Abstract
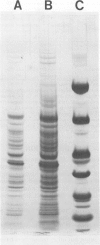
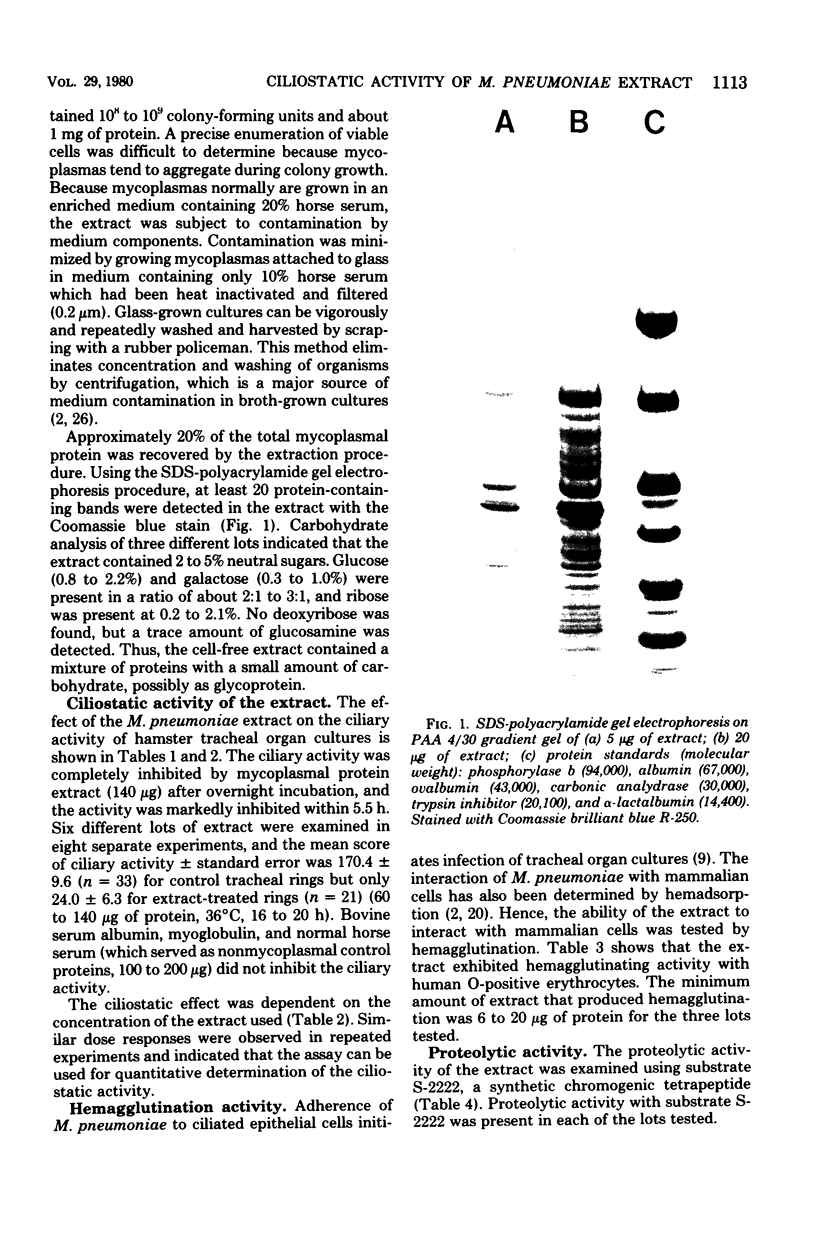
An extract of Mycoplasma pneumoniae, prepared from glass-grown organisms by extraction with 2 M NaCl, followed by freeze-thaw, ultracentrifugation, dialysis, and lyophilization, yielded approximately 20% of the total mycoplasmal protein. The extract contained at least 20 protein bands on sodium dodecyl sulfate-polyacrylamide gels and 2 to 5% carbohydrate and inhibited 70 to 100% of the ciliary activity of hamster tracheal organ cultures (ciliostasis). The extent of ciliostasis was dependent on the concentration of the extract. The extract also produced hemagglutination of human O-positive erythrocytes and showed proteolytic activity with a synthetic tetrapeptide substrate, S-2222. These in vitro tissue-damaging activities may be associated with the virulence of the mycoplasmas and with the pathogenesis of M. pneumoniae disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergström K., Blombäck M. Determination of plasma prothrombin with a reaction rate analyzer using a synthetic substrate. Thromb Res. 1974 Jun;4(6):719–729. doi: 10.1016/0049-3848(74)90016-4. [DOI] [PubMed] [Google Scholar]
- Boykins R. A., Liu T. Y. Automatic analysis of neutral sugar components in glycoproteins and complex carbohydrates. J Biochem Biophys Methods. 1980 Jan-Feb;2(1):71–78. doi: 10.1016/0165-022x(80)90075-5. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson J. L., Collier A. M., Clyde W. A., Jr Ciliary membrane alterations occurring in experimental Mycoplasma pneumoniae infection. Science. 1979 Oct 19;206(4416):349–351. doi: 10.1126/science.113877. [DOI] [PubMed] [Google Scholar]
- Chavin S. I. Isolation and study of functional membrane proteins Present status and future prospects. FEBS Lett. 1971 May 20;14(5):269–282. doi: 10.1016/0014-5793(71)80278-8. [DOI] [PubMed] [Google Scholar]
- Clyde W. A., Jr Immunopathology of experimental Mycoplasma pneumoniae disease. Infect Immun. 1971 Dec;4(6):757–763. doi: 10.1128/iai.4.6.757-763.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Appearance of Mycoplasma pneumoniae in lungs of experimentally infected hamsters and sputum from patients with natural disease. Am Rev Respir Dis. 1974 Dec;110(6):765–773. doi: 10.1164/arrd.1974.110.6P1.765. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald G. W., Collier A. M., Clyde W. A., Jr Respiratory infections due to Mycoplasma pneumoniae in infants and children. Pediatrics. 1975 Mar;55(3):327–335. [PubMed] [Google Scholar]
- Foy H. M., Kenny G. E., Cooney M. K., Allan I. D. Long-term epidemiology of infections with Mycoplasma pneumoniae. J Infect Dis. 1979 Jun;139(6):681–687. doi: 10.1093/infdis/139.6.681. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Agee C. C., Cameron A. M. Differential distribution of ciliated epithelial cells in the trachea of hamsters: implications for studies of pathogenesis. J Infect Dis. 1977 Jan;135(1):9–19. doi: 10.1093/infdis/135.1.9. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Intracellular levels of adenosine triphosphate in hamster trachea organ cultures exposed to Mycoplasma pneumoniae cells or membranes. In Vitro. 1977 Aug;13(8):510–516. doi: 10.1007/BF02615144. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Isolation of mutants of Mycoplasma pneumoniae defective in hemadsorption. Infect Immun. 1979 Mar;23(3):903–906. doi: 10.1128/iai.23.3.903-906.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Male C. J. Immunoglobulin A1 protease production by Haemophilus influenzae and Streptococcus pneumoniae. Infect Immun. 1979 Oct;26(1):254–261. doi: 10.1128/iai.26.1.254-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulks M. H., Plaut A. G. IgA protease production as a characteristic distinguishing pathogenic from harmless neisseriaceae. N Engl J Med. 1978 Nov 2;299(18):973–976. doi: 10.1056/NEJM197811022991802. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Guidotti G. Fractionation of the protein components of human erythrocyte membranes. J Biol Chem. 1969 Oct 10;244(19):5118–5124. [PubMed] [Google Scholar]
- Somerson N. L., James W. D., Walls B. E., Chanock R. M. Growth of Mycoplasma pneumoniae on a glass surface. Ann N Y Acad Sci. 1967 Jul 28;143(1):384–389. doi: 10.1111/j.1749-6632.1967.tb27680.x. [DOI] [PubMed] [Google Scholar]