Abstract
Despite abundant clinical evidence linking metabolic abnormalities to diabetic vasculopathy, the molecular basis of individual susceptibility to diabetic vascular complications is still largely undetermined. Endothelial dysfunction in diabetes-associated vascular complications is considered an early stage of vasculopathy and has attracted considerable research interests. Type 2 diabetes is characterized by metabolic abnormalities, such as hyperglycemia, excess liberation of free fatty acids (FFA), insulin resistance and hyperinsulinemia. These abnormalities exert pathological impact on endothelial function by attenuating endothelium-mediated vasomotor function, enhancing endothelial apoptosis, stimulating endothelium activation/endothelium–monocyte adhesion, promoting an atherogenic response and suppressing barrier function. There are multiple signaling pathways contributing to the adverse effects of glucotoxicity on endothelial function. Insulin maintains the normal balance for release of several factors with vasoactive properties. Abnormal insulin signaling in the endothelium does not affect the whole-body glucose metabolism, but impairs endothelial response to insulin and accelerates atherosclerosis. Excessive level of FFA is implicated in the pathogenesis of insulin resistance. FFA induces endothelial oxidative stress, apoptosis and inflammatory response, and inhibits insulin signaling. Although hyperglycemia, insulin resistance, hyperinsulinemia and dyslipidemia independently contribute to endothelial dysfunction via various distinct mechanisms, the mutual interactions may synergistically accelerate their adverse effects. Oxidative stress and inflammation are predicted to be among the first alterations which may trigger other downstream mediators in diabetes associated with endothelial dysfunction. These mechanisms may provide insights into potential therapeutic targets that can delay or reverse diabetic vasculopathy.
Keywords: Endothelial function, Dyslipidemia, Hyperglycemia, Insulin resistance, Inflammation, Oxidative stress
Introduction
The increased prevalence of obesity is closely associated with the rising incidence of cardiovascular diseases and type 2 diabetes [30, 100]. Diabetes creates an environment adverse to vascular function through a wide variety of metabolic assaults [65], and is linked to macro- and microvasculopathy [35]. Macrovascular complications include coronary artery disease, stroke and peripheral vascular disease. Microvascular consequences include retinopathy and nephropathy, which are regarded as major causes of blindness and end-stage renal failure [51, 60]. Obesity-related insulin resistance, which when severe is Type 2 diabetes, is associated with progression of endothelial impairment [7]. Endothelial dysfunction is a key event in the pathogenesis of diabetic micro- and macrovasculopathy and has gained increasing attention in the study of diabetes-associated cardiovascular complications.
The contributing factors underlying impaired endothelial function in diabetes are varied and commonly include metabolic abnormalities such as hyperglycemia, excess liberation of free fatty acids (FFA) and insulin resistance (see Ref. [71] for review). This review will focus on the current knowledge regarding mechanisms of metabolic abnormalities in type 2 diabetes that drive endothelial dysfunction.
Endothelium and vasomotor function in diabetes
The endothelium releases various contracting and relaxing factors that are responsible for control of blood vessel tone and balance between vasodilation and vasoconstriction (see Ref. [79] for review).
Endothelium-dependent vasoconstriction is exacerbated in diabetes [80]. Endothelin-1 (ET-1), a potent vasoconstricting peptide released from endothelial cells, plays critical roles in diabetes-associated vascular complication (see Ref. [19] for review). ET-1 expression is increased in microvascular endothelial cells isolated from subcutaneous adipose tissue of type 2 diabetic subjects, accompanied by increased basal mitogen-activated protein kinase (MAPK) activity [28]. In cultured endothelial cells, activation of extracellular signal-regulated kinase 5 (ERK5) [89] or inhibition of the janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway [42] suppresses high glucose-induced ET-1 expression. The endothelium also produces cyclooxygenase (COX)-dependent vasoactive factors [20, 88], including the vasoconstrictors, prostaglandin H2 (PGH2) and thromboxane A2 (TXA2), and the vasodilator, prostacyclin (PGI2). Indomethacin, a nonselective inhibitor of COX, abolished hypoxia-induced dilation of skeletal muscle resistance arterioles in obese Zucker rats, while blockade of PGH2/TXA2 receptors and the inhibition of thromboxane synthase increased hypoxia-induced dilation. Moreover, the TXA2 level was higher in the arterioles of obese rats. Together, these data suggest that impaired hypoxia-induced dilation in obese rats may be due in part to an increased vascular production of TXA2 which competes against the vasodilator influences of PGI2 [29]. In intramyocardial arteries of obese Zucker rats, COX-1 inhibition enhanced arachidonic acid (AA)-induced vasorelaxation and inhibited serotonin-induced vasoconstriction, but COX-2 inhibition reduced AA-induced vasorelaxation without modifying serotonin-induced response [64]. These results suggest that COX-2-mediated vasorelaxation in coronary arteries from insulin-resistant obese Zucker rats is enhanced, which may represent a compensatory mechanism.
Factors contributing to vasodilation include nitric oxide(NO), PGI2 and endothelium-derived hyperpolarizing factors (EDHF). Among all the factors, NO is the major factor in regulating endothelium-dependent relaxation. S-Nitrosylation of soluble guanylyl cyclase (sGC) by endothelial NO was recently identified as a mechanism that may compensate for moderate reduction of vascular NO bio-availability [54]. NO-mediated endothelium-dependent vasodilation is impaired in type 2 diabetic mice (db/db), which is attributed to reduced expression and/or phosphorylation (Ser1177) of endothelial nitric oxide synthase (eNOS) [56, 102], enhanced eNOS uncoupling [55] and increased inactivation of NO by reactive oxygen species (ROS) [11]. Superoxide ( ), hydrogen peroxide (H2O2) and peroxynitrate (ONOO−) are significant ROS in vasculopathy. There are multiple cellular sources of , including NAD(P)H oxidase, xanthine oxidase, the mitochondrial respiratory chain, the AA cascade (including lipoxygenase and COX) and uncoupled eNOS [66]. NAD(P)H oxidase is a known key source of in the vasculature (see Ref. [15] for review). One of the NAD(P)H oxidase isoforms, Nox2, is especially abundant in the endothelium. Endothelium-specific overexpression of Nox2 exacerbated angiotensin II-induced oxidative stress and attenuated endothelium-dependent vasorelaxation [50]. Increased intracellular production of derived from NADPH oxidase does not inhibit eNOS activity directly, but instead prevents the extracellular actions of NO by producing ONOO− [104] leading to protein tyrosine nitration and the generation of nitrotyrosine. NAD(P)H oxidase activity, production and nitrotyrosine levels are increased in coronary microvessels and aortae of db/db mice, accompanied by impaired endothelium-dependent vasodilation [23, 102].
In addition to NO, EDHF is also an important mediator of vascular tone and reactivity in diabetes, especially in small resistance vessels (see Ref. [21, 24] for review). A recent study suggests that both NO and EDHF-mediated vasodilation is impaired in mesenteric arteries of Otsuka Long-Evans Tokushima fatty (OLETF) type 2 diabetic rats [45]. Our work shows in coronary arterioles of db/db mice, NO-mediated vasodilation is significantly reduced, but a preserved EDHF function contributes to endothelium-dependent vasodilation [56]. Soluble epoxide hydrolase (s-EH) rapidly hydrolyzes certain epoxylipids (e.g., EET) to less bioactive diols (DHET), thereby attenuating the evoked vasodilator effects. In db/db mice, oral administration of s-EH inhibitors prevented endothelial dysfunction, and the effects were not affected by incubating mesenteric arteries with L-NAME and indomethacin [103].
Thus, diabetes-associated vasodilatory dysfunction is associated with increased production or sensitivity to vasoconctrictors, as well as decreased production or increased degradation of endogenous vasodilators. In addition, in diabetes the relative importance of the endogenous vasodilatory mechanisms are altered and exhibit compensatory dilatory pathways.
Role of hyperglycemia in diabetes-associated endothelial dysfunction
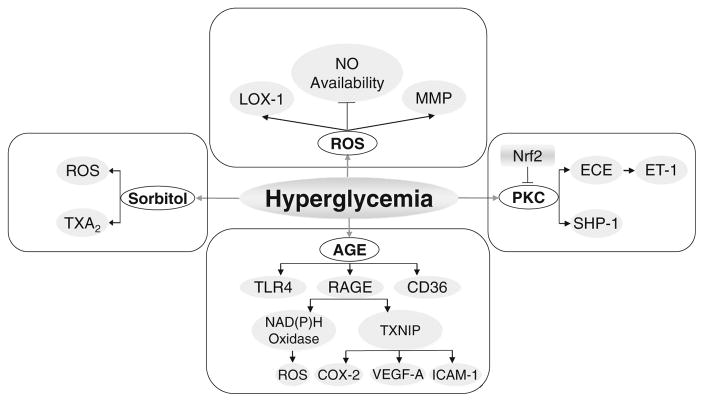
In diabetes, the progression of vasculopathy is highly dependent upon the degree of hyperglycemia [4]. Prior work proposed various biochemical mechanisms which address how hyperglycemia leads to diabetic endothelial dysfunction [65]. This section will primarily focus on the newly identified signaling pathways by which hyperglycemia-induced metabolites exert adverse effects on endothelial function (Fig. 1).
Fig. 1.
The impact of hyperglycemia on endothelial dysfunction. Hyperglycemia causes an increase in toxic metabolites resulting in increased production of ROS, advanced glycation end products (AGE), production of sorbitol and stimulation of protein kinase C (PKC). Activation of these pathways promotes increased vascular oxidative stress, inflammation, apoptosis, atherogenesis and impaired endothelial function. See text for details. AGE advanced glycation end products, COX-2 cyclooxygenase-2, ECE endothelin converting enzyme, ET-1 endothelin-1, LOX-1 lectin-like oxLDL receptor-1, MMP matrix metalloproteinase, NO nitric oxide, Nrf2 transcription factor NF-E2-related factor-2, PKC protein kinase C, RAGE receptor of AGE, ROS reactive oxygen species, SHP-1 Src homology-2 domain-containing phosphatase-1, TLR4 toll-like receptor 4, TXA2 thromboxane A2, TXNIP thioredoxin-interacting protein
The increased production of diacylglycerol (DAG) through glycolysis increases activation of protein kinase C (PKC), which is associated with vascular abnormalities in permeability, contractility, extracellular matrix synthesis, cell growth, apoptosis, angiogenesis, leukocyte adhesion, and cytokine activation and inhibition (see Ref. [27] for review). In cultured human microvascular endothelial cells, hyperglycemia-induced increase in PKC activity can be reversed by activation of transcription factor NF-E2-related factor-2 (nrf2), which regulates antioxidant defense responses [90]. The distinctive role of different PKC isoforms and the therapeutic implications require further investigation. In high glucose-treated primary human umbilical vein endothelial cells (HUVEC), the expression of endothelin converting enzyme-1 (ECE-1) increased as was ET-1 production, which was abolished by inhibiting PKC-delta, but not PKC-alpha, and PKC-beta [36]. In a rat model of type 2 diabetes and hypertension, the PKC-beta inhibitor, ruboxistaurin, restored endothelium-dependent vascular relaxation and suppressed vascular contraction [41]. In addition to the direct effects on endothelial cells, hyperglycemia affects ‘cross talk’ of vascular endothelial cells and pericytes through activating PKC-delta, which increases both expression of Src homology-2 domain-containing phosphatase-1 (SHP-1) and pericyte apoptosis, critical factors in development of diabetic retinopathy [26].
Another area of great interest focused on glucose-induced formation of non-enzymatic advanced glycation end products (AGE) [65]. AGE signaling is mediated through the receptor of AGE (RAGE) or other targets such as the toll-like receptor 4 (TLR4) and CD36. Deleterious vascular effects by AGE can occur by receptor-independent or dependent pathways [32]. In HUVEC, glycated albumin, a precursor of AGE, up-regulates NADPH oxidase and enhances oxidative stress [63]. In human aortic endothelial cells (HAEC), high glucose increased the expression of RAGE. High glucose-induced RAGE expression was normalized by overexpression of either uncoupling protein 1 (UCP1), superoxide dismutase 2 (SOD2) or glyoxalase 1(GLO1) [93]. In retinal endothelial cells, RAGE activation by hyperglycemia induces the expression of thioredoxin-interacting protein (TXNIP, an endogenous inhibitor of the antioxidant thioredoxin) and inflammatory genes such as COX-2, vascular endothelial growth factor-A (VEGF-A) and intercellular adhesion molecule-1 (ICAM-1) [58]. In isolated rat mesenteric arteries, methylglyoxal, an AGE precursor, impaired endothelial function and increased nitrotyrosine expression [9]. In isolated rat retina, AGE also caused increased capillary permeability since pretreatment with anti-RAGE antibodies prevented the abnormalities [85]. Lastly, administration of soluble form of RAGE (sRAGE) partially restored coronary endothelial function in db/db mice [25]. These studies highlight the critical importance of AGE in the pathogenesis of vascular dysfunction in diabetes.
Sorbitol, another toxic compound produced by abnormal metabolic pathways in diabetes, results from increased activity of the polyol pathway. In isolated, pressurized rat gracilis muscle arterioles, the aldose reductase (AR) inhibitor, zopolrestat, attenuated hyperglycemia-induced impairment of flow-mediated vasodilation [76]. Increasing doses of sorbitol elicited dose-dependent constrictions, which were abolished by endothelium removal, SQ-29548 or superoxide dismutase (SOD) plus catalase [76]. The sorbitol pathway serves as an important mechanism for diabetic retinopathy [40]. AR levels are lower in endothelial cells compared to pericytes. Hyperglycemia induces significant polyol accumulation in pericytes, which can be inhibited by AR inhibitors, but little or no accumulation in endothelial cells [34].
Increased ROS serves as a final common pathway of hyperglycemia-induced vascular dysfunction through a multitude of mechanisms. In addition to the inactivation of NO, hyperglycemia-induced ROS production may directly promote vascular apoptosis and remodeling. Hyperglycemia-induced endothelial apoptosis of HAEC was decreased by C-peptide, which reduces RAC-1 translocation to the membrane and NAD(P)H oxidase activation [13]. High glucose increases lectin-like oxLDL receptor-1 (LOX-1) expression and reduces eNOS expression in HUVEC, which is reversed by NAD(P)H oxidase inhibition [73]. Hyperglycemia-stimulated vascular matrix metalloproteinase (MMP) activation in bovine aortic endothelial cells can be reduced by treatment with an antioxidant, but not an inhibitor to PKC [77]. Mesentery artery remodeling and expression of MMP-9, MMP-12 and tissue inhibitors of matrix metalloproteinase (TIMP)-1 and TIMP-2 are increased in db/db arteries [67]. In BAEC, normalizing levels of mitochondrial ROS by manganese SOD (MnSOD) and an inhibitor of electron transport chain complex II prevent glucose-induced activation of PKC, formation of AGE, sorbitol accumulation and nuclear factor-kappa B (NFκB) activation [53]. Thus, hyperglycemia-induced biochemical sequelae lead to enhanced oxidative stress. Interventions that reduce oxidative stress also block the production and action of the adverse biochemical sequelae in hyperglycemia.
It is also noteworthy that glycocalyx, a layer of proteoglycans covering the endothelium, is involved in constituting the vascular barrier together with endothelial cells [6]. Acute hyperglycemia reduced glycocalyx volume and induced endothelial dysfunction in healthy human subjects, indicating a potential role for glycocalyx perturbation in mediating vascular dysfunction during hyperglycemia [52].
Therefore, hyperglycemia may induce chronic vascular complications via formation of toxic metabolites such as ROS, AGE, increased sorbitol and persistent activation of PKC. The interactions among various metabolites may further perpetuate the adverse effects of hyperglycemia. Intensive glycemic control, as well as inhibiting the downstream signaling by various metabolites, may serve as potential therapeutic targets for diabetes-induced vascular dysfunction.
Role of insulin resistance in diabetes-associated endothelial dysfunction
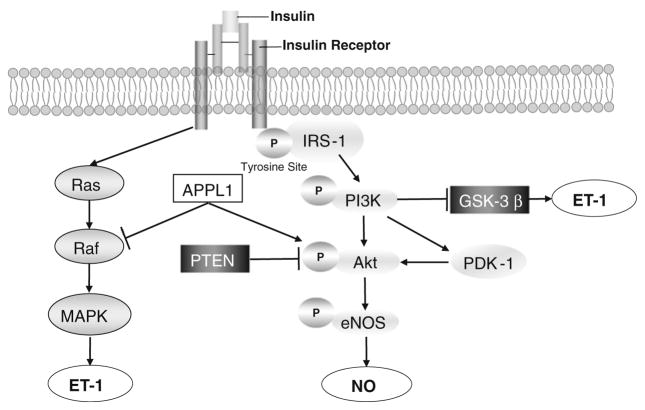
The onset of hyperglycemia and diabetes is often preceded by insulin resistance from many years to decades. The role of insulin resistance and subsequent hyperinsulinemia at the level of endothelial cells in vasculopathy has been extensively studied (Fig. 2). In vitro experiments using cultured endothelial cells suggest that insulin can induce the concurrent release of ET-1 and NO, two substances with opposing vasoactive properties. Insulin stimulates ET-1 gene expression and secretion in endothelial cells via a phosphoinositide-3 kinase (PI3K)-dependent inactivation of glycogen synthase kinase-3beta (GSK-3beta) and Ras (an abbreviation of RAt Sarcoma)-MAPK activation [92, 95]. Insulin induces NO production via activation of an insulin receptor tyrosine kinase that phosphorylates insulin receptor substrate-1 (IRS-1), leading to binding and activation of PI3K, phophoinositide-dependent protein kinase-1 (PDK-1) and protein kinase B (Akt)/eNOS pathway [47, 48, 96]. The multidomain adaptor protein, APPL1, modulates the dual vascular effects of insulin. APPL1 potentiates insulin-stimulated Akt activation by competing with the Akt inhibitor Tribble-3 and suppressing ERK1/2 signaling by altering the phosphorylation status of its upstream kinase Raf-1 (RAF proto-oncogene serine/threonine-protein kinase) in HUVEC [84]. In microvascular endothelial cells isolated from type 2 diabetic subjects, IRS-1/Akt phosphorylation was reduced while ERK1/2 activation was increased, suggesting the presence of endothelial cell insulin resistance [28]. Further evidence suggests that insulin is a double-edged sword in the treatment of diabetics. Physiological concentrations of insulin (10−10 M) preserve telomere length, reduce p53 and vascular cell adhesion molecule-1 (VCAM-1) expression, and delays endothelial senescence under high glucose conditions through an NO-dependent mechanism. However, supra-physiological concentrations of insulin (10−7–10−6 M) in the presence of high glucose promote cellular senescence in an eNOS-independent manner [44].
Fig. 2.
Role of insulin resistance in endothelial dysfunction. Insulin regulates endothelial function through both Ras-MAPK and PI3K-Akt-eNOS signaling pathways to maintain the balance between production of vasodilator mechanisms and vasoconstrictor mechanisms. Akt protein kinase B, eNOS endothelial nitric oxide synthase, GSK3β glycogen synthase kinase-3beta, IRS-1 insulin receptor substrate-1, MAPK mitogen-activated protein kinase, PDK-1 phophoinositide-dependent protein kinase-1, PTEN phosphatase and tensin homolog, Ras rat sarcoma
Animal studies suggest that endothelium-specific insulin resistance does not cause changes in whole-body glucose tolerance, circulating insulin concentrations or insulin sensitivity [17, 81]. Endothelium-dependent vasorelaxation was not examined in endothelium-specific insulin receptor knockout mice; but in transgenic mice with a mutant insulin receptor targeted to endothelium, aortic endothelial function was impaired. Furthermore, in ApoE KO mice with a specific endothelial cell knockout of insulin receptors, atherosclerotic lesion formation is accelerated along with impaired endothelium-dependent vasodilation of carotid arteries, and enhanced VCAM-1 expression and mononuclear cell adhesion [61]. In mice with a genetic deletion of the insulin receptor in all vascular tissues, basal vascular eNOS phosphorylation, endothelial function and blood pressure are normal, despite absent insulin-mediated eNOS phosphorylation [70]. Additionally, knockout of insulin receptors in cardiomyocytes attenuates coronary arterial dysfunction induced by pressure overload, implicating a compensatory mechanism [69]. These studies suggest that the concept of selective insulin resistance is more complex and variable than previously thought [22].
Although in vitro experiments strongly suggest that insulin regulates NO release by endothelial cells, endothelium-intact isolated arteries (mouse aortae and mesenteric arteries) do not relax following the administration of insulin (unpublished work). There is also controversy as to whether insulin-induced relaxation of resistance vessels and increase in blood flow to skeletal muscles occur at physiological exposure time and concentrations of insulin [5]. A clinical study shows that although troglitazone increased whole-body and forearm glucose uptake, and improved insulin sensitivity, it had no effects on insulin-induced vasodilatory function in obese subjects [72].
Thus, mechanisms underlying the association between insulin resistance and endothelial dysfunction, and therapeutic implications of improving insulin sensitivity in the vasculature warrant further investigations.
Role of FFA in diabetes-associated endothelial dysfunction
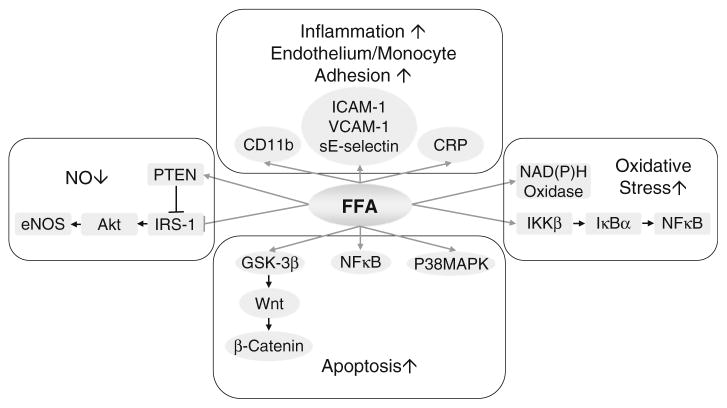
Free fatty acids in excess is implicated in the pathogenesis of insulin resistance [33]. As is seen with hyperglycemia-induced glucotoxicity, lipotoxicity from FFA may promote endothelial dysfunction by a number of related mechanisms (Fig. 3).
Fig. 3.
Role of free fatty acids in endothelial dysfunction. Free fatty acids (FFA) stimulate endothelial apoptosis, augment vascular oxidative stress, reduce NO availability, enhance endothelial and monocyte activation and increase inflammatory responses. CRP C-reactive protein, ICAM-1 intercellular adhesion molecule-1, IκBα inhibitory subunit of NFκB, IKKβ IκB kinase-β, NFκB nuclear factor-kappa B, sE-selectin soluble E-selectin, VCAM-1 vascular cell adhesion molecule-1
Endothelial damage by FFA occurs due to a decrease in Bcl-2/Bax ratio, which augments endothelial apoptosis [59]. FFA’s apoptotic effects are associated with reduced Akt/eNOS phosphorylation and enhanced caspase-9 activation in HUVEC, which can be prevented by insulin (10−8 M) treatment [59]. FFA-induced apoptosis also involves p38 MAPK signaling [10], NFκB activation [68] and GSK-3beta/Wnt/beta-catenin signaling [107]. FFA inhibits insulin-mediated tyrosine phosphorylation of IRS-1, serine phosphorylation of Akt and eNOS, and NO production, while it increases IKKβ (IκB kinase-β) activity and phosphatase and tensin homolog (PTEN) expression [37, 83]. Exposure to FFA enhances the expression of NAD(P)H oxidase subunit, stimulating ROS production [12] and reducing mitochondrial membrane potential [106]. Elevated concentrations of non-esterified fatty acids (NEFA) increase monocyte expression of CD11b, intracellular ROS formation and adhesion to endothelial cells, which can be inhibited by antioxidants, NAD(P)H oxidase inhibitors and PKC inhibitors [105]. FFA increase A disintegrin and metalloproteinase (ADAM)-mediated substrate cleavage resulting in functional effects on cell proliferation, cell migration and endothelial permeability [62]. Moreover, saturated versus unsaturated FFA-induced endothelial apoptosis may be mediated via different mechanisms, and the impact of FFA on endothelial cells depends on vascular origin and growth/proliferation status of the vascular cellular elements. Therefore, it is important to examine the effects of FFA on target tissues that are known to be affected in diabetes, such as human aortic and retinal endothelial cells [2].
Studies on animal models also support the detrimental vascular effects of FFA. In the rabbit, in vitro incubation with FFA impaired endothelial function of isolated aortic rings, which was accompanied by reduced NO levels and enhanced oxidative stress [18]. In Sprague–Dawley rats, FFA infusion increases blood pressure, reduces eNOS and PGI2 synthase activity and impairs aortic endothelial function [16, 82]. In obese Zucker rats and high fat diet-induced obese mice, inhibiting FFA release from adipose and inhibiting rate-limiting enzyme for fatty acid oxidation in mitochondria reduced aortic ROS production and prevented eNOS and PGI2 synthase inactivation [16].
In addition to animal models, the adverse effects of FFA on endothelial function are demonstrated in healthy humans and diabetic patients. Infusion of FFA impairs endothelial function, which can be reversed by an inhibitor of the renin–angiotensin system (RAS) [86] or in the presence of rosiglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist [46]. Lipid infusion blocked insulin-mediated increases in microvascular blood velocity and microvascular blood flow in both cardiac and skeletal muscle of healthy young adults [39]. A 48-h physiological increase in plasma FFA to levels of obesity and diabetes in a group of healthy subjects enhanced leukocyte activation and the angiotensin II-forming activity in human mononuclear and polymorpho-nuclear cells [3]. Elevated FFA also increases plasma markers of endothelial activation, such as ICAM-1, VCAM-1 and soluble E-selectin (sE-selectin), and increases plasma levels of myeloperoxidase (MPO) and tissue-type plasminogen activator inhibitor-1 (tPAI-1, an indicator of a prothrombotic state) [43]. In obese subjects with type 2 diabetes, a lipid infusion results in a rapid and sustained elevation in blood pressure, impaired flow-mediated dilatation and increases in C-reactive protein (CRP), but does not change plasma renin and aldosterone levels [78].
In type 2 diabetic subjects, postprandial lipidemia is exaggerated and prolonged. Prolonged postprandial lipidemia is associated with prolonged endothelial dysfunction likely through the effects described above from FFA. This underscores the importance of dietary compliance with low-fat meals for type 2 diabetic patients [1].
Perspectives
Many studies suggest that intensive control of blood glucose delays the onset and retards the progression of diabetic vascular complications. However, to date, the effectiveness of intensive glucose control on the prevention of major cardiovascular events is still inconclusive [14].
The molecular mechanisms by which hyperglycemia, insulin resistance, hyperinsulinemia and dyslipidemia result in endothelial dysfunction overlap and make it difficult to tease out the specific molecular mechanisms. Among the various pathogenic features induced by metabolic abnormalities in diabetes, oxidative stress and inflammatory responses appear to be the first abnormalities which trigger several other mechanisms in diabetes-associated endothelial dysfunction [8]. Although the role of oxidative stress as a contributing mechanism to diabetes-induced endothelial dysfunction is supported by a large body of experimental and clinical studies, antioxidant supplementation (mostly with vitamin E) has not been shown to improve the pathological consequence. In contrast, substances such as statins, activators of peroxisome proliferator-activated receptors and inhibitors of renin–angiotensin–aldosterone system, which possess indirect antioxidant properties, show improved endothelial function in preclinical and clinical studies as well as reducing the incidence of cardiovascular events in diabetic patients [38]. Factors that may contribute to the apparent discrepancy in these studies include patient selection with diseases that differ in extent of oxidative stress as well as the administered dose and type of antioxidant therapy used. Therefore, individualized assessment of the level of oxidative stress and the potential underlying mechanism of oxidative stress before treatment may provide insight into the appropriate therapeutic approach, which may improve the individual’s condition and resolve this antioxidant paradox. The development of novel, potent antioxidant strategies and early intervention in the process of vascular dysfunction and disease development may also produce benefits in clinical outcomes [49, 71].
In addition to oxidative stress, metabolic abnormalities are correlated directly with markers of inflammation [74, 87]. Chronic low-grade inflammation can be both a cause and consequence of endothelial dysfunction, and the two appear to be tightly linked [65]. Since type 2 diabetes is highly associated with obesity, the metabolic role of adipose tissue potentiates the adverse effects on the vasculature [31, 98, 99]. Adipose tissue secretes a range of proinflammatory molecules, which lead to systemic inflammation and participate in the cross talk between adipose stores and the vascular wall (see Ref. 100 and 101 for review). Local inflammation in the vasculature is attributed to effects by the inflammatory cytokines/chemokines and leukocyte adhesion molecules expressed and released by the endothelium [97, 100]. Anti-inflammatory treatment by neutralizing antibodies to TNFα, MCP-1 and IFNγ effectively attenuated endothelial dysfunction in db/db mice without significantly affecting body weight and glucose metabolism [23, 91, 98]. This suggests that vasoprotection by anti-inflammatory therapies can be independent of their metabolic effects. Thus, newer anti-diabetic agents should not only achieve superior glycemic control, but also improve cardiovascular outcomes [94]. Therapies that combine salutary effects on vascular inflammation and oxidative stress potentially delay or reverse diabetic vasculopathy [57, 99].
Conclusion
Endothelial dysfunction is characterized by a number of functional alterations in the vascular endothelium, which include changes in vasomotor function, enhanced generation of ROS and inflammation resulting in a proatherogenic response, apoptosis, remodeling, and altered barrier function. Impaired endothelial function is a key event associated with subsequent progression of cardiovascular complications in diabetes. Although normal insulin signaling provides protection from glucotoxicity in endothelial cells, hyperinsulinemia further exacerbates hyperglycemia-induced endothelial injury. Insulin resistance leads to enhanced FFA production, which inhibits insulin signaling and accelerates vascular insulin resistance. Thus, glucotoxicity, lipotoxicity, insulin resistance and a mutual interaction between these factors occur to promote the development and progression of endothelial dysfunction in type 2 diabetes. Conventional therapies to reduce hyperglycemia, dyslipidemia and insulin resistance represent important clinical options to improve endothelial function and delay the progression of vascular complications. Therapeutic approaches targeting intracellular mechanisms underlying metabolic alterations, such as inhibiting AGE formation and signaling, suppressing PKC activation, inhibiting the cannabinoid receptor CB(1)-R [75], preventing or decreasing inflammatory responses and restoring the redox balance of the endothelium, are thought to be promising strategies to prevent endothelial dysfunction in the diabetic state. In animal models, to date, these insights are partially established with evidence of favorable effects. Since therapy addressing a single metabolic abnormality has not been beneficial (e.g., vitamin E), to reduce cardiovascular complications in type 2 diabetes may require simultaneous interventions within multiple metabolic and signaling pathways. It may take a multi-component approach such as reducing hyperglycemia, oxidative stress, inflammation and insulin resistance to ameliorate the adverse effects that progress to diabetic vasculopathy.
Therefore, clinical trials targeting multiple therapeutic targets are urgently needed to validate their effectiveness in ameliorating diabetic vascular complications. Combination therapy that simultaneously targets multiple pathways in the pathogenesis of endothelial dysfunction is an attractive emerging concept for slowing progression of diabetic vascular complications.
Acknowledgments
This study was supported by NIH grants (RO1-HL077566 and RO1-HL085119, to C.Z.) and the American Heart Association Predoctoral Fellowship (10PRE4300043 to H.Z.).
Contributor Information
Hanrui Zhang, Departments of Internal Medicine, Medical Pharmacology & Physiology and Nutritional Sciences, Dalton Cardiovascular Research Center, University of Missouri-Columbia, Columbia, MO 65211, USA.
Kevin C. Dellsperger, Departments of Internal Medicine and Medical Pharmacology and Physiology, University of Missouri-Columbia, Columbia, MO 65211, USA
Cuihua Zhang, Departments of Internal Medicine, Medical Pharmacology & Physiology and Nutritional Sciences, Dalton Cardiovascular Research Center, University of Missouri-Columbia, Columbia, MO 65211, USA.
References
- 1.Anderson RA, Evans LM, Ellis GR, Khan N, Morris K, Jackson SK, Rees A, Lewis MJ, Frenneaux MP. Prolonged deterioration of endothelial dysfunction in response to postprandial lipaemia is attenuated by vitamin C in type 2 diabetes. Diabet Med. 2006;23:258–264. doi: 10.1111/j.1464-5491.2005.01767.x. [DOI] [PubMed] [Google Scholar]
- 2.Artwohl M, Lindenmair A, Sexl V, Maier C, Rainer G, Freudenthaler A, Huttary N, Wolzt M, Nowotny P, Luger A, Baumgartner-Parzer SM. Different mechanisms of saturated versus polyunsaturated FFA-induced apoptosis in human endothelial cells. J Lipid Res. 2008;49:2627–2640. doi: 10.1194/jlr.M800393-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Azekoshi Y, Yasu T, Watanabe S, Tagawa T, Abe S, Yamakawa K, Uehara Y, Momomura S, Urata H, Ueda S. Free fatty acid causes leukocyte activation and resultant endothelial dysfunction through enhanced angiotensin II production in mono-nuclear and polymorphonuclear cells. Hypertension. 2010;56:136–142. doi: 10.1161/HYPERTENSIONAHA.110.153056. [DOI] [PubMed] [Google Scholar]
- 4.Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165–189. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 5.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687–701. doi: 10.1007/s00395-010-0118-z. [DOI] [PubMed] [Google Scholar]
- 7.Belin de Chantemele EJ, Ali MI, Mintz J, Stepp DW. Obesity induced insulin resistance causes endothelial dysfunction without reducing the vascular response to hindlimb ischemia. Basic Res Cardiol. 2009;104:707–717. doi: 10.1007/s00395-009-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belin de Chantemele EJ, Stepp DW. Influence of obesity and metabolic dysfunction on the endothelial control in the coronary circulation. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai W, Liu Z. p38 mitogen-activated protein kinase mediates palmitate-induced apoptosis but not inhibitor of nuclear factor-kappaB degradation in human coronary artery endothelial cells. Endocrinology. 2007;148:1622–1628. doi: 10.1210/en.2006-1068. [DOI] [PubMed] [Google Scholar]
- 11.Cheang WS, Wong WT, Tian XY, Yang Q, Lee HK, He GW, Yao X, Huang Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res. 2011;92:267–275. doi: 10.1093/cvr/cvr233. [DOI] [PubMed] [Google Scholar]
- 12.Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007;148:160–165. doi: 10.1210/en.2006-1132. [DOI] [PubMed] [Google Scholar]
- 13.Cifarelli V, Geng X, Styche A, Lakomy R, Trucco M, Luppi P. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia. 2011;54:2702–2712. doi: 10.1007/s00125-011-2251-0. [DOI] [PubMed] [Google Scholar]
- 14.Conget I, Gimenez M. Glucose control and cardiovascular disease: is it important? No Diabetes Care. 2009;32(Suppl 2):S334–336. doi: 10.2337/dc09-S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan ER, Crossey PA, Walker S, Anilkumar N, Poston L, Douglas G, Ezzat VA, Wheatcroft SB, Shah AM, Kearney MT. Effect of endothelium-specific insulin resistance on endothelial function in vivo. Diabetes. 2008;57:3307–3314. doi: 10.2337/db07-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci (Lond) 2006;111:145–151. doi: 10.1042/CS20060001. [DOI] [PubMed] [Google Scholar]
- 19.Ergul A. Endothelin-1 and diabetic complications: focus on the vasculature. Pharmacol Res. 2011;63:477–482. doi: 10.1016/j.phrs.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feletou M, Huang Y, Vanhoutte PM. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol. 2011;164:894–912. doi: 10.1111/j.1476-5381.2011.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009;117:139–155. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- 22.Fulton DJ. Mechanisms of vascular insulin resistance: a substitute Akt? Circ Res. 2009;104:1035–1037. doi: 10.1161/CIRCRESAHA.109.198028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Martinez-Lemus LA, Zhang C. Endothelium-derived hyperpolarizing factor and diabetes. World J Cardiol. 2011;3:25–31. doi: 10.4330/wjc.v3.i1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X, Zhang H, Schmidt AM, Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2008;295:H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwill AG, James ME, Frisbee JC. Increased vascular thromboxane generation impairs dilation of skeletal muscle arterioles of obese Zucker rats with reduced oxygen tension. Am J Physiol Heart Circ Physiol. 2008;295:H1522–H1528. doi: 10.1152/ajpheart.00596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 31.Heusch G. Obesity and inflammatory vasculopathy: a surgical solution as ultima ratio? Arterioscler Thromb Vasc Biol. 2011;31:1953–1954. doi: 10.1161/ATVBAHA.111.232264. [DOI] [PubMed] [Google Scholar]
- 32.Hodgkinson CP, Laxton RC, Patel K, Ye S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:2275–2281. doi: 10.1161/ATVBAHA.108.175992. [DOI] [PubMed] [Google Scholar]
- 33.Jellinger PS. Metabolic consequences of hyperglycemia and insulin resistance. Clin Cornerstone. 2007;8(Suppl 7):S30–42. doi: 10.1016/S1098-3597(07)80019-6. [DOI] [PubMed] [Google Scholar]
- 34.Kador PF, Randazzo J, Blessing K, Makita J, Zhang P, Yu K, Hosoya K, Terasaki T. Polyol formation in cell lines of rat retinal capillary pericytes and endothelial cells (TR-rPCT and TR-iBRB) J Ocul Pharmacol Ther. 2009;25:299–308. doi: 10.1089/jop.2008.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz PS, Trask AJ, Souza-Smith FM, Hutchinson KR, Galantowicz ML, Lord KC, Stewart JA, Jr, Cismowski MJ, Varner KJ, Lucchesi PA. Coronary arterioles in type 2 diabetic (db/db) mice undergo a distinct pattern of remodeling associated with decreased vessel stiffness. Basic Res Cardiol. 2011;106:1123–1134. doi: 10.1007/s00395-011-0201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khamaisi M, Dahan R, Hamed S, Abassi Z, Heyman SN, Raz I. Role of protein kinase C in the expression of endothelin converting enzyme-1. Endocrinology. 2009;150:1440–1449. doi: 10.1210/en.2008-0524. [DOI] [PubMed] [Google Scholar]
- 37.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 38.Koh KK, Oh PC, Quon MJ. Does reversal of oxidative stress and inflammation provide vascular protection? Cardiovasc Res. 2009;81:649–659. doi: 10.1093/cvr/cvn354. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab. 2011;96:438–446. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X, Bean JS, Kassab GS, Rekhter MD. Protein kinase C inhibition ameliorates functional endothelial insulin resistance and vascular smooth muscle cell hypersensitivity to insulin in diabetic hypertensive rats. Cardiovasc Diabetol. 2011;10:48. doi: 10.1186/1475-2840-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manea SA, Manea A, Heltianu C. Inhibition of JAK/STAT signaling pathway prevents high-glucose-induced increase in endothelin-1 synthesis in human endothelial cells. Cell Tissue Res. 2010;340:71–79. doi: 10.1007/s00441-010-0936-1. [DOI] [PubMed] [Google Scholar]
- 43.Mathew M, Tay E, Cusi K. Elevated plasma free fatty acids increase cardiovascular risk by inducing plasma bio-markers of endothelial activation, myeloperoxidase and PAI-1 in healthy subjects. Cardiovasc Diabetol. 2010;9:9. doi: 10.1186/1475-2840-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui-Hirai H, Hayashi T, Yamamoto S, Ina K, Maeda M, Kotani H, Iguchi A, Ignarro LJ, Hattori Y. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: a relationship between telomeres and nitric oxide. J Pharmacol Exp Ther. 2011;337:591–599. doi: 10.1124/jpet.110.177584. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Ishida K, Nakayama N, Taguchi K, Kobayashi T, Kamata K. Mechanisms underlying the losartan treatment-induced improvement in the endothelial dysfunction seen in mesenteric arteries from type 2 diabetic rats. Pharmacol Res. 2010;62:271–281. doi: 10.1016/j.phrs.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Mittermayer F, Schaller G, Pleiner J, Krzyzanowska K, Kapiotis S, Roden M, Wolzt M. Rosiglitazone prevents free fatty acid-induced vascular endothelial dysfunction. J Clin Endocrinol Metab. 2007;92:2574–2580. doi: 10.1210/jc.2006-2130. [DOI] [PubMed] [Google Scholar]
- 47.Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem. 2002;277:1794–1799. doi: 10.1074/jbc.M103728200. [DOI] [PubMed] [Google Scholar]
- 48.Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol. 2002;16:1931–1942. doi: 10.1210/me.2002-0074. [DOI] [PubMed] [Google Scholar]
- 49.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 50.Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M, Walker S, Yu B, Brewer A, Shah AM. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa T, Tanabe K, Croker BP, Johnson RJ, Grant MB, Kosugi T, Li Q. Endothelial dysfunction as a potential contributor in diabetic nephropathy. Nat Rev Nephrol. 2011;7:36–44. doi: 10.1038/nrneph.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, Meijers JC, Holleman F, Hoekstra JB, Vink H, Kastelein JJ, Stroes ES. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 53.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 54.Oppermann M, Suvorava T, Freudenberger T, Dao VT, Fischer JW, Weber M, Kojda G. Regulation of vascular guanylyl cyclase by endothelial nitric oxide-dependent posttranslational modification. Basic Res Cardiol. 2011;106:539–549. doi: 10.1007/s00395-011-0160-5. [DOI] [PubMed] [Google Scholar]
- 55.Pannirselvam M, Verma S, Anderson TJ, Triggle CR. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;295:H1982–H1988. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park Y, Yang J, Zhang H, Chen X, Zhang C. Effect of PAR2 in regulating TNF-alpha and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol. 2011;106:111–123. doi: 10.1007/s00395-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perrone L, Devi TS, Hosoya K, Terasaki T, Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 59.Piro S, Spampinato D, Spadaro L, Oliveri CE, Purrello F, Rabuazzo AM. Direct apoptotic effects of free fatty acids on human endothelial cells. Nutr Metab Cardiovasc Dis. 2008;18:96–104. doi: 10.1016/j.numecd.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 60.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 61.Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–389. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiss K, Cornelsen I, Husmann M, Gimpl G, Bhakdi S. Unsaturated fatty acids drive disintegrin and metalloproteinase (ADAM)-dependent cell adhesion, proliferation, and migration by modulating membrane fluidity. J Biol Chem. 2011;286:26931–26942. doi: 10.1074/jbc.M111.243485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodino-Janeiro BK, Gonzalez-Peteiro M, Ucieda-Somoza R, Gonzalez-Juanatey JR, Alvarez E. Glycated albumin, a precursor of advanced glycation end-products, up-regulates NADPH oxidase and enhances oxidative stress in human endothelial cells: molecular correlate of diabetic vasculopathy. Diabetes Metab Res Rev. 2010;26:550–558. doi: 10.1002/dmrr.1117. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez A, Contreras C, Martinez P, Villalba N, Benedito S, Garcia-Sacristan A, Salaices M, Hernandez M, Prieto D. Enhanced cyclooxygenase 2-mediated vasorelaxation in coronary arteries from insulin-resistant obese Zucker rats. Atherosclerosis. 2010;213:392–399. doi: 10.1016/j.atherosclerosis.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 65.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109:143–159. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 66.Shi Y, Vanhoutte PM. Reactive oxygen-derived free radicals are key to the endothelial dysfunction of diabetes. J Diabetes. 2009;1:151–162. doi: 10.1111/j.1753-0407.2009.00030.x. [DOI] [PubMed] [Google Scholar]
- 67.Souza-Smith FM, Katz PS, Trask AJ, Stewart JA, Jr, Lord KC, Varner KJ, Vassallo DV, Lucchesi PA. Mesenteric resistance arteries in Type 2 diabetic db/db mice undergo outward remodeling. PLoS One. 2011;6:e23337. doi: 10.1371/journal.pone.0023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staiger K, Staiger H, Weigert C, Haas C, Haring HU, Kellerer M. Saturated, but not unsaturated, fatty acids induce apoptosis of human coronary artery endothelial cells via nuclear factor-kappaB activation. Diabetes. 2006;55:3121–3126. doi: 10.2337/db06-0188. [DOI] [PubMed] [Google Scholar]
- 69.Symons JD, Hu P, Yang Y, Wang X, Zhang QJ, Wende AR, Sloan CL, Sena S, Abel ED, Litwin SE. Knockout of insulin receptors in cardiomyocytes attenuates coronary arterial dysfunction induced by pressure overload. Am J Physiol Heart Circ Physiol. 2011;300:H374–H381. doi: 10.1152/ajpheart.01200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tack CJ, Ong MK, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia. 1998;41:569–576. doi: 10.1007/s001250050948. [DOI] [PubMed] [Google Scholar]
- 73.Taye A, Saad AH, Kumar AH, Morawietz H. Effect of apocynin on NADPH oxidase-mediated oxidative stress-LOX-1-eNOS pathway in human endothelial cells exposed to high glucose. Eur J Pharmacol. 2010;627:42–48. doi: 10.1016/j.ejphar.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 74.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tiyerili V, Zimmer S, Jung S, Wassmann K, Naehle CP, Lutjohann D, Zimmer A, Nickenig G, Wassmann S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res Cardiol. 2010;105:465–477. doi: 10.1007/s00395-010-0090-7. [DOI] [PubMed] [Google Scholar]
- 76.Toth E, Racz A, Toth J, Kaminski PM, Wolin MS, Bagi Z, Koller A. Contribution of polyol pathway to arteriolar dysfunction in hyperglycemia. Role of oxidative stress, reduced NO, and enhanced PGH(2)/TXA(2) mediation. Am J Physiol Heart Circ Physiol. 2007;293:H3096–3104. doi: 10.1152/ajpheart.01335.2006. [DOI] [PubMed] [Google Scholar]
- 77.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS. Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res. 2001;88:1291–1298. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 78.Umpierrez GE, Smiley D, Robalino G, Peng L, Kitabchi AE, Khan B, Le A, Quyyumi A, Brown V, Phillips LS. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:609–614. doi: 10.1210/jc.2008-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanhoutte PM, Shimokawa H, Tang EH, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 80.Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, Li H, Hou Z, Pan L, Shen X, Li G. Role of oxidative stress in elevated blood pressure induced by high free fatty acids. Hypertens Res. 2009;32:152–158. doi: 10.1038/hr.2008.35. [DOI] [PubMed] [Google Scholar]
- 83.Wang XL, Zhang L, Youker K, Zhang MX, Wang J, LeMaire SA, Coselli JS, Shen YH. Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes. 2006;55:2301–2310. doi: 10.2337/db05-1574. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Cheng KK, Lam KS, Wu D, Huang Y, Vanhoutte PM, Sweeney G, Li Y, Xu A. APPL1 counteracts obesity-induced vascular insulin resistance and endothelial dysfunction by modulating the endothelial production of nitric oxide and endothelin-1 in mice. Diabetes. 2011;60:3044–3054. doi: 10.2337/db11-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warboys CM, Toh HB, Fraser PA. Role of NADPH oxidase in retinal microvascular permeability increase by RAGE activation. Invest Ophthalmol Vis Sci. 2009;50:1319–1328. doi: 10.1167/iovs.08-2730. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe S, Tagawa T, Yamakawa K, Shimabukuro M, Ueda S. Inhibition of the renin–angiotensin system prevents free fatty acid-induced acute endothelial dysfunction in humans. Arterioscler Thromb Vasc Biol. 2005;25:2376–2380. doi: 10.1161/01.ATV.0000187465.55507.85. [DOI] [PubMed] [Google Scholar]
- 87.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong MS, Vanhoutte PM. COX-mediated endothelium-dependent contractions: from the past to recent discoveries. Acta Pharmacol Sin. 2010;31:1095–1102. doi: 10.1038/aps.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Y, Feng B, Chen S, Zuo Y, Chakrabarti S. Glucose-induced endothelin-1 expression is regulated by ERK5 in the endothelial cells and retina of diabetic rats. Can J Physiol Pharmacol. 2010;88:607–615. doi: 10.1139/Y10-033. [DOI] [PubMed] [Google Scholar]
- 90.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Park Y, Zhang H, Gao X, Wilson E, Zimmer W, Abbott L, Zhang C. Role of MCP-1 in tumor necrosis factor-alpha-induced endothelial dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;297:H1208–H1216. doi: 10.1152/ajpheart.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Z, Li JC. Stimulation of endothelin-1 gene expression by insulin via phosphoinositide-3 kinase-glycogen synthase kinase-3beta signaling in endothelial cells. Life Sci. 2008;82:512–518. doi: 10.1016/j.lfs.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye Y, Perez-Polo JR, Aguilar D, Birnbaum Y. The potential effects of anti-diabetic medications on myocardial ischemia–reperfusion injury. Basic Res Cardiol. 2011;106:925–952. doi: 10.1007/s00395-011-0216-6. [DOI] [PubMed] [Google Scholar]
- 95.Yu Q, Gao F, Ma XL. Insulin says NO to cardiovascular disease. Cardiovasc Res. 2011;89:516–524. doi: 10.1093/cvr/cvq349. [DOI] [PubMed] [Google Scholar]
- 96.Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 97.Zhang C, Wu J, Xu X, Potter BJ, Gao X. Direct relationship between levels of TNF-alpha expression and endothelial dysfunction in reperfusion injury. Basic Res Cardiol. 2010;105:453–464. doi: 10.1007/s00395-010-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang H, Potter BJ, Cao JM, Zhang C. Interferon-gamma induced adipose tissue inflammation is linked to endothelial dysfunction in type 2 diabetic mice. Basic Res Cardiol. 2011;106:1135–1145. doi: 10.1007/s00395-011-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–2069. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Zhang C. Adipose “talks” to distant organs to regulate insulin sensitivity and vascular function. Obesity (Silver Spring) 2010;18:2071–2076. doi: 10.1038/oby.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang H, Zhang C. Regulation of microvascular function by adipose tissue in obesity and Type 2 diabetes: evidence of an adipose-vascular loop. Am J Biomed Sci. 2009;1:133–142. doi: 10.5099/aj090200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang LN, Vincelette J, Chen D, Gless RD, Anandan SK, Rubanyi GM, Webb HK, MacIntyre DE, Wang YX. Inhibition of soluble epoxide hydrolase attenuates endothelial dysfunction in animal models of diabetes, obesity and hypertension. Eur J Pharmacol. 2011;654:68–74. doi: 10.1016/j.ejphar.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol. 2008;28:1627–1633. doi: 10.1161/ATVBAHA.108.168278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang WY, Schwartz E, Wang Y, Attrep J, Li Z, Reaven P. Elevated concentrations of nonesterified fatty acids increase monocyte expression of CD11b and adhesion to endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:514–519. doi: 10.1161/01.ATV.0000200226.53994.09. [DOI] [PubMed] [Google Scholar]
- 106.Zhou H, Liu X, Liu L, Yang Z, Zhang S, Tang M, Tang Y, Dong Q, Hu R. Oxidative stress and apoptosis of human brain microvascular endothelial cells induced by free fatty acids. J Int Med Res. 2009;37:1897–1903. doi: 10.1177/147323000903700627. [DOI] [PubMed] [Google Scholar]
- 107.Zhu P, Chen G, You T, Yao J, Jiang Q, Lin X, Shen X, Qiao Y, Lin L. High FFA-induced proliferation and apoptosis in human umbilical vein endothelial cell partly through Wnt/beta-catenin signal pathway. Mol Cell Biochem. 2010;338:123–131. doi: 10.1007/s11010-009-0345-5. [DOI] [PubMed] [Google Scholar]