Summary
The Fenton-chemistry generating properties of copper ions are considered a potent phagolysosome defense against pathogenic microbes, yet our understanding of underlying host/microbe dynamics remains unclear. We address this issue in invasive aspergillosis and demonstrate that host and fungal responses inextricably connect copper and reactive oxygen intermediate (ROI) mechanisms. Loss of the copper-binding transcription factor AceA yields an A. fumigatus strain displaying increased sensitivity to copper and ROI in vitro, increased intracellular copper concentrations, decreased survival in challenge with murine alveolar macrophages and reduced virulence in a non-neutropenic murine model. ΔaceA survival is remediated by dampening of host ROI (chemically or genetically) or enhancement of copper-exporting activity (CrpA) in A. fumigatus. Our study exposes a complex host/microbe multifactorial interplay that highlights the importance of host immune status and reveals key targetable A. fumigatus counter-defenses.
In Brief
Wiemann et al. find that Aspergillus fumigatus employs the copper-sensing transcription factor AceA to express the copper exporter CrpA as a defense mechanism against macrophages. Copper and reactive oxygen intermediate attack and defense are inextricably connected on the side of both host and pathogen during infection.
Introduction
The ubiquitous, saprophytic mold Aspergillus fumigatus forms and releases asexual airborne spores (conidia) (Latgé, 1999). In the immunocompetent individual, inhalation of conidia does not usually cause disease, as professional phagocytes such as alveolar macrophages (AMΦ) and neutrophils prevent the development of aspergillosis Dagenais and Keller, 2009; Gilbert et al., 2015; Heinekamp et al., 2015). However, a spectrum of immune deficiencies in the population render patients susceptible to invasive growth. The first line of defense is phagocytosis of inhaled conidia by AMΦ and neutrophils. AMΦ reside beneath the alveolar surfactant film where they represent 90% of the resident leucocytes in the lung (Hasenberg et al., 2011). Molecular mechanisms by which AMΦ and neutrophils destroy inhaled A. fumigatus spores are only partially understood. Together, these data imply that transition metal homeostasis (mainly iron, copper, and zinc) and production of reactive oxygen intermediates (ROI) are the major strategies employed to kill A. fumigatus conidia (Clark et al., 2016; Dagenais and Keller, 2009; Heinekamp et al., 2015; Kasahara et al., 2016; Lanternier et al., 2013; Park and Mehrad, 2009).
Accumulating evidence suggests that innate phagocyte defense includes not only toxic ROI generated through the phagocyte NADPH oxidase (PHOX) complex, but also utilizes copper as a microbial toxin (Ding et al., 2014; Djoko et al., 2015; García-Santamarina and Thiele, 2015). Similar to iron, copper is a Janus-faced transition metal functioning on the one hand as an essential cofactor for enzymes like cytochrome c oxidase (complex IV), super oxide dismutases (SODs), laccases, and reductive iron transporters and on the other hand as a catalyst in toxic ROI-generating Fenton chemistry. Infection studies with Mycobacterium species, Salmonella typhimurium and Cryptococcus neoformans suggest that macrophages elevate copper levels inside the phagosome by increasing expression of the copper importer Ctr1 and locating the P-type copper ATPase pump (ATP7A) to the phagosomal membrane (Achard et al., 2012; Ding et al., 2013; White et al., 2009).
Fungi utilize several protein classes to regulate copper homeostasis, including copper-binding transcription factors, copper transporters (import and export) and copper-binding metallothioneins (Table 1). Copper-binding transcription factors ensure correct expression of genes required for survival in insufficient or toxic copper environments. In S. cerevisiae, copper deficiency is sensed by the copper-binding transcription factor Mac1p that, in the absence of copper, activates the plasma membrane-localized copper transporters Ctr1p and Ctr3p as well as Fre1p, a metalloreductase which mobilizes copper ions from oxidized copper complexes (Cyert and Philpott, 2013; Graden and Winge, 1997; Jungmann et al., 1993). Copper excess in S. cerevisiae is sensed by the copper-binding transcription factor Ace1p (also called Cup2p) which activates expression of the metallothionein-encoding genes CUP1 and CRS5 (Culotta et al., 1994; Ecker et al., 1986; Thiele, 1988). In addition, Ace1p induces SOD1 (encoding a copper-dependent superoxide dismutase) and metalloreductase-encoding genes (FREs) (Cyert and Philpott, 2013).
Table 1.
Relevant Copper-binding Proteins in A. fumigatus, S. cerevisiae, C. albicans and C. neoformans
| A. fumigatus | S. cerevisiae | C. albicans | C. neoformans | Description | |
|---|---|---|---|---|---|
| ID | name | ||||
| Copper-binding transcription factors | |||||
|
| |||||
| AfuA_6G07780 | AceA | Ace1p (Cup2p) | Cup2 | Copper-toxicity TF | |
| AfuA_1G13190 | MacA | Mac1p (Cua1p) | Mac1 | Copper-starvation TF | |
| Cuf1 | Dual function copper-binding TF | ||||
| AfuA_2G01190 | CufA | Haa1p | Copper-binding TF with specialized function | ||
| Copper transporters | |||||
|
| |||||
| AfuA_6G02810 | CtrA21 | Ctr1p | Ctr1 | Ctr1 | High affinity copper transporter |
| AfuA_2G03730 | CtrC1 | Ctr3p | Ctr4 | Ctr4 | High affinity copper transporter |
| AfuA_3G08180 | Ctr21 | Ctr2p | Ctr2 | Ctr2 | Low Affinity copper transporter |
| AfuA_3G13660 | CtrA11 | Unknown function | |||
| AfuA_4G12620 | CptA2 | Ccc2p | Ccc2 | Ccc2 | Intracellular copper ATPase |
| AfuA_3G12740 | CrpA | Crp1 | Copper exporting ATPase | ||
| Cu metallothioneins | |||||
|
| |||||
| AfuA_4G04318 | CmtA | Cup1p | Cup1 | Cmt1 | Copper metallothioneins |
| Crs5p | Crd2 | Cmt2 | |||
| Superoxide dismutases | |||||
|
| |||||
| AfuA_5G09240 | Sod13 | Sod1p | Sod1 | Sod1 | Cytoplasmic Cu/Zn-SOD |
Human pathogenic fungi follow suit with deviations dependent on species. Physiological studies of the pathogenic ascomycete C. albicans identified a putative homolog of the human ATP7A P-type copper ATPase and S. cerevisiae Ccc2p (Lowe et al., 2004), Crp1p, as critical for copper detoxification with the metallothionein Cup1p responsible for residual copper resistance when CRP1 was deleted and both proteins essential for establishing full virulence (Douglas et al., 2012; Mackie et al., 2016; Schwartz et al., 2013; Weissman et al., 2000) (Table 1). Both CRP1 and CUP1 are induced by elevated copper concentrations through the homolog of Ace1p (Schwartz et al., 2013; Weissman et al., 2000). In the pathogenic basidiomycete C. neoformans, one copper-binding transcription factor, Cuf1, regulates expression of both copper importers Ctr1 and Ctr4 as well as the two metallothioneins Cmt1 and Cmt2 involved in copper detoxification (Ding et al., 2011; Waterman et al., 2007). Deletion of either cuf1 or cmt1/cmt2 results in attenuated virulence of C. neoformans. The copper transporter ctr4 in C. neoformans is essential for establishing full virulence during meningoencephalitis rather than pulmonary infection (Ding et al., 2013; Sun et al., 2014; Waterman et al., 2007; Waterman et al., 2012).
Little is known about copper homeostasis in A. fumigatus. This opportunistic human pathogen encodes four putative copper importers (CtrA1, CtrA2, CtrB, and CtrC) (Table 1) (Park et al., 2014). A double deletion mutant of ctrA2 and ctrC showed reduced SOD and catalase activities but was not altered in virulence in an immunocompromised murine model of invasive aspergillosis (IA) (Park et al., 2014). Complicating an understanding of A. fumigatus virulence factors is the growing realization that host immune status often dictates IA progression. Neutropenic and non-neutropenic populations are both susceptible to IA (Russo et al., 2011) and murine models of these two conditions can display differential outcomes. This is illustrated in a compilation of five studies showing gliotoxin to be a virulence factor only in the murine non-neutropenic IA model (Dagenais and Keller, 2009). Furthermore, some inherited primary immunodeficiencies such as Chronic Granulomatous Disease (CGD), which lack the ROI-generating leukocyte NADPH oxidase, are highly associated with IA development (Lanternier et al., 2013).
Since to date there is no information on how A. fumigatus regulates genes involved in copper acquisition and detoxification, we set out to identify copper-dependent regulators and characterize their role in IA progression. We also assessed the importance of copper mediated defense in a wide breadth of host immune status capabilities using multiple host IA models. We reveal the inextricable interface of copper and ROI mechanisms in both host and microbe and demonstrate that host copper dynamics potentiate ROI stress for A. fumigatus. The copper-binding transcription factor AceA is a virulence factor in a non-neutropenic IA model. Our biochemical and virulence data strongly support a mechanism of an inability of ΔaceA mutants to manage host-derived copper imported by host copper ion transporters. This macrophage sensitivity is corrected by either ΔaceA regain of activity of the putative copper exporter CrpA or the spore specific ROI response bZIP protein AtfA. Furthermore, the inability of the host to mount an ROI defense dampens a copper defense response as demonstrated by equivalent persistence of ΔaceA to that of wild type A. fumigatus in both zebrafish and murine PHOX-deficient hosts.
Results
The genome of Aspergillus fumigatus encodes three putative copper-binding transcription factors
Our interest in copper regulation was originally piqued by microarray data where a putative copper-binding transcription factor encoding gene (AfuA_6G07780) was among the most downregulated transcription factor genes in the reduced virulence ΔlaeA mutant (Perrin et al., 2007). We next identified all proteins encoded in the genome that harbor a conserved copper-fist DNA-binding domain (Jungmann et al., 1993; Szczypka and Thiele, 1989). A domain search in the A. fumigatus Af293 genome database (Cerqueira et al., 2014) using the conserved copper-fist DNA-binding domain C-X2-C-X8-C-X-H (InterPro ID: IPR001083) resulted in two additional hits (AfuA_1G13190 and AfuA_2G01190). Protein alignment using the three A. fumigatus sequences and characterized copper-binding transcription factor sequences from S. cerevisiae and other fungi showed that AfuA_1G13190 groups with the nutritional copper-binding transcription factors including Mac1p from S. cerevisiae and is most closely related to GRISEA from the filamentous ascomycete Podospora anserina (Borghouts and Osiewacz, 1998) and was therefore assigned the name MacA (Figure S1A). Unexpectedly, AfuA_2G01190 and AfuA_6G07780 also group to the Mac1 family and within this group are closest to Cuf1 from C. neoformans (Figure S1A) (Ding et al., 2011). Yeast copper-binding transcription factors involved in copper detoxification including Crf1, Amt1, Cup2 and Cup2p/Ace1p from Yarrowia lipolytica, Candida glabrata, C. albicans, and S. cerevisiae form a distinct group of related proteins (Figure S1 A).
As it was not obvious from phylogeny alone if AfuA_2G01190 or AfuA_6G07780 more likely regulate pathways protecting from copper toxicity, we examined all three proteins in detail for predicted copper regulatory motifs. In addition to the N-terminally located conserved copper-fist Zn(II)- and DNA-binding motif found in all three proteins, MacA/AfuA_1G13190 contains a cysteine-rich motif in its C-terminus that aligns with the cysteine-rich C2 motif of Mac1p (Figure S1B) known to be involved in inactivation of the protein under replete copper conditions in S. cerevisiae (Graden and Winge, 1997; Jensen and Winge, 1998; Keller et al., 2000). The protein sequences of AfuA_2G01190 and AfuA_6G07780 are missing this C-terminally located motif but contain additional cysteine residues in their respective N-termini in proximity to the copper-fist DNA-binding domain (Figure S1B). Of these, AfuA_6G07780 contains all eight cysteine residues required for Ace1p functionality in S. cerevisiae (Hu et al., 1990), and was therefore assigned the name AceA. AfuA_2G01190 is missing four cysteine residues and was named CufA (Figure S1B). This finding is reminiscent of S. cerevisiae Haa1p which has significant homology to Ace1p but is lacking one of the eight conserved cysteine residues (Figure S1).
Copper detoxification by AceA relieves ROI stress
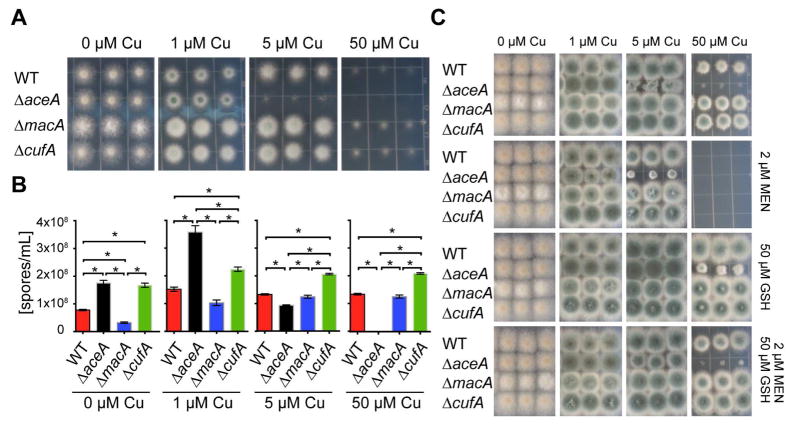
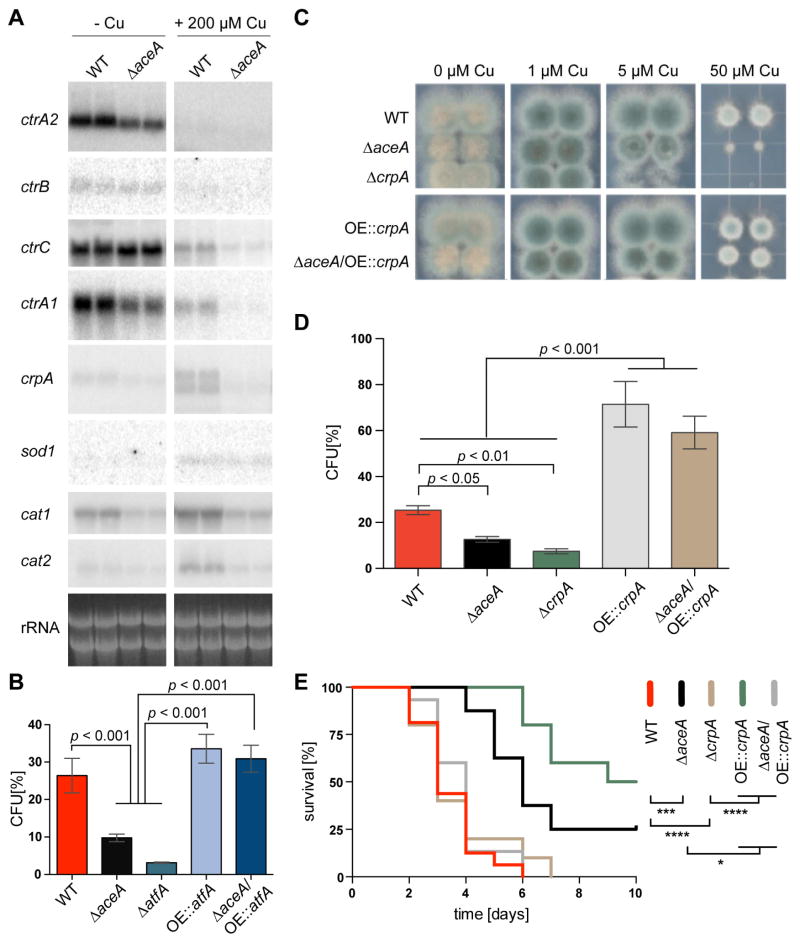
To test if and how the identified copper-fist DNA-binding domain proteins in A. fumigatus affect copper homeostasis in growth studies, we constructed gene deletion mutants of each gene, and – due to phenotypes described below – additionally complemented the ΔaceA mutant with a wild-type gene copy (Figure S2A). The sensitivity of ΔaceA to copper became apparent at 5 μM copper on solidified growth media after two days (Figure 1A and B). This hypersensitivity of the ΔaceA mutant is specific to copper ions, as addition of 100 μM Cd or Fe in copper depleted medium revealed no growth difference between the ΔaceA and wild type strain (Figure S2B). Complementation of ΔaceA with a wild-type aceA copy restored normal growth (Figure S2A). The ΔmacA and ΔcufA strains exhibited milder phenotypes with ΔmacA forming fewer and non-pigmented spores in copper depletion conditions (Figure 1 and Figure S2C). However, when the copper chelator, bathocuproinedisulfonic acid, was added to the medium, the ΔmacA strain showed very sick growth (Figure S2C). Similarly, when media was prepared with trace elements containing the metal ion chelator enthyldiaminetetraacetic acid (EDTA) the ΔmacA strain showed a severe growth reduction on media even when 5 μM copper was added (Figure S2D). Using the same EDTA-containing media, 50 μM copper did not cause any growth reduction of the WT or the ΔaceA strain (Figure S2D), Together these latter results highlight the importance of fungal growth conditions for experimentation.
Fig. 1. Growth and g phenotypes of copper-binding transcription factor encoding gene mutants of A. fumigatus on extreme copper concentrations.
(A) 2000 spores of indicated strains grown on solidified glucose minimal medium (GMM) with indicated concentration of CuSO4 for 48 h at 37°C.
(B) Spores were enumerated from cores taken from overlay cultures of copper-binding transcription factor deletion strains grown on the same media indicated incubated at 37°C for 5 days. Experiments were perf ormed in triplicates, error bars represent standard deviations and asterisks indicate statistical significance, p < 0.01.
(C) Growth assay on solidified GMM for 72 h at 37 °C under indicated copper concentrations plus supplements.
Since on the one hand copper is involved in detoxification of superoxide (O2−) as a cofactor of copper-dependent SODs and on the other hand can contribute to hydroxy radical (·OH) production from hydrogen peroxide (H2O2) by participation in Fenton chemistry, we tested the mutants for synergistic effects of increasing copper and the intracellular O2− generator, menadione (Thor et al., 1982; White and Clark, 1988). When we grew the strains on increasing copper concentrations and 2 μM menadione, we observed a synergistic growth inhibitory effect for all strains that was most severe in the ΔaceA mutant (Figure 1C). When we assessed the sensitivities of the strains towards H2O2 under increasing copper concentrations, we observed the same trend with an even more severe inhibition of growth of the ΔaceA strain (Figure S2F). This copper-dependent growth defect could be alleviated when the reducing agent reduced L-glutathione (GSH) was added to high copper-containing media in all strains (Figure 1C and Figure S2A and E), suggesting that copper increases ROI stress in an AceA-dependent fashion.
AceA contributions to host infection
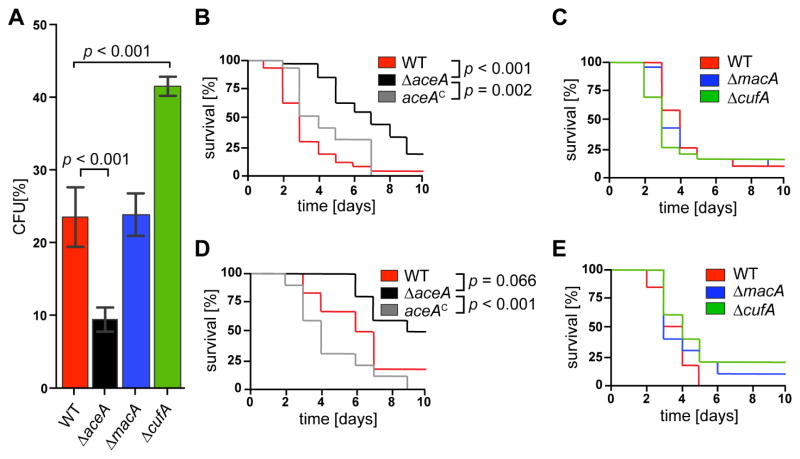
Since A. fumigatus encounters AMΦ as one of the first lines of host defense, we compared the survival rates of the wild type to the ΔmacA, ΔcufA and ΔaceA mutants in murine AMΦ. When challenged with macrophages, the wild type and the ΔmacA strain displayed a survival rate of ~25%, whereas the ΔaceA mutant only showed ~10% survival (Figure 2A). Interestingly, deletion of cufA increased survival of spores when challenged with macrophages (Figure 2A). Next, infection assays were performed using both a non-neutropenic (cortisone acetate) and neutropenic (cyclophosphamide) murine model of IA. The ΔaceA mutant was significantly less virulent than the wild type and reconstituted strains in the non-neutropenic model (Figure 2B). In line with the reduced virulence, the ΔaceA mutant formed less numerous and smaller infection loci compared to the wild type in the infected lung tissue, as assessed by histopathology and colony forming unit (CFU) enumeration (Figure S3 A and B). Levels of TNF-alpha in the lungs showed no differences between the two strains although they were significantly higher than in uninfected mice (Figure S3C). Similar to the assays performed with murine AMΦ, the ΔmacA mutant showed wild-type-like virulence, however, despite the elevated survival rate in the macrophage assay, the ΔcufA strain did not show increased virulence in this model (Figure 2C). Although not significant compared to wild type, analysis of the ΔaceA strain in the neutropenic IA model presented ambiguous results considering the p value (p = 0.0662) and its decreased virulence in comparison to the complemented control (p = 0.0008) (Figure 2D). There was no difference in virulence between ΔcufA or ΔmacA and wild type in this model (Figure 2E).
Fig. 2. Deletion of aceA reduces fungal survival and virulence in immunocompromised mice.
(A) Colony forming units (CFU) of fungal strains after incubation with murine alveolar macrophages for 2h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
(B) Survival rates of mice immunocompromised with cortisone acetate and infected with the A. fumigatus wild type, ΔaceA and the reconstituted strain aceAC, respectively. Statistical significance is indicated by p values. 10 mice were in each group.
(C) Survival rates of mice immunocompromised with cortisone acetate and infected with the A. fumigatus wild type, ΔmacA and ΔcufA strains, respectively. 10 mice were in each group.
(D) Survival rates of mice immunocompromised with cyclophosphamide and infected with the A. fumigatus wild type, ΔaceA and the reconstituted strain aceAC, respectively. Statistical significance is indicated by p values.
(E) Survival rates of mice immunocompromised with cyclophosphamide and infected with the A. fumigatus wild type, ΔmacA and ΔcufA strains, respectively.
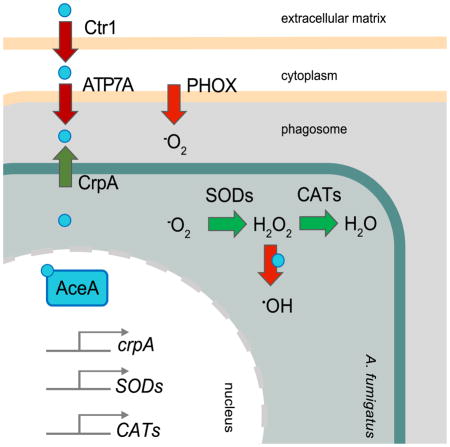
Macrophage copper flux is altered in ΔaceA cells
Activation of macrophage ATP7A copper ATPase coupled with the importer Crt1 are implicated in host mediated copper accumulation in the phagosome during bacterial infections (Wagner et al., 2005; White et al., 2009). Furthermore, murine infections with C. neoformans increased serum copper levels and altered expression of both ATP7A and Ctr1 in murine bronchoalveolar lung cells (Ding et al., 2013). Thus, we reasoned that activity of this conserved defense response could also be induced by A. fumigatus infection and measureable in copper levels in macrophage confrontations between wild type and ΔaceA A. fumigatus strains.
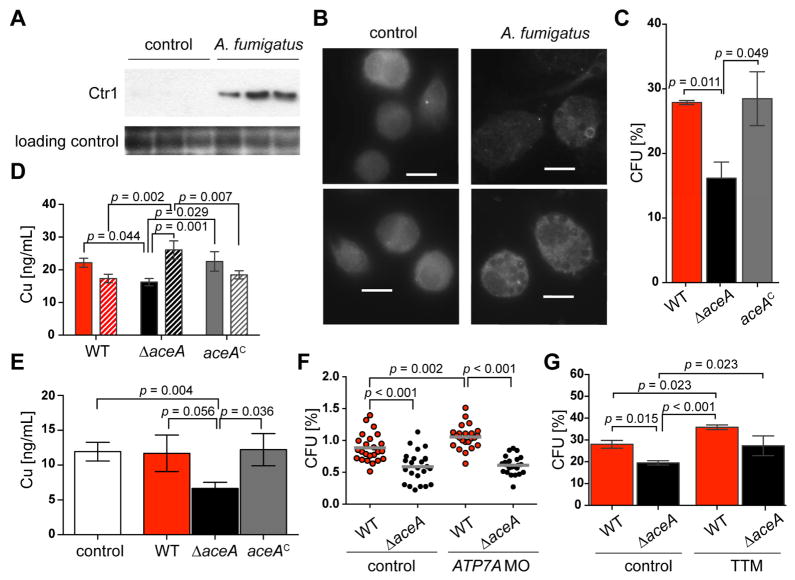
We first examined for any alterations in ATP7A or Ctr1 dynamics. Western blot analysis of non-infected and A. fumigatus challenged GM-CSF activated bone marrow derived murine macrophages (BMDMs) showed a significant induction of Ctr1 of challenged cells compared to non-infected cells (Figure 3A and Figure S3D and E). Immunohistochemistry analysis of the murine copper ATPase ATP7A showed an increased fluorescent signal in A. fumigatus challenged BMDMs that showed aggregation in distinct foci, sometimes distinctly surrounding fungal spores. These signals are distinctively different than the signals observed in non-challenged cells (Figure 3B).
Fig. 3. ΔaceA strains accumulate more copper during macrophage encounters.
(A) Western blot against mouse Ctr1 and GAPDH of murine bone marrow derived macrophages activated with GM-CSF that were unchallenged or challenged with A. fumigatus spores for 2h.
(B) Immuno-staining against mouse ATP7A of murine bone marrow derived macrophages activated with GM-CSF that were unchallenged or challenged with A. fumigatus spores for 2h. Scale bars are 10 μM.
(C) Colony forming units (CFU) of fungal strains after incubation with murine bone marrow derived macrophages activated with GM-CSF for 2h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
(D) Total copper concentration of unchallenged 3 × 107 spores (solid) and 3 × 107 spores incubated with 1 × 107 GM-CSF activated bone marrow derived murine macrophages for 2h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
(E) Total copper concentration of 1 × 107 GM-CSF activated bone marrow derived murine macrophages incubated with 3 × 107 spores of the indicated A. fumigatus strains for 2h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
(F) Colony forming units (CFU) of fungal strains from whole zebrafish larvae at 24 hours post microinjection. Genetic inhibition of ATP7A was obtained with morpholino-mediated knockdown (ATP7AMO). Data shown are pooled from four independent experimental replicates where significance is indicated by p values as determined by a least squares means analysis.
(G) Colony forming units (CFU) of fungal strains after incubation with murine alveolar macrophages supplemented with or without 50 μM tetrathiomolybdate (TTM) for 2 h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
Next, we determined total copper levels in A. fumigatus spores (wild type, ΔaceA and aceAC strains) either unchallenged or challenged with GM-CSF activated BMDMs using inductively coupled plasma mass spectrometry (ICP-MS) (Subramanian Vignesh et al., 2013). Total copper, zinc and iron quantification was also carried out in BMDMs incubated with the A. fumigatus strains. As demonstrated with AMΦs (Figure 2), the ΔaceA mutant had a lower survival rate in BMDMs (Figure 3C). Quantification of total copper ion levels in spores challenged with BMDMs showed an increased copper concentration in ΔaceA spores compared to unchallenged ΔaceA spores (Figure 3D). This increase did not occur in wild type and reconstituted aceA strains (Figure 3D). Quantification of the copper content in macrophages challenged with the different strains showed that the cells incubated with ΔaceA spores had a decreased total copper concentration, suggesting a mobilization of copper to the ΔaceA spores – a trend that was not observed for the wild type or the reconstituted aceA strain (Figure 3E). Importantly, the level of zinc or iron in macrophages incubated with the ΔaceA spores was not decreased relative to the wild type and aceAC strains (Figure S3F and G).
Together, this data strongly supports copper mobilization to fungal tissue as one means of defense. To further examine a role for ATP7A in IA progression of wild type and ΔaceA strains, we compared fungal burden in both immunocompetent and ATP7A-deficient zebrafish larvae using our previously established zebrafish IA model (Knox et al., 2014; Mendelsohn et al., 2006). The larval zebrafish has functionally conserved and competent vertebrate innate immune mechanisms (Harvie and Huttenlocher, 2015; Herbomel et al., 1999; Le Guyader et al., 2008) and previous studies have demonstrated the conserved nature of zebrafish ATP7A to the mammalian ortholog (Madsen et al., 2008). Although we saw a significant increase in wild type burden in the ATP7A morphants (Figure 3F), there was no rescue of wild-type-like growth in the ΔaceA strain in the ATP7A-deficient zebrafish. However, addition of the copper chelator ammonium tetrathiomolybdate (TTM) (Brewer, 2005) showed a similar restoration of ΔaceA survival to wild-type-like levels that were significantly higher than in the untreated ΔaceA infection (Figure 3G).
Depleting host ROI synthesis remediates ΔaceA survival in host tissues
Considering that the ΔaceA strain grew poorly in the ATP7A-deficient larval zebrafish and is sensitive to ROI, we considered an alternative host mechanism in addressing the ΔaceA phenotype. Since macrophages deploy mechanisms of O2− production by the PHOX complex to fight pathogens (Hogan and Wheeler, 2014; Lambeth and Neish, 2014) and our physiological studies (Figure 1C and Figure S2) suggest a copper-dependent ROI-sensitivity of the ΔaceA strain, we asked whether dampening ROI stress would restore ΔaceA survival in AMΦ.
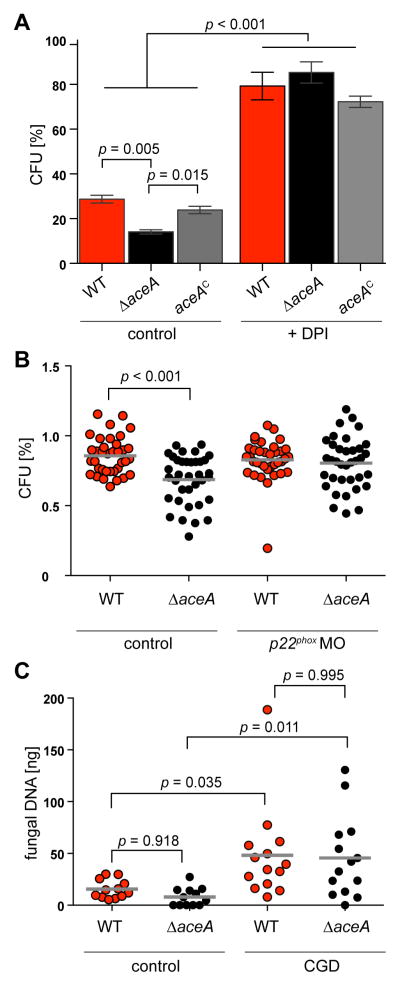
To test if inhibition of O2− production by host immune cells and/or copper limitation by chemical chelation would restore wild-type-like survival rates of the ΔaceA mutant we performed three experiments. First, we used the pharmacological PHOX complex inhibitor diphenyleneiodonium (DPI) (O’Donnell et al., 1993; Philippe et al., 2003) in our AMΦ experiment and observed that survival of the ΔaceA strain returned to wild-type levels (Figure 4A). Second, in an in vivo complementary approach, we compared fungal burden in both immunocompetent and p22phox-deficient zebrafish larvae (Knox et al., 2014; Tauzin et al., 2014). The larval zebrafish has been used to study PHOX activity during C. albicans infection (Brothers et al., 2011) highlighting conserved ROI-generating pathways in this model (Niethammer et al., 2009). Examining wild type and ΔaceA persistence in whole-larval homogenates revealed that attenuated ΔaceA survival was dependent on p22phox expression (Figure 4B). Third, we compared fungal burden of the A. fumigatus wild type and the ΔaceA mutant in a murine model (p91phox-deficient) of CGD and observed a significant increase of fungal burden of both strains in CGD mice compared to immunocompetent mice (Figure 4C and Figure S3L). In contrast to the reduced virulence and fungal burden of the ΔaceA mutant compared to the wild type that we observed in our immunocompromised murine infection model (Figure 2B and Figure S3A, B and C), both strains showed no significant difference in fungal burden in CGD mice (Figure 4C and Figure S3L).
Fig. 4. Inhibition of the Nox complex restores ΔaceA survival.
(A) Colony forming units (CFU) of fungal strains after incubation with murine alveolar macrophages supplemented with or without 25 μM diphenyleneiodonium (DPI) for 2h. Experiments were carried out in triplicates; error bars represent standard deviations and statistical significance is indicated by p values.
(B) Colony forming units (CFU) of fungal strains from whole zebrafish larvae at 24 h post microinjection. Genetic inhibition of p22phox was obtained with morpholino-mediated knockdown (p22MO). Data shown are pooled from four independent experimental replicates where significance is indicated by p values as determined by a least squares means analysis.
(C) Fungal burden from immunocompetent control mice and CGD mice infected with indicated fungal strains. Fungal DNA concentration was determined by qRT-PCR after 24 h post infection (see Material and Methods for details). Data shown are pooled from three independent experimental replicates where significance is indicated by p values as determined by a least squares means analysis.
AceA transcriptionally regulates copper and ROI detoxification genes
The susceptibility of the ΔaceA strain to copper and ROI exposure (Figure 2 and 4), supported a role for AceA in regulating genes involved in both copper and ROI detoxification. We assessed such a possibility by examining gene expression in both wild type and ΔaceA in copper depleted and excess conditions. We tested expression of the four copper importers identified in A. fumigatus (Table 1) as well as genes implicated in copper detoxification. Search of the A. fumigatus genome for putative homologs of the C. albicans copper exporter encoding gene crp1 and the S. cerevisiae copper metallothioneins CUP1 and CRS5 identified one homolog each that we call crpA (Afu3g12740) and cmtA (Afu4g04318), respectively (Table 1; Figure S4A). We also tested for the expression of the copper-dependent superoxide dismutase sod1 and the two mycelial catalases cat1 and cat2 as well as the spore catalase catA.
Our results show that all four copper importers ctrA1, ctrA2, ctrB and ctrC are induced under copper depleted conditions (Figure 5A and Figure S4B). We observed an induction of sod1, cat1 and cat2 (catA was not detectable) by copper addition with cat1 and cat2 also regulated by AceA (Figure 5A). Additionally, we found that the ROI-responsive transcription factor atfA and yap1 were slightly induced under copper surplus conditions in an AceA-dependent manner (Figure S4C). Under the conditions tested, no signal for cmtA was detected (data not shown), whereas crpA, was highly induced by copper addition in an AceA-dependent manner (Figure 5A).
Fig. 5. CrpA is a putative copper-exporting ATPase essential for virulence of A. fumigatus.
(A) Northern blot analysis of indicated strains grown for 24 h in liquid GMM-copper at 37°C. To half of the cultures copper was added t o a final concentration of 200 μM for 1 h before harvesting. Indicated genes were hybridized. rRNA visualization as loading as control. Original image is shown in Fig. S9.
(B) Colony forming units (CFU) of indicated strains from infected mice lungs. Experiments were carried out in triplicate; error bars represent standard deviations and statistical significance is indicated as p value
(C) Growth assay on solidified GMM for 72 h at 37 °C under indicated copper concentrations plus supplements.
(D) Colony forming units (CFU) of fungal strains after incubation with murine alveolar macrophages for 2h. Experiments were carried out in triplicate; error bars represent standard deviations and statistical significance is indicated by p values.
(E) Survival rates of mice immunocompromised with cortisone acetate and infected with the A. fumigatus wild type, ΔaceA, ΔcrpA and the crpA over expressing strain ΔaceA/OE::crpA, respectively. Statistical significance is indicated by asterisks; ****: p < 0.0001, ***: p < 0.0005, *: p < 0.05. 10 mice were in each group.
The putative copper-exporting P-type ATPase CrpA and spore specific ROI defense bZIP transcription factor AtfA remediate ΔaceA macrophage survival
The transcription profiling (Figure 5A, Figure S4B and C) suggested that both ROI degradation pathways and copper export could be contributing to ΔaceA phenotype. To test the former hypothesis, we investigated if constitutive expression of the A. fumigatus bZIP transcription factor encoding gene atfA, that is known for its involvement in spore maturation and spore ROI defense (Hagiwara et al., 2009; Hagiwara et al., 2014; Hagiwara et al., 2016), could restore the ΔaceA survival defect in macrophages (Figure 5B). As previously reported (Pereira Silva et al., 2016), we observed a significant loss of survival in activated BMDMs challenged with an ΔatfA mutant compared to the wild type that was similar to the ΔaceA strain (Figure 5B). Forced expression of atfA brought survival back to wild-type levels in a ΔaceA background (Figure 5B) despite its poor growth phenotype when grown on solidified media (Figure S5A). Since AtfA is suggested to specifically govern spore ROI defense, we tested spore sensitivity towards H2O2 with 5 μM copper present and observed a significant reduction in CFUs of the ΔatfA and ΔaceA strain compared to the wild type (Figure S5B). When atfA was overexpressed in the ΔaceA background, spore viability was significantly increased (Fig. S5B).
Next, we deleted cmtA, crpA and constitutively expressed crpA in both a wild type and ΔaceA background (Figure S5C). Phenotypic analysis on growth media with elevated copper concentrations demonstrated that deletion of cmtA did not affect the growth on elevated copper conditions nor survival when the strain was challenged with murine AMΦ (Fig. S5C and D). In contrast, deletion of crpA resulted in hypersensitivity to copper compared to the wild type and ΔaceA strain (Figure 5C). When crpA is constitutively expressed, copper tolerance exceeds the wild type in both an aceA sufficient and deficient background (Figure S5E). Quantification of copper in mycelia grown in copper-deplete submerged conditions showed no significant difference between the wild type and the ΔcrpA strain (Figure S3H). However, spores collected from solidified media containing 5 μM copper showed a significant increase in copper of the ΔcrpA strain compared to the wild type (Figure S3I).
The relative sensitivity and resistant phenotypes from copper growth plates was also consistent with the observed host interactions. Spore survival assays with murine AMΦ showed significantly reduced viability of the ΔcrpA mutant and increased spore survival in the constitutive crpA expression strains OE::crpA and ΔaceA/OE::crpA compared to the wild type (Figure 5D). Copper quantification from infected activated BMDMs recapitulated our initial experiments and additionally showed ΔaceA-like decreased copper levels in cells challenged with ΔcrpA spores and restoration of wild-type-like copper concentrations when crpA was constitutively expressed in an ΔaceA background (Fig. S3J). As expected ΔaceA/OE::crpA spores from infected cells showed decreased copper concentrations compared to the ΔaceA mutant (Fig. S3K). In the non-neutropenic IA murine model, the ΔcrpA mutant showed significantly decreased virulence similar to the ΔaceA mutant and constitutive expression of crpA in the ΔaceA rescued virulence fully (Figure 5E). Furthermore, the morphology of the fungal lesions of ΔaceA/OE::crpA infected lungs and the fungal burden were restored to that of the wild type strain (Fig. S3A and S5F).
S3E is not mentioned in text.
Discussion
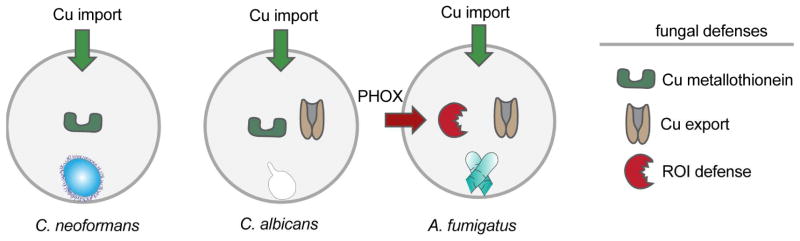
Copper has been suggested to play a major role in innate immune functions against prokaryotic and eukaryotic microbial pathogens (Ding et al., 2014; Djoko et al., 2015; Festa and Thiele, 2012; García-Santamarina and Thiele, 2015; Hodgkinson and Petris, 2012). Studies with bacterial and yeast pathogens have shown that phagocytes isolated from hypocrupemic conditions displayed reduced phagocytotic and antimicrobial activities (Babu and Failla, 1990; Heresi et al., 1985; Xin et al., 1991). In line with these findings, copper pretreatment of phagocytes enhanced intracellular killing of E. coli (White et al., 2009) and copper chelation with a non-permeable chelator increased intracellular survival of S. enterica (Achard et al., 2012). Several studies involving the ascomycete pathogen C. albicans and basidiomycete C. neoformans clearly demonstrate the importance of copper mediated phagocytic killing of these yeasts (Ding et al., 2013; Mackie et al., 2016). While our experimentation with the filamentous fungus A. fumigatus confirms the importance of this host defense mechanism, our work exposes the limitations of copper mediated defense and reveals the inextricable involvement of both host ROI defense and ROI countermeasures in Aspergillus (Fig. 6).
Fig. 6. Copper-defense strategies of the three fungal pathogens C. neoformans, C. albicans and A. fumigatus.
Upon infection, all depicted pathogens activate host copper importers (Ctr1 and ATP7A). Known fungal defense strategies include metallothioneins in C. neoformans and metallothioneins and a copper-exporter in C. albicans. Our results demonstrate that in A. fumigatus the copper-exporter and not the copper-metallothionein is involved in copper-defense. Furthermore, we demonstrate that host PHOX generated ROI is potentiated in strains unable to export copper and that copper-export and ROI-detoxification can remediate virulence of the A. fumigatus ΔaceA mutant. We hypothesize that the existing ROI-detoxification mechanisms of C. neoformans and C. albicans may also be important in copper-regulon interactions of these yeast with host phagocytes in a manner similar to A. fumigatus.
Host mechanisms and immune status underlies importance of copper mediated defense in IA
Although the precise mechanisms of phagocyte copper mobilization remain to be fully explored, studies in E. coli and S. enterica (Achard et al., 2012; White et al., 2009) and recent work on C. neoformans (Ding et al., 2013) have demonstrated that phagocytes respond with upregulation of CTR1 and ATP7A (White et al., 2009). Similarly, we have shown here that macrophages encountering A. fumigatus spores react by upregulation of the copper importer CTR1 and show aggregation of ATP7A in distinct focal points near engulfed spores (Figure 3A and B). However, quantification of copper ions from infected and non-infected macrophages showed no significant difference in the isolate host cell fractions (Figure 3E), reflecting the situation in M. tuberculosis where there was no significant difference in macrophage copper concentration between extra- and intracellular bacteria despite an observed upregulation of host CTR1 (Wagner et al., 2005). However, addition of the permeable copper chelator TTM increased spore survival of A. fumigatus spores when encountering macrophages (Figure 3G) similar to the situation in C. albicans. These data suggest that there might be a difference in biodistribution of copper in whole animals and isolated macrophages in vitro and together, support a conserved host copper transport response to microbes in general.
Efforts to genetically assess the role of ATP7A, however, are difficult. Specific mutations in this protein can cause Menkes’ disease in humans (Woimant and Trocello, 2014) and whereas patients suffering from this hypocupric condition have been reported to suffer from reoccurring urinary tract infections (Tümer and Møller, 2010; Wheeler and Roberts, 1976), ATP7A gene deletion animal models are extremely sick thus in effect precluding their use in infection studies (Madsen et al., 2008; Mercer, 1998). As morpholino technology allows for manageable assessment of nearly lethal mutations in zebrafish, we used this technology to further query a role for this protein in IA, specifically by testing the hypothesis that the reduced colonization the ΔaceA mutant would be restored to wild-type levels in the ATP7A morphant line. Although we found significantly increased growth of wild type A. fumigatus in this zebrafish mutant, this was not the case for ΔaceA (Figure 3F).
While acknowledging that morpholino experimentation has limitations, these results did nevertheless suggest that other host mechanisms were involved and spurred our interest in asking if phagocyte NADPH oxidase (PHOX) activity could also contribute to host dampening of ΔaceA invasion. Phagocytes generate ROI upon infection through activity of the PHOX complex, known as the initial respiratory burst (Hogan and Wheeler, 2014). The complex catalyzes the production of O2− that is subsequently converted to H2O2 (Panday et al., 2015). If copper is mobilized into this environment, it can potentiate the redox potential and can thereby form highly reactive DNA-damaging ·OH via Fenton chemistry (Benov, 2001). Mutations in PHOX are associated with a human disease, CGD, an indicator of susceptibility to IA (Pollock et al., 1995). Using both zebrafish and murine CGD models, we found ΔaceA survival restored to wild-type levels upon inactivation of the PHOX complex (Figure 4). Additionally, biochemical inhibition of host PHOX by DPI support an important role for PHOX in contributing to the phenotype observed in the ΔaceA mutant (Figure 4). Although contribution of ROI detoxification mechanisms on virulence of the two pathogens C. neoformans and C. albicans has been reported (Cox et al., 2003; Frohner et al., 2009; Gleason et al., 2014; Martchenko et al., 2004; Narasipura et al., 2003; Narasipura et al., 2005; Xu et al., 2013), a direct connection to the copper-regulon was not examined in these species. It appears, at least in the host/A. fumigatus interaction, that host ROI and copper responses cannot be clearly separated (Figure 6).
Dual nature of Aspergillus fumigatus countermeasures: copper efflux and ROI defense
Until now, regulation of copper homeostasis in eukaryotic human pathogens has been only explored in two fungi, C. albicans and C. neoformans. In C. albicans, a homolog of Cup1p only detoxifies residual copper when the copper exporting ATPase Crp1 is compromised (Weissman et al., 2000). Deletions of both crp1 and ctr1 resulted in reduced virulence of infected mice (Mackie et al., 2016). In C. neoformans, the metallothioneins Cmt1/2 are important for copper detoxification in the lung (Ding et al., 2013) while the copper importers Ctr1/4 play a major role during infection of the brain, suggesting a tissue-specific host strategy to combat pathogens (Sun et al., 2014). Our studies define yet another tactic taken by the filamentous fungus A. fumigatus in defending from host copper defenses that involves not only copper-binding transcription factor regulation of a copper ATPase transporter but also, critically, regulation of ROI defenses (Figure 6).
Experimentation supported this hypothesis on both fronts. Constitutive expression of either crpA or the transcription factor atfA, shown to govern spore ROI detoxification mechanisms (Hagiwara et al., 2009; Hagiwara et al., 2014; Hagiwara et al., 2016; Pereira Silva et al., 2016), rescued survival of the ΔaceA mutant in confrontations with macrophages (Figure 5) and supports the view that copper mobilized by host cells partially exerts its lethality by potentiating host ROI toxicity. In S. cerevisiae, similar transcriptional control of SOD1 by Ace1p was observed (Gralla et al., 1991). Thus, we show that in contrast to the copper-defense tactics of C. neoformans (metallothionein) and C. albicans (both metallothionein and transporter), AceA regulation of the ATPase CrpA and ROI defense mechanisms are the primary host countermeasures in A. fumigatus (Figure 6). The fact that activation of either mechanism (e.g. CrpA mediated transport or AtfA ROI activation) were sufficient to rescue ΔaceA survival blurs the line between which fungal mechanism is most important and – similar to the intertwined contributions of copper transport and PHOX systems in host response above – reinforces the interconnectedness of both fungal responses to copper extremes. Recent studies in C. albicans show a distinct response of ROI defense mechanism towards different copper-environments during infection (Broxton and Culotta, 2016; Li et al., 2015), suggesting that a similar connection as demonstrated in A. fumigatus in this study could represent a common maneuver in other fungal pathogens.
Considering that P-type ATPase proteins are considered therapeutic targets due to their accessibility on cell membranes, coupled with the recent progress in specifically targeting a microbial P-type ATPase (Kirk, 2015; Novoa-Aponte and Soto Ospina, 2014; Turner, 2016), efforts to target CrpA may hold promise for future work.
Experimental Procedures
Fungal strains and culture conditions
A. fumigatus strains used in this study are listed in Table S1. Strains were grown on solid glucose minimal medium without copper (GMM) at 37 °C with appropriate supplements (Shimizu and Keller, 2001). For pyrG auxotrophs, the growth medium was supplemented with 5 mM uridine and uracil. Conidia were harvested in 0.01% Tween 80 and enumerated using a hemocytometer. For RNA analysis all strains were inoculated into 50 mL of liquid GMM-copper at 5 × 106 conidia/mL in duplicate and grown at 37°C and 250 rpm for 24 h in ambient light conditions. copper was added for 1 h at a final concentration of 200 μM. For growth assays all strains indicated number of conidia were inoculated in 2 μL on solidified (Noble Agar, Difco™, BD, USA) GMM containing indicated supplements, respectively, and incubated for 2–4 days as indicated at 37 °C in the dark. For spore quantification, 1 × 108 were mixed with 10 mL hand warm GMM containing agar and the indicated copper concentration and plated on 10 mL of the same solidified media in petri dishes. For harvesting spores for macrophage survival assays, all strains were grown for 3 days at 37 °C in the dark on GMM + 1 μM copper to ensure comparable growth and melanization of spores. For colony forming unit enumeration, spores were plated on GMM + 1 μM copper and incubated for 2 days at 37 °C in the dark. For zebrafish larvae infection f ungal strains were inoculated onto GMM plates at a concentration of 1 × 106 conidia per plate using an overlay method and grown for 3 days at 37 °C.
Fungal transformation and deletion constructs
Deletion fragments were created by double-joint fusion PCR and transformation was carried out as previously described (Palmer et al., 2008). (d’Enfert, 1996) using primers listed in Table S2. DNA of transformants was isolated as described by (Green and Sambrook, 2012). Integration of the transformation construct was confirmed by diagnostic PCR using primer pairs as indicated in Fig. S6–9. Single integration was confirmed by Southern analysis as described by (Green and Sambrook, 2012) (Figure S6–9).
Gene expression analysis
Mycelia were harvested by filtration through Miracloth (Calbiochem). Total RNA was extracted with TRIzol reagent (Invitrogen), following the manufacturer’s protocol. Northern analysis was performed as described by (Green and Sambrook, 2012). Probes for northern analysis were constructed at regions internal to the gene of interest using primers listed in Table S2 (‘gene’-F/’gene’-R) and labeled with dCTP αP32.
Protein bio- and histochemistry
Infected and non-infected bone marrow derived macrophages (see below) were lysed with 0.5 % SDS on ice for 5 min before an equal volume of PBS was added. Protein concentration was quantifies using an Epoch2 microplate reader (BioTek) and equal amounts were reconstituted in 2x loading dye. Western blotting was performed according to standard procedures (Green and Sambrook, 2012). For fluorescent detection of ATP7A, infected and non-infected bone marrow derived macrophages were cultivated as described below, but on microscopy glass coverslips on the bottom of the wells. Cells were incubated with ATP7A and a fluorescently labeled secondary antibody. Coverslips were mounted onto a pre-cleaned microscope slide. Images were taken with a Zeiss AxioMager A10.
Phylogeny and data analysis
For phylogenetic analysis, reviewed and curated sequences of interest from the Swiss-Prot database (www.uniprot.org) of proteins were retrieved and aligned together with A. fumigatus protein sequences (www.aspergillus.org) (Cerqueira et al., 2014) using MAFFT (http://mafft.cbrc.jp/alignment/software/) (Katoh et al., 2002) and (http://www.microbesonline.org/fasttree/) (Price et al., 2009).
Copper quantification
Quantification of copper was carried out after spores were challenged with activated murine bone marrow macrophages for 2 hours. Cells were permeabilized with 0.5% SDS as described below. Spores were separated from cell lysate by centrifugation. Cell lysates were sterile filtered before analysis. Remaining spore pellets were reconstituted in 500 μL deionized water and enumerated using a hemocytometer. Equal amount of spores were sonicated for 30 min before analysis. An Agilent 8800 ICP-MS was used to quantify copper in the samples after an acid digestion with nitric acid and further dilution with doubly deionized water. Sc was used as internal standard at 10 ng/ml to quantify by the external calibration method with reagent blank correction (less than 0.1 ng/ml) as previously described (Subramanian Vignesh et al., 2013).
Murine Alveolar Macrophage Isolation
Specific pathogen-free C57BL/6J and Swiss ICR mice (8–12 weeks old, equal ratio of female and male) were used in this study purchased from Harlan Laboratories Inc.. Bronchoalveolar lavage fluid (BALF) were collected from 12–20 mice, pooled and seeded at a density of 1×105 cells/well and allowed to rest overnight in a 37°C humidified incubator (5% CO2) prior to use.
Murine Bone Marrow Macrophage Differentiation and Activation
Bone marrow was obtained by aseptically flushing the femurs and tibias of 8–10 week old C57BL/6J mice (equal ratio of female and male). Cells were incubated for seven days in a 37°C humidified incubator (5% CO2) with media replacement and removal of non-adherent cells performed every 2–3 days before use.
Murine alveolar and bone marrow derived macrophage killing assays
For metal quantification (1 × 107 cells/well) and killing assays (1 × 105 cells/well) spores were incubated with cells in a 3:1 (spore:cell ratio) plus indicated supplements in complete alveolar macrophage media. Cells and spores were centrifuged at 300 g for 5 min before incubation for 1 h at 37 °C in a cell in cubator. After 1 h the media was aspirated and non-adherent spores were then washed away with PBS before fresh media plus indicated supplements was added to the cells and incubated for 1 h at 37 °C in a cell incubator. Cells were washed, lysed and spores were enumerated. From each well, spores were plated in three 1:1 serial dilutions in 200 μL in duplicate, starting with 500 spores per plate as the highest amount of spores. The initial spore solution in complete macrophage media was enumerated and plated in a similar fashion starting with 100 spores per plate in duplicate.
Murine infection model
Six week old ICR female mice were used in this assay. In the non-neutropenic (cortisone acetate) model, mice were injected subcutaneously with cortisone acetate (300 mg/kg) 3 days prior to infection, on the day of their infection, 3, 7 and 11 days post infection. In the neutropenic (cyclophosphamide) model, mice were injected subcutaneously with cyclophosphamide (150 mg/kg) and cortisone acetate (150 mg/kg) 3 days prior to infection, and with cyclophosphamide (150 mg/kg) on the day of their infection, 3 and 6 days post infection. The mice were infected intranasally with 5 × 105 dormant conidia. Mortality was monitored for 21 days. For histopathology, mice were sacrificed two days after infection and their lungs were removed staining with Grocott’s methenamine silver stain (GMS; fungal staining) and hematoxylin and eosin (H&E; tissue and nuclear staining). For fungal burden, infected mice were sacrificed on the second day post infection, their lungs were removed and homogenized, and the homogenates were plated on YAG. TNF-α levels were measured two days post infection by ELISA of the supernatant from whole lung homogenates.
CGD infection model
C57Bl/6J mice were purchased from The Jackson Laboratory. Mice with an inactivation of X-linked Cybb (X-CGD mice) in the C57Bl/6J (backcrossed >15 generations) and wild type littermates controls were obtained from in-house colonies (Pollock et al., 1995). Mice were used between 10–21 weeks of age. Mice received 30,000 conidia via nasopharyngeal installation. Mice were sacrificed after 24 h and lungs were then homogenized and plated for CFU on GMM for 2 days at 37°C. To quantitate total fungal DNA, homogenized lungs were further bead beaten with acid washed glass beads and DNA was extracted with the DNeasy Blood & Tissue Kit (Qiagen-69504). All DNA quantity and quality were assessed with BioTek Gen5 microplate reader (BioTek Instruments, Inc.,) previously described (Li et al., 2011).
Zebrafish care and maintenance
Adult zebrafish were housed on a system with regulated water temperature at 28.5 °C, pH, and conductivity in a room programmed with a light/dark cycle of 14 hours and 10 hours, respectively, and fed twice daily.
Larval zebrafish infection model
All larval zebrafish infection experiments were performed as described (Knox et al., 2014). Morpholino-mediated genetic knockdown of p22phox or atp7a was obtained as previously described (Tauzin et al., 2014). Immediately following microinjection, 8–12 randomly selected larvae from each condition were individually homogenized and spread evenly on GMM agar plates containing 1 μM copper for time zero CFU enumeration. Similarly, at 24 hours post infection (hpi) 8–12 larvae were randomly selected and processed in a similar manner.
Statistical analyses
Statistical differences of data were analyzed using the GraphPad Prism 5 software package (GraphPad Software, Inc, San Diego, CA). For fungal CFU forming experiments from macrophages, spore counting from fungal growth plates, diameter measurements in H2O2 stress tests and copper quantification, p values were calculated with one-way ANOVA for multiple comparisons and adjusted with Bonferroni’s or Holm Sidak correction and non-paired Student’s t test where two groups were compared. All error bars given represent standard deviations. For larval zebrafish CFU experiments, data from four independent replicates were pooled and significance determined with analysis of variance with results summarized using least squares adjusted means and standard errors.
Ethics Statement
All animal experiments were carried out in strict accordance to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Experiments were performed with the approval of the Institutional Animal Care and Use Committee (IACUC) of Tel Aviv University, the University of Wisconsin-Madison, and Washington University in St. Louis, respectively. All efforts were made to minimize the number of animals used and animal suffering.
Supplementary Material
Highlights.
Aspergillus fumigatus infection activates the host copper (Cu) transporter Ctr1
AceA is the A. fumigatus transcription factor coordinating Cu-dependent defense
A. fumigatus detoxifies high copper levels through the P-type ATPase CrpA
Activation of copper export restores virulence of aceA deficient strains
Acknowledgments
This work was supported by the USDA Hatch Formula Fund (WIS01710) and NIH grant R01 AI065728-01 to NPK, the National Science Foundation-Emerging Frontiers in Research and Innovation-MIKS (Grant 1136903) to AH and NPK, Israel Ministry of Health Infect-ERA (Grant 11080) to NO and by an award from the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital to MCD. We thank Agilent Technologies for ICP-MS instrumental support.
Footnotes
Author Contributions
PW, NO and NPK conceived and designed the study. PW, AP, FYL, YS, BPK, MN, TC, AJS, RAI and JALF performed experiments. JALF, MW, BSK, AH, MCD, NO and NPK provided materials and equipment. PW, NO and NPK wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard ME, Stafford SL, Bokil NJ, Chartres J, Bernhardt PV, Schembri MA, Sweet MJ, McEwan AG. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem J. 2012;444:51–57. doi: 10.1042/BJ20112180. [DOI] [PubMed] [Google Scholar]
- Babu U, Failla ML. Respiratory burst and candidacidal activity of peritoneal macrophages are impaired in copper-deficient rats. J Nutr. 1990;120:1692–1699. doi: 10.1093/jn/120.12.1692. [DOI] [PubMed] [Google Scholar]
- Benov L. How superoxide radical damages the cell. Protoplasma. 2001;217:33–36. doi: 10.1007/BF01289410. [DOI] [PubMed] [Google Scholar]
- Borghouts C, Osiewacz HD. GRISEA, a copper-modulated transcription factor from Podospora anserina involved in senescence and morphogenesis, is an ortholog of MAC1 in Saccharomyces cerevisiae. Mol Gen Genet. 1998;260:492–502. doi: 10.1007/s004380050922. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Copper lowering therapy with tetrathiomolybdate as an antiangiogenic strategy in cancer. Curr Cancer Drug Targets. 2005;5:195–202. doi: 10.2174/1568009053765807. [DOI] [PubMed] [Google Scholar]
- Brothers KM, Newman ZR, Wheeler RT. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 2011;10:932–944. doi: 10.1128/EC.05005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxton CN, Culotta VC. An adaptation to low copper in Candida albicans involving SOD enzymes and the alternative oxidase. PLoS One. 2016;11:e0168400. doi: 10.1371/journal.pone.0168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira GC, Arnaud MB, Inglis DO, Skrzypek MS, Binkley G, Simison M, Miyasato SR, Binkley J, Orvis J, Shah P, Wymore F, Sherlock G, Wortman JR. The Aspergillus Genome Database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res. 2014;42:D705–10. doi: 10.1093/nar/gkt1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. Zinc and manganese chelation by neutrophil S100A8/A9 (Calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol. 2016;196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Howard WR, Liu XF. CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J Biol Chem. 1994;269:25295–25302. [PubMed] [Google Scholar]
- Cyert MS, Philpott CC. Regulation of cation balance in Saccharomyces cerevisiae. Genetics. 2013;193:677–713. doi: 10.1534/genetics.112.147207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Enfert C. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5′-decarboxylase gene, pyrG, as a unique transformation marker. Current Genetics. 1996;30:76–82. doi: 10.1007/s002940050103. [DOI] [PubMed] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Festa RA, Chen YL, Espart A, Palacios Ò, Espín J, Capdevila M, Atrian S, Heitman J, Thiele DJ. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe. 2013;13:265–276. doi: 10.1016/j.chom.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Festa RA, Sun TS, Wang ZY. Iron and copper as virulence modulators in human fungal pathogens. Mol Microbiol. 2014;93:10–23. doi: 10.1111/mmi.12653. [DOI] [PubMed] [Google Scholar]
- Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol. 2011;81:1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djoko KY, Ong CL, Walker MJ, McEwan AG. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J Biol Chem. 2015;290:18954–18961. doi: 10.1074/jbc.R115.647099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas LM, Wang HX, Keppler-Ross S, Dean N, Konopka JB. Sur7 promotes plasma membrane organization and is needed for resistance to stressful conditions and to the invasive growth and virulence of Candida albicans. MBio. 2012;3:e00254–11. doi: 10.1128/mBio.00254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker DJ, Butt TR, Sternberg EJ, Neeper MP, Debouck C, Gorman JA, Crooke ST. Yeast metallothionein function in metal ion detoxification. J Biol Chem. 1986;261:16895–16900. [PubMed] [Google Scholar]
- Festa RA, Thiele DJ. Copper at the front line of the host-pathogen battle. PLoS Pathog. 2012;8:e1002887. doi: 10.1371/journal.ppat.1002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol Microbiol. 2009;71:240–252. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Santamarina S, Thiele DJ. Copper at the fungal pathogen-host axis. J Biol Chem. 2015;290:18945–18953. doi: 10.1074/jbc.R115.649129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AS, Wheeler RT, May RC. Fungal pathogens: Survival and replication within macrophages. Cold Spring Harb Perspect Med. 2015;5:a019661. doi: 10.1101/cshperspect.a019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JE, Galaleldeen A, Peterson RL, Taylor AB, Holloway SP, Waninger-Saroni J, Cormack BP, Cabelli DE, Hart PJ, Culotta VC. Candida albicans SOD5 represents the prototype of an unprecedented class of Cu-only superoxide dismutases required for pathogen defense. Proc Natl Acad Sci U S A. 2014;111:5866–5871. doi: 10.1073/pnas.1400137111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graden JA, Winge DR. Copper-mediated repression of the activation domain in the yeast Mac1p transcription factor. Proc Natl Acad Sci U S A. 1997;94:5550–5555. doi: 10.1073/pnas.94.11.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla EB, Thiele DJ, Silar P, Valentine JS. ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. Proc Natl Acad Sci U S A. 1991;88:8558–8562. doi: 10.1073/pnas.88.19.8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- Hagiwara D, Asano Y, Marui J, Yoshimi A, Mizuno T, Abe K. Transcriptional profiling for Aspergillus nidulans HogA MAPK signaling pathway in response to fludioxonil and osmotic stress. Fungal Genet Biol. 2009;46:868–878. doi: 10.1016/j.fgb.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Hagiwara D, Suzuki S, Kamei K, Gonoi T, Kawamoto S. The role of AtfA and HOG MAPK pathway in stress tolerance in conidia of Aspergillus fumigatus. Fungal Genet Biol. 2014;73:138–149. doi: 10.1016/j.fgb.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Hagiwara D, Takahashi H, Kusuya Y, Kawamoto S, Kamei K, Gonoi T. Comparative transcriptome analysis revealing dormant conidia and germination associated genes in Aspergillus species: an essential role for AtfA in conidial dormancy. BMC Genomics. 2016;17:358. doi: 10.1186/s12864-016-2689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie EA, Huttenlocher A. Neutrophils in host defense: New insights from zebrafish. J Leukoc Biol. 2015;98:523–537. doi: 10.1189/jlb.4MR1114-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenberg M, Behnsen J, Krappmann S, Brakhage A, Gunzer M. Phagocyte responses towards Aspergillus fumigatus. Int J Med Microbiol. 2011;301:436–444. doi: 10.1016/j.ijmm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Heinekamp T, Schmidt H, Lapp K, Pähtz V, Shopova I, Köster-Eiserfunke N, Krüger T, Kniemeyer O, Brakhage AA. Interference of Aspergillus fumigatus with the immune response. Semin Immunopathol. 2015;37:141–152. doi: 10.1007/s00281-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- Heresi G, Castillo-Durán C, Muñoz C, Arévalo… M. Phagocytosis and immunoglobulin levels in hypocupremic infants. Nutrition Research 1985 [Google Scholar]
- Hodgkinson V, Petris MJ. Copper homeostasis at the host-pathogen interface. J Biol Chem. 2012;287:13549–13555. doi: 10.1074/jbc.R111.316406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan D, Wheeler RT. The complex roles of NADPH oxidases in fungal infection. Cell Microbiol. 2014;16:1156–1167. doi: 10.1111/cmi.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Fürst P, Hamer D. The DNA and Cu binding functions of ACE1 are interdigitated within a single domain. New Biol. 1990;2:544–555. [PubMed] [Google Scholar]
- Jensen LT, Winge DR. Identification of a copper-induced intramolecular interaction in the transcription factor Mac1 from Saccharomyces cerevisiae. EMBO J. 1998;17:5400–5408. doi: 10.1093/emboj/17.18.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J, Reins HA, Lee J, Romeo A, Hassett R, Kosman D, Jentsch S. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J. 1993;12:5051–5056. doi: 10.1002/j.1460-2075.1993.tb06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara S, Jhingran A, Dhingra S, Salem A, Cramer RA, Hohl TM. Role of granulocyte-macrophage colony-stimulating factor signaling in regulating neutrophil antifungal activity and the oxidative burst during respiratory fungal challenge. J Infect Dis. 2016;213:1289–1298. doi: 10.1093/infdis/jiw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G, Gross C, Kelleher M, Winge DR. Functional independence of the two cysteine-rich activation domains in the yeast Mac1 transcription factor. J Biol Chem. 2000;275:29193–29199. doi: 10.1074/jbc.M001552200. [DOI] [PubMed] [Google Scholar]
- Kirk K. Ion regulation in the malaria parasite. Annu Rev Microbiol. 2015;69:341–359. doi: 10.1146/annurev-micro-091014-104506. [DOI] [PubMed] [Google Scholar]
- Knox BP, Deng Q, Rood M, Eickhoff JC, Keller NP, Huttenlocher A. Distinct innate immune phagocyte responses to Aspergillus fumigatus conidia and hyphae in zebrafish larvae. Eukaryot Cell. 2014 doi: 10.1128/EC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25:736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, Mordelet E, Zapata A, Shinomiya H, Herbomel P. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP, Culotta VC. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc Natl Acad Sci U S A. 2015;112:E5336–42. doi: 10.1073/pnas.1513447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Barker BM, Grahl N, Puttikamonkul S, Bell JD, Craven KD, Cramer RA. The small GTPase RacA mediates intracellular reactive oxygen species production, polarized growth, and virulence in the human fungal pathogen Aspergillus fumigatus. Eukaryot Cell. 2011;10:174–186. doi: 10.1128/EC.00288-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J, Vieyra A, Catty P, Guillain F, Mintz E, Cuillel M. A mutational study in the transmembrane domain of Ccc2p, the yeast Cu(I)-ATPase, shows different roles for each Cys-Pro-Cys cysteine. J Biol Chem. 2004;279:25986–25994. doi: 10.1074/jbc.M308736200. [DOI] [PubMed] [Google Scholar]
- Mackie J, Szabo EK, Urgast DS, Ballou ER, Childers DS, MacCallum DM, Feldmann J, Brown AJ. Host-imposed copper poisoning impacts fungal micronutrient acquisition during systemic Candida albicans infections. PLoS One. 2016;11:e0158683. doi: 10.1371/journal.pone.0158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen EC, Morcos PA, Mendelsohn BA, Gitlin JD. In vivo correction of a Menkes disease model using antisense oligonucleotides. Proc Natl Acad Sci U S A. 2008;105:3909–3914. doi: 10.1073/pnas.0710865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: Transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15:456–467. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Mercer JF. Menkes syndrome and animal models. Am J Clin Nutr. 1998;67:1022S–1028S. doi: 10.1093/ajcn/67.5.1022S. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Ault JG, Behr MJ, Chaturvedi V, Chaturvedi S. Characterization of Cu,Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: Role in biology and virulence. Mol Microbiol. 2003;47:1681–1694. doi: 10.1046/j.1365-2958.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Chaturvedi V, Chaturvedi S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol. 2005;55:1782–1800. doi: 10.1111/j.1365-2958.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa-Aponte L, Soto Ospina CY. Mycobacterium tuberculosis P-type ATPases: Possible targets for drug or vaccine development. Biomed Res Int. 2014;2014:296986. doi: 10.1155/2014/296986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, Perrin RM, Dagenais TR, Keller NP. H3K9 methylation regulates growth and development in Aspergillus fumigatus. Eukaryot Cell. 2008;7:2052–2060. doi: 10.1128/EC.00224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Mehrad B. Innate immunity to Aspergillus species. Clin Microbiol Rev. 2009;22:535–551. doi: 10.1128/CMR.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YS, Lian H, Chang M, Kang CM, Yun CW. Identification of high-affinity copper transporters in Aspergillus fumigatus. Fungal Genet Biol. 2014;73:29–38. doi: 10.1016/j.fgb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Pereira Silva L, Alves de Castro P, Reis TF, Paziani MH, Von Zeska Kress MR, Riaño-Pachón DM, Hagiwara D, Ries LN, Brown NA, Goldman GH. Genome-wide transcriptome analysis of Aspergillus fumigatus exposed to osmotic stress reveals regulators of osmotic and cell wall stresses that are SakA(HOG1) and MpkC dependent. Cell Microbiol. 2016 doi: 10.1111/cmi.12681. [DOI] [PubMed] [Google Scholar]
- Perrin RM, Fedorova ND, Bok JW, Cramer RA, Wortman JR, Kim HS, Nierman WC, Keller NP. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3:e50. doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A, Latgé JP. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003;71:3034–3042. doi: 10.1128/IAI.71.6.3034-3042.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Falcone M, Vena A, Venditti C, Mancini C, Morelli A, Venditti M. Invasive pulmonary aspergillosis in non-neutropenic patients: Analysis of a 14-month prospective clinical experience. J Chemother. 2011;23:290–294. doi: 10.1179/joc.2011.23.5.290. [DOI] [PubMed] [Google Scholar]
- Schwartz JA, Olarte KT, Michalek JL, Jandu GS, Michel SL, Bruno VM. Regulation of copper toxicity by Candida albicans GPA2. Eukaryot Cell. 2013;12:954–961. doi: 10.1128/EC.00344-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Keller NP. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TS, Ju X, Gao HL, Wang T, Thiele DJ, Li JY, Wang ZY, Ding C. Reciprocal functions of Cryptococcus neoformans copper homeostasis machinery during pulmonary infection and meningoencephalitis. Nat Commun. 2014;5:5550. doi: 10.1038/ncomms6550. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Thiele DJ. A cysteine-rich nuclear protein activates yeast metallothionein gene transcription. Mol Cell Biol. 1989;9:421–429. doi: 10.1128/mcb.9.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin S, Starnes TW, Becker FB, Lam PY, Huttenlocher A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol. 2014;207:589–598. doi: 10.1083/jcb.201408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele DJ. ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol. 1988;8:2745–2752. doi: 10.1128/mcb.8.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- Tümer Z, Møller LB. Menkes disease. Eur J Hum Genet. 2010;18:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H. Spiroindolone NITD609 is a novel antimalarial drug that targets the P-type ATPase PfATP4. Future Med Chem. 2016;8:227–238. doi: 10.4155/fmc.15.177. [DOI] [PubMed] [Google Scholar]
- Wagner D, Maser J, Lai B, Cai Z, Barry CE, Höner Zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- Waterman SR, Hacham M, Hu G, Zhu X, Park YD, Shin S, Panepinto J, Valyi-Nagy T, Beam C, Husain S, Singh N, Williamson PR. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest. 2007;117:794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman SR, Park YD, Raja M, Qiu J, Hammoud DA, O’Halloran TV, Williamson PR. Role of CTR4 in the Virulence of Cryptococcus neoformans. MBio. 2012;3 doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman Z, Berdicevsky I, Cavari BZ, Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci U S A. 2000;97:3520–3525. doi: 10.1073/pnas.97.7.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler EM, Roberts PF. Menkes’s steely hair syndrome. Arch Dis Child. 1976;51:269–274. doi: 10.1136/adc.51.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EJ, Clark JB. Menadione-treated synaptosomes as a model for post-ischaemic neuronal damage. Biochem J. 1988;253:425–433. doi: 10.1042/bj2530425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woimant F, Trocello JM. Disorders of heavy metals. Handb Clin Neurol. 2014;120:851–864. doi: 10.1016/B978-0-7020-4087-0.00057-7. [DOI] [PubMed] [Google Scholar]
- Xin Z, Waterman DF, Hemken RW, Harmon RJ. Effects of copper status on neutrophil function, superoxide dismutase, and copper distribution in steers. J Dairy Sci. 1991;74:3078–3085. doi: 10.3168/jds.S0022-0302(91)78493-2. [DOI] [PubMed] [Google Scholar]
- Xu N, Cheng X, Yu Q, Qian K, Ding X, Liu R, Zhang B, Xing L, Li M. Aft2, a novel transcription regulator, is required for iron metabolism, oxidative stress, surface adhesion and hyphal development in Candida albicans. PLoS One. 2013;8:e62367. doi: 10.1371/journal.pone.0062367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.