of our cohort.
A major strength of this study is the prospective design where the AHI results were blinded to the care providers, investigators and participants. This limited the possibility of ascertainment bias. Our sleep disordered breathing ascertainment was optimized using an independent and blinded central reading center. We were able to control for important confounding factors including BMI and weight gain, and we evaluated the interaction between AHI and BMI. We did observe a lower than expected rate of sleep disordered breathing in early pregnancy (early pregnancy 3.6% observed vs. 5% expected, mid-pregnancy 8.3% vs. 10%), but despite the lower rates we detected statistically significant differences in our primary outcome. The observed differences were substantially greater than what we powered to detect. Nonetheless, given the observational and voluntary nature of the study and moderate adjusted odds ratios (particularly for association with hypertensive disorders), the possibility of residual confounding due to selection bias and unmeasured or unknown confounders cannot be definitively excluded. Finally, we recognize the limitations of utilizing a Level 3 home sleep apnea test for measuring AHI. While a full in-laboratory polysomnogram is considered the gold standard for the objective measurement of sleep-related breathing disorders, it is often not feasible to use full polysomnogram for large sleep-related studies given the cost and limited availability of sleep laboratory space as well as difficulties recruiting a larger number of participants in research requiring such in-laboratory monitoring. Data indicate that unattended home sleep testing can reliably detect sleep disordered breathing at substantively lower cost compared to in-laboratory polysomnogram.27 Furthermore, most insurers now routinely require home sleep tests as the first line diagnostic modality for the majority of patients with suspected sleep disordered breathing and without certain co-morbidities.28,29 Unattended home sleep testing may modestly under-estimate the AHI due to this over-estimation of sleep time.30 However, studies were scored after editing movement and artifact, reducing the impact of wake time on the AHI estimates.
Although we found an association with sleep disordered breathing preceding the development of both pregnancy-related hypertensive disorders and GDM, we cannot conclude that universal screening for, and treatment of sleep disordered breathing in pregnancy would reduce the risks of these adverse outcomes. The most widely prescribed treatment for sleep disordered breathing is continuous positive air pressure (CPAP) during sleep. The benefit of treatment with CPAP has been consistently demonstrated when excessive daytime sleepiness and sleep quality are used as endpoints.31,32 However, even in non-pregnant populations, data conflict regarding whether treatment of sleep disordered breathing can reduce the risk of developing hypertension or diabetes.33–35 This is especially true for milder forms of sleep disordered breathing (AHI<30), which our study confirms represents the vast majority of sleep disordered breathing cases in young pregnant women. Pregnancy is an ideal scenario in which to better understand the role of CPAP as a preventative strategy for reducing cardio-metabolic morbidity as the time frame needed to measure outcomes after initiating therapy is significantly contracted. To date, studies examining the effect of CPAP treatment on pregnancy have been small and limited in the scope of endpoints.36–42
In summary, in this prospective analysis of objectively assessed sleep disordered breathing in pregnancy, the prevalence of sleep disordered breathing was 3.6% in early pregnancy and increased to 8.3% in mid-pregnancy. The majority of sleep disordered breathing cases identified were mild. Our data demonstrate that even modest elevations of AHI in pregnancy are associated with an increased risk of developing hypertensive disorders and an increased incidence of GDM. These findings are important because sleep disordered breathing is a risk factor that is amenable to therapeutic intervention. The underlying mechanistic pathways linking sleep disordered breathing and adverse pregnancy outcomes are likely multifactorial. sleep disordered breathing is linked to oxidative stress, autonomic dysfunction, inflammation, endothelial damage and altered hormonal regulation of energy expenditure.11 These same biological pathways have been associated with adverse pregnancy outcomes.12 Further research should help to establish whether screening for and treating sleep disordered breathing in pregnancy can mitigate the risk and consequences of hypertensive disorders of pregnancy and GDM.
Supplementary Material
Acknowledgments
Supported by grant funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Heart Lung and Blood Institute: U10 HD063036, Research Triangle Institute; U10 HD063072, Case Western Reserve University; U10 HD063047, Columbia University; U10 HD063037, Indiana University; U10 HD063041, Magee-Women’s Hospital; U10 HD063020, Northwestern University; U10 HD063046, University of California Irvine; U10 HD063048, University of Pennsylvania; and U10 HD063053, University of Utah.
Footnotes
Presentation:
Presented at the Society for Maternal Fetal Medicine 2015 annual meeting, San Diego, February 02–07, 2015.
For a list of the Sleep Disordered Breathing Study within the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-be (nuMoM2b) Network, please see Appendix 1 online at http://links.lww.com/xxx.
Comments and views of the author(s) do not necessarily represent the views of the NICHD.
Financial Disclosure
Phyllis Zee has served as a consultant for Aptalis, Merck, EISAI, Philips, Vanda, and Pernix. She has received research support (grants to Northwestern University) from Jazz, Eisai, Philips, and Technogel. She also owns stock in Teva. The other authors did not report any potential conflicts of interest.
References
- 1.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2012 Oct 15;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England journal of medicine. 1993 Apr 29;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine. 2003 May 1;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 4.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004 Nov 1;27(7):1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 5.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005 Oct 1;28(10):1299–1305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 6.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010 Jan;115(1):77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 7.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. The New England journal of medicine. 2000 May 11;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 8.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. American journal of epidemiology. 2004 Sep 15;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 9.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. American journal of respiratory and critical care medicine. 2005 Dec 15;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008 Oct;18(7):253–260. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010 Jan;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero R, Badr MS. A role for sleep disorders in pregnancy complications: challenges and opportunities. American journal of obstetrics and gynecology. 2014 Jan;210(1):3–11. doi: 10.1016/j.ajog.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. American journal of obstetrics and gynecology. 2012 Sep 7; doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. American journal of obstetrics and gynecology. 2012 Feb;206(2):136 e131–135. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010 Oct;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 16.Louis J, Auckley D, Miladinovic B, et al. Perinatal Outcomes Associated With Obstructive Sleep Apnea in Obese Pregnant Women. Obstet Gynecol. 2012 Sep 21; doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Facco FL, Ouyang DW, Zee PC, et al. Implications of sleep-disordered breathing in pregnancy. American journal of obstetrics and gynecology. 2013 Dec 25; doi: 10.1016/j.ajog.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014 Apr;69(4):371–377. doi: 10.1136/thoraxjnl-2012-202718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gestational diabetes mellitus. Practice Bulletin No. 137. American College of Obstetricians and Gynecologists. Obstetrics and gynecology. 2013 Aug;122:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 20.Facco FL, Parker CB, Reddy UM, et al. NuMoM2b Sleep-Disordered Breathing study: objectives and methods. American journal of obstetrics and gynecology. 2015 Apr;212(4):542 e541–127. doi: 10.1016/j.ajog.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas DM, Parker CB, Wing DA, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b) American journal of obstetrics and gynecology. 2015 Apr;212(4):539 e531–539 e524. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of O Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013 Nov;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JM, Myatt L, Spong CY, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. The New England journal of medicine. 2010 Apr 8;362(14):1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. American journal of respiratory and critical care medicine. 2002 May 1;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 25.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. Bmj. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2013 Aug 2; doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012 Jun;35(6):757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed M, Patel NP, Rosen I. Portable monitors in the diagnosis of obstructive sleep apnea. Chest. 2007 Nov;132(5):1672–1677. doi: 10.1378/chest.06-2793. [DOI] [PubMed] [Google Scholar]
- 29.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007 Dec 15;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 30.Chai-Coetzer CL, Antic NA, Rowland LS, et al. A simplified model of screening questionnaire and home monitoring for obstructive sleep apnoea in primary care. Thorax. 2011 Mar;66(3):213–219. doi: 10.1136/thx.2010.152801. [DOI] [PubMed] [Google Scholar]
- 31.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. American journal of respiratory and critical care medicine. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999 Jun 19;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 33.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Archives of internal medicine. 2007 Apr 23;167(8):757–764. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 34.Bratton DJ, Stradling JR, Barbe F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014 Dec;69(12):1128–1135. doi: 10.1136/thoraxjnl-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. Jama. 2012 May 23;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 36.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27(1):79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep. 2013 Jan;36(1):15–21. doi: 10.5665/sleep.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guilleminault C, Kreutzer M, Chang JL. Pregnancy, sleep disordered breathing and treatment with nasal continuous positive airway pressure. Sleep Med. 2004 Jan;5(1):43–51. doi: 10.1016/j.sleep.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Guilleminault C, Palombini L, Poyares D, Takaoka S, Huynh NT, El-Sayed Y. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings. Sleep Med. 2007 Dec;9(1):9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007 Dec;9(1):15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Reid J, Taylor-Gjevre R, Gjevre J, et al. Can gestational hypertension be modified by treating nocturnal airflow limitation? Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2013 Apr 15;9(4):311–317. doi: 10.5664/jcsm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. American journal of respiratory and critical care medicine. 2000 Jul;162(1):252–257. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.