Abstract
The Notch pathway is a contact-dependent, or juxtacrine, signaling system that is conserved in metazoan organisms and is important in many developmental processes. Recent investigations have demonstrated that the Notch pathway is active in both the embryonic and postnatal ovary and plays important roles in events including follicle assembly and growth, meiotic maturation, ovarian vasculogenesis, and steroid hormone production. Ovarian pathologies resulting from disruption of the Notch pathway in mice affect meiotic spindle assembly, follicle histogenesis, granulosa cell proliferation and survival, corpora luteal function, and ovarian neovascularization. These aberrations result in abnormal folliculogenesis and reduced fertility. Knowledge of the cellular interactions facilitated by the Notch pathway is an important area for continuing research and future studies are expected to enhance our understanding of ovarian function and provide critical insights for improving reproductive health. This review focuses on the expression of Notch pathway components in the ovary, and on the multiple functions of Notch signaling in follicle assembly, maturation, and development. We focus on the mouse, where genetic investigations are possible, and relate this information to the human ovary.
Keywords: Notch signaling, ovary, follicle, germ cells, granulosa cells
Introduction
The mammalian ovary is a female organ critical for reproductive function that contains and supports the development of the female gamete, the oocyte. The functional unit of the ovary, the ovarian follicle, forms a specialized niche necessary for the development of a mature oocyte and is also essential for the production of steroid hormones that drive reproductive function and support female health. During embryonic development, primordial germ cells (PGCs), which arise within the proximal epiblast, migrate to the genital ridges of the bipotential gonad, at which time, under the influence of signals from surrounding somatic cells, they develop into either sperm or oocytes (McLaren 1984, Saitou & Yamaji 2012). During ovarian development, PGCs undergo several rounds of synchronous mitotic division without complete cytokinesis to form clusters of interconnected germ cells called germ cell syncytia, also known as cysts or nests (Pepling & Spradling 1998). Though the function of germ cell syncytia has not been established, these structures provide a shared local environment to respond to stimuli and to allow the exchange of macromolecular resources (Pepling & Spradling 1998). Following retinoic acid induced expression of stimulated by retinoic acid gene 8 (Stra8), germ cells within the developing female gonad cease mitotic division and enter meiosis in an anterior to posterior wave across the ovary, arresting at the diplotene stage of meiotic prophase I (Menke et al. 2003, Koubova et al. 2006, Anderson et al. 2008).
Interactions between germ cells and somatic pregranulosa cells are critical for the formation of ovarian follicles. Perinatally in mice, and embryonically in humans, somatic pregranulosa cells send cellular projections around individual germ cells within fragmenting syncytia to encapsulate and form primordial follicles through the coordination of syncytial breakdown and follicle assembly in a process known as follicle histogenesis. This process involves bidirectional communication between germ cells and somatic support cells and has been shown to be regulated by steroid hormone signaling, neurotrophins, KITL/KIT signaling, and members of the transforming growth factor-beta (TGFβ) superfamily. Follicle histogenesis is also associated with significant levels of germ cell loss (Pepling 2012), which may serve to eliminate defective germ cells or provide a means to fragment syncytia (Pepling & Spradling 2001). Aberrations in follicle histogenesis can result in the formation of multi-oocytic follicles, which are a consequence of incomplete fragmentation of syncytia, and in oocyte or follicle loss due to errors in follicle assembly (Silva-Santos & Seneda 2011). Notably, follicle histogenesis results in the formation of the primordial follicle pool, which represents the entirety of oocytes available during a female’s reproductive life.
Following puberty, and during each estrous cycle in rodents or menstrual cycle in humans, a cohort of follicles is recruited to undergo further growth and maturation. Early stages of follicle activation and development are generally considered to be gonadotropin-independent, while later stages, including antrum formation, cumulus expansion, and ovulation, require follicle stimulating hormone (FSH) and luteinizing hormone (LH). Following the release of a meiotically competent oocyte, remaining granulosa and theca cells of a follicle luteinize to form a corpus luteum, which is essential for the production of steroid hormones needed to prepare the uterus for pregnancy. In addition, there is rapid neovascularization of blood vessels within the theca layer to support this process.
The Notch pathway is one of the most conserved signaling systems in multicellular organisms, and perturbations in Notch signaling are responsible for a number of inherited human diseases and cancers (Maillard & Pear 2003, Leong & Karsan 2006, Penton et al. 2012). While the function of the Notch pathway in ovarian development has been extensively studied in model organisms such as Drosophila and C. elegans (Andersson et al. 2011, Greenwald & Kovall 2013), there is limited information available on the function of Notch signaling in the mammalian gonad, and only recently have reports become available implicating Notch signaling in ovarian (Manosalva et al. 2013, Xu & Gridley 2013, Vanorny et al. 2014) or testicular (Tang et al. 2008, Garcia & Hofmann 2013, Huang et al. 2013) development. Within the mammalian ovary, Notch receptors, ligands, modulators, and target/effector genes are expressed and dynamically regulated during follicular development (Table 1). Activation of the Notch pathway has been described in both germline and somatic cell populations, and new studies involving the use of transgenic reporter mice and conditional knockout mouse models suggest that productive Notch signaling occurs between germ cells and granulosa cells, between adjacent granulosa cells, and between cells of the ovarian vasculature.
Table 1. Expression of Notch signaling components in the mammalian ovary.
Abbreviations: primordial follicles (Prim), primary follicles (1°), secondary follicles (2°), corpora lutea (CL), vasculature (vasc), and ovarian surface epithelium (OSE).
References: (1) Guo et al. 2012, (2) Feng et al. 2014, (3) Hernandez et al. 2011, (4) Murta et al. 2014, (5) Accialini et al. 2015, (6) Johnson et al. 2001, (7) Vorontchikhina et al. 2005, (8) Jovanovic et al. 2013, (9) Garcia-Pascual et al. 2013, (10) Pan et al. 2015, (11) Trombly et al. 2009, (12) Zhang et al. 2011, (13) Wang et al. 2014, (14) Vanorny et al. 2014, (15) Wang et al. 2015, (16) Dorfman et al. 2011, (17) Manosalva et al. 2013, and (18) Hahn et al. 2005.
| Gene | Oocyte | Prim | 1° | 2° | Antral | CL | Theca | Vasc | OSE |
|---|---|---|---|---|---|---|---|---|---|
| Notch1 | 1,2 | 1 | 3–5 | 3,6–9 | 10 | ||||
|
|
|||||||||
| Notch2 | 4,11–13 | 4,11,13,14 | 4,11–14 | 4,6,8,12–14 | 4,6,8,12–14 | 4 | 4 | ||
|
|
|||||||||
| Notch3 | 4 | 4,6 | 4,6 | 4,6,15 | 4 | ||||
|
|
|||||||||
| Notch4 | 7 | 7 | 7 | 7 | 3,7 | 3,6,7 | 7 | ||
|
|
|||||||||
| Jag1 | 4,6,11–14,16 | 4 | 4 | 4,13 | 4,15 | 7 | |||
|
|
|||||||||
| Jag2 | 1 | 6 | 6 | ||||||
|
|
|||||||||
| Dll1 | 4 | ||||||||
|
|
|||||||||
| Dll3 | |||||||||
|
|
|||||||||
| Dll4 | 4 | 4 | 4 | 4 | 4 | 3,4 | 3,9 | ||
|
|
|||||||||
| Hes1 | 4,11 | 4,11 | 4 | 4 | 4 | 4 | 10 | ||
|
|
|||||||||
| Hes5 | 4 | 4 | 4 | 4,6 | 4,6 | 4 | 10 | ||
|
|
|||||||||
| Hes7 | |||||||||
|
|
|||||||||
| Hey1 | 12,17 | 17 | 12 | 6,12 | 6,12 | ||||
|
|
|||||||||
| Hey2 | 11,12 | 11 | 12 | 6,12 | 6,12 | ||||
|
|
|||||||||
| Heyl | 6 | 6 | |||||||
|
|
|||||||||
| Lfng | 18 | 18 | 18 | ||||||
|
|
|||||||||
| Mfng | 18 | ||||||||
|
|
|||||||||
| Rfng | 18 | ||||||||
Morphogenesis of the mammalian ovary requires the precise spatial and temporal organization and function of multiple cell types, and is coordinated by endocrine, paracrine, autocrine, and juxtacrine signaling mechanisms. The actions and regulation of paracrine and endocrine hormone signaling within the ovary has been extensively studied (Albertini et al. 2001, Knight & Glister 2006, Edson et al. 2009, Conti et al. 2012); however, the roles of juxtacrine signaling within the ovary remain largely unexplored. This review will focus on the Notch pathway, a juxtacrine or contact-dependent signaling system, during ovarian and follicular development.
Notch pathway signal transduction and regulation
The Notch signaling pathway (Figure 1) is a highly conserved and broadly used molecular transduction system for processes including cell-fate specification (Lindsell et al. 1996, Weijzen et al. 2002, Chau et al. 2006, Raetzman et al. 2006, Hayashi & Kume 2008, Doetzlhofer et al. 2009), cell migration (Jordan et al. 2000), mesenchymal/epithelial transition (Li et al. 1998), cell survival/death (Nickoloff et al. 2002, Sainson et al. 2005, Choi et al. 2008), cell division (Sainson et al. 2005, Kolev et al. 2008, Monahan et al. 2009), and cell adhesion (Choi et al. 2008). Disruption of Notch signaling results in multiple human diseases including Alagille syndrome (Oda et al. 1997), familial aortic valve disease (Garg et al. 2005), Adams-Oliver syndrome (Stittrich et al. 2014), Hajdu-Cheney syndrome (Simpson et al. 2011), spondylocostal dysostosis (Bulman et al. 2000), cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) (Joutel et al. 1996), Alzheimer disease (Kopan & Goate 2000), and cancers including T-cell acute lymphoblastic leukemia (Weng et al. 2004) and pancreatic (Mullendore et al. 2009), colon (Reedijk et al. 2008), and ovarian cancers (Groeneweg et al. 2014).
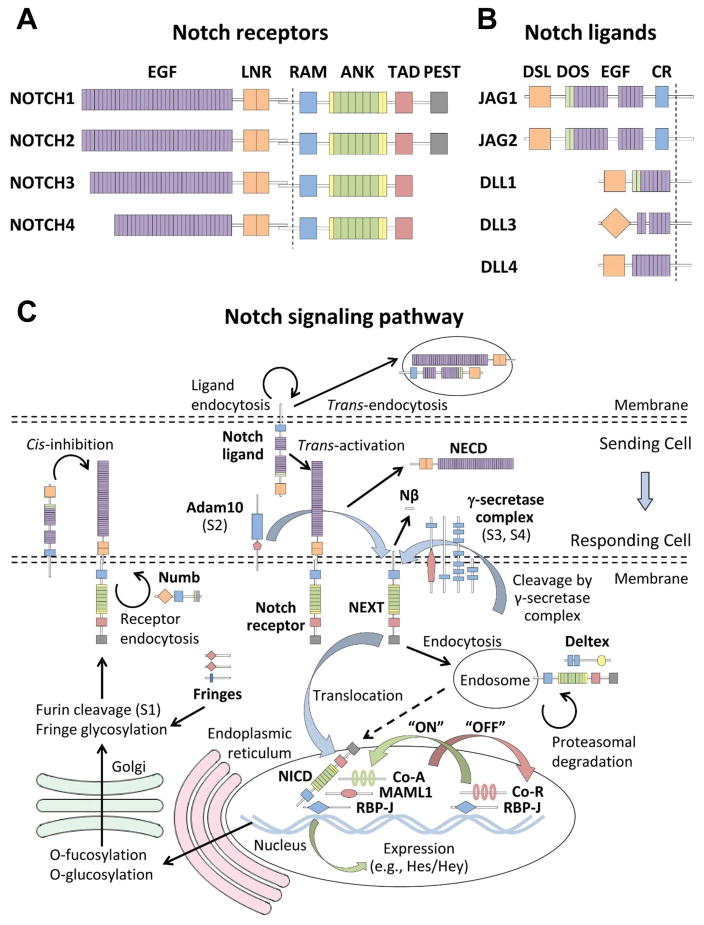
Figure 1. The Notch signaling pathway.
In mammals, there are (A) four Notch receptors and (B) five Notch ligands. Extracellular interactions between receptors and ligands expressed on juxtaposed cells are mediated by several conserved domains. Among these are epidermal growth factor (EGF) repeats found on both ligands and receptors, LIN-12/Notch repeat (LNR) domains that are found on all receptors, and a highly conserved Delta-Serrate-Lag2 (DSL) domain found on ligands. Notably, the DSL domain of DLL3 is structurally divergent from that of other Notch ligands and does not promote Notch receptor activation. JAG1 and JAG2 also contain a cysteine-rich (CR) domain, while DLL3 and DLL4 lack a Delta and OSM-11 (DOS) domain composed of atypical EGF repeats. (C) Productive interactions between juxtaposed cells lead to cleavage of Notch receptors at the juxtamembrane region by the γ-secretase complex. Nuclear localization signals (yellow bars) within the intracellular domain of Notch receptors (NICD) allow for nuclear translocation following receptor cleavage. The RBP-J associated molecule (RAM) domain facilitates interactions between the intracellular domain and the DNA-binding factor RBP-J. Binding of the NICD with RBP-J displaces corepressors (Co-R) and allows for the recruitment of transcriptional coactivators (Co-A) via interactions with CDC10/ankyrin (ANK) repeats and a trans-activation domain (TAD). Target or effector genes of the Notch pathway include the Hes and Hey families of transcriptional repressors. Notch signaling is terminated by ubiquitination of a proline-glutamate-serine-threonine (PEST) domain that targets the intercellular domain for proteasomal degradation.
Notch signaling is frequently used to select amongst pre-existing cellular potentials to specify cell fate decisions. The two classical mechanisms by which Notch signaling functions are lateral inhibition and inductive signaling (Flores et al. 2000, Haines & Irvine 2003). Lateral inhibition is a mechanism by which a cell that adopts a particular fate inhibits neighboring cells from developing in a similar manner. By contrast, inductive signaling is a mechanism by which one cell, or group of cells, remains in its current state and signals to and causes a neighboring cell, or group of cells, to differentiate.
In mammals, the Notch pathway (Artavanis-Tsakonas et al. 1999, Mumm & Kopan 2000, Kopan 2002, Schweisguth 2004, Ehebauer et al. 2006) involves the interaction of one of four hetero-oligomer (Blaumueller et al. 1997) single-pass type I transmembrane receptors (NOTCH1, NOTCH2, NOTCH3, and NOTCH4) with one of five single-pass type I transmembrane ligands (JAG1, JAG2, DLL1, DLL3, and DLL4). Notch receptors contain 29–36 N-terminal epidermal growth factor (EGF) repeats, of which EGF repeats 11–12 are essential for ligand binding, while Notch ligands contain a conserved N-terminal Delta-Serrate-Lag2 (DSL) domain, which is important for mediating interactions with Notch receptors. Notably, transmission of Notch signaling utilizes a unique mechanism mediated through a series of sequential proteolytic cleavage events that does not involve amplification by classical intracellular secondary messengers.
Following their synthesis, Notch receptors are post-translationally modified through fucosylation by protein O-fucosyltransferase 1 (POFUT1) within the endoplasmic reticulum. The receptors are further modified within the Golgi by the GlcNAc-transferase proteins lunatic (LFNG), manic (MFNG), and radical fringe (RFNG), which function to modulate receptor-ligand interactions. During their transit through the Golgi, Notch receptors are cleaved by the furin-like protein proprotein convertase subtilisin/kexin type 5 (PCSK5) at site 1 (S1) during exocytosis, which regulates receptor trafficking and signaling activity (Logeat et al. 1998). The two receptor cleavage products remain associated at the cell surface as a heterodimer through non-covalent, calcium-dependent interactions (Gridley 2003). Importantly, the S1 cleavage site is contained within a critical negative regulatory region (NRR) of the receptor that is comprised of three highly conserved Lin-12/Notch repeat (LNR) domains and a heterodimerization domain (HD), which cooperate to prevent premature signaling in the absence of a ligand (Gordon et al. 2009).
Productive interactions between the extracellular domains of Notch ligands and receptors occur intercellularly in trans (i.e., trans-activation) between juxtaposed cells, whereas inhibitory interactions occur between receptors and ligands coexpressed along the membrane of the same cell in cis (i.e., cis-inhibition). As a consequence of trans-activating interactions, Notch receptors undergo a conformational change that exposes the site 2 (S2) cleavage moiety that is recognized by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10). The remaining membrane-anchored Notch extracellular truncation (NEXT) fragment is recognized by the γ-secretase complex, an enzymatic assemblage composed of presenilin 1 (PSEN1), nicastrin (NCSTN), presenilin enhancer 2 (PEN2), and anterior pharynx-defective 1 (APH1) (Saxena et al. 2001). Further proteolytic processing at the juxtamembrane region of the Notch receptor by the γ-secretase complex at site 3 (S3) and site 4 (S4) results in the release of the Notch intracellular domain (NICD) and Nβ peptide, which allows the NICD to translocate to the nucleus where it acts as a modulator of transcription (Fortini 2002).
Within the nucleus, the NICD interacts with the DNA-binding transcriptional repressor recombination signal binding protein for immunoglobulin kappa j region (RBP-J), also known as CBF1/Su(H), through its RBP-J associated molecule (RAM) domain (Lubman et al. 2007). Subsequently, this interaction positions CDC10/ankyrin repeats within the NICD to recruit transcriptional activators, such as mastermind-like proteins (MAMLs), to the promoters of Notch target genes. Classic target genes of Notch signaling include the hairy/enhancer-of-spilt (Hes) and hairy/enhancer-of-split related with YRPW motif protein (Hey) genes, which are members of the basic helix-loop-helix (bHLH) family and function as transcriptional repressors. The ankyrin repeats of Notch receptors also facilitate interactions with NUMB and Deltex homologs, which are important cytosolic factors that regulate the Notch pathway. Downregulation of Notch signaling requires phosphorylation of NICD on a C-terminal proline, glutamic acid, serine, and threonine (PEST) domain, and further modification by E3 ubiquitin ligases, such as F-box/WD repeat-containing protein 7 (FBW7), which promote proteasomal degradation of NICD. In the absence of NICD, RBP-J is associated with co-repressor (Co-R) proteins and histone deacetylases (HDACs), which act to repress the transcription of target genes; thus, activation of the Notch pathway involves a switch from a repressed state to an activated state.
Emerging evidence also indicates that the Notch pathway can be regulated through several non-canonical mechanisms (D’Souza et al. 2010, Heitzler 2010, Andersen et al. 2012). These include activation by membrane ligands such as delta-like 1 homolog (DLK1) (Baladron et al. 2005) and delta- and notch-like epidermal growth factor-related receptor (DNER) (Eiraku et al. 2005), GPI-linked ligands including F3/contactin 1 (CNTN1) (Hu et al. 2003) and NB3/contactin 6 (CNTN6) (Cui et al. 2004), and secreted ligands such as connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed gene 3 (CCN3) (Sakamoto et al. 2002), microfibril-associated glycoprotein family 1 (MAGP1) and 2 (MAGP2) (Miyamoto et al. 2006), thrombospondin 2 (TSP2) (Meng et al. 2009), Y-box protein 1 (YB1) (Rauen et al. 2009), and EGF-like domain 7 (EGFL7) (Schmidt et al. 2009). Some of these ligands impact Notch signaling by functioning as activators or inhibitors in either trans or cis, and some of them can also regulate Notch activation independently of γ-secretase proteolysis by causing dissociation of the Notch receptor heterodimer, as with MAGP2, or by facilitating interactions with other downstream modulators at the cell surface, including Deltex and β-Arrestin (Mukherjee et al. 2005). Importantly, many of the observations regarding non-canonical Notch signaling have yet to be proven within in vivo systems; thus, the significance of these findings is still unknown, and non-canonical signaling has not been well-studied within the ovary.
Notch pathway in the developing ovary
During embryonic ovarian development, Notch receptors, ligands, and target genes are expressed (Table 1). NOTCH2, which is localized to somatic pregranulosa cells, is the most abundantly expressed receptor within the embryonic ovary, while the Notch ligands JAG1 and JAG2, which are found in germ cells of the embryonic ovary, are the most abundantly expressed ligands (Vanorny et al. 2014). Use of a summative Notch-responsive fluorescent reporter mouse line demonstrates that activation of the Notch pathway in the embryonic ovary is first observed around embryonic day 15.5 (E15.5) and is restricted to somatic cells (Vanorny et al. 2014). Furthermore, Notch activation within the embryonic mouse ovary is concomitant with a significant increase in the expression of the ligands, Jag1 and Jag2, the receptor, Notch2, and the Notch target genes Hes1 and Hey2 (Vanorny et al. 2014). At E18.5, Notch active pregranulosa cells can be observed undergoing dramatic reorganization to form an intricate network around ovigerous cords containing partially fragmented germ cell nests (Figure 2A,B). Additionally, Notch active pregranulosa cells, which are organized along collagen fibrils, begin to invade nests and can be seen sending cellular projections around and encapsulating individual germ cells to form primordial follicles (Vanorny et al. 2014). With these data, a model begins to emerge in which upregulation of Jag1 and Jag2 in the oocyte, signals through Notch2 expressed in pregranulosa cells to facilitate the resolution of germ cell syncytia and assembly of primordial follicles.
Figure 2. Notch activity during follicle assembly, growth, and maturation.
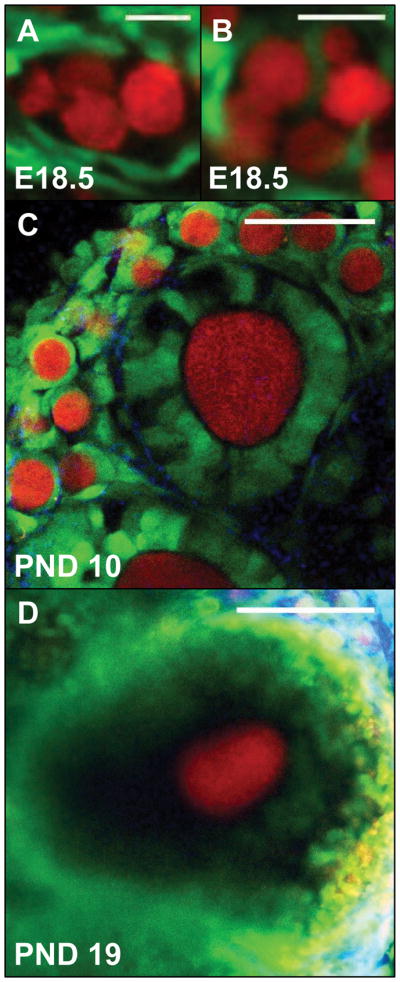
A Notch-responsive GFP reporter mouse line, used to label Notch active cells (green), was crossed with a Vasa-Cre mouse and conditional tdTomato reporter line, to label germ cells (red). (A) Notch active pregranulosa cells surround clusters of germ cells in an embryonic day 18.5 (E18.5) ovary (scale bar = 20 microns). (B) Notch active cells can also be seen invading germ cell syncytia and sending projections around individual germ cells to form primordial follicles (scale bar = 20 microns). (C) Notch active pregranulosa cells are observed within primordial follicles at the ovarian cortex of a postnatal day 8 (PND8) ovary, while granulosa cells within primary follicles demonstrate variable Notch reporter activity. Collagen fibrils (blue) were detected by second harmonic generation (scale bar = 50 microns). (D) Within antral follicles, both cumulus and mural granulosa cells are Notch active (scale bar = 100 microns).
Functional studies in embryonic ovaries demonstrate that the Notch pathway is important for germ cell syncytia formation and meiotic entry. Inhibition of Notch signaling in embryonic ovarian explants using the pharmacological γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) results in suppression of retinoic acid induced stimulation of Stra8 expression and a reduction in downstream targets deleted in azoospermia-like (Dazl), disrupted meiotic cDNA protein 1 (Dmc1), and meiotic recombination protein 8 (Rec8) (Feng et al. 2014). Furthermore, siRNA knockdown of Notch1 leads to markedly reduced expression of both Stra8 and synaptonemal complex protein 3 (Scp3) (Figure 3A). Reduction in Stra8 expression following Notch inhibition is correlated with increased methylation of the Stra8 proximal promoter region, suggesting that Notch signaling regulates the epigenetic status of the Stra8 promoter. These changes cause delayed meiotic progression, defective oocyte growth, and aberrant primordial follicle assembly resulting in the formation of multi-oocytic follicles within renal grafts of embryonic ovarian tissue exposed to γ-secretase inhibitors (Feng et al. 2014), consistent with a relationship between meiotic progression and follicle assembly.
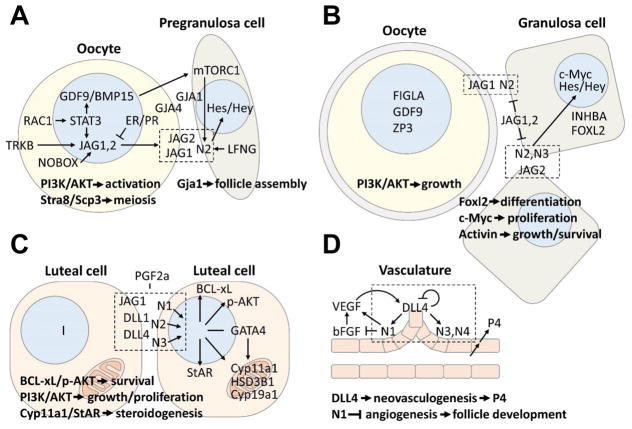
Figure 3. Regulation and function of Notch signaling during ovarian and follicle development.
The Notch pathway has multiple roles during follicle assembly and growth, steroidogenesis, and ovarian vascular development. (A) Bidirectional communication between germ cells and somatic support cells is critical for follicle assembly. In the mammalian ovary, this process appears to be regulated in large part by the modulation of Jag1, and possibly Jag2, transcription in germ cells, which is regulated by NOBOX, STAT3, TRKB, and steroid hormone action. Upregulation of Notch ligand expression in oocytes facilitates interactions with adjacent pregranulosa cells expressing Notch receptor that is supported by growth factors (i.e., GDF9 and BMP15) from the oocyte. Notch signaling between germ cells and somatic pregranulosa cells promotes follicle assembly and has non-cell autonomous roles on oocyte development including the regulation of meiosis and oocyte activation. (B) Notch signaling supports follicular growth and maturation by regulating granulosa cell differentiation, proliferation, and survival. Formation of zona pellucida in oocytes may impede productive Notch signaling between oocytes and granulosa cells; thus, within multi-laminar follicles, continued trans-activing interactions between Notch receptors and ligands likely occur between juxtaposed granulosa cells. The expression of Notch receptors and ligands in these granulosa cells, therefore, must facilitate both bidirectional inductive signaling yet overcome cis-inhibition due to receptor ligand co-expression. Functionally, continued Notch signaling in growing follicles promotes Foxl2 expression, activin action, and has non-cell autonomous effects of oocyte growth and maturation. (C) Steroid production in luteal cells is modulated by Notch signaling through transcriptional regulation of enzymes involved in steroid biosynthesis. Bidirectional inductive signaling and cis-inhibitory mechanisms likely coordinate these processes. Notch signaling also functions of prevent luteolysis by regulating the expression of the anti-apoptotic factor BCL-xL and the pro-survival factor p-AKT. (D) Notch signaling, mediated predominately through the actions of Notch1, 3, and 4, regulates bFGF-induced VEGF-mediated angiogenesis and the differentiation and activity of endothelial tips cells that express DLL4. These events modulate neovascularization and vascular density to support follicle growth and progesterone (P4) production. (A–C) The schematic representations of cells used in these figures were spatially separated for clarity.
Pharmacological inhibition of Notch signaling with the γ-secretase inhibitors DAPT and L-685,458 in neonatal ovaries using an ex vivo ovary culture system results in delayed syncytial breakdown (Trombly et al. 2009b, Chen et al. 2014), fewer primordial follicles (Trombly et al. 2009b, Chen et al. 2014), decreased granulosa cell proliferation (Terauchi et al. 2016), increased numbers of degenerative oocytes (Terauchi et al. 2016), and decreased expression of the germ cell markers newborn ovary homeobox protein (Nobox), factor in the germline alpha (Figla), LIM/homeobox protein 8 (Lhx8), and spermatogenesis and oogenesis specific basic helix-loop-helix 1 (Sohlh2) (Chen et al. 2014). Interestingly, deletion of Nobox in mice causes markedly delayed syncytial breakdown, accelerated postnatal oocyte loss, and decreased ovarian expression of Jag1 (Figure 3A) (Rajkovic et al. 2004), while Figla null mice display aberrant follicle assembly and postnatal oocyte loss due to the failure of pregranulosa cells to associate properly with germ cells (Soyal et al. 2000). Disruption of Notch signaling using DAPT is also associated with decreased recruitment of granulosa precursors and aberrant follicle assembly, which is similar to the phenotype observed with pharmacological inhibition or conditional knockout of the cell surface sheddase Adam10 (Feng et al. 2016). In addition, RNAi-mediated knockdown of Jag2 or Notch1 also leads to disrupted follicle assembly in cultured ovarian explants (Guo et al. 2012).
Multiple observations suggest that maternally-derived steroid hormones are important for the maintenance of germ cell syncytia in the ovaries of fetal rodents, and that the subsequent loss of exposure to these hormones at the time of birth leads to the initiation of follicle histogenesis. Additionally, treatment with estrogens (Iguchi et al. 1990, Chen et al. 2007, Chen et al. 2009) or progestins (Iguchi et al. 2001, Nilsson et al. 2006) leads to an increased incidence of multi-oocytic follicles. Progesterone has also been shown to directly disrupt follicle histogenesis (Iguchi 1992, Kezele & Skinner 2003, Chen et al. 2007) and result in the suppression of Jag2, Notch1, and Hey2 expression in both in vivo and in vitro systems (Guo et al. 2012). Surprisingly, treatment of neonatal ovaries with the progesterone antagonist RU486 also leads to suppression of Jag2, Notch1, and Hey2 expression (Guo et al. 2012), suggesting that an appropriate steroid hormone environment is required for the accurate regulation of Notch components. Despite cross-talk between steroid hormone signaling and the Notch pathway, the formation of multi-oocytic follicles in models of altered steroid hormone action may have distinct etiologies from those resulting from disrupted Notch signaling. Unlike treatment with DES, exposure of neonatal ovaries to DAPT does not result in increased numbers of multi-oocytic follicles when examined histologically after renal grafting (Terauchi et al. 2016). This is in contrast to similar studies that did report the formation of multi-oocytic follicles following treatment with DAPT using embryonic ovaries (Feng et al. 2014). These findings indicate that alternative mechanisms or windows of sensitivity may exist in which DES exposure or Notch inhibition can cause perturbations to follicle assembly.
Genetic models of Notch pathway disruption in the ovary
Given that Notch1 and Notch4 expression is largely restricted to the ovarian vasculature (Table 1), and that mice with global deletion of Notch3 (Krebs et al. 2003), Notch4 (Krebs et al. 2000), or both Notch3 and Notch4 (Xu 2011) have no overt reproductive phenotype, the expression of Notch2 in granulosa cells has the greatest potential to mediate the actions of Notch signaling during follicle assembly and growth. Moreover, JAG1 has been shown to productively interact with NOTCH2 (Shimizu et al. 1999, Shimizu et al. 2000a, Shimizu et al. 2000b); thus, it is hypothesized that JAG1, and possibly JAG2, expressed in germ cells of perinatal ovaries signals through NOTCH2 expressed in granulosa cells to facilitate germ and somatic cell interactions important for follicle formation and development. Importantly, however, since global deletion of either Jag1 or Notch2 results in embryonic lethality (Xue et al. 1999, McCright et al. 2006), tissue-specific approaches are required to investigate the role of these genes within the developing ovary.
Conditional deletion of the Notch ligand, Jag1, in germ cells (J1KO) (Vanorny et al. 2014) or the Notch receptor, Notch2, in somatic pregranulosa cells (N2KO) (Xu & Gridley 2013, Vanorny et al. 2014) produces a phenotype characterized by decreased numbers of primordial follicles, persistence of germ cell syncytia at the ovarian cortex, uncoordinated follicle growth, and aberrant folliculogenesis (Figure 4). The most striking feature of these genetic models is the presence of numerous multi-oocytic follicles, which is evidence of significantly disturbed ovigerous cord fragmentation or syncytial breakdown. Interestingly, in the Drosophila ovariole, disruption of the ligand Delta in the germ line, the receptor Notch in follicle cells, or the Lfng homolog, Fringe in somatic polar cells, results in the production of fused and enlarged egg chambers containing multiple oocytes (Grammont & Irvine 2001, Torres et al. 2003), suggesting functional conservation of Notch signaling across metazoan ovarian development.
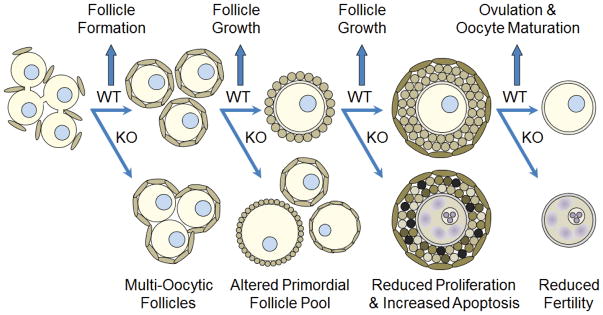
Figure 4. Summary of observed phenotypes in Notch pathway knockout mice during ovarian follicle development.
Disruption of Notch signaling between oocytes and somatic pregranulosa cells, as observed in J1KO and N2KO mice, leads to perturbations in follicle assembly characterized by the presence of multi-oocytic follicles, which represent a failure to completely resolve germ cell syncytia. Loss of Notch signaling also leads to changes in follicle dynamics that adversely impact the primordial follicle pool and development of antral follicles, which is accompanied by decreased proliferation and increased apoptosis of granulosa cells. As a consequence of these defects, female Notch knockout mice are subfertile. These mouse models demonstrate important functions for Notch signaling in the resolution of germ cell syncytia, coordination of somatic and germ cell growth within follicles, and oocyte maturation.
In postnatal day 1 (PND1) N2KO ovaries, decreased germ and pregranulosa cell apoptosis is observed (Xu & Gridley 2013), possibly due to delayed syncytial breakdown, which is a process that normally results in massive germ cell loss. In contrast, at PND19, there is a significant increase in somatic cell apoptosis and a decrease in granulosa cell proliferation within growing follicles of both J1KO and N2KO ovaries (Figure 4) (Vanorny et al. 2014). Both knockout models also contain follicles in which there is oocyte expansion in the absence of granulosa cell growth, which is consistent with a role for Notch signaling in somatic cell proliferation and maturation. N2KO ovaries also contain increased numbers of atretic follicles and follicles that become cystic, hemorrhagic, and contain trapped oocytes (Xu & Gridley 2013), similar to mouse genetic models of disturbed estrogen (Couse & Korach 1999, Britt et al. 2000), activin (McMullen et al. 2001, Bristol-Gould et al. 2005, Pangas et al. 2007), and gonadotropin signaling (Risma et al. 1995, Risma et al. 1997, Kumar et al. 1999, Keri et al. 2000, Matzuk et al. 2003, Meehan et al. 2005). In addition, both conditional knockout models contain reduced numbers of antral follicles, while J1KO ovaries also contain fewer corpora lutea, consistent with a reduction in ovarian follicle growth and ovulation capacity (Vanorny et al. 2014).
Interestingly, the transcript levels of Notch2 are elevated in J1KO ovaries, and conversely, the levels of Jag1 are elevated in N2KO ovaries, signifying a mechanism of compensatory expression between Jag1 and Notch2 (Vanorny et al. 2014). In addition, the expression of Jag2 and Notch1 are increased in J1KO ovaries. These data indicate that the effects of Jag1 or Notch2 deletion might be masked through compensation or functional complementation by the expression of other Notch ligands or receptors. Transcript levels of growth differentiation factor 9 (Gdf9), zona pellucida glycoprotein 3 (Zp3), and Figla are elevated in both J1KO and N2KO ovaries, consistent with the observation of follicles with uncoordinated growth between the oocyte and somatic layer (Figure 3B). Additionally, within J1KO ovaries, the expression of inhibin beta A (Inhba) and B (Inhbb) is decreased, consistent with the phenotype of altered granulosa cell proliferation and decreased ovarian reserve, respectively (Figure 3B).
Histological examination of ovaries in which Jag1 and Notch2 are both deleted in the germ and somatic cell compartments reveals that the effect of combined deletion of Jag1 and Notch2 produces an ovarian phenotype similar to the conditional deletion of either gene individually (Vanorny et al. 2014). This finding is consistent with a model in which Jag1 in germ cells directly signals through Notch2 in granulosa cells. As a consequence of the multiple defects resulting from disrupted Notch signaling between germ cells and granulosa cells, both J1KO (Vanorny et al. 2014) and N2KO (Xu & Gridley 2013) models display reduced fertility, as measured by a reduction in litter size and total progeny, and some female J1KO and N2KO mice appear to undergo premature reproductive senescence (Vanorny et al. 2014). Furthermore, preliminary studies suggest that the impaired fertility observed in J1KO females may result from a combination of reduced oocyte quality and a trend toward reduced ovulatory capacity, whereas N2KO females have reduced oocyte quality, but preserved ovulatory potential (Vanorny 2016).
Constitutive expression of Notch1 intracellular domain (NICD1) through induction with Amhr2-Cre leads to multiple abnormalities within the female reproductive tract of mice (Manosalva et al. 2013, Ferguson et al. 2016). Importantly, NICD1 has been shown to fully complement the actions of the Notch2 intracellular domain (NICD2) (Liu et al. 2015); thus, overexpression of NICD1 is functionally equivalent to constitutive activation of Notch2. The predominant ovarian phenotype displayed in these animals includes the presence of hemorrhagic blood vessels and cystic lesions (Ferguson et al. 2016), and an increase number of mature oocytes and a decrease number of pregranulosa cells (Manosalva et al. 2013). Importantly, these mice have significant extra-ovarian pathologies consisting of aberrant coiling and canalization of the oviducts, and the presence of hemorrhagic blood vessels and cystic lesions in the uteri and oviducts. These features, which are fully penetrant in older females (Ferguson et al. 2016), are similar to the phenotypes observed in mouse models with disruption of Wnt family members 4 (Wnt4) and 7a (Wnt7a), β-catenin, and Dicer (Vainio et al. 1999, Nagaraja et al. 2008, Hernandez Gifford et al. 2009). Although gain-of-function NICD1 female mice are subfertile (Manosalva et al. 2013, Ferguson et al. 2016), follicular development appears to be largely normal in these mice (Ferguson et al. 2016), suggesting that the altered fertility observed in these animals is possibly extra-ovarian in origin.
Ovaries from traditional or conditional Hes1 knockout mice contain germ cells with decreased cellular diameters and fewer total germ cells, similar to those treated with DAPT, due to increased apoptosis resulting from the failure to suppress the pro-apoptotic gene Inhbb (Manosalva et al. 2013). In addition, Hes1 knockout ovaries contain germ cells with reduced markers of oocyte maturity, as measured by the expression of Kit, which has been shown to promote primordial follicle activation and survival of late-stage follicles (Hutt et al. 2006). Interestingly, despite the presence of fewer germ cells, there are increased numbers of pregranulosa cells in Hes1 knockouts, which is correlated with increased levels of forkhead box protein L2 (Foxl2) and follistatin (Fst) expression (Manosalva et al. 2013). Notably, in either Hes1 conditional or global knockouts, female mice are subfertile; thus, suppression of the Notch effector gene within granulosa cells leads to impaired follicular function that impacts female fertility.
Disruption of the fucose-specific glycosyltransferase Lfng leads to complete infertility in a large subset of female mice that survive into adulthood (Hahn et al. 2005), although some survivors can have productive matings (Xu et al. 2006). Mutation of Lfng is also associated with abnormal folliculogenesis characterized by the presence of multi-oocytic follicles and follicles with disorganized thecal layers. Unexpectedly, given the poor fertility of Lfng knockouts, follicles of all developmental stages are present within mutant ovaries at roughly equivalent numbers and percentages in comparison to controls (Hahn et al. 2005). Additionally, corpora lutea are observed in Lfng null ovaries, indicating that mutant mice are capable of cycling and ovulating naturally; however, many of the corpora lutea contain trapped oocytes indicative of defective ovulation. Interestingly, despite altered folliculogenesis and diminished fertility, no difference is observed in the number of oocytes ovulated following gonadotropin stimulation. Analysis of ovulated oocytes, however, demonstrates significantly impaired meiotic maturation resulting in the failure of oocytes to complete meiosis due to aberrant meiotic spindle assembly (Hahn et al. 2005). Notably, ovarian expression of both Jag1 and Notch2 is reduced in Lfng null mice, and mutant ovaries share many of the phenotypic features observed in J1KO and N2KO ovaries.
Regulation of Notch pathway expression and action during follicle development
Suppression of Notch signaling in ex-vivo cultured ovarian explants using the γ-secretase inhibitors DAPT or L-685,458 results in the presence of fewer primordial follicles and delayed follicle recruitment, culminating in fewer growing and total follicles (Wang et al. 2014). This reduction in total follicle numbers is associated with decreased granulosa cell proliferation, increased germ cell apoptosis, and decreased expression of germ cell-specific transcription factors. Interestingly, Notch inhibition appears to be coupled with increased expression of PTEN, suggesting that suppression of Notch signaling in pregranulosa cells may disrupt follicle development by preventing PI3K/AKT mediated germ cell activation (Figure 3A), consistent with studies demonstrating a role of pregranulosa cells in primordial follicle activation (Zhang & Liu 2015).
Secondary ovarian follicles cultured in a Notch suppressive environment with γ-secretase inhibitors DAPT and L-685,458 have been shown to display arrested growth, exhibit granulosa cell detachment, and contain degenerative oocytes (Zhang et al. 2011). Furthermore, γ-secretase inhibitor treatment leads to a reduction in granulosa cell proliferation that is concomitant with decreased c-Myc expression. Surprisingly, gain-of-function experiments using lentiviral-mediated expression of NICD2 within cultured granulosa cells show a restoration of c-Myc expression and rescue of granulosa cell proliferation in the presence of DAPT (Zhang et al. 2011). This demonstrates that the Notch effector c-Myc plays a critical role in the maintenance and function of granulosa cells.
Ovarian follicles require the formation of gap junctions through the expression of gap junction protein alpha 1 (Gja1) in granulosa cells and gap junction protein alpha 4 (Gja4) in oocytes to prevent premature meiotic resumption (Simon et al. 1997, Juneja et al. 1999). Pharmacological disruption of gap junction complexes in cultured ovarian explants leads to a marked decrease in the expression of Notch2 in pregranulosa cells resulting in reduced somatic cell proliferation (Teng et al. 2016). In addition, germ cells remain in syncytial structures and fail to appropriately assemble into follicular units. Inhibition of Notch signaling using DAPT results in a reciprocal decrease in the expression of multiple gap junction proteins and the key granulosa cell transcription factor Foxl2, supporting an important and complementary relationship between productive Notch signaling and gap junction formation that is required for primordial follicle formation and maintenance (Figure 3A).
The small GTPase, Rac1, has been shown to play an indispensable role in primordial follicle formation by controlling nuclear import of STAT3 in germ cells, which facilitates the transcription of the Notch ligand, Jag1, and the expression of Gdf9, bone morphogenetic protein 15 (Bmp15), and Nobox (Figure 3A) (Zhao et al. 2016). Increased expression of the oocyte-specific growth factors leads to increased translation of NOTCH2 in granulosa cells, via mammalian target of rapamycin complex 1 (mTORC1) modulation (Figure 3A), which is subsequently activated by juxtaposed germ cells expressing JAG1. Similar to models of suppressed Notch signaling, disruption of Rac1 action through pharmacological inhibition or siRNA knockdown leads to delayed syncytial breakdown, reduced numbers of primordial follicles, and increased numbers of multi-oocytic follicles. Conversely, overexpression of Jag1 in oocytes rescues Rac1 inhibition and leads to accelerated syncytial breakdown and follicle assembly through activation of Notch2 in juxtaposed pregranulosa cells.
The tyrosine kinase receptor tropomyosin-related kinase receptor B (TrkB), which supports the actions of neurotrophin 4/5 (Nt-4) and brain-derived neurotrophic factor (Bdnf) during folliculogenesis, has also been shown to impact Notch signaling within the developing ovary (Dorfman et al. 2011). Similar to Rac1, TrkB expression within the ovary is specific to germ cells where it is suspected of being a direct regulator of Jag1 transcription (Figure 3A). In addition to Jag1, the expression of Hes1 and Hey2 are reduced in TrkB null ovaries, suggesting that the inability to express Jag1 within the oocyte leads to suppressed Notch signaling within adjacent granulosa cells expressing Notch2. Deletion of TrkB also leads to a reduction in granulosa cell proliferation, delayed follicular growth, and reduced ovarian follicle survival, consistent with findings from other reports of disrupted Notch signaling within the ovary and studies using blocking antibodies against BDNF and NT-4 (Spears et al. 2003). Interestingly, lentiviral-mediated expression of Jag1 targeted to germ cells of TrkB null ovaries restores Notch activation and rescues the expression of the downstream Notch effector c-Myc (Figure 3B). Notably, although lacking in TrkB null ovaries, mice with disruption of the neurotrophin nerve growth factor (Ngf) exhibit multi-oocytic follicles in addition to reduced numbers of primary and secondary follicles (Dissen et al. 2001).
Once formed, primordial follicles remain quiescent until select cohorts are recruited each reproductive cycle to transition from primordial to primary follicles. This process, termed follicle activation, results in morphological and physiological changes in both the oocyte and somatic cells. Oocytes within activated follicles begin to grow, while squamous pregranulosa cells transition into cuboidal granulosa cells, become proliferative, and later steroidogenic and responsive to pituitary gonadotropins. Within this growing pool of follicles, components of the Notch pathway are dynamically expressed (Table 1) (Johnson et al. 2001, Hahn et al. 2005, Trombly et al. 2009a, Vanorny et al. 2014). The ligand Jag2 and the receptors Notch2 and Notch3 are localized to granulosa cells, the ligand Jag1 is found in oocytes, and the target genes Hes1 and Hey2 are expressed in granulosa cells. Interestingly, Hes1 is localized to both somatic and germ cells of embryonic and postnatal ovaries (Trombly et al. 2009b, Zhang et al. 2011, Manosalva et al. 2013), suggesting an important bidirectional role for the Notch pathway during folliculogenesis. However, deletion of Rbp-j in oocytes under the direction of TNAP-Cre does not have any impact on follicle development or fertility (Manosalva et al. 2013), confirming that canonical Notch activity within the oocyte is dispensable for normal ovarian function and that the actions of Notch signaling are mediated through its function in somatic cell types.
Within a developing follicle, physical contact between the inner layer of granulosa cells and oocyte is maintained via the formation of transzonal projections (TZPs), which are thin cellular extensions that emanate from granulosa cells and penetrate the zona pellucida (Li & Albertini 2013). However, as granulosa cells proliferate to form multi-laminar follicles, they consequently lose direct contact with the oocyte, which may preclude continued juxtacrine signaling between the two cell types. Accordingly, continued Notch activation within granulosa cells of developing follicles requires the interactions of juxtaposed granulosa cells. Examination of a Notch fluorescent reporter demonstrates continued, though variable, Notch activity within granulosa cells of secondary follicles (Figure 2C) and within both mural and cumulus granulosa cells of antral follicles (Figure 2D) that persists through ovulation and luteinization (Vanorny et al. 2014, Vanorny 2016). It is interesting to consider whether a change in the source of Notch ligand during this transition may play a role in follicle activation and in the new cellular processes acquired by granulosa cells.
Change in the source of ligand used for continued Notch activation following follicle recruitment also requires the conversion from Jag1, in oocytes, to Jag2, in granulosa cells. Although the specific role of Jag2 in the ovary has not been determined, a naturally occurring hypomorphic allele for Jag2 reportedly has poor female fertility (Gruneberg 1956), signifying that this ligand may be critical for ovarian function. Additionally, the expression pattern of Jag2 resembles that of the Notch ligand Delta, which is expressed in germline cells and later in somatic follicle cells of Drosophila ovarioles (Lopez-Schier & St Johnston 2001, Torres et al. 2003), again suggesting an evolutionarily conserved role for Notch signaling in metazoan follicle development.
Notch signaling during luteinization
Following stimulation by pituitary gonadotropins, granulosa and thecal cells that remain within follicles that ovulate undergo luteinization to form corpora lutea, which provide a source of steroid hormones throughout pregnancy. The Notch ligands Jag1, Dll1, and Dll4 and the Notch receptors Notch1-4 have all been shown to be expressed within small and large luteal cells (Table 1) (Johnson et al. 2001, Vorontchikhina et al. 2005, Hernandez et al. 2011, Murta et al. 2014, Accialini et al. 2015, Wang et al. 2015), consistent with a role for Notch signaling in their function. Following in vivo treatment with prostaglandin F2 alpha (PGF2a), a uterine-derived factor that initiates luteolysis in rodents, transcripts for Notch1, Notch4, and Dll4 are reduced, as are cleaved forms of the receptors (Figure 3C) (Hernandez et al. 2011). Additionally, suppression of Notch signaling through intrabursal injection with DAPT leads to decreased progesterone production and increased luteal cell apoptosis. These findings suggest that the luteolytic effects of PGF2a may be in part an effect of decreased Notch activation, which is consistent with a luteotropic role for Notch signaling.
Treatment of cultured mouse luteal cells with DAPT or L-658,458 results in a reduction in both basal and hCG-induced progesterone production (Wang et al. 2015). In addition, the inhibitory effects on progesterone levels observed in luteal cells treated with DAPT have been shown to arise from the suppression of cytochrome P450 family 11 subfamily A member 1 (Cyp11a1) expression. In contrast, overexpression of the intracellular domain of Notch3 (NICD3) causes in an increase in both basal and hCG-induced progesterone secretion through the regulation of Cyp11a1 and steroidogenic acute regulatory protein (StAR), which controls the rate-limiting step in steroid biosynthesis (Figure 3C). These results indicate that activation of Notch signaling and stimulation with hCG have synergistic effects on progesterone production in luteal cells. Interestingly, overexpression of NICD3 has no observable effect on estrogen levels despite regulation of upstream enzymes in the steroid synthesis pathway.
Mechanically-isolated rat corpora lutea cultured in the presence of DAPT also have decreased levels of progesterone production as a consequence of reduced expression of Cyp11a1 (Accialini et al. 2015). Furthermore, suppression of Notch signaling is able to reverse progesterone-induced expression of StAR. Consistent with in vivo studies, cultured corpora lutea treated with DAPT have increased markers of apoptosis, as a consequence of decreased levels of the anti-apoptotic protein BCL-xL and the pro-survival factor phosphorylated AKT (Figure 3C). Moreover, suppression of Notch activation is able to reverse the survival effects that progesterone has on luteal cells. Similar to treatments with DAPT, corpora lutea incubated with an inactivating DLL4 antibody have decreased progesterone production. These findings support a novel role for the Notch pathway in the regulation of luteal function in response to progesterone stimulation, and indicate an important relationship between the actions of progesterone and Notch signaling.
In contrast to the findings above, transfection of a gonadotropin-responsive mouse cell line with NICD1 or NICD2, leads to a decrease in progesterone production, implying that Notch activation normally functions to suppress steroidogenesis (George et al. 2015). In addition, the expression of StAR, Cyp19a1, and HSD3b1 are significantly upregulated following pharmacological suppression of Notch signaling in cultured preantral follicles and downregulated with transient transfection of NICD1-3. Furthermore, activation of the Notch pathway represses the ability of GATA4 to induce the expression of these genes through Notch target gene expression and promoter binding, rather than through dimerization of Notch target genes with GATA4 (Figure 3C) (George et al. 2015). These findings, together with studies employing DLL4 and γ-secretase inhibitors discussed above, indicate that alteration in the levels of basal Notch activity negatively impact progesterone production in steroidogenically active cells, suggesting complex regulatory effects of steroid hormone production by Notch signaling.
Notch pathway in the ovarian vasculature
The Notch pathway has previously been shown to be critical for multiple aspects of vasculogenesis and angiogenesis including arteriovenous specification, endothelial and vascular smooth muscle cell differentiation, and the regulation of vessel sprouting and branching (Table 1 and Figure 3D) (Krebs et al. 2000, Gridley 2007, Gridley 2010). Several Notch components are expressed within the ovarian vasculature including Dll4 located at the tip of endothelial cells within the thecal layer (Jovanovic et al. 2013) and growing luteal vessels (Garcia-Pascual et al. 2013); Notch3 within endothelial cells and vascular smooth muscle cells (Jovanovic et al. 2013); and Notch1, Notch4, and Jag1 within thecal layer endothelial and vascular smooth muscle cells (Vorontchikhina et al. 2005, Jovanovic et al. 2013).
The Notch ligand, Dll4, is primarily localized to vascular endothelial cells where it acts to regulate the differentiation and activity of tips cells, which are located at the growing edge of the ovarian neovasculature. Treatment of marmosets during the periovulatory period with a neutralizing DLL4 antibody demonstrates that Dll4 acts as a negative regulator of vascular endothelial growth factor (VEGF) mediated sprouting and branching within the ovarian vasculature (Figure 3D) (Fraser et al. 2012). In addition, inhibition of Dll4 induces luteal hypervascularization, including increased angiogenesis and vascular density, which is associated with a decrease in progesterone production. Furthermore, primate ovaries in which Dll4 is perturbed are reduced in size and weight, and corpora lutea undergo enhanced degeneration due to increased cellular apoptosis. Interestingly, inhibition of Dll4 during the mid-luteal phase has only a minimal impact on progesterone production and does not affect follicular development.
In immature female mice treated with PMSG and hCG, to stimulate follicle development, injection of an inhibitory DLL4 antibody promotes promiscuous expression of Dll4 leading to increased, but non-functional, vascularization that is associated with decreased progesterone production (Figure 3D) (Garcia-Pascual et al. 2013). In contrast, suppression of Notch signaling using a pan-Notch inhibitor, compound E ((s,s)-2-(3,5-Difluorophenyl)-acetylamino]-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-propionamide), causes a reduction in ovarian weight and estrogen production as a result of uninhibited, but highly disorganized, vascular proliferation that culminates in blocked follicle development at the preovulatory stage (Jovanovic et al. 2013). Thus, while the blockade of Notch signaling using a DLL4 inhibitory antibody has an effect on the organization of the follicular vasculature, it appears to have little or no impact on gonadotropin-dependent folliculogenesis, suggesting the involvement of other Notch ligands in this process. These studies demonstrate a specific role for Dll4 in suppressing vascular overgrowth and in organizing ovarian neovasculature during luteal formation.
The role of Notch signaling within the ovarian vasculature of mice has also been examined using a gain-of-function genetic model (Liu et al. 2014). Constitutive activation of the Notch pathway in vascular endothelial cells by induction of NICD1 expression under the control of a tamoxifen-inducible Tie2-Cre allele results in severely altered female fertility, and ovaries from these mice lack mature ovarian follicles. The specific mechanism behind the interrupted development of advanced follicles is attributed to inhibition of bFGF-induced angiogenesis (Figure 3D), supporting a critical role for Notch signaling in vascular development during ovarian follicle maturation.
Role of Notch signaling in the human ovary
Transcriptional profiling of human preantral follicles demonstrates that Notch components are expressed and dynamically regulated during follicle growth in humans (Kristensen et al. 2015). Abundantly expressed Notch pathway transcripts in human preantral follicles include Jag1, Hes1, and Hey2, whereas low to moderately expressed transcripts include Notch1, Notch2, Notch3, Jag2, and Hes5. Notch signaling components with no discernable expression in human preantral follicles include Notch4, Dll1, Dll3, Dll4, and Hes7. Additionally, expression of the Notch target gene Hey1 is significantly reduced with increasing preantral follicle size. In human cumulus granulosa cells obtained by follicle aspiration during IVF, all four Notch receptors and the Notch ligands JAG1 and JAG2 are expressed (Tanriverdi et al. 2013). When comparing poor and normal responders to IVF treatments, there is a significant reduction in the expression of NOTCH2 in the cumulus cells of the poor responders and the expression of both NOTCH2 and NOTCH3 are positively correlated with IVF response.
Polycystic ovarian syndrome (PCOS) is the most common endocrine disorder in females of reproductive age and is characterized by hyperandrogenism, insulin resistance, chronic anovulation, and infertility (Fauser et al. 2012). Ovarian follicles in PCOS patients frequently exhibit arrest of follicle growth at the antral stage leading to the appearance of ovaries containing multiple cysts on diagnostic imaging studies. In addition, there is a reduction in granulosa cell proliferation, hyperplasia of the theca interna, and disrupted ovulation. Profiling of miRNA transcripts in women with PCOS demonstrates that Notch3 is a target of miRNAs that are differentially expressed in the cumulus cells of these patients (Xu et al. 2015). The miRNA hsa-miR-483-5p has been specifically shown to regulate the expression of both Notch3 and mitogen-activated protein kinase (Mapk), suggesting that women with PCOS may have alterations in granulosa cell proliferation and function in part through decreased Notch action.
Each year more than 20,000 women in the United States are diagnosed with, and approximately 14,000 die from, ovarian cancer, making it the leading cause of death of gynecologic malignancy (Group 2014). The overall five-year survival rate for ovarian cancer is approximately 31% (Jemal et al. 2009), and this high rate of mortality can be largely explained by the frequent occurrence of patients with an advanced-stage of disease at the time of diagnosis (Koonings et al. 1989). Notably, genomic profiling has revealed that as many as 22% of high-grade serous ovarian carcinomas contain mutations in components of the Notch pathway with more than 50% of those cases involving Notch3 (Cancer Genome Atlas Research 2011), which is highly associated with poor clinical outcomes (Hu et al. 2014). In addition, the Notch ligands Jag1 (Choi et al. 2008, Cancer Genome Atlas Research 2011) and Jag2 (Euer et al. 2005, Cancer Genome Atlas Research 2011) are also commonly amplified in these cancers, resulting in enhanced Notch signaling, likely through a Notch3 juxtacrine loop, which supports ovarian tumor growth and cellular adhesion (Choi et al. 2008). Though Notch3 is the receptor most frequently involved in ovarian carcinogenesis, the activity of Notch1 has also been shown to be elevated in a subset of these cancers (Rose et al. 2010).
Alterations in the expression of Notch pathway genes has also been correlated with ovarian cancers that develop resistance to treatment (Groeneweg et al. 2014); however, it is unclear by what means aberrant Notch signaling may facilitate the survival of cancer cells. Difficulty in treating ovarian tumors has at least in part been attributed to the formation or maintenance of cancer stem cells (Jordan et al. 2006, Shah et al. 2013, Walters Haygood et al. 2014). Stem cell factors such as Nanog and Oct4 have been shown to be upregulated by the overexpression of NICD3 in cells of the ovarian surface epithelium, suggesting a potential role in supporting an undifferentiated cellular state (Park et al. 2010). In addition, aberrant Notch signaling may cause loss of an epithelial phenotype through an epithelial to mesenchymal transition; thus, allowing tumor cells to acquire a stem cell-like phenotype and resistance to certain therapies (Espinoza & Miele 2013).
Notch signaling has also been shown to play important roles in tumor angiogenesis of other cancers (Li et al. 2007, Benedito et al. 2009), though only a few studies have specifically examined an angiogenic role in ovarian cancer. In one report, Jag1 was shown to be upregulated in ovarian tumor epithelial cells, and disruption of the gene led to a reduction in blood vessel formation and endothelial migration (Lu et al. 2007). In a second study (Hu et al. 2011), Dll4 was found to be upregulated in more than half of ovarian cancer samples that were analyzed, and increased levels of Dll4 expression were correlated with overall poor survival. New treatments designed to target the Notch pathway will hopefully provide additional means by which to counter the various mechanisms by which altered Notch signaling promotes the establishment and maintenance of tumors, especially those that are resistant to current therapeutic options (Rose 2009, Domingo-Domenech et al. 2012, Sahebjam et al. 2013, Groeneweg et al. 2014).
Conclusions
The Notch pathway supports juxtacrine signaling between families of membrane-bound ligands and receptors, and plays a fundamental role in numerous cellular processes during metazoan development. Notch signaling is active in the embryonic mouse ovary and is strongly upregulated at birth in somatic cells that surround germ cell syncytia, coincident with the resolution of these syncytia and establishment of the primordial follicle pool. Communication between juxtaposed germ cells and somatic support cells of the ovary is critical for the formation of follicles and for the establishment of the follicular niche in which the oocyte will develop through ovulation. In addition, the Notch pathway promotes the growth and maturation of ovarian follicles through interactions with juxtaposed follicular cells. Cellular interactions between other populations of cell types within the developing ovary also facilitate productive Notch signaling that is critical for ovarian processes including luteinization and vascular development, which are central to the production and delivery of critical secreted hormonal factors that support pregnancy and female health.
The phenotypes observed in models of disrupted Notch signaling are consistent with the broad functions ascribed to Notch signaling in the regulation of cellular processes that include proliferation, cell death or survival, adhesion and migration, and differentiation and cell-fate specification. These diverse roles for Notch signaling are highly context-dependent and consistent with the concept that the ovary and its functional unit, the follicle, represent a highly complex and dynamic continuum of developmental stages. To address the pathophysiology underlying the considerable histological derangements observed in ovaries with disrupted Notch signaling, additional studies are needed to identify new genes and interactions with other signaling pathways involved in the downstream regulatory actions of the Notch pathway. Furthermore, new strategies to culture and visualize ovarian tissues should help to elucidate the dynamic cellular behaviors and relationships involved in the coordination of ovarian and follicle development.
This review and emerging evidence indicates that the Notch pathway is critical for the establishment of a microenvironment where the female germ cell develops and has distinct regulatory roles throughout the development of the ovarian follicle to support female reproductive function. Understanding the juxtacrine signaling mechanisms by which ovarian follicles serve as a critical environmental niche for the maturation of female germ cells is vital to addressing human infertility and for the establishment of new reproductive technologies to improve oocyte quality and preserve reproductive function and fertility. Furthermore, examination of the interplay between multiple signaling modalities within the ovary and reproductive axis should allow for greater recognition of essential signaling and regulatory networks for the development of new therapeutic paradigms for the improvement of women’s health.
Acknowledgments
Funding
The original research reported in this review was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (P01 HD021921) to KEM. DAV received support from the Cellular and Molecular Basis of Disease Training Program through a grant from the National Institutes of Health, Institute of General Medical Sciences (T32 GM08061). The Northwestern University Multi-Photon Core received support through a grant from the National Institute of Neurological Disorders and Stroke (P30 NS054850).
The authors would like to thank Dr. Pamela Monahan, Rexxi Prasasya, and Nisan Hubbard for their critical evaluation of this review.
Footnotes
Disclosure Summary
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
- Accialini P, Hernandez SF, Bas D, Pazos MC, Irusta G, Abramovich D, Tesone M. A link between Notch and progesterone maintains the functionality of the rat corpus luteum. Reproduction. 2015;149:1–10. doi: 10.1530/REP-14-0449. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- Andersen P, Uosaki H, Shenje LT, Kwon C. Non-canonical Notch signaling: emerging role and mechanism. Trends Cell Biol. 2012;22:257–265. doi: 10.1016/j.tcb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Baladron V, Ruiz-Hidalgo MJ, Nueda ML, Diaz-Guerra MJ, Garcia-Ramirez JJ, Bonvini E, Gubina E, Laborda J. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp Cell Res. 2005;303:343–359. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Blaumueller CM, Qi H, Zagouras P, Artavanis-Tsakonas S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell. 1997;90:281–291. doi: 10.1016/s0092-8674(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Bristol-Gould SK, Hutten CG, Sturgis C, Kilen SM, Mayo KE, Woodruff TK. The development of a mouse model of ovarian endosalpingiosis. Endocrinology. 2005;146:5228–5236. doi: 10.1210/en.2005-0697. [DOI] [PubMed] [Google Scholar]
- Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, Simpson ER, Findlay JK. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–2623. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24:438–441. doi: 10.1038/74307. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MD, Tuft R, Fogarty K, Bao ZZ. Notch signaling plays a key role in cardiac cell differentiation. Mech Dev. 2006;123:626–640. doi: 10.1016/j.mod.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Fu XF, Wang LQ, Wang JJ, Ma HG, Cheng SF, Hou ZM, Ma JM, Quan GB, Shen W, Li L. Primordial follicle assembly was regulated by Notch signaling pathway in the mice. Mol Biol Rep. 2014;41:1891–1899. doi: 10.1007/s11033-014-3038-4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202:407–417. doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Choi JH, Park JT, Davidson B, Morin PJ, Shih Ie M, Wang TL. Jagged-1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716–5723. doi: 10.1158/0008-5472.CAN-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, Sun L, Hu WP, Karsak M, Duka T, Takeda Y, Ou LY, Dawe GS, Yu FG, Ahmed S, Jin LH, Schachner M, Watanabe K, Arsenijevic Y, Xiao ZC. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem. 2004;279:25858–25865. doi: 10.1074/jbc.M313505200. [DOI] [PubMed] [Google Scholar]
- D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman MD, Kerr B, Garcia-Rudaz C, Paredes AH, Dissen GA, Ojeda SR. Neurotrophins acting via TRKB receptors activate the JAGGED1-NOTCH2 cell-cell communication pathway to facilitate early ovarian development. Endocrinology. 2011;152:5005–5016. doi: 10.1210/en.2011-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehebauer M, Hayward P, Martinez-Arias A. Notch signaling pathway. Sci STKE. 2006:cm7. doi: 10.1126/stke.3642006cm7. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–880. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Euer NI, Kaul S, Deissler H, Mobus VJ, Zeillinger R, Weidle UH. Identification of L1CAM, Jagged2 and Neuromedin U as ovarian cancer-associated antigens. Oncol Rep. 2005;13:375–387. [PubMed] [Google Scholar]
- Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. e25. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Feng L, Wang Y, Cai H, Sun G, Niu W, Xin Q, Tang X, Zhang J, Wang C, Zhang H, Xia G. ADAM10-Notch signaling governs the recruitment of ovarian pregranulosa cells and controls folliculogenesis in mice. J Cell Sci. 2016;129:2202–2212. doi: 10.1242/jcs.184267. [DOI] [PubMed] [Google Scholar]
- Feng YM, Liang GJ, Pan B, Qin XS, Zhang XF, Chen CL, Li L, Cheng SF, De Felici M, Shen W. Notch pathway regulates female germ cell meiosis progression and early oogenesis events in fetal mouse. Cell Cycle. 2014;13:782–791. doi: 10.4161/cc.27708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson L, Kaftanovskaya EM, Manresa C, Barbara AM, Poppiti RJ, Tan Y, Agoulnik AI. Constitutive Notch Signaling Causes Abnormal Development of the Oviducts, Abnormal Angiogenesis, and Cyst Formation in Mouse Female Reproductive Tract. Biol Reprod. 2016 doi: 10.1095/biolreprod.115.134569. [DOI] [PubMed] [Google Scholar]
- Flores GV, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U. Combinatorial signaling in the specification of unique cell fates. Cell. 2000;103:75–85. doi: 10.1016/s0092-8674(00)00106-9. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Hastings JM, Allan D, Morris KD, Rudge JS, Wiegand SJ. Inhibition of delta-like ligand 4 induces luteal hypervascularization followed by functional and structural luteolysis in the primate ovary. Endocrinology. 2012;153:1972–1983. doi: 10.1210/en.2011-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pascual CM, Zimmermann RC, Ferrero H, Shawber CJ, Kitajewski J, Simon C, Pellicer A, Gomez R. Delta-like ligand 4 regulates vascular endothelial growth factor receptor 2-driven luteal angiogenesis through induction of a tip/stalk phenotype in proliferating endothelial cells. Fertil Steril. 2013;100:1768–1776. e1761. doi: 10.1016/j.fertnstert.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Garcia TX, Hofmann MC. NOTCH signaling in Sertoli cells regulates gonocyte fate. Cell Cycle. 2013;12:2538–2545. doi: 10.4161/cc.25627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- George RM, Hahn KL, Rawls A, Viger RS, Wilson-Rawls J. Notch signaling represses GATA4-induced expression of genes involved in steroid biosynthesis. Reproduction. 2015;150:383–394. doi: 10.1530/REP-15-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Vardar-Ulu D, L’Heureux S, Ashworth T, Malecki MJ, Sanchez-Irizarry C, McArthur DG, Histen G, Mitchell JL, Aster JC, Blacklow SC. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One. 2009;4:e6613. doi: 10.1371/journal.pone.0006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammont M, Irvine KD. fringe and Notch specify polar cell fate during Drosophila oogenesis. Development. 2001;128:2243–2253. doi: 10.1242/dev.128.12.2243. [DOI] [PubMed] [Google Scholar]
- Greenwald I, Kovall R. Notch signaling: genetics and structure. WormBook. 2013:1–28. doi: 10.1895/wormbook.1.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003;12(Spec No 1):R9–13. doi: 10.1093/hmg/ddg052. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling in the vasculature. Curr Top Dev Biol. 2010;92:277–309. doi: 10.1016/S0070-2153(10)92009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg JW, Foster R, Growdon WB, Verheijen RH, Rueda BR. Notch signaling in serous ovarian cancer. J Ovarian Res. 2014;7:95. doi: 10.1186/s13048-014-0095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group UCSW 2014 United States Cancer Statistics: 1999–2011 incidence and mortality web-based report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; [Google Scholar]
- Gruneberg H. Genetical studies on the skeleton of the mouse. XVIII. Three genes for syndactylism. In. J Genet. 1956:113–145. [Google Scholar]
- Guo M, Zhang H, Bian F, Li G, Mu X, Wen J, Mao G, Teng Z, Xia G, Zhang M. P4 down-regulates Jagged2 and Notch1 expression during primordial folliculogenesis. Front Biosci (Elite Ed) 2012;4:2731–2744. doi: 10.2741/e579. [DOI] [PubMed] [Google Scholar]
- Hahn KL, Johnson J, Beres BJ, Howard S, Wilson-Rawls J. Lunatic fringe null female mice are infertile due to defects in meiotic maturation. Development. 2005;132:817–828. doi: 10.1242/dev.01601. [DOI] [PubMed] [Google Scholar]
- Haines N, Irvine KD. Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol. 2003;4:786–797. doi: 10.1038/nrm1228. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzler P. Biodiversity and noncanonical Notch signaling. Curr Top Dev Biol. 2010;92:457–481. doi: 10.1016/S0070-2153(10)92014-0. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Peluffo MC, Stouffer RL, Irusta G, Tesone M. Role of the DLL4-NOTCH system in PGF2alpha-induced luteolysis in the pregnant rat. Biol Reprod. 2011;84:859–865. doi: 10.1095/biolreprod.110.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Gifford JA, Hunzicker-Dunn ME, Nilson JH. Conditional deletion of beta-catenin mediated by Amhr2cre in mice causes female infertility. Biol Reprod. 2009;80:1282–1292. doi: 10.1095/biolreprod.108.072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Hu W, Liu T, Ivan C, Sun Y, Huang J, Mangala LS, Miyake T, Dalton HJ, Pradeep S, Rupaimoole R, Previs RA, Han HD, Bottsford-Miller J, Zand B, Kang Y, Pecot CV, Nick AM, Wu SY, Lee JS, Sehgal V, Ram P, Liu J, Tucker SL, Lopez-Berestein G, Baggerly KA, Coleman RL, Sood AK. Notch3 pathway alterations in ovarian cancer. Cancer Res. 2014;74:3282–3293. doi: 10.1158/0008-5472.CAN-13-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu C, Dong HH, Huang J, Shen DY, Stone RL, Nick AM, Shahzad MM, Mora E, Jennings NB, Lee SJ, Roh JW, Matsuo K, Nishimura M, Goodman BW, Jaffe RB, Langley RR, Deavers MT, Lopez-Berestein G, Coleman RL, Sood AK. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–6039. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Rivas B, Agoulnik AI. NOTCH1 Gain of Function in Germ Cells Causes Failure of Spermatogenesis in Male Mice. PLoS One. 2013;8:e71213, 71211–71210. doi: 10.1371/journal.pone.0071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt KJ, McLaughlin EA, Holland MK. KIT/KIT ligand in mammalian oogenesis and folliculogenesis: roles in rabbit and murine ovarian follicle activation and oocyte growth. Biol Reprod. 2006;75:421–433. doi: 10.1095/biolreprod.106.051516. [DOI] [PubMed] [Google Scholar]
- Iguchi T. Cellular effects of early exposure to sex hormones and antihormones. Int Rev Cytol. 1992;139:1–57. doi: 10.1016/s0074-7696(08)61409-6. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Watanabe H, Katsu Y. Developmental effects of estrogenic agents on mice, fish, and frogs: a mini-review. Horm Behav. 2001;40:248–251. doi: 10.1006/hbeh.2001.1675. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- Johnson J, Espinoza T, McGaughey RW, Rawls A, Wilson-Rawls J. Notch pathway genes are expressed in mammalian ovarian follicles. Mech Dev. 2001;109:355–361. doi: 10.1016/s0925-4773(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- Jordan KC, Clegg NJ, Blasi JA, Morimoto AM, Sen J, Stein D, McNeill H, Deng WM, Tworoger M, Ruohola-Baker H. The homeobox gene mirror links EGF signalling to embryonic dorso-ventral axis formation through notch activation. Nat Genet. 2000;24:429–433. doi: 10.1038/74294. [DOI] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Jovanovic VP, Sauer CM, Shawber CJ, Gomez R, Wang X, Sauer MV, Kitajewski J, Zimmermann RC. Intraovarian regulation of gonadotropin-dependent folliculogenesis depends on notch receptor signaling pathways not involving Delta-like ligand 4 (Dll4) Reprod Biol Endocrinol. 2013;11:43. doi: 10.1186/1477-7827-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja SC, Barr KJ, Enders GC, Kidder GM. Defects in the germ line and gonads of mice lacking connexin43. Biol Reprod. 1999;60:1263–1270. doi: 10.1095/biolreprod60.5.1263. [DOI] [PubMed] [Google Scholar]
- Keri RA, Lozada KL, Abdul-Karim FW, Nadeau JH, Nilson JH. Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proc Natl Acad Sci U S A. 2000;97:383–387. doi: 10.1073/pnas.97.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Kolev V, Mandinova A, Guinea-Viniegra J, Hu B, Lefort K, Lambertini C, Neel V, Dummer R, Wagner EF, Dotto GP. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat Cell Biol. 2008;10:902–911. doi: 10.1038/ncb1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonings PP, Campbell K, Mishell DR, Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989;74:921–926. [PubMed] [Google Scholar]
- Kopan R. Notch: a membrane-bound transcription factor. J Cell Sci. 2002;115:1095–1097. doi: 10.1242/jcs.115.6.1095. [DOI] [PubMed] [Google Scholar]
- Kopan R, Goate A. A common enzyme connects notch signaling and Alzheimer’s disease. Genes Dev. 2000;14:2799–2806. doi: 10.1101/gad.836900. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol. 2015;401:189–201. doi: 10.1016/j.mce.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 2006;107:2223–2233. doi: 10.1182/blood-2005-08-3329. [DOI] [PubMed] [Google Scholar]
- Li JL, Sainson RC, Shi W, Leek R, Harrington LS, Preusser M, Biswas S, Turley H, Heikamp E, Hainfellner JA, Harris AL. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007;67:11244–11253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- Li L, Milner LA, Deng Y, Iwata M, Banta A, Graf L, Marcovina S, Friedman C, Trask BJ, Hood L, Torok-Storb B. The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity. 1998;8:43–55. doi: 10.1016/s1074-7613(00)80457-4. [DOI] [PubMed] [Google Scholar]
- Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–152. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8:14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- Liu J, Deutsch U, Jeong J, Lobe CG. Constitutive notch signaling in adult transgenic mice inhibits bFGF-induced angiogenesis and blocks ovarian follicle development. Genesis. 2014;52:809–816. doi: 10.1002/dvg.22790. [DOI] [PubMed] [Google Scholar]
- Liu Z, Brunskill E, Varnum-Finney B, Zhang C, Zhang A, Jay PY, Bernstein I, Morimoto M, Kopan R. The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development. 2015;142:2452–2463. doi: 10.1242/dev.125492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeat F, Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Schier H, St Johnston D. Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 2001;15:1393–1405. doi: 10.1101/gad.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, Coleman RL, Gershenson DM, Jaffe RB, Birrer MJ, Sood AK. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67:1757–1768. doi: 10.1158/0008-5472.CAN-06-3700. [DOI] [PubMed] [Google Scholar]
- Lubman OY, Ilagan MX, Kopan R, Barrick D. Quantitative dissection of the Notch:CSL interaction: insights into the Notch-mediated transcriptional switch. J Mol Biol. 2007;365:577–589. doi: 10.1016/j.jmb.2006.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard I, Pear WS. Notch and cancer: best to avoid the ups and downs. Cancer Cell. 2003;3:203–205. doi: 10.1016/s1535-6108(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Manosalva I, Gonzalez A, Kageyama R. Hes1 in the somatic cells of the murine ovary is necessary for oocyte survival and maturation. Dev Biol. 2013;375:140–151. doi: 10.1016/j.ydbio.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, DeMayo FJ, Hadsell LA, Kumar TR. Overexpression of human chorionic gonadotropin causes multiple reproductive defects in transgenic mice. Biol Reprod. 2003;69:338–346. doi: 10.1095/biolreprod.102.013953. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- McMullen ML, Cho BN, Yates CJ, Mayo KE. Gonadal pathologies in transgenic mice expressing the rat inhibin alpha-subunit. Endocrinology. 2001;142:5005–5014. doi: 10.1210/endo.142.11.8472. [DOI] [PubMed] [Google Scholar]
- Meehan TP, Harmon BG, Overcast ME, Yu KK, Camper SA, Puett D, Narayan P. Gonadal defects and hormonal alterations in transgenic mice expressing a single chain human chorionic gonadotropin-lutropin receptor complex. J Mol Endocrinol. 2005;34:489–503. doi: 10.1677/jme.1.01669. [DOI] [PubMed] [Google Scholar]
- Meng H, Zhang X, Hankenson KD, Wang MM. Thrombospondin 2 potentiates notch3/jagged1 signaling. J Biol Chem. 2009;284:7866–7874. doi: 10.1074/jbc.M803650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Lau R, Hein PW, Shipley JM, Weinmaster G. Microfibrillar proteins MAGP-1 and MAGP-2 induce Notch1 extracellular domain dissociation and receptor activation. J Biol Chem. 2006;281:10089–10097. doi: 10.1074/jbc.M600298200. [DOI] [PubMed] [Google Scholar]
- Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150:4386–4394. doi: 10.1210/en.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Veraksa A, Bauer A, Rosse C, Camonis J, Artavanis-Tsakonas S. Regulation of Notch signalling by non-visual beta-arrestin. Nat Cell Biol. 2005;7:1191–1201. doi: 10.1038/ncb1327. [DOI] [PubMed] [Google Scholar]
- Mullendore ME, Koorstra JB, Li YM, Offerhaus GJ, Fan X, Henderson CM, Matsui W, Eberhart CG, Maitra A, Feldmann G. Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clin Cancer Res. 2009;15:2291–2301. doi: 10.1158/1078-0432.CCR-08-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Murta D, Batista M, Silva E, Trindade A, Mateus L, Duarte A, Lopes-da-Costa L. Differential expression of Notch component and effector genes during ovarian follicle and corpus luteum development during the oestrous cycle. Reprod Fertil Dev. 2014 doi: 10.1071/RD13399. [DOI] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM. Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol. 2008;22:2336–2352. doi: 10.1210/me.2008-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction. 2006;132:877–886. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Jorgez CJ, Tran M, Agno J, Li X, Brown CW, Kumar TR, Matzuk MM. Intraovarian activins are required for female fertility. Mol Endocrinol. 2007;21:2458–2471. doi: 10.1210/me.2007-0146. [DOI] [PubMed] [Google Scholar]
- Park JT, Chen X, Trope CG, Davidson B, Shih Ie M, Wang TL. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am J Pathol. 2010;177:1087–1094. doi: 10.2353/ajpath.2010.100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23:450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143:139–149. doi: 10.1530/REP-11-0299. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Raetzman LT, Wheeler BS, Ross SA, Thomas PQ, Camper SA. Persistent expression of Notch2 delays gonadotrope differentiation. Mol Endocrinol. 2006;20:2898–2908. doi: 10.1210/me.2005-0394. [DOI] [PubMed] [Google Scholar]
- Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305:1157–1159. doi: 10.1126/science.1099755. [DOI] [PubMed] [Google Scholar]
- Rauen T, Raffetseder U, Frye BC, Djudjaj S, Muhlenberg PJ, Eitner F, Lendahl U, Bernhagen J, Dooley S, Mertens PR. YB-1 acts as a ligand for Notch-3 receptors and modulates receptor activation. J Biol Chem. 2009;284:26928–26940. doi: 10.1074/jbc.M109.046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, Dickson BC, Lockwood G, Gallinger S, Egan SE. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33:1223–1229. doi: 10.3892/ijo_00000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci U S A. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risma KA, Hirshfield AN, Nilson JH. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;138:3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- Rose SL. Notch signaling pathway in ovarian cancer. Int J Gynecol Cancer. 2009;19:564–566. doi: 10.1111/IGC.0b013e3181a12ed2. [DOI] [PubMed] [Google Scholar]
- Rose SL, Kunnimalaiyaan M, Drenzek J, Seiler N. Notch 1 signaling is active in ovarian cancer. Gynecol Oncol. 2010;117:130–133. doi: 10.1016/j.ygyno.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Sahebjam S, Bedard PL, Castonguay V, Chen Z, Reedijk M, Liu G, Cohen B, Zhang WJ, Clarke B, Zhang T, Kamel-Reid S, Chen H, Ivy SP, Razak AR, Oza AM, Chen EX, Hirte HW, McGarrity A, Wang L, Siu LL, Hotte SJ. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours (PJC-004/NCI 8503) Br J Cancer. 2013;109:943–949. doi: 10.1038/bjc.2013.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. FASEB J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- Saitou M, Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, Li CL, Perbal B, Katsube K. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notch1 extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- Saxena MT, Schroeter EH, Mumm JS, Kopan R. Murine notch homologs (N1-4) undergo presenilin-dependent proteolysis. J Biol Chem. 2001;276:40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- Schmidt MH, Bicker F, Nikolic I, Meister J, Babuke T, Picuric S, Muller-Esterl W, Plate KH, Dikic I. Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat Cell Biol. 2009;11:873–880. doi: 10.1038/ncb1896. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Regulation of notch signaling activity. Curr Biol. 2004;14:R129–138. [PubMed] [Google Scholar]
- Shah MM, Zerlin M, Li BY, Herzog TJ, Kitajewski JK, Wright JD. The role of Notch and gamma-secretase inhibition in an ovarian cancer model. Anticancer Res. 2013;33:801–808. [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Hosoya N, Kumano K, Saito T, Kurokawa M, Kanda Y, Hamada Y, Hirai H. Binding of Delta1, Jagged1, and Jagged2 to Notch2 rapidly induces cleavage, nuclear translocation, and hyperphosphorylation of Notch2. Mol Cell Biol. 2000a;20:6913–6922. doi: 10.1128/mcb.20.18.6913-6922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Kumano K, Hosoya N, Takahashi T, Kanda Y, Hamada Y, Yazaki Y, Hirai H. Mouse jagged1 physically interacts with notch2 and other notch receptors. Assessment by quantitative methods. J Biol Chem. 1999;274:32961–32969. doi: 10.1074/jbc.274.46.32961. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Chiba S, Saito T, Kumano K, Hirai H. Physical interaction of Delta1, Jagged1, and Jagged2 with Notch1 and Notch3 receptors. Biochem Biophys Res Commun. 2000b;276:385–389. doi: 10.1006/bbrc.2000.3469. [DOI] [PubMed] [Google Scholar]
- Silva-Santos K, Seneda M. Multioocyte follicles in adult mammalian ovaries. Anim Reprod. 2011;8:58–67. [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Simpson MA, Irving MD, Asilmaz E, Gray MJ, Dafou D, Elmslie FV, Mansour S, Holder SE, Brain CE, Burton BK, Kim KH, Pauli RM, Aftimos S, Stewart H, Kim CA, Holder-Espinasse M, Robertson SP, Drake WM, Trembath RC. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nat Genet. 2011;43:303–305. doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- Soyal SM, Amleh A, Dean J. FIGalpha, a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–4654. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, Telfer EE, Anderson RA, Price DJ. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development. 2003;130:5481–5491. doi: 10.1242/dev.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stittrich AB, Lehman A, Bodian DL, Ashworth J, Zong Z, Li H, Lam P, Khromykh A, Iyer RK, Vockley JG, Baveja R, Silva ES, Dixon J, Leon EL, Solomon BD, Glusman G, Niederhuber JE, Roach JC, Patel MS. Mutations in NOTCH1 cause Adams-Oliver syndrome. Am J Hum Genet. 2014;95:275–284. doi: 10.1016/j.ajhg.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Brennan J, Karl J, Hamada Y, Raetzman L, Capel B. Notch signaling maintains Leydig progenitor cells in the mouse testis. Development. 2008;135:3745–3753. doi: 10.1242/dev.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi G, Denir S, Ayla S, Bilir A, Oktar H, Cepni I, Irez T. Notch signaling pathway in cumulus cells can be a novel marker to identify poor and normal responder IVF patients. J Assist Reprod Genet. 2013;30:1319–1326. doi: 10.1007/s10815-013-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Z, Wang C, Wang Y, Huang K, Xiang X, Niu W, Feng L, Zhao L, Yan H, Zhang H. Gap junctions are essential for murine primordial follicle assembly immediately before birth. Reproduction. 2016;151:105–115. doi: 10.1530/REP-15-0282. [DOI] [PubMed] [Google Scholar]
- Terauchi KJ, Shigeta Y, Iguchi T, Sato T. Role of Notch signaling in granulosa cell proliferation and polyovular follicle induction during folliculogenesis in mouse ovary. Cell Tissue Res. 2016;365:197–208. doi: 10.1007/s00441-016-2371-4. [DOI] [PubMed] [Google Scholar]
- Torres IL, Lopez-Schier H, St Johnston D. A Notch/Delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev Cell. 2003;5:547–558. doi: 10.1016/s1534-5807(03)00272-7. [DOI] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009a;27:14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology. 2009b;150:1014–1024. doi: 10.1210/en.2008-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vanorny DA. Notch Signaling during Ovarian Follicle Formation and Development. 2016. [Google Scholar]
- Vanorny DA, Prasasya RD, Chalpe AJ, Kilen SM, Mayo KE. Notch signaling regulates ovarian follicle formation and coordinates follicular growth. Mol Endocrinol. 2014;28:499–511. doi: 10.1210/me.2013-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorontchikhina MA, Zimmermann RC, Shawber CJ, Tang H, Kitajewski J. Unique patterns of Notch1, Notch4 and Jagged1 expression in ovarian vessels during folliculogenesis and corpus luteum formation. Gene Expr Patterns. 2005;5:701–709. doi: 10.1016/j.modgep.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Walters Haygood CL, Arend RC, Straughn JM, Buchsbaum DJ. Ovarian cancer stem cells: Can targeted therapy lead to improved progression-free survival? World J Stem Cells. 2014;6:441–447. doi: 10.4252/wjsc.v6.i4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu S, Peng L, Dong Q, Bao R, Lv Q, Tang M, Hu C, Li G, Liang S, Zhang C. Notch Signaling Pathway Regulates Progesterone Secretion in Murine Luteal Cells. Reprod Sci. 2015 doi: 10.1177/1933719115572480. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Liu JC, Chen CL, Cheng SF, Sun XF, Zhao Y, Yin S, Hou ZM, Pan B, Ding C, Shen W, Zhang XF. Regulation of primordial follicle recruitment by cross-talk between the Notch and phosphatase and tensin homologue (PTEN)/AKT pathways. Reprod Fertil Dev. 2014 doi: 10.1071/RD14212. [DOI] [PubMed] [Google Scholar]
- Weijzen S, Velders MP, Elmishad AG, Bacon PE, Panella JR, Nickoloff BJ, Miele L, Kast WM. The Notch ligand Jagged-1 is able to induce maturation of monocyte-derived human dendritic cells. J Immunol. 2002;169:4273–4278. doi: 10.4049/jimmunol.169.8.4273. [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang YW, Tong XH, Liu YS. Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS. Mol Cell Endocrinol. 2015;404:26–36. doi: 10.1016/j.mce.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Xu J. Notch Function in Mouse Folliculogenesis. 2011. [Google Scholar]
- Xu J, Gridley T. Notch2 is required in somatic cells for breakdown of ovarian germ-cell nests and formation of primordial follicles. BMC Biol. 2013;11:13. doi: 10.1186/1741-7007-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Norton CR, Gridley T. Not all lunatic fringe null female mice are infertile. Development. 2006;133:579. doi: 10.1242/dev.02221. author reply 579–580. [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- Zhang CP, Yang JL, Zhang J, Li L, Huang L, Ji SY, Hu ZY, Gao F, Liu YX. Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology. 2011;152:2437–2447. doi: 10.1210/en.2010-1182. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu K. Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood. Hum Reprod Update. 2015 doi: 10.1093/humupd/dmv037. [DOI] [PubMed] [Google Scholar]
- Zhao L, Du X, Huang K, Zhang T, Teng Z, Niu W, Wang C, Xia G. Rac1 modulates the formation of primordial follicles by facilitating STAT3-directed Jagged1, GDF9 and BMP15 transcription in mice. Sci Rep. 2016;6:23972. doi: 10.1038/srep23972. [DOI] [PMC free article] [PubMed] [Google Scholar]