Abstract
During a visceral leishmaniasis outbreak in an area of Madrid, Spain, the incidence of disease among solid organ transplant recipients was 10.3% (7/68). Being a black person from sub-Saharan Africa, undergoing transplantation during the outbreak, and residing <1,000 m from the epidemic focus were risk factors for posttransplant visceral leishmaniasis.
Keywords: solid organ transplant, solid organ transplantation, visceral leishmaniasis, epidemiology, risk factors, urban outbreak, parasites, Spain, Switzerland, vector-borne infections, Leishmania infantum, leishmaniasis
Visceral leishmaniasis (VL) is an uncommon but potentially fatal complication for solid organ transplant (SOT) recipients (1,2). Beginning in July 2009, an outbreak of leishmaniasis affected the southwest area of Madrid (3). The outbreak was primarily located in Fuenlabrada, which has an annual VL incidence of 21.1 cases/100,000 population (4), notably higher than that estimated for the general population in Spain (0.5 cases/100,000 population) (5).
Spatial analysis revealed that the highest concentration of cases was in the residential area bordering the park (Bosque Sur) (6). A large population of Lepus granatensis hares, which serve as a reservoir for Leishmania infantum, was present in the area (7,8), and the Phlebotomus perniciosus sand fly in Spain can act as a vector and take blood meals from these hares (6,8,9). Thus, the parkland facilitated the transmission of the leishmaniasis pathogen, which led to the outbreak. This large, urban outbreak provided us the opportunity to analyze the incidence and specific risk factors of VL among SOT recipients.
The Study
The University Hospital 12 de Octubre in Madrid, Spain, acts as the reference hospital for SOT in South Madrid. We performed a retrospective study of all consecutive adult patients who underwent kidney, liver, or heart transplantation during January 1, 2005–January 1, 2013, and lived in the outbreak area. Patients who underwent SOT before January 1, 2005, were excluded because of the difficulty of ensuing long-term follow-up and the potential of heterogeneity in posttransplant practices. Patients who died or had moved to a different place of residence before outbreak onset were excluded (Technical Appendix Figure 1).
The primary study outcome was the occurrence of VL, the diagnosis of which required confirmation of parasitemia (online Technical Appendix) (10). We recorded pretransplant, peritransplant and posttransplant variables and collected various environmental factors prospectively by unblinded, direct interview with the patients. Patients were considered to have frequent contact with dogs if patients reported having dogs at home or taking care of dogs and to have the habit of visiting the park if they reported visiting once a year. The distance between the place of residence and the park was obtained by locating the patient’s home address and measuring the shortest linear distance to the nearest border of the parkland by means of an online mapping tool (Google Maps; Google Inc., Mountain View, CA, USA).
The beginning of the exposure period was set as July 2009 (outbreak onset) for patients who underwent SOT before the outbreak and as the transplant date for those who underwent SOT after outbreak onset. In both cases, the exposure period extended to the date of diagnosis of VL, death, or December 2013. We chose to end the study in December 2013 because the incidence of leishmaniasis decreased thereafter because of the implementation of control measures. The clinical research ethics committee of the University Hospital 12 de Octubre approved the study, and participants provided informed consent.
We analyzed 68 patients (Table 1) for a median follow-up of 4.4 (interquartile range 2.39–6.95) years. VL was diagnosed in 7 patients, yielding a cumulative incidence of 10.3% (95% CI 3.1%–17.5%) and an annual incidence of 2,997 (95% CI 1,213–6,161) cases per 100,000 population. Details on disease pathology and therapy were recorded (Table 2). The mean interval between transplant and diagnosis was 1.34 ± 0.89 years. No patients had visited highly VL-endemic countries.
Table 1. Baseline and clinical characteristics of solid organ transplant recipients in study of risk factors for VL, Madrid, Spain, January 1, 2005–January 1, 2013*.
| Characteristics | Overall cohort, n = 68 | VL, n = 7 | No VL, n = 61 | p value† |
|---|---|---|---|---|
| Recipient age, y, mean ± SD | 51.1 ± 14.2 | 53.0 ± 15.5 | 51.0 ± 13.5 | 0.721 |
| Male sex, no. (%) |
48 (70.6) |
6 (85.7) |
42 (68.9) |
0.664 |
| Race, no. (%) | ||||
| White | 62 (91.2) | 5 (71.4) | 57 (93.4) | 0.112 |
| Black, sub-Saharan African | 4 (5.9) | 2 (28.6)‡ | 2 (3.3) | 0.049 |
| Other |
2 (2.9) |
0 (0) |
2 (3.3) |
1.000 |
| Type of SOT, no. (%) | ||||
| Kidney | 57 (83.8) | 6 (85.7) | 51 (83.6) | 1.000 |
| Liver | 8 (11.8) | 0 (0) | 8 (13.1) | 0.587 |
| Heart |
3 (4.4) |
1 (14.3) |
2 (3.3) |
0.282 |
| Donor age, y, mean ± SD | 46.1 ± 16.2 | 49.4 ± 17.4 | 46.3 ± 16.3 | 0.596 |
| Cold ischemia time, min, median (IQR) | 1,005 (630–1,354) | 795 (371–1,365) | 1,020 (660–1,360) | 0.370 |
| No. HLA mismatches, mean ± SD | 4.0 ± 1.2 | 5.0 ± 1.0 | 4.0 ± 1.2 | 0.265 |
| DCD donor, no. (%) | 18 (26.5) | 3 (42.8) | 15 (24.6) | 0.370 |
| Transplant during the outbreak, no. (%) |
41 (60.3) |
7 (100.0) |
34 (55.7) |
0.037
|
| Induction therapy, no. (%) | ||||
| Basiliximab | 22 (32.4) | 0 (0) | 22 (36.1) | 0.087 |
| Antithymocyte globulin | 24 (35.3) | 4 (57.1) | 20 (32.8) | 0.233 |
| None |
22 (32.4) |
3 (42.8) |
19 (31.1) |
0.673 |
| Maintenance immunosuppression, no. (%) | ||||
| Steroids | 56 (82.4) | 7 (100.0) | 49 (80.3) | 0.338 |
| Calcineurin inhibitors | 60 (88.2) | 6 (85.7) | 54 (88.5) | 1.000 |
| Mycophenolate mofetil/mycophenolic acid | 47 (61.8) | 4 (57.1) | 43 (70.5) | 0.668 |
| mTOR inhibitors |
10 (14.7) |
1 (14.3) |
9 (14.8) |
1.000 |
| Complications in the first year after SOT, no. (%) | ||||
| Acute graft rejection | 19 (27.9) | 2 (28.6) | 17 (27.9) | 1.000 |
| CMV infection | 21 (30.9) | 4 (57.1) | 17 (27.9) | 0.189 |
| Bacterial infection |
60 (88.2) |
6 (85.7) |
54 (88.5) |
0.190 |
| Environmental factors | ||||
| Frequent contact with dogs, no. (%) | 26 (38.2) | 3 (42.8) | 23 (37.7) | 1.000 |
| Habit of visiting the park, no. (%)§ | 19 (31.7) | 3 (50.0) | 16 (29.6) | 0.369 |
| Distance from patient’s residence to park, m, median (IQR) | 1,220 (849–1,865) | 399 (261–985) | 1,370 (974–1,880) | 0.001 |
*CMV, cytomegalovirus; DCD, donation after circulatory death; HLA, human leukocyte antigen; IQR, interquartile range; mTOR, mammalian target of rapamycin; SOT, solid organ transplant; VL, visceral leishmaniasis. †The p values refer to the comparison between patients with and without visceral leishmaniasis. Significant values are in bold. ‡The country of birth of both patients with posttransplant VL was Equatorial Guinea. §Data available for 60 patients.
Table 2. Disease characteristics, demographics, clinical characteristics, therapy, and outcomes of 7 solid organ transplant recipients with VL, Madrid, Spain, January 1, 2005–January 1, 2013*.
| Characteristics | Patient no. |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Sex | M | M | M | M | M | F | M |
| Race | Black | Black | White | White | White | White | White |
| Linear distance from patient’s residence to park, m | 794 | 399 | 261 | 1,240 | 985 | 233 | 358 |
| Age at transplant, y | 35 | 34 | 76 | 55 | 68 | 49 | 52 |
| Type of SOT | Kidney | Kidney | Kidney | Kidney | Kidney | Heart | Kidney |
| Donor Leishmania spp. serostatus | Negative | NP | Negative | NP | Negative | Negative | Negative |
| Pretransplant recipient Leishmania spp. serostatus | NP | NP | Negative | Positive | Negative | Negative | Negative |
| Date of transplant | 2011 Feb 11 | 2010 Jan 22 | 2010 Mar 10 | 2010 Jul 7 | 2009 Dec 29 | 2010 Sep 5 | 2010 Apr 15 |
| Interval from transplant to VL diagnosis, y | 1.17 | 2.44 | 0.25 | 1.4 | 0.17 | 1.81 | 2.21 |
| Fever at admission | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Pancytopenia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Splenomegaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Serologic testing results for Leishmania spp. | Positive | Negative | Negative | Positive | Negative | Positive | Negative |
| Presence of amastigote forms in bone marrow sample | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| PCR assay results of bone marrow sample | NP | NP | NP | Negative | NP | NP | NP |
| NNN culture results of bone marrow sample | Positive | NP | Positive | Negative | Negative | Positive | Negative |
| Initial therapy | L-AmB | L-AmB | L-AmB | L-AmB | L-AmB | L-AmB | L-AmB |
| Relapse | Yes | No | Yes | Yes | No | No | No |
| Outcome | Renal failure | Graft loss | Cured | Graft loss | Cured | Cured | Cured |
*L-AmB, liposomal amphotericin B; NNN, Novy-McNeal-Nicolle; NP, not performed; SOT, solid organ transplant; VL, visceral leishmaniasis.
Black sub-Saharan African SOT recipients were more likely than other recipients to become affected by VL (relative risk 6.40, 95% CI 1.76–23.29, p = 0.049) (Table 1). All 7 episodes of VL occurred in patients who underwent transplantation during the outbreak period (Figure 1).
Figure 1.
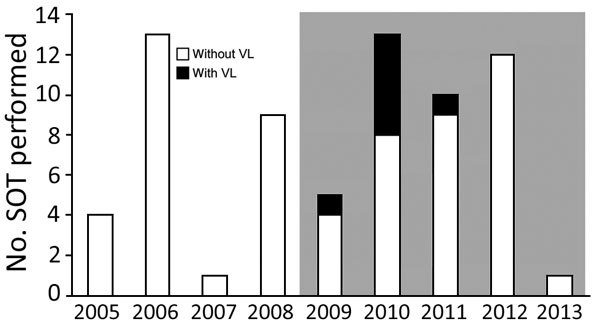
Distribution of VL among solid organ transplant recipients, Madrid, Spain, January 1, 2005–January 1, 2013. Columns represent the number of solid organ transplant procedures performed each year at the University Hospital 12 de Octubre among patients permanently residing in Fuenlabrada, the nearby city affected by the outbreak. Gray shading indicates outbreak period. SOT, solid organ transplant; VL, visceral leishmaniasis.
The median distance between the place of residence and the park was significantly shorter for recipients with VL (399 m) than for those without (1,370 m; p = 0.001) (Figure 2; Technical Appendix Figure 2). We explored the predictive accuracy of this variable by establishing the optimal cutoff value with the area under the receiving operating characteristic curve analysis. Recipients living <1,000 m from the park (26.1%, 6/23) had a higher incidence of VL than recipients living >1,000 m away (2.2%, 1/45; relative risk, 11.74, 95% CI 1.50–91.78; p = 0.005). At 4 years, a lower percentage of the SOT recipients living <1,000 m from the park were free from VL than those living >1,000 m away (61.0% vs 98%; p = 0.001 by log-rank test) (Technical Appendix Figure 3).
Figure 2.
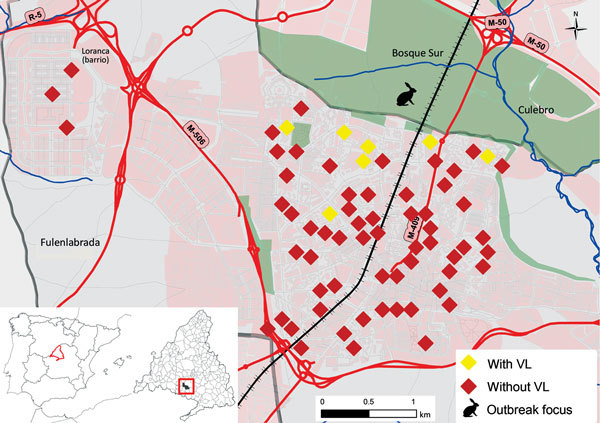
Spatial distribution of solid organ transplant recipients in the southwest area of Madrid, Spain, in relation to park that was focus of visceral leishmaniasis (VL) outbreak, January 1, 2005–January 1, 2013. Map inset shows the location of the outbreak in relation to the rest of Spain. VL, visceral leishmaniasis.
Our study suggests that the incidence of VL in SOT recipients is notably higher than that in the general population (11). Acquisition of the parasite most likely occurred posttransplant because all but 1 recipient affected with VL (from whom serum samples could be recovered) were seronegative for Leishmania spp. before transplantation.
Our findings suggest that environmental factors might be crucial in modulating the incidence of VL in immunocompromised hosts, such as SOT recipients; the distance from the patient’s residence to the focus of the outbreak (6,7) emerged as a key risk factor. The median distance between the park and the homes of recipients with posttransplant VL was <500 m; the maximum flight distance of female sand flies is 600 m (12,13). Therefore, persons living within this radius had a higher chance of being bitten by the VL vector. A similar association was described for the general population during this outbreak (6).
Undergoing transplantation during the outbreak period was another risk factor for VL. This finding suggests that, in the case of an outbreak in a country with low baseline incidence, pretransplant screening of patients listed for SOT for VL-specific antibodies should be considered and repeated during the posttransplant period for the prompt detection of de novo infection. Recipients should also receive specific counseling to reduce the risk of being bitten by sand flies. In addition, treating physicians must maintain a low threshold of suspicion for VL for persons on immunosuppressive therapy during a VL outbreak.
We found that 28% of posttransplant VL cases occurred in black recipients born in sub-Saharan Africa, even though this subgroup only represented 2.4% of the overall population in the affected area (14). An association between sub-Saharan African ethnicity and VL has also been reported in the general population (4). No apparent relationship was found between the race of the patient and the frequency of parkland visits. Both black recipients in question came from Equatorial Guinea, a country not considered endemic for leishmaniasis by the World Health Organization (15). Therefore, the potential association between genetic susceptibility and posttransplant VL warrants further investigation.
Limitations of this study include the small sample size and that interviewers were not blinded to the diagnosis of VL. However, the objective nature of the questionnaire minimized the potential risk for bias. When assessing degree of exposure to sand flies, we used only indirect variables (i.e., distance between the patient’s residence and park, habit of visiting the park) as surrogate measures. Regarding the distance from the park, only linear distances were assessed without considering the potential presence of physical obstacles in the sand fly flight trajectory. Because of these limitations, our findings must be interpreted with caution.
Conclusions
Our study indicates several risk factors (being black and from sub-Saharan Africa, having an SOT during the outbreak, and living <1,000 m from the outbreak focus) useful for helping physicians treat SOT recipients during a VL outbreak. Doctors should select the patients with these risk factors for counseling to minimize their exposure to vectors and active monitoring for prompt diagnosis.
Methods used to confirm visceral leishmaniasis (VL) diagnosis, flow chart of solid organ transplant (SOT) recipients included in the study, box-whisker plot comparing linear distances from outbreak focus area (park) to residences of the SOT recipients with and without VL, and Kaplan-Meier curve analysis showing differences in VL disease onset in SOT recipients on the basis of distance from park.
Acknowledgment
We thank Emiliano Aránguez Ruiz for his kind help providing the map included in this paper.
This study was co-funded by the World Health Organization (APW-2012/271093-O), the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III (Proyecto Integrado de Excelencia 13/00045), and the European Regional Development Fund. M.F.R. holds a clinical research contract Juan Rodés (JR14/00036) from the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III.
Biography
Dr. Carrasco-Antón is a specialist in internal medicine currently working at the Fundación Jiménez Díaz in Madrid, Spain. Her research interests are leishmaniasis and other infections in immunocompromised hosts.
Footnotes
Suggested citation for this article: Carrasco-Antón N, López-Medrano F, Fernández-Ruiz M, Carrillo E, Moreno J, García-Reyne A, et al. Environmental factors as key determinants for visceral leishmaniasis in solid organ transplant recipients, Madrid, Spain. Emerg Infect Dis. 2017 Jul [date cited]. https://dx.doi.org/10.3201/eid2307.151251
References
- 1.Clemente W, Vidal E, Girão E, Ramos AS, Govedic F, Merino E, et al. Risk factors, clinical features and outcomes of visceral leishmaniasis in solid-organ transplant recipients: a retrospective multicenter case-control study. Clin Microbiol Infect. 2015;21:89–95. 10.1016/j.cmi.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Alves da Silva A, Pacheco-Silva A, de Castro Cintra Sesso R, Esmeraldo RM, Costa de Oliveira CM, Fernandes PF, et al. The risk factors for and effects of visceral leishmaniasis in graft and renal transplant recipients. Transplantation. 2013;95:721–7. 10.1097/TP.0b013e31827c16e2 [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Barroso D, Herrador Z, San Martin JV, Gherasim A, Aguado M, Romero-Mate A, et al. Spatial distribution and cluster analysis of a leishmaniasis outbreak in the south-western Madrid region, Spain, September 2009 to April 2013. Euro Surveill. 2015;20:11–20. 10.2807/1560-7917.ES2015.20.7.21037 [DOI] [PubMed] [Google Scholar]
- 4.Horrillo L, San Martín JV, Molina L, Madroñal E, Matía B, Castro A, et al. Atypical presentation in adults in the largest community outbreak of leishmaniasis in Europe (Fuenlabrada, Spain). Clin Microbiol Infect. 2015;21:269–73. 10.1016/j.cmi.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 5.Herrador Z, Gherasim A, Jimenez BC, Granados M, San Martín JV, Aparicio P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997-2011: raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl Trop Dis. 2015;9:e0003594. 10.1371/journal.pntd.0003594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aránguez Ruiz E, Arce Arnáez A, Moratilla Monzo L, Estirado Gómez A, Iriso Calle A, De la Fuente Ureña S, et al. Spatial analysis of an outbreak of leishmaniasis in the south of Madrid’s metropolitan area. 2009–2013 [in Spanish]. Rev Salud Ambient. 2014;14:39–53. [Google Scholar]
- 7.Molina R, Jiménez MI, Cruz I, Iriso A, Martín-Martín I, Sevillano O, et al. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol. 2012;190:268–71. 10.1016/j.vetpar.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 8.Jiménez M, González E, Iriso A, Marco E, Alegret A, Fúster F, et al. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniasis in Madrid, Spain. Parasitol Res. 2013;112:2453–9. 10.1007/s00436-013-3406-3 [DOI] [PubMed] [Google Scholar]
- 9.Suárez Rodríguez B, Isidoro Fernández B, Santos Sanz S, Sierra Moros MJ, Molina Moreno R, Astray Mochales J, et al. [Review of the current situation and the risk factors of Leishmania infantum in Spain] [in Spanish]. Rev Esp Salud Publica. 2012;86:555–64. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–16 March 2010. Geneva: WHO Press; 2010.
- 11.Arce A, Estirado A, Ordobas M, Sevilla S, García N, Moratilla L, et al. Re-emergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013;18:20546. 10.2807/1560-7917.ES2013.18.30.20546 [DOI] [PubMed] [Google Scholar]
- 12.Dergacheva TI, Strelkova MV, Lapin VA, Karulin BE, Chabovskiĭ AV. [The use of radioisotope labelling for studying the feeding of sandflies (Phlebotominae) and their move into colonies of the greater gerbil (Rhombomys opimus Licht.)]. Med Parazitol (Mosk). 1996; (3):11–4. [PubMed] [Google Scholar]
- 13.Kamhawi S, Abdel-Hafez SK, Molyneux DH. The behaviour and dispersal of sandflies in Ras el Naqb, south Jordan with particular emphasis on Phlebotomus kazeruni. Parassitologia. 1991;33(Suppl):307–14. [PubMed] [Google Scholar]
- 14.City Council of Fuenlabrada. Population in Fuenlabrada, by sex and country of origin [Spanish]. 2016. Jan 5 [cited 2016 Mar 23]. http://ayto-fuenlabrada.es/index.do?MP=3&MS=27&MN=2&TR=A&IDR=1&iddocumento=17132
- 15.World Health Organization. Global Health Observatory data repository. Leishmaniasis [cited 2016 March 23]. http://apps.who.int/gho/data/node.main.NTDLEISH?lang=en
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods used to confirm visceral leishmaniasis (VL) diagnosis, flow chart of solid organ transplant (SOT) recipients included in the study, box-whisker plot comparing linear distances from outbreak focus area (park) to residences of the SOT recipients with and without VL, and Kaplan-Meier curve analysis showing differences in VL disease onset in SOT recipients on the basis of distance from park.


