“We shall not cease from exploration. And the end of all our exploring will be to arrive where we started and know the place for the first time.”
T.S. Eliot
The recent 2014 World Health Organization (WHO) Classification of Tumours of the Female Reproductive Organs introduced a new category of ovarian neoplasm designated “seromucinous tumours”. The recognition of this distinctive group of tumors is an important addition to the classification of epithelial ovarian tumors but the term “seromucinous” has some serious flaws that obscures the nature of these neoplasms and which we believe need to be addressed. Like other epithelial ovarian tumors this group subsumes adenomas, atypical proliferative (borderline) tumors and invasive carcinomas (1). The fact that this is a recent addition to the classification of ovarian tumors, belies its long and somewhat tortured history that began, to the best of our knowledge, in 1976 when Fox and Langley (2) introduced the term “seromucinous tumor” to describe a tumor composed of endocervical-type mucinous epithelium and serous-type cells. This was followed by a hiatus of 12 years during which time these tumors were essentially ignored. Then in 1988 Rutgers and Scully published two papers on the subject dividing similar appearing borderline tumors into two categories. One, composed of pure endocervical-type epithelium was classified as “ovarian” müllerian mucinous cystadenomas of borderline malignancy” (3) and another,composed of a mixture of endocervical-type mucinous, serous, endometrioid and indifferent cells with abundant eosinophilic cytoplasm, was classified “ovarian mixed-epithelial papillary cystadenomas of borderline malignancy” (4). Later, in 1993 Hendrickson and Kempson (5) resurrected the term “seromucinous tumor,” a term that Shappell et al. adopted in 2002 (6). Shappell and colleagues found that virtually all of these neoplasms were composed of a mixture of different cell types and furthermore, as there were no clinically relevant differences between the two categories combined them into a single group. In addition to the previously described borderline tumors (Fig. 1) we included, in the Shappell study, a group of tumors with similar morphology but that displayed stromal invasion, characterized by a confluent glandular growth pattern, and therefore classified these as carcinomas (Fig.2). The latter were closely associated with atypical proliferative seromucinous tumors strongly suggesting that they were precursors of the invasive carcinomas (6).
Figure 1.
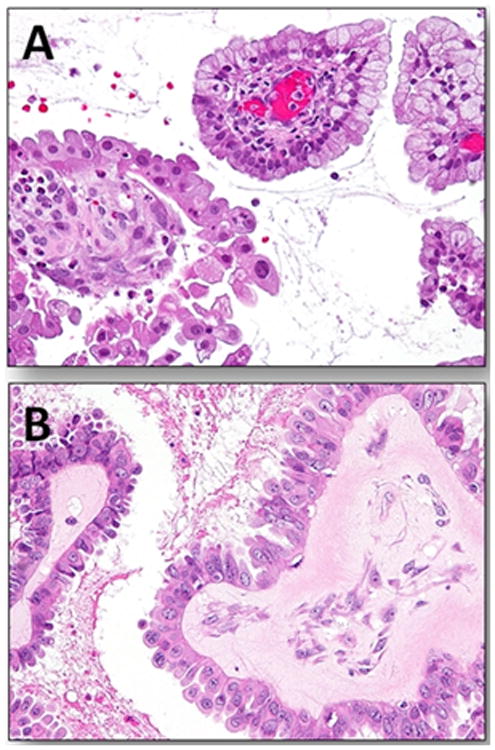
Atypical proliferative (borderline) seromucinous tumor. A. Papillary architecture with papillae lined by stratified mucinous-type cells and hobnail shaped cells containing abundant eosinophilic cytoplasm. An inflammatory infiltrate within the stroma of the papillae is frequently present in these tumors. B. Higher magnification shows that the cells displays minimal cytologic atypia.
Figure 2.
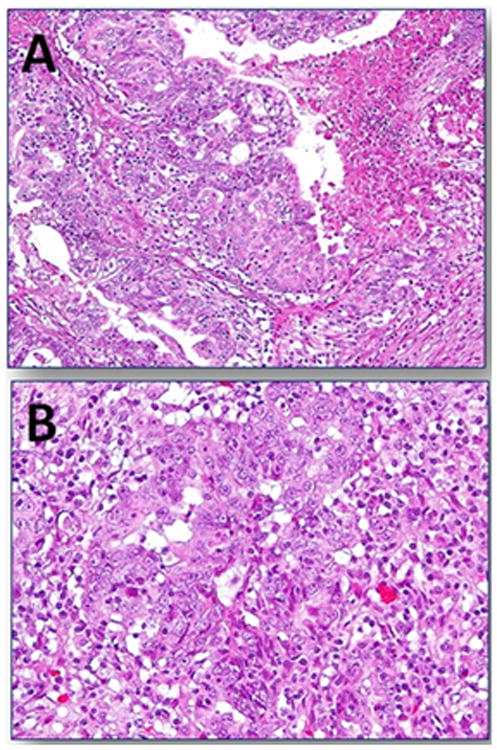
Seromucinous carcinoma. A. Stromal invasion characterized by masses of epithelium displaying a confluent pattern associated with a focus of necrosis. B. Higher magnification reveals cells with enlarged nuclei displaying prominent nucleoli and abundant eosinophilic cytoplasm surrounded by an inflammatory infiltrate.
Rutgers and Scully reported that about a third of the tumors, which were all borderline in their series, were associated with endometriosis, a finding that we confirmed, and which is in sharp contrast to gastrointestinal-type mucinous tumors that are rarely associated with endometriosis (3-6).
In 2010 two research groups independently reported that ARID1A, a tumor suppressor gene involved in chromatin remodeling, was mutated in half of ovarian clear cell carcinomas and 30% of endometrioid carcinomas but not in any of the high-grade serous carcinomas that were analyzed (7,8). The gene encodes the protein BAF250a, which participates in forming switch/sucrose nonfermentable chromatin remodeling complexes. Most ARID1A mutations are nonsense, frame-shift, and in-frame mutations, leading to loss of expression of BAF250a. Accordingly, the loss of expression of BAF250a immunoreactivity can be used as a marker for ARID1A-inactivating mutations in formalin fixed, paraffin-embedded tissue (9). The loss of ARID1A (BAF250a) expression was subsequently reported by Ayhan et al. in 66% of ovarian endometrioid and clear cell carcinomas (10). Importantly, molecular genetic and immunohistochemical studies showed loss of expression in the endometriotic epithelium immediately adjacent to the carcinoma but not in endometriotic tissue more distant from the tumors (7,10) thereby providing strong evidence that endometriosis is a likely precursor of these tumors. Similar findings were subsequently reported by Wu et al. in an immunohistochemical study of atypical proliferative seromucinous tumors using an antibody against BAF250a (11). A detailed molecular genetic analysis of seromucinous carcinomas has not yet been performed but given the morphologic and immunohistochemical (see below) similarity of the atypical proliferative tumors to the carcinomas it is very likely they will demonstrate similar findings. Based on these data we recently expanded the dualistic model of ovarian carcinoma and added a subcategory designated “endometriosis-associated neoplasms” to the type I group of tumors which includes seromucinous along with endometrioid and clear cell carcinomas (12).
The immunohistochemical profile of atypical proliferative seromucinous tumors reported by Vang et al (13) reveals frequent expression of ER (100%), PR (67%), CA125 (92%), infrequent expression of WT1 (8%) and lack of expression of CK20 and CDX2, an immunostaining pattern consistent with a “müllerian” immunophenotype. A recently reported study by Taylor and McCluggage described the almost identical immunoprofile in a series of seromucinous carcinomas. Specifically, they found consistent positive expression for CK7, hormone receptors, CA125, PAX8 and CA19.9 but only a minor proportion of the tumors were positive for WT1. None of the tumors were positive for CK20 and CDX2. (14). In addition, they reported that the clinicopathologic features of the carcinomas were similar to those of the atypical proliferative tumors that were previously reported, including the presence of associated endometriosis in over half of their cases.
Based on the morphologic, immunohistochemical and molecular genetic findings it is now evident that these tumors do not show serous-type differentiation and the implication that they are composed solely of serous and mucinous epithelium is erroneous. The papillary architecture and the presence of cilia suggest serous differentiation but a papillary architecture is not specific for serous differentiation and cilia are present on the surface of the endometrium. Mucinous (endocervical-type) differentiation is frequently observed in ovarian endometrioid tumors and is very often present in endometrial proliferative lesions, both hyperplasia and carcinoma. Serous tumors are not generally associated with endometriosis and furthermore the very limited expression of WT1 in seromucinous tumors does not support a relationship to serous neoplasms. Even more persuasive evidence linking seromucinous tumors to endometrioid and clear cell neoplasms is the loss of ARID1A expression presumably due to ARID1A mutations in a high proportion of seromucinous tumors, similar to that of endometrioid and clear cell tumors, and in sharp contrast to serous tumors which do not lose ARID1A expression or harbor this mutation. The designation “seromucinous” for a group of tumors that show no relationship to serous tumors based on morphologic, immunohistochemical and molecular genetic findings is therefore inaccurate and misleading. What characterizes seromucinous tumors is an admixture of various cell types including endocervical-type mucinous, endometrioid and squamous type epithelium, an immunophenotype which is “müllerian” and clinical and molecular features demonstrating a close relationship with endometriosis similar to that of endometrioid and clear cell tumors. Accordingly, a more appropriate term for this group of tumors is “mixed müllerian tumors” which can be subcategorized as “mixed müllerian cystadenomas”, “mixed müllerian atypical proliferative (borderline) tumors” and “mixed müllerian carcinomas”. Admittedly, this designation can potentially lead to confusion with the “malignant mixed mesodermal (müllerian) tumor”. This latter term, which is used to describe a highly malignant biphasic neoplasm, is not ideal and, in fact, the recent 2104 WHO classification prefers the term “carcinosarcoma” (1).Thus, the continuing saga of the terminology of this interesting and relatively uncommon group of tumors has come full circle as it is a hybrid of the terms “mixed-epithelial papillary cystadenomas of borderline malignancy” and “müllerian mucinous cystadenomas of borderline malignancy”as previously proposed by Rutgers and Scully for a subset of these neoplasms (3,4).
Acknowledgments
This work was supported by grants from the Department of Defense CDMRP grant OC 100517 Grant # W81XWH-11-2-0230 and NIH/NC CA165807. The authors thank Emily Gerry for her editorial assistance.
Literature Cited
- 1.Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO classification of tumours of female reproductive organs. 4th. IARC; 2014. [Google Scholar]
- 2.Fox H, Langley FA. Tumors of the Ovary. Chicago: William Heinemann/Year Book; 1976. This is a book so I am not sure how we can get it. [Google Scholar]
- 3.Rutgers JL, Scully RE. Ovarian müllerian mucinous cystadenomas of borderline malignancy. Cancer. 1988;61:340–8. doi: 10.1002/1097-0142(19880115)61:2<340::aid-cncr2820610225>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Rutgers JL, Scully RE. Ovarian mixed-epithelial papillary cystadenomas of borderline malignancy. Cancer. 1988;61:546–54. doi: 10.1002/1097-0142(19880201)61:3<546::aid-cncr2820610321>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson MR, Kempson RL. Well differentiated mucinous neoplasms of the ovary. Pathology: State of the Arts Reviews. 1993;1:307–34. [PubMed] [Google Scholar]
- 6.Shappell HW, Riopel MA, Smith Sehdev AE, Ronnett BM, Kurman RJ. Diagnostic criteria and behavior of ovarian seromucinous (endocervical-type mucinous and mixed cell-type) tumors: atypical proliferative (borderline) tumors, intraepithelial, microinvasive, and invasive carcinomas. Am J Surg Pathol. 2002;26:1529–41. doi: 10.1097/00000478-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih Ie M, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A mutations in endometriosis-associated ovarian carcinomas. New Eng J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Shih IeM. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011:625–32. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayhan A, Mao TL, Seckin T, Wu CH, Guan B, Ogawa H, Futagami M, Mizukami H, Yokoyama Y, Kurman RJ, Shih Ie M. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gyn Cancer. 2012;22:1310–5. doi: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CH, Mao TL, Vang R, Ayhan A, Wang TL, Kurman RJ, Shih IM. Endocervical-type mucinous borderline tumors are related to endometrioid tumors based on mutation and loss of expression of ARID1A. Int J Gyn Pathol. 2012;31:297–303. doi: 10.1097/PGP.0b013e31823f8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurman RJ, Shih IM. Am J Pathol. In press. [Google Scholar]
- 13.Vang R, Gown AM, Barry TS, Wheeler DT, Ronnett BM. Ovarian atypical proliferative (borderline) mucinous tumors: gastrointestinal and seromucinous (endocervical-like) types are immunophenotypically distinctive. Int J Gynecol Pathol. 2006;25:83–9. doi: 10.1097/01.pgp.0000177125.31046.fd. [DOI] [PubMed] [Google Scholar]
- 14.Taylor J, McCluggage WG. Ovarian seromucinous carcinoma: Report of a series of a newly categorized and uncommon neoplasm. Am J Surg Pathol. 2015;39:983–92. doi: 10.1097/PAS.0000000000000405. [DOI] [PubMed] [Google Scholar]


