Abstract
Previous research suggests that age-related differences in attention reflect the interaction of top-down and bottom-up processes, but the cognitive and neural mechanisms underlying this interaction remain an active area of research. Here, within a sample of community-dwelling adults 19–78 years of age, we used diffusion reaction time (RT) modeling and multivariate functional connectivity to investigate the behavioral components and whole-brain functional networks, respectively, underlying bottom-up and top-down attentional processes during conjunction visual search. During functional MRI scanning, participants completed a conjunction visual search task in which each display contained one item that was larger than the other items (i.e., a size singleton) but was not informative regarding target identity. This design allowed us to examine in the RT components and functional network measures the influence of (a) additional bottom-up guidance when the target served as the size singleton, relative to when the distractor served as the size singleton (i.e., size singleton effect) and (b) top-down processes during target detection (i.e., target detection effect; target present vs. absent trials). We found that the size singleton effect (i.e., increased bottom-up guidance) was associated with RT components related to decision and nondecision processes, but these effects did not vary with age. Also, a modularity analysis revealed that frontoparietal module connectivity was important for both the size singleton and target detection effects, but this module became central to the networks through different mechanisms for each effect. Lastly, participants 42 years of age and older, in service of the target detection effect, relied more on between-frontoparietal module connections. Our results further elucidate mechanisms through which frontoparietal regions support attentional control and how these mechanisms vary in relation to adult age.
Keywords: aging, attentional control, diffusion modeling, functional connectivity, graph theory
Graphical abstract
1.0 INTRODUCTION
Visual attention is hypothesized to be controlled by two processes – top-down and bottom-up (Connor et al., 2004; Yantis, 1998; 2005; however, for an opposing view see Awh, Belopolsky, & Theeuwes, 2012). Top-down attention refers to the goal-oriented, voluntary allocation of attention to an object or spatial location, whereas bottom-up attention refers to a less voluntary, stimulus-driven capture of attention (Theeuwes, 2010; Yantis, 1998). Although the relative contributions of top-down and bottom-up attention have been under investigation for several decades, exactly how these two processes interact is a topic of active investigation. This issue is especially relevant for conjunction visual search, which requires observers to detect a target that is a conjunction of nontarget (distractor) features (e.g., a right-tilted blue bar target among left-tilted blue bars and right-tilted green bars). Historically, conjunction search has been viewed as relying predominantly on top-down attention, because when salience is relatively constant across displays, a correct response relies on the observer’s knowledge of the particular combination of features that define a target (Bacon and Egeth, 1997; Eckstein, 2011; Kristjansson and Campana, 2010; Kristjansson et al., 2002; Treisman, 1988; Wolfe, 1998). Some evidence, however, indicates that bottom-up processes may also influence conjunction search (Kaptein et al., 1995; Proulx, 2007).
This issue is further complicated in aging, where, in general, increased age is associated with a decline in visual search performance, especially when the task relies on visual sensory functioning (Hommel et al., 2004; Madden and Whiting, 2004). The specific processes underlying age-related decline in visual search performance, however, are still poorly understood. Several studies have demonstrated that top-down attentional processes are relatively preserved with age (Madden et al., 2004; Madden et al., 2005b; McAvinue et al., 2012; Whiting et al., 2005), but bottom-up attentional processes in aging remain understudied and may be a source of age-related decline in cognitive performance (Baltes and Lindenberger, 1997; Monge and Madden, 2016; Schneider and Pichora-Fuller, 2000).
1.1 Bottom-up Guidance during Conjunction Search
The overall goal of the current study was to investigate age-related differences in the interaction of top-down and bottom-up attentional processes during conjunction search, using both behavioral and event-related functional MRI (fMRI) measures. We used a conjunction search task, adapted from Proulx (2007), in which participants searched for a Color × Orientation conjunction target among distractors (e.g., a right-tilted blue bar among left-tilted blue bars and right-tilted green bars). Each display also included a salient feature (a size singleton) that was not part of the target definition and was not informative regarding target presence. Thus, size was a potential source of bottom-up guidance, but the size singleton was no more likely to be the target than nonsingleton display items. This size singleton effect is expressed as a decrease in reaction time (RT) for singleton target trials, relative to nonsingleton target trials, demonstrating that participants appeared to use salience (i.e., bottom-up processing) as the basis for their search strategy. In contrast, the target detection effect, defined as the increase in RT for nonsingleton target (i.e., target present) trials, relative to target-absent trials, primarily represents top-down processes of target identification and response selection, as salience does not provide any guidance or support on these trials.
1.2 Neural Mechanisms Underlying Visual Attention in Aging
Researchers have attempted to further elucidate the role of top-down and bottom-up processes in visual search by examining the neural mechanisms underlying these processes. A large neuroimaging literature has demonstrated that top-down attentional processes rely more on a dorsal frontoparietal network, whereas bottom-up attentional processes rely more on a ventral frontoparietal network (Corbetta and Shulman, 2002; Miller and Buschman, 2013; Noudoost et al., 2010; Riddoch et al., 2010; Shipp, 2004; Shulman et al., 2004), but these network components are highly interconnected (Egner et al., 2008; Monge et al., 2016; Treue, 2003; Vossel et al., 2014). However, with regard to conjunction visual search, this interaction remains poorly understood, in terms of both (a) the extent of this interaction and (b) the neural mechanisms underlying this interaction.
Regarding aging, it appears that the previously described preservation of top-down attentional processes in older adults is often associated with increased activation in dorsal frontoparietal regions (Allen and Payne, 2012; Eyler et al., 2011; Madden et al., 2005a; Spreng et al., 2010), but how bottom-up attentional processes may influence dorsal frontoparietal functional properties in aging is largely unknown. Madden et al. (2017) recently explored this issue using the conjunction visual search task adapted from Proulx (2007), and found, in the examination of brain-behavior relations, that individuals 35 years of age and older (vs. relatively younger adults) exhibited greater engagement of the left frontal eye field (FEF) in service of the size singleton effect (i.e., increased bottom-up guidance). Thus, preliminary evidence indicates that age does have an effect on the neural mechanisms supporting bottom-up attentional guidance.
1.3 Modularity and Centrality Measures of Attentional Networks
The Madden et al. (2017) study, as the majority of neuroimaging studies of attention, used univariate, event-related fMRI activation analyses. These univariate analyses, however, are limited in that they do not indicate how brain regions interact with each other in the context of whole-brain networks. A useful approach in this regard is multivariate functional connectivity (Bullmore and Sporns, 2009; van den Heuvel and Sporns, 2013). In the current study, we used multivariate functional connectivity to investigate the whole-brain functional networks underlying top-down and bottom-up attentional control during conjunction search in aging. We specifically used a multivariate framework, graph theory, which views the brain as a network consisting of discrete nodes (brain regions) and the edges between nodes (functional connections between brain regions). The analysis of the edges between nodes allows for the characterization of the topological properties of a functional network; these unique properties cannot be revealed with more traditional analysis approaches such as univariate activation or bivariate functional connectivity. Within the current study, we focused on two types of graph metrics – modularity and centrality.
Functional and structural brain networks have been found to consist of distinct clusters of interconnected nodes (i.e., modules; for reviews, see Mišić and Sporns, 2016; Sporns and Betzel, 2016). Modularity describes the degree to which sets of nodes may be segregated into modules in a data-driven manner. It is hypothesized that modules allow the brain to efficiently adapt to cognitive demands of the environment (Crossley et al., 2013; Mišić and Sporns, 2016), making understanding the functional role of modules in service of cognition of great importance. The functional role of modules is especially important in the study of cognitive aging because of preliminary evidence, predominantly from resting-state functional connectivity analyses, indicating that with increased age, modules become less segregated and that this decreased segregation may have cognitive consequences (Betzel et al., 2014; Cao et al., 2014; Chan et al., 2014; Onoda and Yamaguchi, 2013; Song et al., 2014). However, since resting-state functional connectivity may not correspond to task-based connectivity (Campbell and Schacter, 2016; Cohen and D’Esposito, 2016; Davis et al., 2016), these studies do not inform how the modular topology of the brain adapts to cognitive demands of the environment. Task-based functional connectivity analyses, therefore, are necessary. Only two studies, to our knowledge, have examined modularity in aging using task-based functional connectivity, and found that (a) the modular topology of the brain adapts to executive function and cognitive control demands, and (b) older adults relied more on between-module connections in service of cognition (Gallen et al., 2016; Schlesinger et al., 2017)1. These studies, however, are limited in that they only examined extreme age groups (older vs. younger adults). Also, it is unknown how the modular architecture of the aging brain changes in service of other cognitive processes important in the study of cognitive aging, such as visual attention. These ambiguities indicate the importance of further studying the modular architecture of the aging brain.
Centrality refers to the relative importance of a node within a network (Bullmore and Sporns, 2009; van den Heuvel and Sporns, 2013), which may also be used to assess the centrality of modules. Furthermore, the examination of different centrality measures may determine different mechanisms through which a module becomes central to a network. Within this study we were interested in examining modules that may become central to a network through (a) maintaining strong functional connections, (b) becoming more reliant on highly central nodes in other modules, or (c) both. To investigate these properties, we examined two centrality measures – degree centrality and PageRank centrality. Degree centrality measures the overall strength of the functional connections maintained by a node. A node with high degree centrality maintains many strong, functional connections. PageRank centrality is based on the importance (specifically the PageRank centrality) of a node’s neighbors. A node with high PageRank centrality may not necessarily maintain many strong, functional connections (i.e., have high degree centrality) but maintains functional connections with neighboring nodes that are highly central to the network (Boldi et al., 2009; Morrison et al., 2005); it should be noted that similar centrality measures (e.g., eigenvector centrality, betweenness centrality) may also similarly examine this property but we chose PageRank centrality (vs. eigenvector centrality or betweenness centrality) because it has been shown to be more sensitive to age-related effects (Khazaee et al., 2016; Zuo et al., 2012). The use of these two graph metrics allowed us to examine the three previously described ways a module may become central to a network, which, again, may be through (a) maintaining strong functional connections (high degree centrality but low PageRank centrality), (b) becoming more reliant on highly central nodes in other modules (high PageRank centrality but low degree centrality), or (c) both (high degree centrality and PageRank centrality).
1.4 The Current Study: Behavioral Components of Visual Attention, Functional Connectivity, and Aging
In the current study, we applied to the Madden et al. (2017) data set these multivariate functional connectivity measures, as well as other novel behavior measures, to address several questions. First, is bottom-up attentional guidance related specifically to decision processes or instead to nondecision processes (e.g., perceptual processing/motor speed)? The vast majority of neuroimaging investigations of attention, including the Madden et al. study, have used mean RT and error rate as the outcome measures. Here, we used estimates of RT components from Ratcliff’s diffusion model of RT (Ratcliff, 1979; Ratcliff and McKoon, 2008; Ratcliff et al., 2016) to distinguish decisional and nondecisional aspects of search performance. As described in the Methods (Statistical Analyses), the diffusion model uses information from each participant’s RT distributions to differentiate several components: drift rate, which is the rate at which information is accumulated to make a decision; nondecision time, which is the time spent on nondecision processes, such as perceptual processing and the motor response; and boundary separation, which is the amount of information required for a decision (i.e., cautiousness). Thus, if bottom-up attentional guidance (i.e., the size singleton effect) influences the decision process associated with target detection, rather than nondecision processes, then salience should lead to an increased drift rate for singleton targets, relative to nonsingleton targets.
Second, how do the behavioral components underlying the size singleton and target detection effects vary with age? Regarding the diffusion model parameters in aging, the most pronounced effect of adult age is typically an increase in nondecision time (reflecting slower perceptual processing and motor speed) and boundary separation (reflecting increased cautiousness; Ratcliff et al., 2004; Ratcliff et al., 2001, 2003; Thapar et al., 2003), but some studies also observe a decrease in drift rate (Madden et al., 2009; Spaniol et al., 2006; Yang et al., 2015), reflecting a slower rate of information acquisition. As accumulating evidence indicates that deficient bottom-up processes in older adults may directly cause some cognitive deficiencies (Monge and Madden, 2016), we predicted that bottom-up guidance would be more helpful to older adults’ performance than to younger adults’ performance, which would be reflected as increased age being associated with the size singleton effect, specifically for drift rate and nondecision time.
Third, how are the size singleton and target detection effects, in drift rate and nondecision time, related to centrality of the modules underlying visual attention? To address this question, we first conducted a modularity analysis on the nonsingleton target functional network. We predicted that this network would contain a frontoparietal module, among other modules, in light of abundant evidence indicating the importance of frontoparietal regions in visual attention. To characterize the centrality of the modules, we calculated both degree centrality and PageRank centrality. We then examined the relation between the behavioral size singleton and target detection effects, as expressed in drift rate and nondecision time, and module centrality. For the size singleton effect, we predicted that increases in the size singleton effect for drift rate and nondecision time (i.e., higher drift rates and lower nondecision times for size singleton targets relative to nonsingleton targets) would be associated with decreased centrality within the frontoparietal module, reflecting the size singleton target requiring less frontoparietal, attentional processing. For the target detection effect, since previous work has demonstrated that, in the presence of a size singleton, target-present responses can be slower than target-absent responses (Madden et al., 2017; Proulx, 2007), we predicted that increases in the target detection effect for drift rate and nondecision time (i.e., lower drift rates and higher nondecision times for nonsingleton target-present trials, relative to target-absent trials), would be associated with increased frontoparietal module centrality, reflecting greater attentional processing for target detection. We examined both degree and PageRank centrality to further characterize the mechanisms through which modules may become central to a network.
Fourth, how does the relation between drift rate/nondecision time and module centrality, for the size singleton and target detection effects, vary as a function of age? To investigate this question, for each of the modules that significantly predicted either the drift rate/nondecision time (size singleton or target detection effects), we conducted linear regressions to examine if Age × Module Centrality interactions could predict the drift rate/nondecision time size singleton or target detection effects. Based on evidence demonstrating increased frontoparietal activation in older adults in service of visual attention (e.g., Allen and Payne, 2012; Madden et al., 2005a), particularly during bottom-up processing (Madden et al., 2017), we predicted that increased frontoparietal centrality may become more prominent with increasing age, particularly with regard to bottom-up attentional guidance and associated effects in the RT component measures.
2.0 METHODS
2.1 Study Participants
Our study sample included 68 community-dwelling adults between the ages of 19 and 78 years (Table 1), in which 23 participants were between 19 and 39 years of age, 24 were between 40 and 59 years of age, and 21 were between 60 and 78 years of age. Participants met the following inclusion criteria: corrected visual acuity greater than or equal to 20/40 (Bach, 1996); Mini-Mental State Examination (Folstein et al., 1975) score 27 or greater; a Beck Depression Inventory (Beck, 1978) score less than or equal to 10; a Vocabulary subtest of the Wechsler Adult Intelligence Scale III (Wechsler, 1997) scaled score greater than or equal to the 50th percentile; a Dvorine color vision test (Dvorine, 1963) score greater than or equal to 12; and at least 75% accuracy on the practice version of the visual search task administered during screening. For more details on the exclusion criteria and screening process, see Madden et al. (2017). Also, all participants were right-handed and completed at least 12 years of education (M = 16.5, SD = 2.1 years), and 55.9% were female. Participants self-reported to be free of significant health problems (including atherosclerosis, neurological and psychiatric disorders), and not taking medications known to affect cognitive function or cerebral blood flow (except antihypertensive agents). The Duke University Institutional Review Board approved all experimental procedures, and participants provided informed consent prior to testing. After study completion, participants were monetarily compensated for their time.
Table 1.
Participant Characteristics
| Variable | M | SD | r with Age |
|---|---|---|---|
| Education (years) | 16.5 | 2.1 | 0.31* |
| Color Vision | 13.9 | 0.4 | −0.19 |
| Visual Acuity | −0.0534 | 0.1311 | 0.32** |
| BDI | 2.3 | 2.7 | 0.18 |
| MMSE | 29.1 | 1.0 | −0.23 |
| Vocabulary | 56.8 | 5.9 | 0.03 |
Note. n = 68. Color Vision = score on Dvorine color plates (Dvorine, 1963); Visual Acuity = logarithm of the minimum angle to resolution (MAR), for the Freiburg Visual Acuity Test (Bach, 1996); BDI = score on Beck Depression Inventory (Beck, 1978); MMSE = raw score on Mini-Mental State Examination (Folstein et al., 1975); Vocabulary = raw score on the vocabulary subtest of the Wechsler Adult Intelligence Scale III (Wechsler, 1997);
= p < .05;
= p < .01.
2.2 Visual Search Task
In the scanner, participants performed a conjunction visual search task, modified from Proulx (2007). Participants decided whether a target bar was present or not present among nontarget (distractor) bars (Figure 1). The target was defined as a conjunction of color (blue or green) and orientation (45° left- or 45° right-titled). Each distractor shared only one feature with the target; for example, a blue, right-titled target would be accompanied by two blue, left-tilted bars and two green, right-titled bars. The display always contained five items that were distributed within an approximately 15° diameter circular area. Items were distributed in an irregular pattern wherein the center-to-center distance between all pairs of items ranged from 5.4° to 10.6°, and the edges between adjacent items were no closer than 1°. The distance between the center of the display and the center of each display item ranged from 3.3° to 7.5°. The color bars in the display were isoluminant and presented on a black background.
Figure 1. Conjunction visual search task.
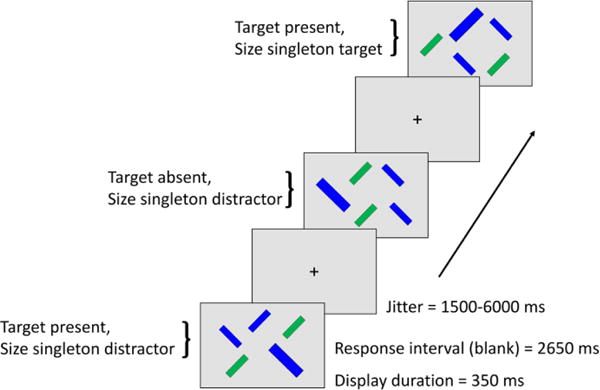
Participants performed a conjunction visual search task in which the target shared one feature (either color or orientation) with each of the nontarget (distractor) items. The target could be either (a) a right tilted, blue bar among right-tilted green bars and left-titled blue bars (distractors); or (b) a left-titled green bar among left-tilted blue bars and right-titled green bars; in the scanner, display items were presented against a black background. The target was constant within participants and counterbalanced across participants. Display size was constant at five items. Participants made a yes/no response on each trial regarding the presence of the target. In each display, one of the distractors was 50% larger than the other items (i.e., a size singleton). On one-fifth of the target-present trials, the size singleton coincided with the target.
Each participant completed 350 trials, in which half the trials contained one target and four distractors (target-present, 175 trials) and the other half five distractors (target-absent, 175 trials). For each participant, the search target (e.g., blue, right-tilted bar) was constant, and the values of color and orientation defining the target were counterbalanced across participants. Each display contained four bars of equal size (0.8° × 3.2°), and one bar that was 50% larger (i.e., a size singleton; 1.2° × 4.8°). On 1/5 of the trials (i.e., 35 trials) the size singleton corresponded to the target and on the rest of the trials the size singleton corresponded to a distractor. Therefore, a size singleton was present on every trial and provided a bottom-up form of visual salience, but was not informative regarding the target location. This design yielded three trial types of interest: trials in which the target was present and the size singleton was a distractor (nonsingleton target trials), trials in which the target was present and was also the size singleton (singleton target trials), and trials in which the target was absent and the size singleton was a distractor (target absent trials). When the size singleton was a distractor, within both the target-present and target-absent trials, it contained each target-relevant value of color and orientation an approximately equal number of times. For each trial type, the size singleton/target location was approximately equally distributed between the top versus bottom, and left versus right halves of the screen.
Participants completed five event-related, functional imaging runs of 70 trials each – a total of 350 trials. The 70 trials per run contained a randomly ordered sequence of 35 target-present trials (including seven size-singleton target trials) and 35 target-absent trials. For each trial, participants indicated whether the target was present or absent via a button-press response, using their right index and middle fingers and two buttons on a hand-held, fiber optic response box (Current Designs, Philadelphia, PA, USA); the assignment of the button corresponding to a target-present trial was balanced across participants. Participants were instructed to respond as quickly as possible without sacrificing accuracy.
Each trial started with a white fixation cross displayed for a variable duration (jitter), followed by the five-item display for a duration of 350 ms, then a 2650 ms response period, during which the display was black. We measured RT from display onset. After the response period, the fixation cross returned to begin the next trial. Participants did not receive feedback regarding accuracy. The jitter duration was varied among the values of 1500, 3000, 4500, and 6000 ms defined by multiples of the fMRI repetition time (TR) value (1500 ms). The jitter values and trial order were randomized and optimized using Optseq2 (Dale, 1999; http://surfer.mnr.mgh.harvard.edu/optseq). Task presentation and response recordings were controlled by E-Prime 2.0 (Psychology Software Tools, Sharpsburg, PA, USA).
2.3 MRI Data Acquisition
A General Electric 3T MR750 whole-body 60 cm bore MRI scanner (GE Healthcare, Waukesha, WI, USA) and 8-chanel head coil were used to collect functional and anatomical images. Participants wore earplugs to reduce scanner noise, and foam pads were used to reduce head motion. The imaging session started with a localizer scan, in which 3-plane (straight axial/coronal/sagittal) localizer fast spin echo (FSE) images were collected. A semi-automated high-order shimming program ensured global field homogeneity. For the task event-related, functional imaging runs, 29 contiguous slices were acquired at an axial oblique orientation, parallel to the AC-PC plane, in an interleaved order (TR = 1500 ms, TE = 27 ms, FOV = 240 mm, flip angle = 77°, voxel size = 3.75 mm × 3.75 mm × 4 mm, 64 × 64 matrix, sensitivity encoding [SENSE] factor = 1); for each run, a total of 252 brain volumes were acquired.
After functional imaging acquisition, a high-resolution, anatomical image was collected. For this image, 166 straight axial slices T1-weighted images were acquired with a 3D fast inverse-recovery-prepared spoiled gradient recalled (SPGR) sequence (TR = 8.10 ms, TE = 3.18 ms, inversion recovery time [TI] = 450 ms, FOV = 256 mm, flip angle = 12°, voxel size = 1 × 1 × 1 mm, 256 × 256 matrix, SENSE factor = 2, using the array spatial sensitivity encoding technique and extended dynamic range).
2.4 fMRI Analysis
2.4.1 Preprocessing
All preprocessing and first-level analyses of the functional data were conducted within FSL 5.0.5 (Smith et al., 2004; http://www.fmrib.ox.ac.uk/fsl) and FEAT 6.0. The T1-weighted images were skull-striped using the FSL Brain Extraction Tool (BET; Smith, 2002). For the functional images, the first four volumes of each run were discarded to allow for scanner equilibrium. All images were slice-time corrected and corrected for head motion using FSL MCFLIRT (Jenkinson et al., 2002); the motion parameters (6 rigid-body transformations) were included as nuisance covariates in the first-level general linear models (GLMs). To further correct for motion, events that overlapped with trials in which the participant moved more than 2.5 mm in any direction were later excluded from functional network construction; this additional correction was only applicable for one participant (eight events). Following, functional images were co-registered to the participants’ 3D-FSPGR and subsequently normalized to MNI space (Montreal Neurological Institute, Montreal, Canada) using a combination of affine and non-linear registrations (Greve and Fischl, 2009; Jenkinson et al., 2002; Jenkinson and Smith, 2001). Lastly, images were spatially smoothed with a 5 mm Gaussian kernel and temporally smoothed with a high-pass filter (cut off = 90.0 s) to correct for scanner drift.
2.4.2 Functional Network Construction
The input data for the modularity analyses are functional networks, representing all pairwise correlations of regional task-related activation. We specifically used a beta time series approach (Cisler et al., 2014; Rissman et al., 2004), which considers two regions to be functionally connected if the beta time series of the regions are correlated (Fornito et al., 2011a; Fornito et al., 2011b; Geib et al., 2017; Geib et al., 2015; Schedlbauer et al., 2014). To obtain the beta values for each trial, we modeled every trial individually (a total of 70 regressors for each run and a total of five runs) with a stick function at stimulus onset convolved with a standard hemodynamic response function. These beta values were then sorted by the three trial types of interest – nonsingleton target, singleton target, and target absent trials.
In graph theory, a network is viewed as consisting of nodes (here, brain regions of interest; ROIs) and edges (here, the functional connections between nodes). For functional network construction, we used a total of 397 ROIs, comprising a subparcellated version of the Harvard-Oxford Atlas (Tzourio-Mazoyer et al., 2002), excluding the cerebral white matter, lateral ventricles and brain-stem ROIs (see Supplementary Table 1 for a full list of ROIs). For each ROI, for each trial type of interest, we extracted the beta time series, and correlated the beta time series of each ROI with every other ROI. This resulted in three undirected, weighted connectivity matrices for each trial type representing the pairwise Pearson correlations between the mean beta time series of each ROI with every other ROI within the network (397 × 397 matrices). The correlation values between each ROI represent the functional connectivity strength between ROIs. The three connectivity matrices represent the functional networks for each trial type – nonsingleton target, singleton target, and target absent networks. Negative connections were removed from the connectivity matrices because of our specific interest in PageRank centrality which may not be estimated in the presence of negative connections (Telesford et al., 2011), and values within the diagonal of each connectivity matrix were set to zero.
2.4.3 Modularity
After network construction, we conducted a modularity analysis on the nonsingleton target network. The nonsingleton target network was used for the modularity analyses because this condition served as the reference trial type when examining the size singleton (singleton target – nonsingleton target) and target detection (nonsingleton target – target absent) effects. To conduct the modularity analysis, we first averaged each participant’s nonsingleton target connectivity matrix to yield an averaged, nonsingleton target connectivity matrix (collapsed across all ages). We then ran a Louvain algorithm (Blondel et al., 2008), contained within the Brain Connectivity Toolbox (Rubinov and Sporns, 2010; https://sites.google.com/site/bctnet/), on the averaged nonsingleton target connectivity matrix. We applied the Louvain algorithm on the nonsingleton target network ten times because network modularity is an optimization algorithm, and identifying the perfect partition is a non-deterministic polynomial-time hard problem (Brandes et al., 2006). The run that produced the highest Q value was considered the best partition and the accompanying community assignments were used for subsequent analyses herein. Similar to Chan et al. (2014), the same community assignments were used across all ages. All results overlaid on brain surfaces were created with BrainNet Viewer (Xia et al., 2013) and are shown overlaid onto BrainMesh_ICBM152 within MNI standard space (neurological convention).
2.5 Centrality Measures
After running the modularity analysis on the nonsingleton target network, we calculated degree centrality and PageRank centrality for each of the nodes in all three networks of interest (Rubinov and Sporns, 2010). Degree centrality was calculated by summing the total weights maintained by a node. PageRank centrality was calculated using a damping value of 0.85 (Boldi et al., 2009). Both of these measures have previously been used to assess the centrality of nodes within functional networks (e.g., Geib et al., 2017; Geib et al., 2015; Zuo et al., 2012). For each module within each network, we averaged the centrality measures across the ROIs (separately for degree and PageRank centrality) to create a composite degree and PageRank centrality score for each module (e.g., frontoparietal module degree centrality within the singleton target network).
2.6 Statistical Analyses/Diffusion Model of Reaction Time
All statistical analyses were conducted in SAS 9.4 (Cary, NC, USA). For analysis, we excluded trials in which either the participant did not respond or responded in less than 250 ms (1.07% of all trials). As noted in the Introduction, we used a version of Ratcliff’s diffusion model of RT to distinguish different components of RT for two-choice decisions (Ratcliff, 1979; Ratcliff and McKoon, 2008; Ratcliff et al., 2016). This model (Figure 2) assumes that the accumulation of information toward one of two decision boundaries is a diffusion process and that the decisional and nondecisional components of RT can be estimated from the distributions of RT for correct and incorrect responses. We used a reduced version of the model, the EZ Diffusion Model (Wagenmakers et al., 2008; Wagenmakers et al., 2007), which estimates the RT components from mean RT for correct decisions (MRT), variance of response times for correct decisions (VRT), and proportion of correct responses (Pc). The estimated components are: drift rate, which is the rate at which information is accumulated to make a decision; nondecision time, which is the time for processes not considered part of the decision, such as perceptual processing/motor speed; and boundary separation, which is the amount of information required for a decision, or cautiousness. The EZ Diffusion Model does not estimate all of the parameters associated with the full model; however, the drift rate, nondecision time, and boundary separation parameters were of central interest to the present analyses, and this model is appropriate for data with relatively few error trials (Dutilh and Donkin, 2016; Siciliano et al., 2017; van Ravenzwaaij and Oberauer, 2009; Voss et al., 2013; Wagenmakers et al., 2007), as in the present experiment. We ensured that the EZ Diffusion Model was appropriate for our data by checking the shape of the RT distributions and whether the starting point was unbiased. For more details on how these parameters were estimated, see the Supplementary Methods.
Figure 2. Diffusion model of reaction time.
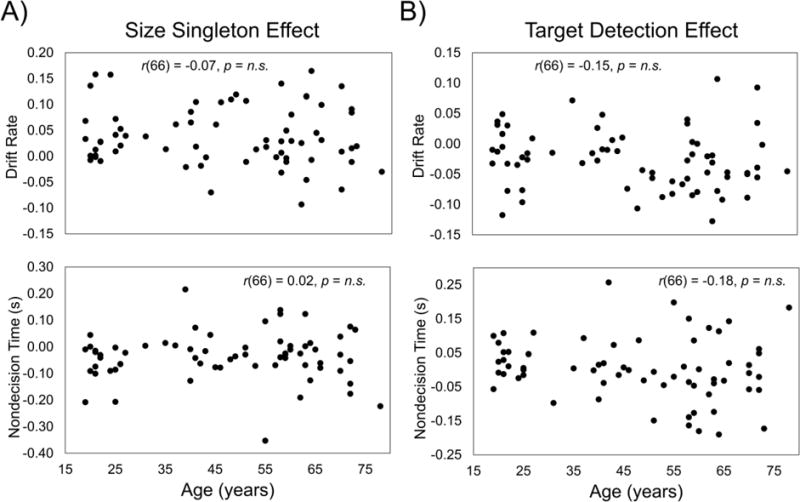
The diffusion model of two-choice RT developed by Ratcliff and colleagues (Ratcliff, 1979; Ratcliff and McKoon, 2008; Ratcliff et al., 2016) uses information from each participant’s RT distributions for correct and incorrect responses, to differentiate several components of the decision process. The model yields quantitative estimates of the following components: drift rate (v), the rate at which information is accumulated to make a decision; nondecision time (Ter), the time spent on nondecision processes such as perceptual processing/motor speed; and boundary separation (a), the amount of information required for a decision, or cautiousness. z = the starting point of the decision.
3.0 RESULTS
3.1 Behavioral Components Underlying Visual Search
On the visual search task, participants achieved an average accuracy of 97.4% (SD = 2.3%). Our first question was is bottom-up attentional guidance related specifically to decision processes or instead to nondecision processes (e.g., perceptual processing/motor speed)? The mean correct RT, drift rate, and nondecision time for each trial type are presented in Table 2, along with boundary separation; note that boundary separation is estimated at the level of participants rather than trials because the diffusion model assumes that cautiousness is constant as long as speed-accuracy instructions are constant. As we were predominately interested in the diffusion model parameters, our analyses will focus on these measures.
Table 2.
Conjunction Visual Search Task Performance
| Trial Type (s) | RT (ms) Boundary Separation |
Accuracy | Drift Rate | Nondecision Time |
|---|---|---|---|---|
| Nonsingleton target | 885 (143) | 0.970 (0.030) | 0.246 (0.047) | 0.556 (0.081) |
| – | ||||
| Singleton target | 850 (145) | 0.980 (0.0267) | 0.285 (0.071) | 0.520 (0.095) |
| – | ||||
| Target absent | 852 (127) | 0.977 (0.027) | 0.272 (0.054) | 0.552 (0.084) |
| – | ||||
| Across all conditions | 865 (129) | 0.974 (0.023) | 0.253 (0.043) | 0.550 (0.068) |
| 0.162 (0.068) |
Note. Values represent M (SD).
For the size singleton effects, consistent with our prediction, participants exhibited higher drift rates, t(67) = 5.62, p < .0001, and lower nondecision times, t(67) = 3.37, p = .0012, for singleton targets relative to nonsingleton targets, indicating that bottom-up attentional guidance is associated with both (a) an increased rate of information accumulation toward a decision and (b) a reduction in the time required for nondecision-related processes. We also examined target detection effects and found that participants exhibited lower drift rates, t(67) = 4.39, p < .0001, but similar nondecision times, t(67) = 0.44, p = .66, for nonsingleton target-present trials (relative to target-absent trials), indicating slower decisional processing but similar processing time for nondecision components, respectively, when the target was present. In sum, we demonstrated a size singleton effect within both drift rate and nondecision time, and a target detection effect within drift rate.
3.2 Behavioral Components Underlying Visual Search in Aging
Our second question was how do the behavioral components underlying the size singleton and target detection effects vary with age? We found that age did not correlate with the drift rate/nondecision time size singleton and target detection effects (Figure 3), the absolute values of r(66) were < 0.19, p > .13 (uncorrected). We also examined the relation between age and the diffusion parameters for each of the three trial types; we used a corrected alpha level of .05/3 = .0167. Age did not correlate with drift rate in any of the three trial types; the absolute value of r(66) was < 0.26, p > .032 (uncorrected). We did find, however, that increased age was associated with increased nondecision time within the target absent trials, r(66) = 0.41, p = .0005 (uncorrected); for the other two trial types, the absolute values of r(66) were < 0.23, p > .060 (uncorrected). Boundary separation, estimated across all of the trials, did not correlate significantly with age, r(66) = 0.16, p = .18 (Supplementary Figure 1).
Figure 3. Drift rate and nondecision time size singleton and target detection effects in aging.
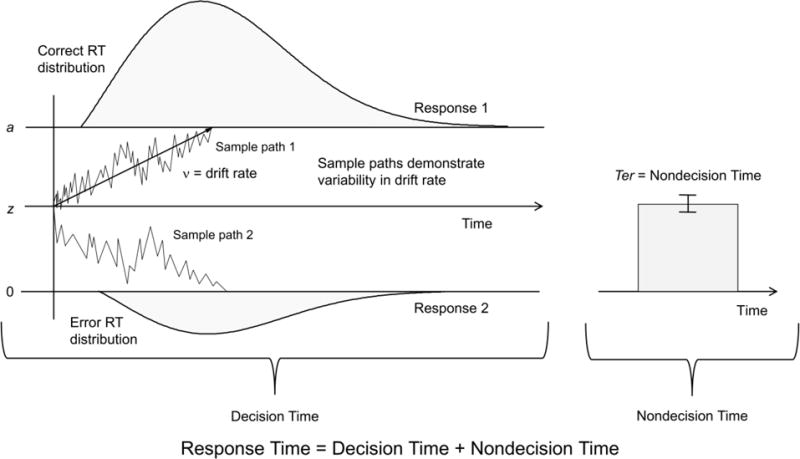
We conducted correlations between age and the drift rate/nondecision size singleton (singleton target – nonsingleton target) and target detection (nonsingleton target – target absent) effects. Age did not correlate with either effects, all p-values > .05.
3.3 Relation between Module Centrality and Task Performance
Our third question was how are the size singleton and target detection effects, in drift rate and nondecision time, related to centrality of the modules underlying visual attention? To address this question, we first determined whether the nonsingleton target network contained a frontoparietal module. The modularity algorithm revealed that the nonsingleton target network contained five modules (Figure 4). Consistent with our prediction, one of the modules was comprised predominantly of frontoparietal regions. Four other modules comprised of regions that we categorized as sensorimotor, visual, subcortical, and anterior temporal/ventral prefrontal cortex (PFC) modules. These were used as modules of interest for all subsequent analyses.
Figure 4. Visual attention modularity analysis.
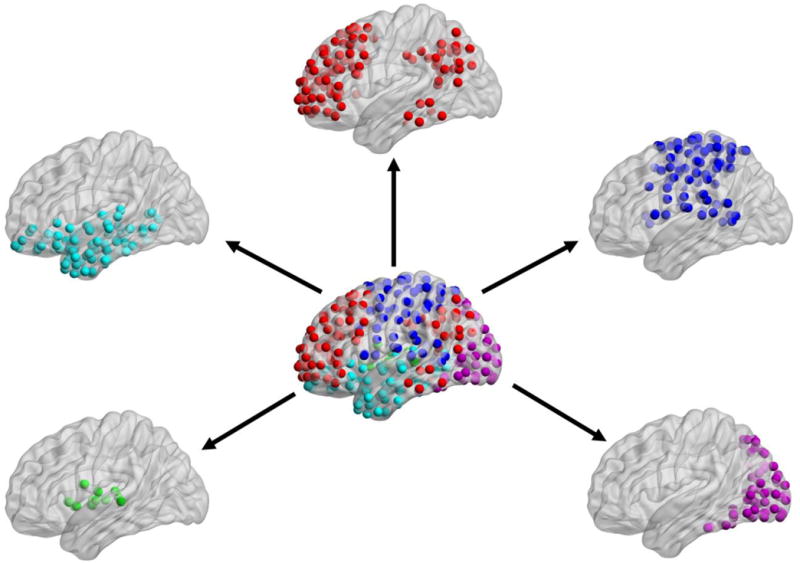
To determine the modular architecture underlying visual attention, we conducted a modularity analysis on the nonsingleton target network. The modularity algorithm revealed that this network contained five modules, which we named the frontoparietal (red, top-center), sensorimotor (dark blue, right-top), visual (pink, right-bottom), subcortical (green, left-bottom), and anterior temporal/ventral PFC (light blue, left-top) modules. Spheres of the same color represent nodes from the same module. PFC = prefrontal cortex.
To confirm that the nonsingleton target network exhibited modular properties, we ran the modularity algorithm 1,000 times, and then compared the Q values from the randomized vs. non-randomized networks; randomized networks were constructed via a rewiring algorithm (Rubinov and Sporns, 2010), which preserved the overall density of the networks. The non-randomized network contained higher Q values than the randomized network, t-test p < .0001, confirming that the nonsingleton target network exhibited modular proprieties. In addition, since we used modules derived from the nonsingleton target network to examine network properties within the singleton target and target absent networks, we ran the modularity algorithm on these other two networks. Qualitatively, all three networks exhibited similar community partitions (Supplementary Figure 2).
Since we confirmed that the nonsingleton target network contained a frontoparietal module, we were able to address our third question. For each of the modules within each of the three networks, we calculated degree and PageRank centrality, and then calculated size singleton (singleton target – nonsingleton target) and target detection (nonsingleton target – target absent) effects within each module. We then conducted stepwise regressions (criterion for entering model p < .05, criterion for leaving model p > .10) for each centrality measure and behavioral measure (size singleton and target detection effects), in which the behavioral measure was the outcome variable and the predictor variables were centrality for each of the modules. The stepwise regressions were conducted separately for the size singleton and target detection effects. To correct for multiple comparisons, we used a false discovery rate procedure (Benjamini and Hochberg, 1995); this procedure was conducted separately for the size singleton and target detection effects, and only stepwise regressions that allowed variables to enter the model were included.
3.3.1 Size Singleton Effects
For degree centrality (Figure 5A), the frontoparietal module was the only significant predictor of the drift rate-size singleton effect, F(1, 66) = 9.49, p = .0030 (uncorrected), reflecting an increased magnitude of the drift rate-size singleton effect (i.e., faster rate of information accumulation when the target was the size singleton) as a function of increasing degree centrality in the frontoparietal module. The stepwise regression with the nondecision time-size singleton effect did not yield any of the modules as significant predictors.
Figure 5. Relation between module centrality and behavioral size singleton effects.
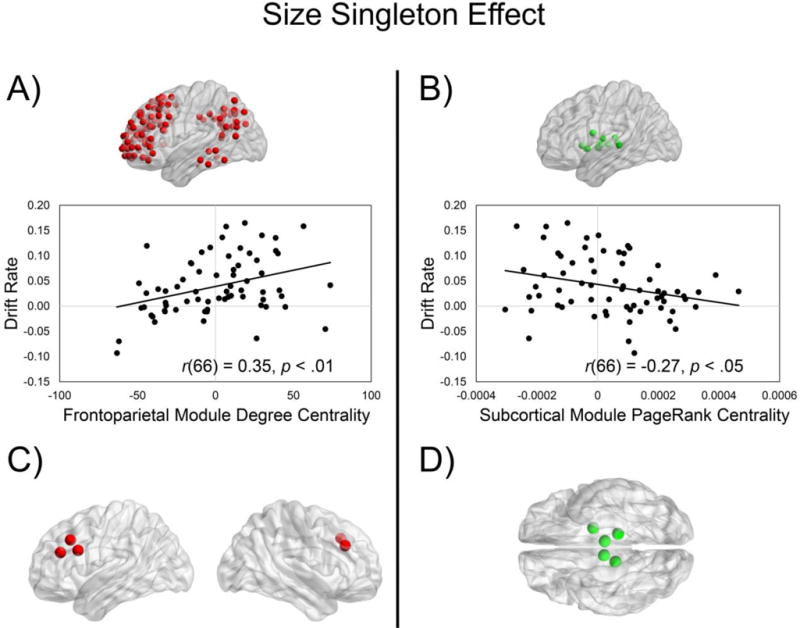
We examined the relation between the module centrality and drift rate/nondecision time size singleton effects (singleton target – nonsingleton target). We found that increased magnitude of the drift rate-size singleton effect was associated with (A) increased frontoparietal module degree centrality (size singleton effect) and (B) decreased subcortical module PageRank centrality (size singleton effect). Exploratory analyses revealed that within the frontoparietal module, the ROIs with the strongest drift rate-degree centrality (size singleton effects) were predominately within the middle frontal gyrus (C). Exploratory analyses also revealed that within the subcortical module, the ROIs with the strongest drift rate-PageRank centrality (size singleton effects) were within left pallidum and bilateral thalamus (D).
For PageRank centrality (Figure 5B), the subcortical module was the only significant predictor of the drift rate-size singleton effect, F(1, 66) = 5.35, p = .0238 (uncorrected), reflecting an increased magnitude of the drift rate-size singleton effect (i.e., faster rate of information accumulation when the target was the size singleton) as a function of decreasing PageRank centrality in the subcortical module. The stepwise regression with the nondecision time-size singleton effect did not yield any of the modules as significant predictors.
To further explore these analyses, we examined the five ROIs contained within the frontoparietal (degree centrality) and subcortical (PageRank centrality) modules with the strongest relations to the drift rate size singleton effect. For the frontoparietal module (degree centrality)-drift rate size singleton relation, we found that the ROIs with the strongest relations were contained within bilateral middle frontal gyrus, left inferior frontal gyrus, and left paracingulate gyrus (Figure 5C; Supplementary Table 2). For the subcortical module (PageRank centrality)-drift rate size singleton relation, we found that the ROIs with the strongest relations were contained within the left pallidum and bilateral thalamus (Figure 5D; Supplementary Table 3).
3.3.2 Target Detection Effects
For the target detection effects, we found that for PageRank centrality, the frontoparietal module was the only significant predictor of the nondecision time-target detection effect, F(1, 66) = 5.07, p = .0277 (uncorrected), reflecting an increased magnitude of the nondecision time-target detection effect (i.e., greater time processing nondecision components) as a function of increasing PageRank centrality in the frontoparietal module (Figure 6A). The stepwise regression with PageRank centrality predicting drift rate-target detection effect did not find any of the modules as significant predictors. Also, we did not find that degree centrality-target detection effects in any of the modules significantly predicted the drift rate/nondecision time-target detection effect.
Figure 6. Relation between module centrality and behavioral target detection effects.
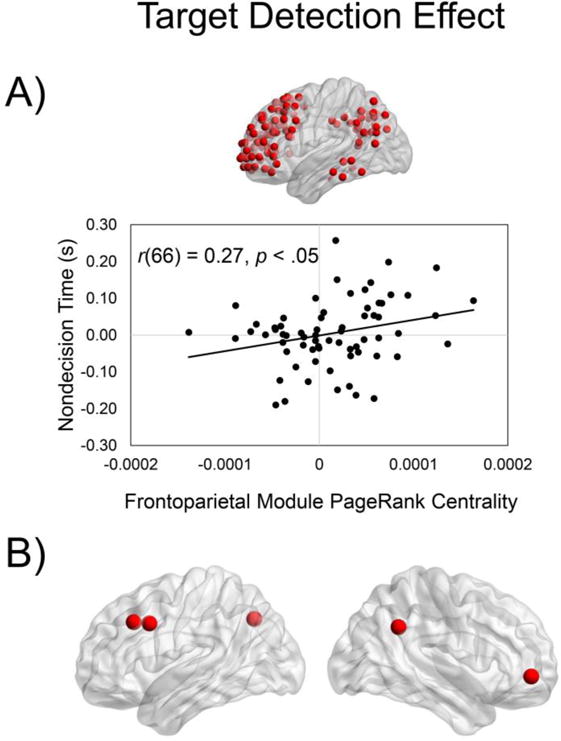
We examined the relation between the module centrality and drift rate/nondecision time target detection effects (nonsingleton – target absent). We found that increased magnitude of the nondecsion time-target detection effect was associated with increased frontoparietal module PageRank centrality target detection effect (A). Exploratory analyses revealed that within the frontoparietal module, the ROIs with the strongest nondecision time-PageRank centrality (target detection effects) were within right angular gyrus, right frontal pole, left middle frontal gyrus, left paracingulate gyrus, and left superior, lateral occipital cortex (B).
To further explore this analysis, we examined the five ROIs contained within the frontoparietal module (PageRank centrality) with the strongest relations to the nondecision time-target detection effect. We found that the ROIs with the strongest relations were contained within the right angular gyrus, right frontal pole, left middle frontal gyrus, and left superior, lateral occipital cortex (Figure 6B; Supplementary Table 4).
3.4 Age-Related Differences in Modular Architecture Underlying Visual Attention
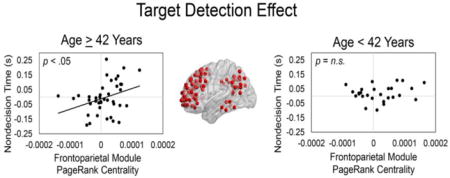
Our fourth question was how does the relation between drift rate/nondecision time and module network properties, for the size singleton and target detection effects, vary with age? To address this question, for each of the modules that significantly predicted either the drift rate/nondecision time size singleton or target detection effects, we conducted additional linear regressions to examine whether the Age × Module Centrality (size singleton or target detection effect) interactions significantly predicted drift rate/nondecision time (size singleton or target detection effect). Only the Age × Frontoparietal Module PageRank Centrality-Target Detection Effect interaction was trending toward predicting the nondecision time-target detection effect, β = 19.6, t(64) = 2.00, p = .05. To examine if whether PageRank centrality-target detection effect transitioned from a nonsignificant to significant predictor of the nondecision time-target detection effect at a specific age, we used the Johnson-Neyman technique (Bauer and Curran, 2005; Johnson and Fay, 1950), as implemented in the PROCESS macro for SAS (Hayes, 2013). We found that the frontoparietal module PageRank centrality-target detection effect transitioned from a nonsignificant to significant predictor of the nondecision time-target detection effect, p < .05, at approximately 42 years of age (Figure 7 A & B; Supplementary Table 5).
Figure 7. Age effects on modular architecture-task performance relations.
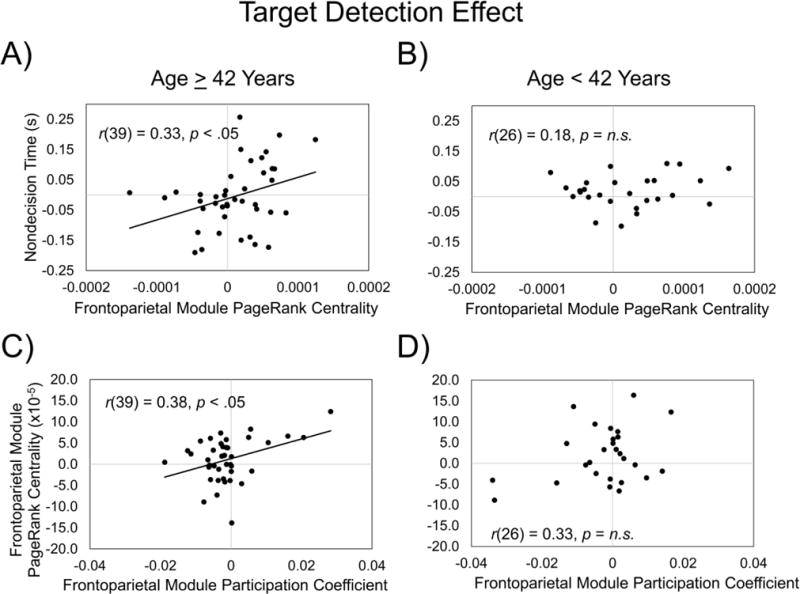
We further explored the Age × Frontoparietal Module PageRank Centrality-Target Detection Effect interaction, and found that the frontoparietal module PageRank centrality-target detection effect transitioned from a nonsignificant to significant predictor of the nondecision time-target detection effect (p < .05) at approximately 42 years of age (A & B). To further investigate this finding, we examined the relation between frontoparietal module PageRank centrality and participation coefficient target detection effects. We found that within participants 42 years or older (C), but not younger than 42 years (D), increased magnitude of the PageRank centrality-target detection effect was associated with increased between-module connections (i.e., higher participation coefficients) in the frontoparietal module.
Because only PageRank centrality, and not degree centrality, was associated with the age effects, this suggests that the frontoparietal module became more central to the network, in service of the target detection effect, by relying more on highly central nodes in other modules. Therefore, we examined in participants 42 years of age and older and participants younger than 42 years of age if the frontoparietal PageRank centrality target-detection effect was related to the relative strength of between- to within-frontoparietal module functional connections (i.e., participation coefficients; Guimera and Amaral, 2005). Indeed, within participants 42 years of age and older, the participation coefficient-target detection effect in the frontoparietal module was associated with the PageRank centrality-target detection effect in the frontoparietal module, r(39) = 0.38, p = .014 (Figure 7C), but not in individuals younger than 42 years of age, r(25) = 0.33, p = .10 (Figure 7D); however, a direct comparison of the correlation coefficients for these two groups was not statistically significant, z = 0.22, p = .83, Cohen’s q = 0.057. Thus, within the individuals 42 years of age and older, an increased magnitude of the PageRank centrality-target detection effect was associated with increased strength of between-module connections in the frontoparietal module.
4.0 DISCUSSION
The overall goal of the current study was to investigate age-related differences in the interaction of top-down and bottom-up attentional processes during conjunction search. We took a novel approach to address this issue with our use of diffusion modeling and multivariate functional connectivity. This study yielded four main findings. First, the size singleton and target detection effects were related to specific RT components. Second, although age was associated with an overall slowing of nondecision time (on target-absent trials), the drift rate/nondecision time-size singleton and -target detection effects did not vary with age. Third, the RT component effects were associated with module centrality: increased magnitude of the drift rate-size singleton effect was associated with increased frontoparietal degree centrality but decreased subcortical PageRank centrality; and increased magnitude of the nondecision time-target detection effect was associated with increased frontoparietal PageRank centrality. Fourth, at approximately 42 years of age, we observed a specific transition to greater dependence on between-module connections in service of the target detection effect. These findings are discussed in greater detail below.
4.1 Behavioral Components Underlying Effects of Interest
Our first question sought to examine if bottom-up attention guidance was related specifically to decision processes or instead to nondecision processes (e.g., perceptual processing/motor response). For the size singleton effect, we found that a more salient target was associated with an increased accumulation rate of target-relevant information (drift rate) and decreased time on nondecision processes (nondecision time), relative to a nonsingleton target. Thus, the non-informative feature of size provided a relatively broad form of bottom-up guidance for target detection, affecting both decision and nondecision processes. For the target detection effect, in contrast, the nonsingleton target trials (relative to target-absent trials) exhibited a decreased drift rate but no difference in nondecision time. The target detection effect was more top-down in nature, as no bottom-up featural information distinguished the nonsingleton target trials and target-absent trials. This effect may represent the time required to shift attention away from the more salient, nontarget size singleton, to confirm that another one of the nonsingleton display items was the target. Consistent with this interpretation, the target detection effect was specific to drift rate and not evident in nondecision time.
Only one previous study, to our knowledge, has fitted a diffusion model to a conjunction visual search task (Ward and McClelland, 1989), in which it was found that manipulating different properties of the display (e.g., number of targets, display size) varied different diffusion parameters, such as boundary separation; however, this study was limited in that they did not examine nondecision time. Although the questions of interests and design of Ward and McClelland (1989) was different from the current study, our finding extends this previous study by demonstrating that the target detection effect for conjunction visual search is related to the rate of information accumulation rather than nondecision processes.
4.2 Behavioral Components Underlying Effects of Interest in Aging
Our second question was what are the potential age-related differences in the decisional and nondecisional components of the size singleton and target detection effects? We found that the size singleton and target detection effects, for both drift rate and nondecision time, did not vary significantly with age (Figure 3), but we observed an age-related increase in overall nondecision time (though limited to the target-absent trials). This is consistent with previous studies indicating that increased age is associated with a decline in many measures of perceptual processing/motor speed (Monge and Madden, 2016; Salthouse, 2000), including an increase with age of the diffusion model measure of nondecision time (e.g., Ratcliff, 2008; Ratcliff et al., 2006).
For the size singleton effect, we hypothesized that relatively older adults would exhibit greater benefits from bottom-up guidance due to evidence indicating that deficient bottom-up processes in older adults may directly cause some cognitive deficiencies (for a review, see Monge and Madden, 2016). The age-related constancy of the effects in Figure 3 did not support this hypothesis. As noted in the Introduction, Proulx (2007) viewed the size singleton effect as an interactive influence of bottom-up and top-down effects, and thus the present effects are not purely bottom-up. As top-down attention stays relatively preserved with age (e.g., Madden et al., 2004; McAvinue et al., 2012), perhaps this finding indicates that the relatively older adults still relied heavily on top-down processes in service of the size singleton effect. It is also possible that that an age-related decline in bottom-up processing may not be apparent until stimulus presentation is closer to a sensory threshold, which future studies could determine by varying the relative size of the nonsingleton to singleton display items. The target detection effect, reflecting primarily top-down detection and response selection processes was also constant with age, which is consistent with previous literature indicating that top-down attentional control is preserved with age.
4.3 Relation between Module Centrality and Task Performance
Our third question examined if the drift rate/nondecision time-size singleton and -target detection effects are related to properties of the whole-brain functional networks underlying visual attention. The modularity analysis on the nonsingleton target network, consistent with our prediction, revealed that this network contained a frontoparietal module along with a few other modules (Figure 4). Although traditionally frontoparietal regions underlying visual attention have been divided into dorsal components, subserving top-down attentional control, and ventral components, subserving bottom-up attentional control (e.g., Corbetta and Shulman, 2002), the dorsal and ventral components of the frontoparietal network are highly interconnected (Egner et al., 2008; Monge et al., 2016; Treue, 2003; Vossel et al., 2014). Therefore, it is not surprising that our frontoparietal module contained both dorsal and ventral frontoparietal regions. It should also be noted that some work suggests that this top-down vs. bottom-up dichotomy is not as robust as historically viewed (for a review, see Awh et al., 2012); perhaps the frontoparietal module containing both dorsal and ventral components is a reflection of this.
In the examination of the relation between size singleton effects in module centrality and the behavioral measures, we found that increased magnitude of the drift rate-size singleton effect (i.e., greater bottom-up guidance) was associated with increased degree centrality within the frontoparietal module (Figure 5A). This is partially inconsistent with our hypothesis that increased magnitude of the drift rate-size singleton effect would be associated with decreased centrality within the frontoparietal module. Frontoparietal regions have previously been hypothesized to contain a visual salience map (Jerde et al., 2012; Ptak, 2012; Serences and Yantis, 2007), which are derived from top-down and bottom-up inputs and encode the relative salience of stimuli (Bichot and Schall, 1999; Motter and Belky, 1998; Thompson et al., 2005). Perhaps our finding reflects a larger “peak” in this frontoparietal salience map with greater bottom-up guidance.
In our exploratory analysis, we found that nodes predominantly located within the middle frontal gyrus had the strongest degree centrality-drift rate size singleton effect relations (Figure 5C). Previous work has indicated that the middle frontal gyrus may link the dorsal and ventral attention networks by acting as a “circuit breaker” (Fox et al., 2006; Japee et al., 2015), perhaps indicating the importance of interactions between dorsal and ventral frontoparietal regions in service of the size singleton effect.
Also for the size singleton effect, we found that increased magnitude of the drift rate-size singleton effect was associated with decreased PageRank centrality in the subcortical module (Figure 5B). Since only subcortical PageRank centrality, and not degree centrality, was associated with the size singleton effect, this indicates that subcortical regions became less central to the network via decreased reliance on highly central nodes in other modules. Although we did not originally hypothesize that subcortical regions would be associated with the size singleton effect, this may indicate that less sensory integration is necessary with increased bottom-up guidance (Brown et al., 1997; Lidsky et al., 1985).
Lastly, for the size singleton effect, even though we were able to demonstrate the size singleton effect within nondecision time (Table 2), we did not find that the nondecision time-size singleton effect was associated with module centrality. These null findings indicate that our brain-behavior results were selective to the decision, and not nondecision processes underlying the size singleton effect.
For the target detection effect, we found that increased magnitude of the nondecision time-target detection effect was associated with increased PageRank centrality within the frontoparietal module (Figure 6A). Overall, we were not able to demonstrate the target detection effect in nondecision time (Table 2), indicating that nondecision time appears to be important for the target detection effect only in the examination of individual differences. This work extends a large literature, including Madden et al. (2017), indicating the importance of frontoparietal regions during target detection by demonstrating that nondecision processes may be associated with frontoparietal processing. Also, since only frontoparietal PageRank centrality, and not degree centrality, was associated with the target detection effect, this indicates that frontoparietal regions became more central to the network by increasing their reliance on highly central nodes within other modules.
In our exploratory analysis of the target detection effect, we found that nodes predominately located within the ventral PFC, ventral parietal cortex, and a visual processing related region had the strongest PageRank centrality-nondecision time target detection effect relations (Figure 6B). As these relations were only with the nondecision component, it is not surprising that regions typically associated with bottom-up attentional control (e.g., Corbetta and Shulman, 2002; Miller and Buschman, 2013; Noudoost et al., 2010) exhibited the strongest relations. These findings further suggest that even during target detection, which is thought to be associated more with top-down attentional control, bottom-up processes also appear to be important and modulate frontoparietal centrality.
4.4 Age-Related Differences in Modular Architecture Underlying Visual Attention
Our fourth question sought to examine how the relations between drift rate/nondecision time and module centrality, for the size singleton and target detection effects, vary with age. We found that the Age × Frontoparietal Module PageRank Centrality-Target Detection Effect interaction trended toward predicting the nondecision time-target detection effect, and that the frontoparietal PageRank centrality-target detection effect transitioned from a nonsignificant to significant predictor of the nondecision time-target detection effect at approximately 42 years of age (Figure 7A vs. 7B). This supports previous behavioral studies demonstrating that perceptual/motor deficiencies within older adults may impact top-down, cognitive processes (for a review, see Monge and Madden, 2016), and extends these studies by demonstrating that a nondecision component may affect frontoparietal, top-down processing and that this transition begins to occur within middle-age. Also, since this relation was only significant with PageRank centrality, this suggests that frontoparietal regions become central to the network in service of the target detection effect by becoming more reliant on between-module connections, which we further confirmed by examining the relation between target detection effects in PageRank centrality and participation coefficients (Figure 7C vs. Figure 7D).
Furthermore, these findings extend previous univariate activation studies showing that older (vs. younger) adults exhibit greater frontoparietal activity in service of visual search target detection (e.g., Allen and Payne, 2012; Madden et al., 2005a), by demonstrating that in middle-aged and older adults, frontoparietal regions become central to the network by exhibiting more between-module connections. We also improve upon limitations of past studies examining the functional modular topology of the aging brain (e.g., Chan et al., 2014; Gallen et al., 2016; Grady et al., 2016; Schlesinger et al., 2017) by demonstrating that (a) functional modular properties during task undergo changes at approximately 42 years of age, (b) these changes are reflected in middle aged and older adults becoming more reliant on between-module connections in service of cognition, and (c) functional modular properties are associated with visual attention in aging.
Madden et al. (2017) found that the Age × Frontoparietal Activity predicting RT was only significant for the size singleton effect rather than the target detection effect found here. This may be due to our use of diffusion parameters, which examine the components underlying RT. As drift rate is associated with the decision (vs. nondecision), and we found that this component did not vary with age, it may be that nondecision components are relatively more important for visual attention in aging. Perhaps in Madden et al., the RT size singleton effect was being driven by the nondecision component. However, similar to the current study, Madden et al. also found an age-related transition point, where the frontoparietal-RT size singleton effect shifted from statistically nonsignificant to significant in middle-aged adults. Our results extend these findings by demonstrating possible mechanisms underlying these age-related changes in brain activation, where changes in modular topology may underlie this shift.
4.5 Limitations
Although we believe this study advances our knowledge of mechanisms underlying visual attention in aging, it does contain a few limitations. First, it is possible that the study sample included individuals with preclinical neurodegenerative disease. Although we included a standardized neuropsychological assessment to reduce the probability of including these individuals, it may be the case that our neuropsychological assessment did not detect individuals with preclinical neurodegenerative disease. Also, it is possible that some participants exhibited a cognitively normal phenotype, but contained neurodegenerative pathology. This could be revealed by a positron emission tomography tracer, such as the Pittsburgh Compound-B (Klunk et al., 2004), which was not included in our protocol. Second, some researchers have brought into question the validity of calculating centrality based on functional connectivity matrices (Power et al., 2013). Although this issue has only been previously examined within the context of resting-state fMRI and identifying network hubs, further work examining the use of graph metrics based on task-based fMRI connectivity matrices is needed. Third, within our connectivity matrices, negative values were set to zero in order to estimate PageRank centrality. Although this is a widely used technique within the graph theory-fMRI literature (e.g., Chiang et al., 2016; Liao et al., 2017; Reineberg and Banich, 2016; Wang et al., 2017), it is possible that these negative correlation values contained information related to cognition.
4.6 Conclusions
In sum, we used diffusion modeling and multivariate functional connectivity to investigate the behavioral components and whole-brain functional networks, respectively, underlying bottom-up and top-down attentional control during conjunction search in healthy aging. We found that (a) the size singleton effect (i.e., increased bottom-up guidance) was associated with higher drift rate and lower nondecision time, whereas the target detection effect was associated only with lower drift rate; (b) the drift rate/nondecision time-size singleton and -target detection effects did not vary with age; (c) increased magnitude of the drift rate-size singleton effect was associated with increased frontoparietal centrality but decreased subcortical centrality, and increased magnitude of the nondecision time-target detection effect was associated with increased frontoparietal centrality; and (d) the neural mechanisms underlying the target detection effect varied with age, where participants 42 years of age and older exhibited a greater dependence on between-frontoparietal module connections in service of target detection. These results further elucidate mechanisms through which frontoparietal regions support attentional control and how these mechanisms vary in relation to adult age.
Supplementary Material
HIGHLIGHTS.
Bottom-up processes are important for conjunction visual search.
Bottom-up guidance influences both decision and nondecision behavioral processes.
Bottom-up guidance influences frontoparietal and subcortical connectivity.
Nondecision processes influence top-down, frontoparietal connectivity.
Adults 42 years and older utilize different top-down, frontoparietal mechanisms.
Acknowledgments
This research was supported by the National Institutes of Health research grant R01-AG039684 (DJM). The funding agency had no role in the decision to publish or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There was a third study that examined modularity in aging using task-based functional connectivity, but the goal of this study was to estimate the intrinsic (resting-state) component of task-related connectivity and thus task-related connectivity effects were regressed out (Grady et al., 2016).
The authors do not have any conflicts of interest to report.
References
- Allen HA, Payne H. Similar behaviour, different brain patterns: age-related changes in neural signatures of ignoring. Neuroimage. 2012;59:4113–4125. doi: 10.1016/j.neuroimage.2011.10.070. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity test—automatic measurement of visual acuity. Optom Vis Sci. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Bacon WJ, Egeth HE. Goal-directed guidance of attention: Evidence from conjunctive visual search. J Exp Psychol Hum Percept Perform. 1997;23:948–961. doi: 10.1037//0096-1523.23.4.948. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Probing interactions in fixed and multilevel regression: Inferential and graphical techniques. Multivariate Behav Res. 2005;40:373–400. doi: 10.1207/s15327906mbr4003_5. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Beck depression inventory. Psychological Corporation; New York: 1978. [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, Sporns O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 2014;102(Part 2):345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Saccade target selection in macaque during feature and conjunction visual search. Vis Neurosci. 1999;16:81–89. doi: 10.1017/s0952523899161042. [DOI] [PubMed] [Google Scholar]
- Blondel VD, Guillaume JL, Hendrickx JM, de Kerchove C, Lambiotte R. Local leaders in random networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:036114. doi: 10.1103/PhysRevE.77.036114. [DOI] [PubMed] [Google Scholar]
- Boldi P, Santini M, Vigna S. PageRank: functional dependencies. ACM Transactions on Information Systems. 2009;27:1–23. [Google Scholar]
- Brandes U, Delling D, Gaertler M, Goerke R, Hoefer M, Nikoloski Z, Wagner D. Maximizing modularity is hard. arXiv:physics. 2006:0608255. [Google Scholar]
- Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–163. doi: 10.1016/s0959-4388(97)80003-7. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Campbell KL, Schacter DL. Ageing and the resting state: is cognition obsolete? Lang Cogn Neurosci. 2016:1–8. doi: 10.1080/23273798.2016.1227858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, Song XW, Xia MR, Shu N, Dong Q, Milham MP, Castellanos FX, Zuo XN, He Y. Topological organization of the human brain functional connectome across the lifespan. Develop Cogn Neurosci. 2014;7:76–93. doi: 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proc Nat Acad Sci U S A. 2014;111:E4997–E5006. doi: 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S, Cassese A, Guindani M, Vannucci M, Yeh HJ, Haneef Z, Stern JM. Time-dependence of graph theory metrics in functional connectivity analysis. Neuroimage. 2016;125:601–615. doi: 10.1016/j.neuroimage.2015.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1052. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, D’Esposito M. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 2016;36:12083–12094. doi: 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S. Visual attention: Bottom-up versus top-down. Curr Biol. 2004;14:R850–852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Controls of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–214. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vértes PE, Winton-Brown TT, Patel AX, Ginestet CE, McGuire P, Bullmore ET. Cognitive relevance of the community structure of the human brain functional coactivation network. Proc Natl Acad Sci U S A. 2013;110:11583–11588. doi: 10.1073/pnas.1220826110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Stanley ML, Moscovitch M, Cabeza R. Resting-state networks do not determine cognitive function networks: a commentary on Campbell and Schacter 2016. Lang Cogn Neurosci. 2016:1–5. doi: 10.1080/23273798.2016.1252847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilh G, Donkin C. The quality of response time data inference: a blinded, collaborative assessment of the validity of cognitive models. 2016 doi: 10.3758/s13423-017-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorine I. Dvorine pseudo-isochromatic plates. 2nd. Harcourt; New York: 1963. [Google Scholar]
- Eckstein MP. Visual search: A retrospective. J Vis. 2011;11:1–36. doi: 10.1167/11.5.14. [DOI] [PubMed] [Google Scholar]
- Egner T, Monti JM, Trittschuh EH, Wieneke CA, Hirsch J, Mesulam MM. Neural integration of top-down spatial and feature-based information in visual search. J Neurosci. 2008;28:6141–6151. doi: 10.1523/JNEUROSCI.1262-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler LT, Sherzai A, Kaup AR, Jeste DV. A review of functional brain imaging correlates of successful cognitive aging. Biol Psychiatry. 2011;70:115–122. doi: 10.1016/j.biopsych.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011a;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Bassett DS, Meunier D, Ellison-Wright I, Yucel M, Wood SJ, Shaw K, O’Connor J, Nertney D, Mowry BJ, Pantelis C, Bullmore ET. Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci. 2011b;31:3261–3270. doi: 10.1523/JNEUROSCI.4858-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Nat Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallen CL, Turner GR, Adnan A, D’Esposito M. Reconfiguration of brain network architecture to support executive control in aging. Neurobiol Aging. 2016;44:42–52. doi: 10.1016/j.neurobiolaging.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geib BR, Stanley ML, Dennis NA, Woldorff MG, Cabeza R. From hippocampus to whole-brain: the role of integrative processing in episodic memory retrieval. Hum Brain Mapp. 2017 doi: 10.1002/hbm.23518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geib BR, Stanley ML, Wing EA, Laurienti PJ, Cabeza R. Hippocampal contributions to the large-scale episodic memory network predict vivid visual memories. Cereb Cortex. 2015:1–14. doi: 10.1093/cercor/bhv272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Sarraf S, Saverino C, Campbell K. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 2016;41:159–172. doi: 10.1016/j.neurobiolaging.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimera R, Amaral LA. Cartography of complex networks: Modules and universal roles. J Stat Mech. 2005;2005 doi: 10.1088/1742-5468/2005/02/P02001. nihpa35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis. Guilford; New York: 2013. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual search across the life span. Dev Psychol. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Sys Neurosci. 2015;9 doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE. Prioritized maps of space in human frontoparietal cortex. J Neurosci. 2012;32:17382–17390. doi: 10.1523/JNEUROSCI.3810-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, Fay LC. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15:349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Kaptein NA, Theeuwes J, van der Heijden AHC. Search for a conjunctively defined target can be selectively limited to a color-defined subset of elements. J Exp Psychol Hum Percept Perform. 1995;21:1053–1069. [Google Scholar]
- Khazaee A, Ebrahimzadeh A, Babajani-Feremi A. Application of advanced machine learning methods on resting-state fMRI network for identification of mild cognitive impairment and Alzheimer’s disease. Brain Imaging Behav. 2016;10:799–817. doi: 10.1007/s11682-015-9448-7. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kristjansson A, Campana G. Where perception meets memory: A review of repetition priming in visual search tasks. Atten Percept Psychophys. 2010;72:5–18. doi: 10.3758/APP.72.1.5. [DOI] [PubMed] [Google Scholar]
- Kristjansson A, Wang D, Nakayama K. The role of priming in conjunctive visual search. Cognition. 2002;85:37–52. doi: 10.1016/s0010-0277(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Liao X, Cao M, Xia M, He Y. Individual differences and time-varying features of modular brain architecture. Neuroimage. 2017;152:94–107. doi: 10.1016/j.neuroimage.2017.02.066. [DOI] [PubMed] [Google Scholar]
- Lidsky TI, Manetto C, Schneider JS. A consideration of sensory factors involved in motor functions of the basal ganglia. Brain Res Rev. 1985;9:133–146. doi: 10.1016/0165-0173(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Parks EL, Tallman CW, Boylan MA, Hoagey DA, Cocjin SB, Johnson MA, Chou Y-h, Potter GG, Chen N-k, Packard LE, Siciliano RE, Monge ZA, Diaz MT. Frontoparietal activation during visual conjunction search: effects of bottom-up guidance and adult age. Hum Brain Mapp. 2017;38:2128–2149. doi: 10.1002/hbm.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL. Age-related changes in visual attention. In: Costa PT, Siegler IC, editors. Recent advances in psychology and aging. Elsevier; Amsterdam: 2004. pp. 41–88. [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, Huettel SA. Age-related preservation of top-down attentional guidance during visual search. Psychol Aging. 2004;19:304–309. doi: 10.1037/0882-7974.19.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA. Age-related changes in neural activity during visual perception and attention. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: linking cognitive and cerebral aging. Oxford University Press; New York, New York: 2005a. pp. 157–185. [Google Scholar]
- Madden DJ, Whiting WL, Spaniol J, Bucur B. Adult age differences in the implicit and explicit components of top-down attentional guidance during visual search. Psychol Aging. 2005b;20:317–329. doi: 10.1037/0882-7974.20.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvinue LP, Habekost T, Johnson KA, Kyllingsbaek S, Vangkilde S, Bundesen C, Robertson IH. Sustained attention, attentional selectivity, and attentional capacity across the lifespan. Atten Percept Psychophys. 2012;74:1570–1582. doi: 10.3758/s13414-012-0352-6. [DOI] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr Opin Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić B, Sporns O. From regions to connections and networks: new bridges between brain and behavior. Curr Opin Neurobiol. 2016;40:1–7. doi: 10.1016/j.conb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge ZA, Greenwood PM, Parasuraman R, Strenziok M. Individual differences in reasoning and visuospatial attention are associated with prefrontal and parietal white matter tracts in healthy older adults. Neuropsychology. 2016;30:558–567. doi: 10.1037/neu0000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge ZA, Madden DJ. Linking cognitive and visual perceptual decline in healthy aging: the information degradation hypothesis. Neurosci Biobehav Rev. 2016;69:166–173. doi: 10.1016/j.neubiorev.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Breitling R, Higham DJ, Gilbert DR. GeneRank: using search engine technology for the analysis of microarray experiments. BMC Bioinformatics. 2005;6:233. doi: 10.1186/1471-2105-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC, Belky EJ. The guidance of eye movements during active visual search. Vis Res. 1998;38:1805–1815. doi: 10.1016/s0042-6989(97)00349-0. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr Opin Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, Yamaguchi S. Small-worldness and modularity of the resting-state functional brain network decrease with aging. Neurosci Lett. 2013;556:104–108. doi: 10.1016/j.neulet.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Power Jonathan D, Schlaggar Bradley L, Lessov-Schlaggar Christina N, Petersen Steven E. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx MJ. Bottom-up guidance in visual search for conjunctions. J Exp Psychol Hum Percept Perform. 2007;33:48–56. doi: 10.1037/0096-1523.33.1.48. [DOI] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain. The Neuroscientist. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Group reaction time distributions and an analysis of distribution statistics. Psychol Bul. 1979;86:446–461. [PubMed] [Google Scholar]
- Ratcliff R. Modeling aging effects on two-choice tasks: response signal and response time data. Psychol Aging. 2008;23:900–916. doi: 10.1037/a0013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Smith PL, Brown SD, McKoon G. Diffusion decision model: current issues and history. Trends Cogn Sci. 2016;20:260–281. doi: 10.1016/j.tics.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, Gomez P, McKoon G. A diffusion model analysis of the effects of aging in the lexical-decision task. Psychol Aging. 2004;19:278–289. doi: 10.1037/0882-7974.19.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. The effects of aging on reaction time in a signal detection task. Psychol Aging. 2001;16:323–341. [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. A diffusion model analysis of the effects of aging on brightness discrimination. Percept Psychophys. 2003;65:523–535. doi: 10.3758/bf03194580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. Aging and individual differences in rapid two-choice decisions. Psychon Bull Rev. 2006;13:626–635. doi: 10.3758/bf03193973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineberg AE, Banich MT. Functional connectivity at rest is sensitive to individual differences in executive function: a network analysis. Hum Brain Mapp. 2016;37:2959–2975. doi: 10.1002/hbm.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch MJ, Chechlacz M, Mevorach C, Mavritsaki E, Allen H, Humphreys GW. The neural mechanisms of visual selection: The view from neuropsychology. Ann N Y Acad Sci. 2010;1191:156–181. doi: 10.1111/j.1749-6632.2010.05448.x. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. doi: 10.1038/srep06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger KJ, Turner BO, Lopez BA, Miller MB, Carlson JM. Age-dependent changes in task-based modular organization of the human brain. Neuroimage. 2017;146:741–762. doi: 10.1016/j.neuroimage.2016.09.001. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. Implications of perceptual deterioration for cognitive aging research. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 2nd. Lawrence Erlbaum Associates Publishers; Mahwah, New Jersey, United States: 2000. p. 755. [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 2007;17:284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Corbetta M. Two cortical systems for the selection of visual stimuli. In: Posner MI, editor. Cognitive neuroscience of attention. The Guilford Press; New York, NY: 2004. pp. 114–126. [Google Scholar]
- Siciliano RE, Madden DJ, Tallman CW, Boylan MA, Kirste I, Monge ZA, Packard LE, Potter GG, Wang L. Task difficulty modulates brain activation in the emotional oddball task. Brain Research. 2017;1664:74–86. doi: 10.1016/j.brainres.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Song J, Birn RM, Boly M, Meier TB, Nair VA, Meyerand ME, Prabhakaran V. Age-related reorganizational changes in modularity and functional connectivity of human brain networks. Brain Connectivity. 2014;4:662–676. doi: 10.1089/brain.2014.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. J Exp Psychol Learn Mem Cogn. 2006;32:101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng NR, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Telesford QK, Simpson SL, Burdette JH, Hayasaka S, Laurienti PJ. The brain as a complex system: using network science as a tool for understanding the brain. Brain Connect. 2011;1:295–308. doi: 10.1089/brain.2011.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Ratcliff R, McKoon G. A diffusion model analysis of the effects of aging on letter discrimination. Psychol Aging. 2003;18:415–429. doi: 10.1037/0882-7974.18.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Features and objects: the fourteenth Bartlett memorial lecture. Q J Exp Psychol A. 1988;40A:201–237. doi: 10.1080/02724988843000104. [DOI] [PubMed] [Google Scholar]
- Treue S. Visual attention: The where, what, how and why of saliency. Curr Opin Neurobiol. 2003;13:428–432. doi: 10.1016/s0959-4388(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- van Ravenzwaaij D, Oberauer K. How to use the diffusion model: parameter recovery of three methods: EZ, fast-dm, and DMAT. J Math Psychol. 2009;53:463–473. [Google Scholar]
- Voss A, Nagler M, Lerche V. Diffusion models in experimental psychology: A practical introduction. Exp Psychol. 2013;60:385–402. doi: 10.1027/1618-3169/a000218. [DOI] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist. 2014;20:150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers EJ, van der Maas HL, Dolan CV, Grasman RP. EZ does it! Extensions of the EZ-diffusion model. Psychon Bull Rev. 2008;15:1229–1235. doi: 10.3758/PBR.15.6.1229. [DOI] [PubMed] [Google Scholar]
- Wagenmakers EJ, van der Maas HL, Grasman RP. An EZ-diffusion model for response time and accuracy. Psychon Bull Rev. 2007;14:3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Wang J, Ren Y, Hu X, Nguyen VT, Guo L, Han J, Guo CC. Test–retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Hum Brain Mapp. 2017;38:2226–2241. doi: 10.1002/hbm.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R, McClelland JL. Conjunctive search for one and two identical targets. J Exp Psychol Hum Percept Perform. 1989;15:664–672. doi: 10.1037//0096-1523.15.4.664. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Administration and scoring manual: Wechsler adult intelligence scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Whiting WL, Madden DJ, Pierce TW, Allen PA. Searching from the top down: ageing and attentional guidance during singleton detection. Q J Exp Psychol A. 2005;58:72–97. doi: 10.1080/02724980443000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998. pp. 13–73. [Google Scholar]
- Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bender AR, Raz N. Age related differences in reaction time components and diffusion properties of normal-appearing white matter in healthy adults. Neuropsychologia. 2015;66:246–258. doi: 10.1016/j.neuropsychologia.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S. Control of visual attention. In: Pashler H, editor. Attention. Psychology Press; East Sussex, UK: 1998. pp. 223–256. [Google Scholar]
- Yantis S. How visual salience wins the battle for awareness. Nat Neurosci. 2005;8:975–977. doi: 10.1038/nn0805-975. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Ehmke R, Mennes M, Imperati D, Castellanos FX, Sporns O, Milham MP. Network centrality in the human functional connectome. Cereb Cortex. 2012;22:1862–1875. doi: 10.1093/cercor/bhr269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


