Abstract
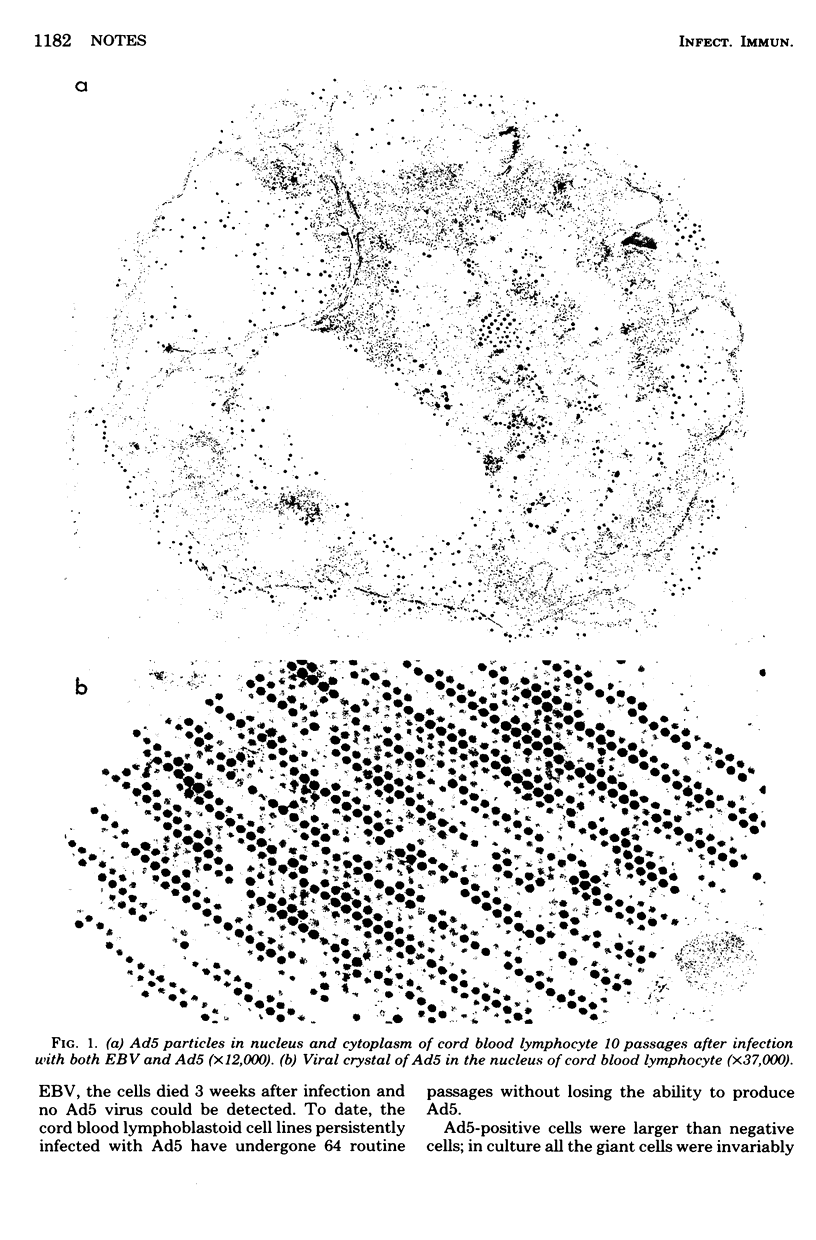
Lymphoblastoid cell lines derived from human cord blood leukocytes were persistently infected with human adenovirus 5. These cell lines expressed the Epstein-Barr nuclear antigen, but no other Epstein-Barr virus-related antigen. They continually produced infectious adenovirus 5 particles, but this production could be inhibited by the presence of specific neutralizing antibody to adenovirus 5. This suggests that the persistent infection might be due to the continual reinfection of susceptible cells by complete virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Bjursell G., Kaschka-Dierich C., Lindahl T. Circular Epstein-Barr virus genomes of reduced size in a human lymphoid cell line of infectious mononucleosis origin. J Virol. 1977 May;22(2):373–380. doi: 10.1128/jvi.22.2.373-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S. Umbilical cord leucocytes transformed by lymphoid cell filtrates from healthy people. Nat New Biol. 1971 Sep 22;233(38):124–124. doi: 10.1038/newbio233124a0. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Lyon M., Soula A. Identification of the adenovirus components during the penetration in HeLa cells by immunofluorescence technique. Arch Virol. 1977;54(3):189–199. doi: 10.1007/BF01314785. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Faucon N., Chardonnet Y., Perrinet M. C., Sohier R. Superinfection with adenovirus of Burkitt's lymphoma cell lines. J Natl Cancer Inst. 1974 Aug;53(2):305–308. doi: 10.1093/jnci/53.2.305. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Klein E., Klein G., Clifford P., Singh S. Immunoglobulin and glucose-6-phosphate dehydrogenase as markers of cellular origin in Burkitt lymphoma. J Exp Med. 1973 Jul 1;138(1):89–102. doi: 10.1084/jem.138.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. E., Black P. H., Wolford R., Huebner R. J. Adenovirus type 12-rat embryo transformation system. J Virol. 1967 Apr;1(2):362–367. doi: 10.1128/jvi.1.2.362-367.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresen K. O., Hausen H. Establishment of EBNA-expressing cell lines by infection of Epstein-Barr virus (EBV)-genome-negative human lymphoma cells with different EBV strains. Int J Cancer. 1976 Feb 15;17(2):161–166. doi: 10.1002/ijc.2910170203. [DOI] [PubMed] [Google Scholar]
- GREEN M. Biochemical studies on adenovirus multiplication. III. Requirement for DNA synthesis. Virology. 1962 Dec;18:601–613. doi: 10.1016/0042-6822(62)90063-6. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Lambriex M., Van der Veen J. Comparison of replication of adenovirus type 2 and type 4 in human lymphocyte cultures. Infect Immun. 1976 Sep;14(3):618–622. doi: 10.1128/iai.14.3.618-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- MCBRIDE W. D., WIENER A. IN VITRO TRANSFORMATION OF HAMSTER KIDNEY CELLS BY HUMAN ADENOVIRUS TYPE 12. Proc Soc Exp Biol Med. 1964 Apr;115:870–874. doi: 10.3181/00379727-115-29060. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Giovanella B. C., Stehlin J. S., Klein G. Tumorigenicity of human hematopoietic cell lines in athymic nude mice. Int J Cancer. 1977 Mar 15;19(3):337–344. doi: 10.1002/ijc.2910190309. [DOI] [PubMed] [Google Scholar]
- Norrby E., Skaaret P. Comparison between soluble components of adenovirus types 3 and 16 and of the intermediate strain 3-16 (the San Carlos agent). Virology. 1968 Oct;36(2):201–211. doi: 10.1016/0042-6822(68)90137-2. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Perrinet M. C., Chardonnet Y., Sohier R. Etude préliminaire de la multiplication in vitro de l'adénovirus type 5 dans des lymphocytes de sang de cordon ombilical. Ann Microbiol (Paris) 1974 Dec;125(4):575–580. [PubMed] [Google Scholar]
- Pope J. H., Scott W., Moss D. J. Human lymphoid cell transformation by Epstein-Barr virus. Nat New Biol. 1973 Dec 5;246(153):140–141. doi: 10.1038/newbio246140a0. [DOI] [PubMed] [Google Scholar]
- ROSEN L. A hemagglutination-inhibition technique for typing adenoviruses. Am J Hyg. 1960 Jan;71:120–128. doi: 10.1093/oxfordjournals.aje.a120085. [DOI] [PubMed] [Google Scholar]
- ROWE W. P., HUEBNER R. J., GILMORE L. K., PARROTT R. H., WARD T. G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953 Dec;84(3):570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Vuillaume M., de Thé G. Nasopharyngeal carcinoma. 3. Ultrastructure of different growths leading to lymphoblastoid transformation in vitro. J Natl Cancer Inst. 1973 Jul;51(1):67–80. doi: 10.1093/jnci/51.1.67. [DOI] [PubMed] [Google Scholar]
- van der Veen J., Lambriex M. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 1973 Apr;7(4):604–609. doi: 10.1128/iai.7.4.604-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]