Abstract
Cardiovascular disease, including atherosclerosis and atherosclerosis-associated complications, is an increasing cause of morbidity and mortality in HIV patients in the post-antiretroviral therapy era. HIV alone accelerates atherosclerosis. Antiretroviral therapy, HIV-associated comorbidities, such as dyslipidemia, drug abuse, opportunistic infections, and lifestyle, are risk factors for HIV-associated atherosclerosis. However, our current understanding of HIV-associated atherogenesis is very limited and largely obtained from clinical observation. There is a pressing need to experimentally unravel the missing link between HIV and atherosclerosis. Understanding these mechanisms will help to better develop and design novel therapeutic interventions for the treatment of HIV-associated cardiovascular disease. HIV mainly infects T cells and macrophages resulting in the induction of oxidative and endoplasmic reticulem stress, the formation of the inflammasome, and the dysregulation of autophagy. These mechanisms may contribute to HIV-associated atherogenesis. In this review, we will summarize our current understanding and propose potential mechanisms of HIV-associated atherosclerosis.
Keywords: caspase-1, immune activation, inflammasome, inflammation, macrophage, Tg26 transgenic mice
INTRODUCTION
The current spectrum of human immunodeficiency virus (HIV) infections has dramatically shifted after the advent of effective antiretroviral therapy (ART). Although ART has successfully suppressed plasma viremia in HIV-infected (HIV+) patients, it is not sufficient to eradicate HIV. Despite a monumental investment in vaccine development, there have been no effective vaccines to date. With the persistence of the virus and the dramatically increased life expectancy of HIV+ patients stabilized on ART, the new challenge is the treatment of HIV-associated comorbidities, including HIV-associated neurocognitive disorders and cardiovascular disease (CVD) (1).
Atherosclerosis-associated CVD, including myocardial infarction (MI) and stroke, is currently one of the leading causes of mortality among HIV+ patients (2–6). There is emerging evidence to indicate that HIV infection and subsequent inflammatory processes in humans accelerate atherogenesis. The study of simian immunodeficiency virus (SIV) infection in primates displays a similar pattern of accelerated atherogenesis, and thus serves as an important model system for HIV pathogenesis. However, the mechanisms for HIV- or SIV-induced atherogenesis remain unclear. HIV-associated atherogenesis may be further complicated by ART, drug abuse, other HIV-associated comorbidities (dyslipidemia, opportunistic infections [OIs], and renal disease), and traditional atherosclerosis risk factors (lifestyle, smoking, and so on) (2). In patients on ART, HIV infection not only mediates immune cell activation and endothelial dysfunction, but also activates an array of cellular pathways, such as inflammasome formation/caspase-1 activation, autophagy, oxidative stress (OS), and endoplasmic reticulum (ER) stress. These mechanisms have an established contribution in the development of traditional atherosclerosis. However, our understanding of the pathogenic roles of HIV infection, immune cell activation, and the cellular and molecular mechanisms underlying HIV-associated atherogenesis is very limited and largely obtained from clinical observation. In this review, we will summarize the current understanding of HIV-associated atherosclerosis, its complications, and its associated risk factors. Then, we will discuss the potential mechanisms underlying HIV-associated atherosclerosis and the use of animal models to further dissect its pathogenesis.
HIV INFECTION INCREASES ATHEROSCLEROSIS-ASSOCIATED CVD IN THE ART ERA
CVD among HIV+ patients has drastically changed with the advent of ART. Early in the HIV epidemic, the predominant presentations of HIV-associated CVD were dilated cardiomyopathy, pericardial disease, pulmonary hypertension, HIV-associated malignancies, and OIs. Since the onset of ART, HIV+ patients are increasingly at risk for more common cardiovascular complications associated with atherosclerosis, including MI, stroke, and heart failure (2–6).
The use of ART has increased the survival of HIV+ patients to close to that of the general population. Although fewer HIV+ patients are dying of AIDS-related complications, the prevalence of non-AIDS-related comorbidities, including atherosclerosis-associated CVD, remains increased compared with HIV− controls. CVD is the second leading cause of non-AIDS-related mortality in the United States and third in Europe among HIV+ patients (6). Data suggest that HIV+ patients have a higher prevalence of traditional risk factors, including hypertension, diabetes, and dyslipidemia (7). There is extensive evidence to suggest that even when controlled for these traditional cardiovascular risk factors, HIV+ patients are still at a higher (1.5- to 3-fold) risk of developing CVD (8,9).
Two landmark clinical trials have provided valuable information in regard to ART and its timing of ART in HIV infection as they relate to CVD: the SMART (Strategies for Management of Antiretroviral Therapy) and START (Strategic Timing of Antiretroviral Treatment) studies (10). The SMART study showed that consistent use of ART in individuals with CD4+ cell counts below 350/µl resulted in a decrease in AIDS-related adverse events and in CVD events (11). For those deferring or interrupting treatment, there was a 70% increased hazard of CVD events, suggesting the need for continuous ART in preventing HIV-associated chronic inflammation and mitigating CVD risk (11). In the START study, a 40% reduction in AIDS-related events was observed when ART was given immediately; however, this did not prevent CVD events (12). Although both SMART and START support the notion that ART on the basis of stricter CD4+ thresholds will likely result in decreased CVD rates, ART is not sufficient to prevent CVD risk in HIV+ patients. Data from the VACS (Veterans Aging Cohort Study) cohort showed that HIV+ patients were at a higher risk of acute MI (hazard ratio, 1.48), even after adjustment for Framingham risk factors, comorbid conditions, and drug use (8). Thus, non-ART interventions are needed to decrease CVD risk among HIV+ populations and to improve immune function.
HIV+ individuals known as “elite controllers,” that is, those who have undetectable plasma viral loads without ART, have increased coronary atherosclerosis and high immune activation, including elevated plasma soluble CD163 (sCD163) (13). In another cohort, HIV+ elite controllers had a higher median carotid intima-media thickness (CIMT) than that observed in uninfected subjects, even after adjustment for traditional cardiovascular risk factors (14). These studies reinforce the role of inflammation, and not ART or virus, as the key mediator of HIV-associated CVD.
HIGH-RISK PLAQUE FEATURES IN HIV CVD
The presence of noncalcified plaques detected by coronary computed tomography angiography (CCTA) in the general population is associated with higher rates of acute coronary syndrome when compared with mixed and calcified plaques (15–17). These noncalcified plaques in HIV+ patients represent an early stage of atherosclerosis, and are more prone to rupture and thrombus formation compared with calcified plaques, likely leading to HIV-associated acute coronary syndrome (15). In well-controlled HIV+ patients, studies show a higher prevalence of subclinical coronary atherosclerosis (16,17) and greater burden of coronary atherosclerotic plaque, particularly noncalcified inflammatory plaques, in HIV+ young men than in HIV− individuals with similar cardiovascular risk factors (16). Imaging studies using CCTA have shown that HIV+ men have 59.0% prevalence of coronary atherosclerosis compared with 34.4% in non-HIV-infected (HIV−) control subjects(16,18). In addition to HIV+ men, HIV+ women had significantly higher percentages of segments with noncalcified plaques (74% vs. 23%) compared with female HIV- controls (19).
The presence of high-risk plaques is substantially associated with increased immune activation, more so than other risk factors. Plaque more prone to rupture have a necrotic core with an overlying thin fibrous cap and numerous macrophages (15). There is an increased prevalence of vulnerable plaque features (low attenuation, positively remodeled and spotty calcification) among young HIV+ patients compared with HIV− controls. HIV+ patients had greater low-attenuation plaque (22.8% vs. 7.3%), positively-remodeled plaque (49.5% vs. 31.7%), and high-risk 3-feature plaque (7.9% vs. 0%)(18). A meta-analysis study showed an association between noncalcified coronary artery plaques and reduced CD4 cell count in HIV+ patients, supporting the notion of systemic inflammatory dysregulation in HIV+ patients contributing to CVD (15). This evidence indicates that systemic inflammation and immune activation in HIV infection contributes to the accelerated atherogenesis seen in HIV+ patients.
HIV-ASSOCIATED ATHEROSCLEROSIS BIOMARKERS
Biomarkers also support the contribution of inflammation and immune activation in HIV CVD. Before ART, clinical studies found that the severity of HIV-associated atherosclerosis correlates with decreased CD4 levels (20,21). Countless studies have also looked for biomarkers, including those involved in the activation of monocytes/macrophages, T cells, endothelial cells, platelets, immune cell senescence, and microbial translocation (Table 1). As evident in Table 1, there is conflicting evidence of correlations between these biomarkers and HIV-associated atherosclerosis. This may be due, in part, to several factors, including differences in cohort size, patient status, lifestyle, ART, comorbidities, and measurement assays.
Table 1.
Correlation of Plasma or Surface Biomarkers for Cell Activation, Dysfunction and Senescence With HIV Atherosclerosis: Evident Monocyte/Macrophage Correlation With HIV-Associated Atherosclerosis, With Few Studies in Opposition
| References | Monocyte Macrophage* |
CD4+ T cell† |
CD8+ T cell† |
Endothelial Cells‡ |
Platelet Coagulation § |
Immune Cell Senescence∥ |
Microbial Translocation¶ |
|---|---|---|---|---|---|---|---|
| Alcaide et al., 2013 (38) | + | + | NC | + | |||
| Barbour et al., 2014 (47) | + | NC | + | ||||
| Burdo et al., 2011 (22) | + | NC | NC | ||||
| Chow et al., 2016 (44) | + | NC | |||||
| D’Abramo et al., 2014 (53) | NC | + | + | ||||
| Duprez et al., 2012 (24) | + | ||||||
| Falcäo et al., 2012 (131) | NC | NC | |||||
| Fitch et al., 2013 (19) | + | ||||||
| Ford et al., 2010 (39) | NC | NC | + | + | |||
| Goulenok et al., 2015 (132) | NC | NC | NC | ||||
| Grome et al., 2016 (52) | NC | NC | + | NC | |||
| Guaraldi et al., 2013 (56) | NC | + | |||||
| Hsu et al., 2016 (25) | + | NC | NC | NC | |||
| Kaplan et al., 2011 (51) | + | + | + | ||||
| Karim et al., 2014 (50) | + | NC | |||||
| Kelesidis et al., 2012 (35) | + | + | |||||
| Kuller et al., 2008 (26) | + | ||||||
| Longenecker et al., 2013 (133) | + | NC | NC | NC | |||
| Longenecker et al., 2013 (54) | NC | + | NC | + | |||
| McKibben et al., 2015 (45) | + | ||||||
| Merlini et al. 2012, (55) | NC | + | + | NC | NC | NC | |
| Papasavvas et al. 2012, (134) | NC | ||||||
| Pereyra et al. 2012, (13) | + | + | + | ||||
| Rönsholt et al., 2012 (135) | NC | NC | |||||
| Ross et al., 2009 (40) | + | ||||||
| Shaked et al., 2014 (36) | + | ||||||
| Siedner et al., 2016 (37) | + | NC | NC | NC | |||
| Subramanian et al., 2012 (23) | + | NC | |||||
| Tawakol et al., 2016 (58) | + | NC | |||||
| Westhorpe et al., 2014 (41) | + | ||||||
| Zanni et al., 2013 (18) | + | NC | NC | NC | |||
| Zungsontiporn et al., 2016 (46) | + | NC | |||||
| Total number +:NC | 17:4 | 4:8 | 8:10 | 3:6 | 4:5 | 2:5 | 2:4 |
sCD163, sCD14, CD14+/CD16+ monocytes, CD11b+ monocytes
CD38/HLA-DR
Soluble vascular cell adhesion molecule-1 (sVCAM-1), P-selectin, endothelial progenitor cells
P-selectin, D-dimer, fibrinogen
CD57+/CD28−
Lipopolysaccharide (LPS)
+ = positive correlation; NC = measured, but no significant correlation
A number of studies have demonstrated that activated monocytes and plasma macrophage-specific markers are correlated to HIV CVD. For example, plasma-soluble CD163 (sCD163), a monocyte/macrophage-specific activation marker, correlated with the presence of noncalcified plaque in a cohort of young HIV+ men (22). Female HIV+ patients also had elevated inflammatory markers, with increased plasma sCD163 and CXCL10, as well as CD14+CD16+ inflammatory monocytes (19). Fluorodeoxyglucose (18F) positron emission tomography (18F-FDG-PET) imaging showed increased arterial inflammation among HIV+ patients matched for traditional coronary risk factors (23). Plasma sCD163 correlated with 18F-FDG-PET imaging data showing increased presence of inflammatory macrophages in the ascending aorta of HIV-infected individuals, whereas C-reactive protein (CRP) and interleukin (IL)-6 did not (23).
In the SMART study, nonspecific immune markers (IL-6, CRP, and D-dimer) were associated with an increased risk of CVD, independent of other CVD risk factors (24). IL-6 and D-dimer were strongly related to all-cause mortality, and suggest that interrupting ART may increase inflammation and further increase the risk of cardiovascular death (25,26).
In summary, clinical evidence shows that even in the presence of ART, there is extensive atherosclerosis-associated CVD driven by HIV-associated immune activation and inflammation, which contribute to the unique pathophysiology of HIV-associated CVD. However, the exact cellular and molecular mechanisms are unknown, and may be further complicated by comorbidities and behavioral risk factors.
CONTRIBUTIONS OF RISK FACTORS AND COMORBIDITIES
Atherosclerosis-associated CVD in HIV+ patients is complicated not only by an increase in traditional cardiovascular risk factors, such as dyslipidemia and smoking, but also by side effects of ART. Primary HIV infection is associated with dyslipidemia, as measured by an increase in serum triglycerides and a decrease in the percentage of cholesterol (27), and further complicated by metabolic alterations introduced by ART. In the D:A:D (Data collection on adverse effects of Anti-HIV Drugs) study, the presence of dyslipidemia was 45.9% (27). Additionally, the ART regimen can be tailored to the patient’s CVD risk.
Behavioral risk factors, including illicit drug use and tobacco smoking, are more common in the HIV+ population and compound the risk of CVD (28). Hepatitis C virus (HCV) infection in the general population is associated with higher prevalence of atherosclerosis, and coinfection in HIV+ patients is associated with an even higher frequency of atherosclerotic plaques, stroke, and MI (29). However when risk factors are taken into account using the Framingham Risk scores and a new risk prediction algorithm, the atherosclerotic cardiovascular disease (ASCVD) risk algorithm, the CVD risk in HIV+ patients is underestimated (30). There is a need to understand how HIV infection contributes to the development of atherosclerosis and to determine how overlying risk factors may accelerate this process. This knowledge will aid in the development of personalized medicine for atherosclerosis and atherosclerotic complications in HIV+ patients.
UNDERLYING CELLULAR MECHANISMS
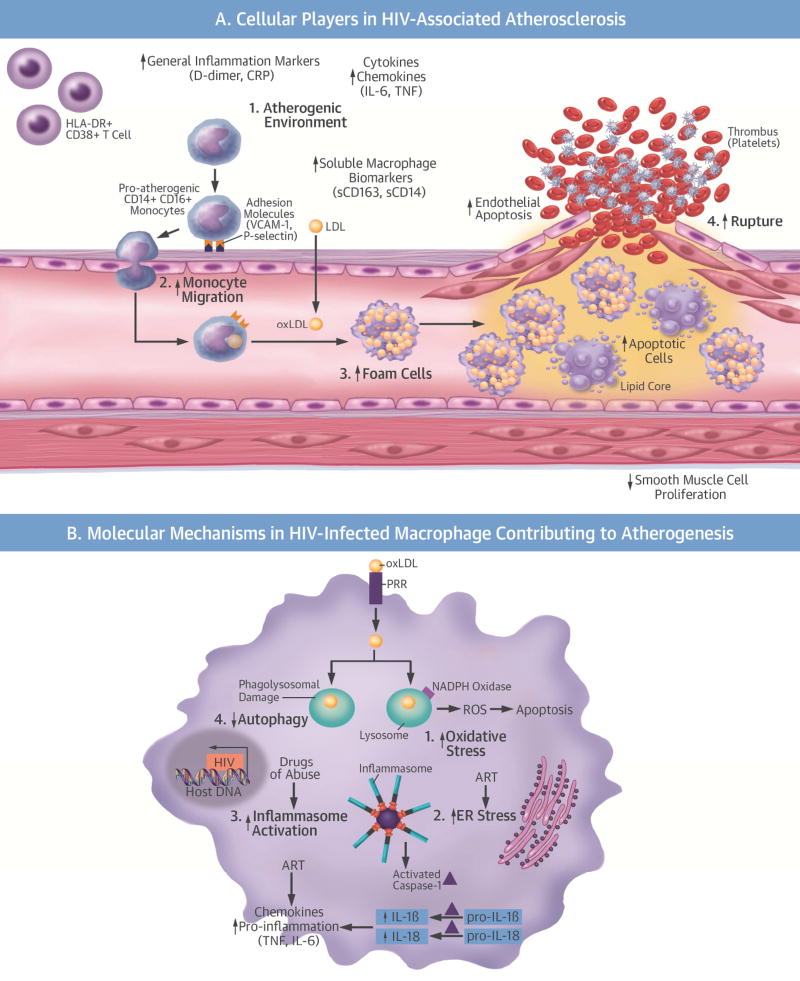
Our current knowledge of HIV-associated atherogenesis has been gained almost exclusively through clinical studies, with few mechanistic experimental studies. As such, our knowledge and understanding of HIV atherosclerosis is limited by what is known of traditional (non-HIV) atherosclerosis. Atherosclerosis is a chronic inflammatory condition in which immune and nonimmune mechanisms induce endothelial dysfunction, the first step in atherogenesis. In turn, endothelial dysfunction induces the expression of prothrombotic and proinflammatory cytokines and adhesion molecules that further propagate the inflammatory response, attracting monocytes and T-cells. Once in the intima, macrophages phagocytize oxidized low-density lipoproteins (oxLDL), leading to foam cell formation and eventually formation of plaques, the hallmark of atherosclerosis. Finally, the rupture of advanced plaques leads to stroke, MI, and thrombosis (Central Illustration, A).
Central Illustration. Cellular and Molecular Mechanisms of HIV Atherogenesis.
(A) Cellular players in the initiation, progression, and plaque rupture of HIV-associated atherosclerosis. 1) HIV enhances the inflammatory environment, causing an increase in atherogenic monocytes, HLA-DR+CD38+ T cells, cytokines, and chemokines. 2) The increase in atherogenic monocytes and chemokines increases monocyte migration into the vasculature. 3) HIV increases foam cell formation. 4) HIV decreases smooth muscle cell proliferation and increases endothelial and foam cell apoptosis, making the plaque more vulnerable to rupture. (B) Molecular mechanisms in an HIV-infected macrophage contributing to atherogenesis: 1) increased OS; 2) increased ER stress; 3) increased inflammasome activation and cytokine production; 4) decreased autophagy. These molecular mechanisms amplify each other and are further complicated by ART and HIV risk factors and comorbidities. ART = antiretroviral therapy; CRP = C-reactive protein; ER = endoplasmic reticulum; HCV = hepatitis C virus; HIV = human immunodeficiency virus; IL = interleukin; LDL = low-density lipoprotein; NADPH = nicotinamide adenine dinucleotide phosphate; OS = oxidative stress; oxLDL= oxidized low-density lipoprotein; PRR = pathogen recognition receptor; ROS = reactive oxygen species; TNF = tumor necrosis factor; VCAM-1 = vascular cell adhesion molecule-1.
A major link in HIV-associated atherosclerosis likely centers on the macrophage and its critical role in the inflammatory process and plaque formation (31). Although T cells are the major cellular reservoir for HIV infection, monocytes and macrophages also support HIV replication, and these cells remain chronically infected. HIV infection persists in patients on stable ART and the virus remains in a latent state in T cells and monocyte/macrophages, where it integrates into the host genome, only to reemerge when ART is discontinued (32). Even in the latent state, where viral loads are low or undetectable in the blood, potent viral regulatory proteins, including Tat, Nef, and others, are produced in T cells and monocytes at low levels, and can alter their function (31,33).
Biomarker studies, as discussed earlier, have provided important insights into the cellular mechanisms underlying HIV-associated atherogenesis. We have summarized these studies with regard to the correlation of cellular markers to the development of HIV-associated CVD (Table 1). These studies reach a consensus that highlights the importance of immune cell activation and inflammation, primarily via monocyte/macrophage activation, in the pathogenesis of HIV-associated atherogenesis. These studies support the hypothesis that the key underlying cellular mechanism appears to be chronic macrophage-mediated inflammation (34–38). From these clinical biomarker studies, there has been little focus on smooth muscle cells (SMC), endothelial cells, or platelet activation, and few correlations to HIV CVD identified. However, a few studies with large cohorts have found a significant correlation between CVD and activation of endothelial cells and platelets. It is important to not overlook their atherogenic roles when elucidating the mechanism (11,39,40).
MACROPHAGES AND FOAM CELLS
The migration and adhesion of monocytes to the subendothelial space and the scavenging of oxLDL, leading to the transformation into foam cells, are critical steps in atherogenesis (41). Chemokines and their receptors are important in driving macrophage migration into the vasculature. Inhibition of their activity (e.g., CCL2, CX3CR1, and CCR5) led to a remarkable reduction in atherogenesis, and atherosclerotic lesion size was highly correlated to the number of circulating monocytes. This indicates that these chemokines and adhesion molecules account for almost all of the monocyte migration and accumulation in atherosclerotic arteries, and highlights the importance of macrophage migration in atherogenesis (42,43). During HIV infection, chemokines are elevated and may play a role in the recruitment of monocytes to the vasculature, thereby facilitating HIV-associated atherogenesis (44–47). The atherogenic roles of monocytes/macrophages prompted the field to dissect the direct effects of HIV infection on macrophage cholesterol metabolism and HIV-associated atherosclerosis. HIV Nef protein, affects normal function of the ATP-binding cassette transporter A1 (ABCA1), impairing cholesterol efflux from infected macrophages (48). This impairment results in the accumulation of lipids and transformation of the macrophages into foam cells in vitro and in vivo (48). Conversely, the efflux capacity from ABCA1+/+ macrophages was increased in serum obtained from ART-treated subjects during acute infection (48). The significant relationship between reduction in viral load and increased cholesterol efflux capacity was independent of changes in high-density lipoprotein, CD4+ T cells, and activation markers. These results suggest that HIV infection dampens monocyte cholesterol metabolism and accelerates foam cell formation, which may be further influenced, or even improved by ART. Interestingly, HIV single-stranded RNAs induced foam cell formation in macrophages in a dose-dependent manner via binding to TLR8 in vitro (49). This finding helps to explain why HIV-accelerated atherogenesis is increased, even in well-controlled patients and elite controllers.
T CELLS
In HIV+ patients with viral suppression, percentages of activated T cells (CD38+ HLA− DR+) were still 2-fold higher compared with HIV− controls (50,51). Even HIV elite controllers have abnormally high T cell activation levels, which may contribute to progressive CD4+ T cell loss, even without measurable viremia (52). Chronic immune activation might contribute to the initiation of endothelial activation and subsequent atherogenesis (53), and these markers of T-cell activation are correlated with atherosclerosis in several studies (50–56). In atherosclerosis, T cells are recruited along with macrophages into the endothelium. However, they are far less abundant in the plaques (57). In the plaques, T cells produce proatherogenic mediators that contribute to lesion growth and exacerbate atherogenesis (57).
The immune cell activation in the periphery or lymph nodes (LNs) may not be reflective of cardiovascular inflammation. A recent study demonstrated that arterial inflammation in HIV+ patients, as measured by 18F-FDG PET, correlated with CRP, IL-6, and CD14+CD16+ monocytes, markers of immune activation, whereas LN inflammation correlated with measures of HIV disease activity (58). Another recent report showed discordant effects of newly-initiated ART in treatment-naïve HIV+ patients, which restored peripheral immune function, but did not reduce arterial inflammation (59). Together, these results indicate that the pathogenesis of HIV-associated vascular inflammation may be different from peripheral blood or LN inflammation. They highlight the importance of further dissecting HIV-mediated immune activation and inflammation, specifically in the vasculature.
Clinical correlation studies stress the importance of monocyte/macrophages, T cells, and inflammatory pathways in HIV-associated atherogenesis. The exact mechanism(s) of HIV-associated atherogenesis has not been established and requires further investigation.
POTENTIAL MOLECULAR MECHANISMS
Our understanding of the molecular mechanisms underlying HIV-associated atherosclerosis is less extensive than that of cellular mechanisms. In HIV+ patients, even those on ART, the interaction of HIV with host immune cells and endothelial cells can trigger several molecular events, including increased OS, ER stress, inflammasome formation, and dysregulation of autophagy (Central Illustration, B). These cellular mechanisms have an established role in traditional atherogenesis. Therefore, we will focus on the potential role of these molecular mechanisms in the development of HIV-associated atherogenesis.
OXIDATIVE STRESS
Reactive oxygen species (ROS) are highly-reactive molecules produced during normal oxidation/reduction reactions in cellular processes, such as oxidative metabolism, degradation of macromolecules and their substrates, and protein folding in the ER (60). Major sources of ROS are produced by the electron transport chain, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes (61), or by the cytochrome P450 system (60). Because ROS are abundant and produced regularly, eukaryotic cells have developed mechanisms of neutralization, repair, and inhibition of production. Neutralization or scavenging of ROS occurs through multiple mechanisms, including small-molecule antioxidants such as glutathione, NADPH reductase enzymes, superoxide dismutases, and heme oxygenases (60,61). However, ROS can become pathological when their production is increased or the antioxidant capacity of the cell is decreased.
ROS play a significant role in the development of atherosclerosis. NAPDH oxidases are highly concentrated in phagocytic cells, including macrophages, where they play a critical role in the host defense of ingested pathogens (62). NADPH oxidase is also expressed in endothelial cells and SMCs (63,64). Under hyperlipidemic conditions, NADPH oxidases are up-regulated, and are critical in the formation of oxLDL in both mice and humans (65). The atherogenic role of the NADPH oxidase enzyme family members has been extensively studied using apolipoprotein E null (ApoE−/−) mice (65). In these studies, they have a critical role in endothelial cell and SMC proliferation (63) and macrophage migration (64).
Both in vitro and in vivo models of HIV infection, as well as primary patient samples, show increased ROS and induced OS (66). ROS levels and its effects (e.g., oxLDL and oxidized nucleic acids) are higher in treatment-naive patients compared with those controlled on ART (67). Increased ROS results from both increased production and decreased antioxidant capacity (68). HIV+ patients with increased OS due to mutations affecting antioxidant capacity have an increased HIV plasma viral load, decreased CD4+ T cell counts, and increased cytotoxicity (69). Many of the HIV proteins, including Nef, increase ROS generation, causing endothelial dysfunction (70). HCV coinfection increases the levels of OS in HIV+ patients (71). Numerous reports also showed that specific ART regimens increased ROS production, mediating endothelial dysfunction, for which mitochondrial-targeted antioxidants and antioxidant treatment are protective (72). These results indicate that increased chronic OS resulting from HIV infection or ART mediates endothelial dysfunction and increases oxLDL production. However, whether the increased ROS production and its intracellular effects, including ER stress, as discussed in the following section, contribute to HIV-associated immune cell activation and atherogenesis still requires further investigation.
ER STRESS
The ER is the site of lipid and protein synthesis and modification, protein folding and maturation, and transport of assembled proteins (1). The ER also functions in calcium regulation and intracellular redox potential. Perturbations in these functions all result in ER stress. ER stress activates the signaling cascade called the unfolded protein response (UPR). The UPR is sensed and initiated by 3 proteins: inositol requiring 1 (IRE1); activating transcription factor 6 (ATF6); and protein kinase RNA-like ER kinase (PERK). Activation of these stress sensors results in compensatory mechanisms to relieve the ER stress, including inhibition of protein translation, induction of chaperone molecules, and increased ER biogenesis (73). With prolonged ER stress activation or induction of other cell stress signaling pathways, such as OS, apoptosis can ensue.
The atherogenic roles of ER stress and ER-induced apoptosis have been extensively investigated (1,73). Activation of the UPR occurs at all stages of atherosclerotic lesion formation, suggesting that ER stress contributes to multiple steps of atherogenesis (74). Blood flow-induced sheer stress induces ER stress in endothelial cells and endothelial cell dysfunction (75). In SMCs, prolonged stress and apoptosis can result in decreased collagen production and fibrous cap formation, resulting in plaque instability and rupture (76). The ER is the site of cholesterol-induced cytotoxicity in macrophages, and ER stress can induce macrophage apoptosis (77). OxLDL can induce ER stress in animal models of atherosclerosis (78). Inhibition of ER stress or deletion of ER stress genes in mouse models of atherosclerosis protected mice against atherosclerosis (79). ER stress also occurs in advanced human plaques (80). As made evident here, ER stress: 1) increases endothelial activation; 2) increases foam cell formation; 3) increases apoptosis in macrophages, contributing to formation of the necrotic lipid core; and 4) decreases SMC proliferation and collagen production, resulting in plaque instability.
Increased ROS, together with the release of soluble cellular and host factors following HIV infection, results in induction of the ER stress response, leading to apoptosis in the vasculature (81). HIV also triggers ER stress through its interaction with host genes, resulting in an imbalance of ER calcium homeostasis (1). Several HIV proteins are known to contribute to calcium imbalance in endothelial cells and can trigger apoptosis through ER stress, thereby leading to endothelial dysfunction (81). Consistently, HIV Tat-mediated induction of human endothelial cell apoptosis involves ER stress and mitochondrial dysfunction. The HIV envelope protein, gp120, induces type 1 programmed cell death through ER stress using the IRE1α, JNK and AP-1 pathways (33). HIV ART induces ER stress, increases the synthesis of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α) and IL-6, significantly increases apoptosis, and promotes foam cell formation in macrophages (82). The integrase inhibitor raltegravir can prevent the HIV protease inhibitor–induced inflammatory response and foam cell formation by inhibiting ER stress, suggesting that incorporation of raltegravir may reduce the ER stress-induced CVD associated with current ART (83).
NLRP3 INFLAMMASOME ACTIVATION
Inflammasome activation is part of the highly-conserved innate immune system’s response to harmful stimuli, including pathogens such as HIV, cell debris, and irritants. In response to these signals, the immune system initiates production of inflammatory cytokines. Overactivation of the inflammasome contributes to autoimmune diseases and chronic inflammatory diseases, including atherosclerosis (84). Inflammasome formation is dependent upon the recognition of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) by pattern recognition receptors (PRR), such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), or absent in melanoma 2 (AIM)-like receptors (ALRs). In response to these signals, NLRs or ALRs oligomerize to form the inflammasome complex. This inflammasome complex then recruits and activates the zymogen pro-caspase-1, which further activates pro-IL-1β and pro-IL-18, leading to generation of the active cytokines, IL-1β and IL-18, and subsequent inflammatory cascades (Central Illustration, B). IL-1β plays a pivotal role in driving inflammation by binding to its receptor and inducing more proinflammatory cytokines, including TNF-α (85).
The NLRP3 inflammasome is activated by danger signals involved in atherogenesis, including oxLDL and cholesterol crystals (86). Extensive clinical and experimental evidence has documented that inflammasome formation/caspase-1 activation plays a crucial role in the initiation and progression of atherosclerosis, including rupture of advanced plaques (87).
Activated levels of caspase-1 increase rapidly during early HIV infection. In those patients with a high CD4 count, caspase-1 levels decreased immediately, and in patients with a low CD4 count, caspase-1 levels remained elevated after 1 year of HIV infection (88). This process may be independent of the viral envelope (89) and increased by HIV-associated comorbidities (2). Furthermore, NLRP3 inflammasome activation in HIV-infected T cells contributes to the pathogenesis of T cell depletion and activation in HIV+ patients (90). A few studies have shown that monocyte/macrophage inflammasome formation may directly promote HIV-associated atherogenesis. HIV infection activates danger signaling cascades in infected immune and endothelial cells, which trigger inflammasome formation. HIV infection mediates the activation of the NLRP3 inflammasome in human macrophages, leading to release of bioactive IL-1β and IL-18 via TLR8-mediated mechanisms in vitro (85,89). IL-1β further induces its own production and the synthesis and expression of other HIV CVD–associated cytokines, such as IL-6 (24,25,91). In SIV-infected rhesus macaques on a high fat/high cholesterol diet, plasma IL-18 levels significantly correlated with atherosclerotic plaque area (92). In the plaques of these animals, IL-18 specifically colocalized with CD68+ macrophages, and not CD3+ T cells (92). These results suggest that the role of NLRP3 inflammasome activation may differ depending on the cell type involved. The atherogenic role of NLRP3 inflammasome activation in HIV infection has not been directly explored. Understanding the mechanism of the inflammasome complex and its contribution to HIV atherosclerosis will help justify ongoing clinical trials targeting this pathway.
AUTOPHAGY INHIBITION
Autophagy is the programmed and controlled clearance of aggregated toxic proteins and degradation of damaged organelles through fusion with lysosomes in promotion of cell survival (87). Autophagy interacts with the 3 previously-mentioned molecular mechanisms in response to pathogens including HIV. This autophagic effect also includes responses to inflammasome activation signaling pathways, and reduces OS and ER stress (87,93). There are 3 forms of autophagy, classified according to their differences in physiological function and patterns of delivery to the lysosome (94). However, these forms all use the same 3 stages wherein autophagy is first initiated, then the damaged protein or organelles are sectioned off in a membrane-bound autophagosome, and finally the autophagosome fuses with the lysosome to aid in degradation and recycling. Autophagy contributes to the development of many diseases, including inflammatory diseases, neurodegeneration, and CVD (95). Studies on the role of autophagy in inflammation support an anti-inflammatory role through the specific capture and degradation of activated inflammasome complexes and cytokines (93). Basal levels of autophagy vary among different cell types, and may also be preferentially regulated through alternative signaling mechanisms (96).
In regard to atherosclerosis, autophagy plays an important role in protecting cells from OS, reducing apoptosis, and enhancing plaque stability (97). Autophagic mechanisms protect endothelial cells from oxLDL-induced cytotoxicity, and prevent the formation of foam cells in vessel walls (98). Autophagy also protects against apoptosis of endothelial cells and SMCs (99,100). Deficiency of autophagy increases necrosis in atherosclerotic plaques, increasing susceptibility to destabilization and rupture in the low-density lipoprotein (LDL) receptor– deficient (LDLR−/−) mouse model of atherosclerosis (101).
Autophagy is a crucial catabolic process for cell survival in response to different stressors (87,93,98). In HIV infection, it provides a protective role for infected immune cells to reduce the increased oxidative and ER stress. Depending on the cell type, the role of autophagy can differ from contributing to loss of T cells to activation of macrophages (102). In dendritic cells, autophagy activation impairs the response of the immune system, and even enhances infection of CD4+ T cells (103). In macrophages, the induction of autophagy is increased; however, maturation of the cycle is clearly inhibited (104), which can contribute to persistent immune activation. The molecular mechanism of autophagy and how it contributes to HIV-associated atherosclerosis can vary depending on the cell type involved. The inhibition of autophagy in macrophages during HIV infection could be a major driving force of HIV-associated atherosclerosis. There is a well-established role of autophagy in HIV infection; however, its exact contribution to HIV-mediated atherosclerosis has not yet been fully explored.
In summary, there is substantial evidence for each of these pathways (OS, ER stress, inflammasome formation, and autophagy) in atherosclerosis and HIV infection alone (Central Illustration, B), but the role that each of these molecular mechanisms play in HIV-mediated atherosclerosis is currently unclear. HIV infection in combination with ART and comorbidities induces OS, leading to ER stress and increased production of UPR, which can lead to inflammasome formation. HIV infection directly activates the inflammasome, mediating the release of inflammatory cytokines, such as IL-1β and IL-18, thus leading to the initiation and progression of atherosclerosis. HIV then inhibits autophagy, further amplifying the stress effects. Further evaluation of these underlying molecular mechanisms should consider that HIV-associated vascular inflammation may be different from HIV-mediated LN inflammation. Animal models are critical for evaluating the roles of each of these mechanisms in HIV-associated atherosclerosis, and the subsequent effects that HIV-associated comorbidities or treatment may have on these mechanisms.
IN VIVO MODELS TO DISSECT HIV-ASSOCIATED ATHEROSCLEROSIS
Animal models of CVD yield important insights into the underlying pathology and provide a model for testing pharmacological treatments. Mice are resistant to the development of atherosclerosis, and therefore an atherogenic background is needed to accelerate atherogenesis. Two hypercholesterolemic models have been extensively used to study atherogenesis: ApoE−/− and LDLR−/− mice (105,106). The primary advantages of mouse models are ease of genetic manipulation and a reasonable time frame for the development of atherogenesis. ApoE−/− and LDLR−/− mice models have helped us better understand the atherogenesis for the last 2 decades. As discussed earlier in the section on cellular mechanisms, the atherogenic roles of CCL2, CX3CR1, and CCR5, as well as the roles of monocyte activation and homing/migration to the vasculature have been examined using the ApoE−/− and LDLR−/− models (42,43). Although these models have been used to examine the effect of ART (105,106), few studies have directly investigated the pathogenesis of HIV-associated atherosclerosis. The mechanism of monocyte activation and vascular homing in HIV-1–associated atherosclerosis can be tested in HIV animal models using chemokine inhibitors, such as maraviroc (CCR5 inhibitor) and centitricor (CCR2 and CCR5 inhibitor). Development of HIV mouse models in an atherogenic background is needed for further elucidating these mechanisms. A number of rodent models have been developed in the past 2 decades to study HIV-associated pathogenesis, including HIV-associated neurocognitive disorder (107–111).
THE TG26 MOUSE, A HIV TRANSGENIC MOUSE
The HIV Tg26 transgenic mouse was originally generated in the FVB genetic background by using a construct containing the genome of HIV NL4-3 with a deletion of 3.1 kb spanning the C-terminus of the Gag and the N-terminus of the Pol genes (107). Although these mice have no productive HIV replication, low-level expression of viral transcripts and proteins has been detected in various tissues before disease onset (107,112). Hemizygous Tg26 mice on the FVB/N background develop many clinical features of HIV infection, including nephropathy, inflammation, B cell lymphomas, cutaneous papillomas, cardiomyopathy, and muscle wasting (112). Using this model, a recent report showed that HIV promotes NLRP3 inflammasome complex activation in HIV-associated nephropathy (113). Tg26 mice on the FVB/N background have impaired aortic endothelial function, increased CIMT, and increased arterial stiffness, although they do not develop any plaque in the aorta (114). Studies have been hampered by early lethality of the Tg26 mice on the FVB/N background, but crossing the mice onto the C57BL/6J (B6) background eliminates the renal disease and improves their survival (115,116). Indeed, Tg26 on an ApoE−/− background (Tg26/ApoE−/−) demonstrated the development of an accelerated atherosclerosis phenotype (116). This mouse model now provides a valuable tool for studying the mechanisms of HIV-associated atherosclerosis.
HIV SINGLE-PROTEIN TRANSGENIC MOUSE MODELS
To explore the cardiac effect of Tat in vivo, a transgenic line was developed that specifically expressed Tat in murine cardiac myocytes (108). Targeted myocardial transgenic expression of HIV Tat caused cardiomyopathy and mitochondrial damage (108), resulting in bradycardia, depressed systolic and diastolic function, and blunted adrenergic responsiveness (109). To characterize the in vivo effects of Nef on lipid metabolism, Nef transgenic mice were generated (110). These mice had increased levels of neutral lipids in the liver and aorta, but no atherosclerotic plaques in the aorta. These results provide direct evidence that Nef promotes cholesterol deposition in tissues (110), which may contribute to HIV-associated atherogenesis. Gp120 transgenic mice have been extensively used for investigating the pathogenesis of HIV-associated neurocognitive disorder. This model has never been examined for its utility in dissecting the pathogenesis of HIV-associated atherogenesis.
HIV-INFECTED MOUSE MODEL
The use of mouse models for HIV pathogenesis has been hampered by the inability of HIV to replicate in rodent cells. This limitation has been overcome in recent years with the establishment of several humanized mouse models with circulating human lymphocytes that support infection with HIV (111). These mice, such as the humanized NOD-SCID-Gamma receptor knockout mouse model (huNSG) or bone-liver-thymus mouse model (BLT), have been shown to maintain persistent HIV infection over several months, with viral loads detectable in peripheral blood. Animals can be treated with ART to reduce viral loads, which can be reversed upon removal of ART. But this model cannot be introduced to an ApoE−/− or LDLR−/− background, which is required for the induction of atherosclerosis.
SIV MONKEY MODEL
The SIV-infected macaque model has been informative when dissecting HIV cardiac pathology. SIV infection induces changes in monocyte subset populations and phenotypes (117). The elevated CD14+CD16+ inflammatory monocytes are likely key to the initiation and progression of atherosclerosis, including homing to the vasculature and differentiation into foam cells. As in HIV-infected patients, both inflammatory markers, sCD14 and D-dimer, are elevated in SIV-infected rhesus macaques. A recent study using antibiotic and anti-inflammatory therapy to lower microbial translation and affect systemic inflammation showed decreases in these immune markers of inflammation and hypercoagulation, potentially influencing CVD (118). SIV-infected rhesus monkeys on an atherogenic diet develop a spectrum of cardiac lesions similar to those seen in HIV+ patients (119). Myocardial macrophage populations are seen in SIV-infected macaques with cardiomyocyte degeneration or necrosis. Results using SIV-infected rhesus macaques showed more than one-half of the animals had cardiac pathology in ventricular tissues, including macrophage infiltration and myocardial degeneration. The extent of fibrosis correlated with the presence of CD163+ M2 alternatively-activated macrophages in ventricle tissue (120), suggesting that macrophage accumulation drives cardiac pathology with SIV infection. Although SIV-infected rhesus macaques have macrophage infiltration, myocardial degeneration, and necrosis in the heart tissue, rhesus monkeys rarely develop atherosclerosis in the absence of an atherogenic diet. One recent report in macaques infected with SIV or simian-human immunodeficiency virus, but not on an atherogenic diet, demonstrated early atherosclerotic changes, including increased vascular inflammation, endothelial dysfunction. and leukocyte adhesion to the descending thoracic aorta (121). Early studies demonstrated that SIV-infected macaques fed an atherogenic diet showed a more rapid disease progression, resulting in an increased risk of SIV-related death (122). The baseline plasma IL-18 level after 6 months of the high-fat diet was predictive of atherosclerotic lesion progression (92,122). However, due to the expensive cost of maintenance, extreme difficulty of genetic manipulation, and long time frame for monitoring atherogenesis in SIV-infected monkeys, it is still very difficult to apply the SIV monkey model to teasing out pathogenesis, except possibly for preclinical testing of highly promising therapeutic approaches.
CLINICAL TRIALS OF HIV-ASSOCIATED ATHEROSCLEROSIS
Data presented in this review support the role of HIV-mediated inflammation as a causative agent of HIV-associated atherosclerosis. More recent studies have focused on therapies that reduce immune activation, as well as cholesterol, which may be of benefit, particularly for HIV+ individuals because the CVD is driven by traditional and inflammatory factors (3,123). Statins, which are both lipid-lowering and anti-inflammatory, were used in the INTREPID (HIV+ Patients and Treatment with Pitavastatin vs Pravastatin for Dyslipidemia) and the current REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV) trial. Data from INTREPID have shown that pitavastatin was superior to pravastatin in LDL reduction after 12 weeks of therapy in HIV+ individuals with dyslipidemia (124). The REPRIEVE trial is ongoing, and recruiting HIV+ patients between 40 and 75 years of age who are taking an antiretroviral regimen, who have no known CVD or substantial kidney or liver disease, and have a CD4+ cell count higher than 100/µl (125). HIV+ patients (6,500) will be randomized to daily pitavastatin or a placebo for an average of 4 years. The primary endpoint is the prevention of major adverse cardiovascular events, including CVD-related mortality, MI, stroke, unstable angina, peripheral artery disease, and cardiac revascularization. A substudy including 800 patients will investigate the mechanism of pitavastatin in reducing inflammation, immune activation, and high-risk plaque using CCTA and biomarker analyses. A pilot study by Hsue et al. (126) will examine the impact of IL-1 inhibition on systemic inflammation, T cell activation, and vascular inflammation among treated and suppressed HIV+ patients. This study is promising, as IL-1 is up-regulated during inflammasome activation in both atherosclerosis and HIV infection.
Recent trials (127) indicate that rosuvastatin decreases oxLDL levels early after initiation and is associated with decreased monocyte activation (3,123). This early improvement in oxLDL is linked with improved CIMT, a measurement for atherosclerotic plaque in treated HIV infection (3,123). There has been a report of a positive effect of rosuvastatin in reducing LDL levels without a substantial modification of activated T cells and levels of CRP, a general immune marker (128). However, this study did not examine monocytes or macrophage immune markers. Future study is needed to determine whether HIV+ patients with higher levels of inflammation or monocyte activation and lower LDL have greater cardiovascular benefit from statin therapy, which will be addressed by the REPRIEVE trial. Together, these clinical trial studies with ART, lipid lowering, and anti-inflammatory approaches will shed light on the role of HIV infection, dyslipidemia, and inflammation in HIV-associated atherogenesis. It is also conceivable that new clinical trials targeting chronic immune activation and inflammation induced by HIV infection for well-controlled HIV+ patients with specific risk factors will emerge in the near future.
CONCLUSIONS AND FUTURE DIRECTIONS
HIV-associated atherosclerosis and its complications are a significant human health burden for which the pathogenesis remains elusive. The distinct pathological features of HIV-induced atherosclerosis are noncalcified and inflammatory plaques that are more vulnerable to rupture, resulting in MI and stroke. The atherogenic role of immune cell activation and inflammation in HIV infection has been recognized. The immune events of atherosclerosis are complicated in HIV+ patients by ART, lifestyle risk factors, and comorbidities. HIV infection, whether productive or latent, mediates an array of molecular signaling pathways, including inflammasome activation, autophagy inhibition, and induction of oxidative and ER stress, all of which have an established pathogenic role in atherosclerosis. The contribution of these pathways to immune cell activation and inflammation in the vasculature, leading to the initiation, progression, and rupture of atherosclerosis, still requires further investigation. Development of appropriate mouse models for examining HIV-associated atherosclerosis will be a key step to tease out the specific cellular and molecular mechanisms underlying the pathogenesis. Introduction of HIV mouse models to atherogenic backgrounds, such as ApoE−/− and LDLR−/−, together with our targeted immune cell ablation tool (129,130) may be very useful for understanding HIV-associated atherogenesis. The SIV monkey model is important for testing mechanism-targeted therapeutic approaches, before establishing clinical trials. These combined approaches will aid in understanding the pathogenesis of HIV-associated atherogenesis and developing better therapeutic and preventative strategies.
Acknowledgments
This work was supported by W.W. Smith Charitable Trust A1502, NIH R01CA166144, R01 HL130233, and R21 AA024984 to Dr. Qin, and by R01 NS082116 to Dr. Burdo. Ms. Kearns was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award 5T32MH079785.
ABBREVIATIONS AND ACRONYMS
- ART
antiretroviral therapy
- CVD
cardiovascular disease
- ER
endoplasmic reticulum
- HIV
human immunodeficiency virus
- HIV+
human immunodeficiency virus-infected
- MI
myocardial infarction
- OS
oxidative stress
- oxLDL
oxidized low-density lipoprotein
- ROS
reactive oxygen species
- SIV
simian immunodeficiency virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Shrestha S, Irvin MR, Grunfeld C, Arnett DK. HIV, inflammation, and calcium in atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:244–50. doi: 10.1161/ATVBAHA.113.302191. [DOI] [PubMed] [Google Scholar]
- 2.Vachiat A, McCutcheon K, Tsabedze N, Zachariah D, Manga P. HIV and ischemic heart disease. J Am Coll Cardiol. 2017;69:73–82. doi: 10.1016/j.jacc.2016.09.979. [DOI] [PubMed] [Google Scholar]
- 3.Gili S, Grosso Marra W, D’Ascenzo F, et al. Comparative safety and efficacy of statins for primary prevention in human immunodeficiency virus-positive patients: a systematic review and meta-analysis. Eur Heart J. 2016;37:3600–9. doi: 10.1093/eurheartj/ehv734. [DOI] [PubMed] [Google Scholar]
- 4.D’Ascenzo F, Quadri G, Cerrato E, et al. A meta-analysis investigating incidence and features of stroke in HIV-infected patients in the highly active antiretroviral therapy era. J Cardiovasc Med (Hagerstown) 2015;16:839–43. doi: 10.2459/JCM.0b013e328365ca31. [DOI] [PubMed] [Google Scholar]
- 5.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33:875–80. doi: 10.1093/eurheartj/ehr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CJ, Ryom L, Weber R, et al. D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–8. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 7.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–41. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 10.Siedner MJ. START or SMART? Timing of antiretroviral therapy initiation and cardiovascular risk for people with human immunodeficiency virus infection. Open Forum Infect Dis. 2016;3 doi: 10.1093/ofid/ofw032. ofw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 12.INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26:2409–12. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Ascenzo F, Cerrato E, Calcagno A, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis. 2015;240:197–204. doi: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–67. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanni MV, Abbara S, Lo J, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–72. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–46. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160–6. doi: 10.1097/QAI.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 21.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Curr Opin HIV AIDS. 2014;9:54–62. doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duprez DA, Neuhaus J, Kuller LH, et al. INSIGHT SMART Study Group. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PloS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu DC, Ma YF, Hur S, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. AIDS. 2016;30:2065–74. doi: 10.1097/QAD.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, et al. INSIGHT SMART Study Group. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friis-Møller N, Weber R, Reiss P, et al. DAD study group. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 28.Beltrán LM, Rubio-Navarro A, Amaro-Villalobos JM, Egido J, García-Puig J, Moreno JA. Influence of immune activation and inflammatory response on cardiovascular risk associated with the human immunodeficiency virus. Vasc Health Risk Manag. 2015;11:35–48. doi: 10.2147/VHRM.S65885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosner P, Wangermez M, Chagneau-Derrode C, Le Moal G, Silvain C. Atherosclerosis risk in HIV-infected patients: the influence of hepatitis C virus co-infection. Atherosclerosis. 2012;222:274–7. doi: 10.1016/j.atherosclerosis.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Shahbaz S, Manicardi M, Guaraldi G, Raggi P. Cardiovascular disease in human immunodeficiency virus infected patients: a true or perceived risk? World J Cardiol. 2015;7:633–44. doi: 10.4330/wjc.v7.i10.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87:589–98. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254:326–42. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah A, Kumar A. HIV-1 gp120-mediated mitochondrial dysfunction and HIV-associated neurological disorders. Neurotox Res. 2016;30:135–7. doi: 10.1007/s12640-016-9619-3. [DOI] [PubMed] [Google Scholar]
- 34.Ipp H, Zemlin A. The paradox of the immune response in HIV infection: when inflammation becomes harmful. Clin Chim Acta. 2013;416:96–9. doi: 10.1016/j.cca.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaked I, Hanna DB, Gleiβner C, et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol. 2014;34:1085–92. doi: 10.1161/ATVBAHA.113.303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siedner MJ, Kim JH, Nakku RS, et al. Persistent immune activation and carotid atherosclerosis in HIV-infected Ugandans receiving antiretroviral therapy. J Infect Dis. 2016;213:370–8. doi: 10.1093/infdis/jiv450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PloS One. 2013;8:e63804. doi: 10.1371/journal.pone.0063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–17. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross AC, Rizk N, O’Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–27. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westhorpe CL, Maisa A, Spelman T, et al. Associations between surface markers on blood monocytes and carotid atherosclerosis in HIV-positive individuals. Immunol Cell Biol. 2014;92:133–8. doi: 10.1038/icb.2013.84. [DOI] [PubMed] [Google Scholar]
- 42.Hamon P, Loyher PL, Baudesson de Chanville C, Licata F, Combadière C, Boissonnas A. CX3CR1-dependent endothelial margination modulates Ly6Chigh monocyte systemic deployment upon inflammation in mice. Blood. 2017;129:1296–307. doi: 10.1182/blood-2016-08-732164. [DOI] [PubMed] [Google Scholar]
- 43.Combadière C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–57. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 44.Chow DC, Kagihara JM, Zhang G, et al. Non-classical monocytes predict progression of carotid artery bifurcation intima-media thickness in HIV-infected individuals on stable antiretroviral therapy. HIV Clin Trials. 2016;17:114–22. doi: 10.1080/15284336.2016.1162386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–28. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zungsontiporn N, Tello RR, Zhang G, et al. Non-classical monocytes and monocyte chemoattractant protein-1 (MCP-1) correlate with coronary artery calcium progression in chronically HIV-1 infected adults on stable antiretroviral therapy. PloS One. 2016;11:e0149143. doi: 10.1371/journal.pone.0149143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbour JD, Jalbert EC, Chow DC, et al. Reduced CD14 expression on classical monocytes and vascular endothelial adhesion markers independently associate with carotid artery intima media thickness in chronically HIV-1 infected adults on virologically suppressive anti-retroviral therapy. Atherosclerosis. 2014;232:52–8. doi: 10.1016/j.atherosclerosis.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pushkarsky T, Shilov E, Kruglova N, et al. Short communication: accumulation of neutral lipids in liver and aorta of Nef-transgenic mice. AIDS Res Hum Retroviruses. 2017;33:57–60. doi: 10.1089/aid.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernard MA, Han X, Inderbitzin S, et al. HIV-derived ssRNA binds to TLR8 to induce inflammation-driven macrophage foam cell formation. PloS One. 2014;9:e104039. doi: 10.1371/journal.pone.0104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karim R, Mack WJ, Kono N, et al. T-cell activation, both pre- and post-HAART levels, correlates with carotid artery stiffness over 6.5 years among HIV-infected women in the WIHS. J Acquir Immune Defic Syndr. 2014;67:349–56. doi: 10.1097/QAI.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grome HN, Barnett L, Hagar CC, Harrison DG, Kalams SA, Koethe JR. Association of T cell and macrophage activation with arterial vascular health in HIV. AIDS Res Hum Retroviruses. 2017;33:181–6. doi: 10.1089/aid.2016.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Abramo A, Zingaropoli MA, Oliva A, et al. Immune activation, immunosenescence, and osteoprotegerin as markers of endothelial dysfunction in subclinical HIV-associated atherosclerosis. Mediators Inflamm. 2014;2014:192594. doi: 10.1155/2014/192594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–90. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merlini E, Luzi K, Suardi E, et al. T-cell phenotypes, apoptosis and inflammation in HIV+ patients on virologically effective cART with early atherosclerosis. PloS One. 2012;7:e46073. doi: 10.1371/journal.pone.0046073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guaraldi G, Luzi K, Bellistri GM, et al. CD8 T-cell activation is associated with lipodystrophy and visceral fat accumulation in antiretroviral therapy-treated virologically suppressed HIV-infected patients. J Acquir Immune Defic Syndr. 2013;64:360–6. doi: 10.1097/QAI.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 57.Hansson GK, Robertson AK, Söderberg-Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 58.Tawakol A, Ishai A, Li D, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. 2017;2:163–71. doi: 10.1001/jamacardio.2016.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zanni MV, Toribio M, Robbins GK, et al. Effects of antiretroviral therapy on immune function and arterial inflammation in treatment-naive patients with human immunodeficiency virus infection. JAMA Cardiol. 2016;1:474–80. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ivanov AV, Valuev-Elliston VT, Ivanova ON, et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jagadeesha DK, Takapoo M, Banfi B, Bhalla RC, Miller FJ., Jr Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovasc Res. 2012;93:406–13. doi: 10.1093/cvr/cvr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller AA, De Silva TM, Judkins CP, Diep H, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–9. doi: 10.1161/STROKEAHA.109.575365. [DOI] [PubMed] [Google Scholar]
- 65.Perrotta I, Aquila S. The role of oxidative stress and autophagy in atherosclerosis. Oxid Med Cell Longev. 2015;2015:130315. doi: 10.1155/2015/130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wanchu A, Rana SV, Pallikkuth S, Sachdeva RK. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Res Hum Retroviruses. 2009;25:1307–11. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 67.Parra S, Coll B, Aragonés G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–31. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe LM, Barbosa Júnior F, Jordão AA, Navarro AM. Influence of HIV infection and the use of antiretroviral therapy on selenium and selenomethionine concentrations and antioxidant protection. Nutrition. 2016;32:1238–42. doi: 10.1016/j.nut.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Parsons M, Campa A, Lai S, et al. Effect of GSTM1-polymorphism on disease progression and oxidative stress in HIV infection: modulation by HIV/HCV co-infection and alcohol consumption. J AIDS Clin Res. 2013;4:10002337. doi: 10.4172/2155-6113.1000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duffy P, Wang X, Lin PH, Yao Q, Chen C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res. 2009;156:257–64. doi: 10.1016/j.jss.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang X, Liang H, Fan X, Zhu L, Shen T. Liver damage in patients with HCV/HIV coinfection is linked to HIV-related oxidative stress. Oxid Med Cell Longev. 2016;2016:8142431. doi: 10.1155/2016/8142431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hulgan T, Morrow J, D’Aquila RT, et al. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–7. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 73.Scull CM, Tabas I. Mechanisms of ER stress-induced apoptosis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2792–7. doi: 10.1161/ATVBAHA.111.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou J, Lhoták S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–21. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 75.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–61. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clarke MC, Figg N, Maguire JJ, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–80. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 77.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 78.Dong Y, Zhang M, Wang S, et al. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–96. doi: 10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erbay E, Babaev VR, Mayers JR, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–33. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 81.Ma R, Yang L, Niu F, Buch S. HIV Tat-mediated induction of human brain microvascular endothelial cell apoptosis involves endoplasmic reticulum stress and mitochondrial dysfunction. Mol Neurobiol. 2016;53:132–42. doi: 10.1007/s12035-014-8991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen L, Jarujaron S, Wu X, et al. HIV protease inhibitor lopinavir-induced TNF-α and IL-6 expression is coupled to the unfolded protein response and ERK signaling pathways in macrophages. Biochem Pharmacol. 2009;78:70–7. doi: 10.1016/j.bcp.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Cao R, Liu R, et al. Reduction of the HIV protease inhibitor-induced ER stress and inflammatory response by raltegravir in macrophages. PloS One. 2014;9:e90856. doi: 10.1371/journal.pone.0090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 85.Guo H, Gao J, Taxman DJ, Ting JP, Su L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem. 2014;289:21716–26. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–87. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–44. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Song J, Jiao Y, Zhang T, et al. Longitudinal changes in plasma Caspase-1 and Caspase-3 during the first 2 years of HIV-1 infection in CD4Low and CD4High patient groups. PloS One. 2015;10:e0121011. doi: 10.1371/journal.pone.0121011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hernandez JC, Latz E, Urcuqui-Inchima S. HIV-1 induces the first signal to activate the NLRP3 inflammasome in monocyte-derived macrophages. Intervirology. 2014;57:36–42. doi: 10.1159/000353902. [DOI] [PubMed] [Google Scholar]
- 90.Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–56. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yearley JH, Xia D, Pearson CB, Carville A, Shannon RP, Mansfield KG. Interleukin-18 predicts atherosclerosis progression in SIV-infected and uninfected rhesus monkeys (Macaca mulatta) on a high-fat/high-cholesterol diet. Lab Invest. 2009;89:657–67. doi: 10.1038/labinvest.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deretic V, Jiang S, Dupont N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012;22:397–406. doi: 10.1016/j.tcb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shao BZ, Han BZ, Zeng YX, Su DF, Liu C. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sinica. 2016;37:150–6. doi: 10.1038/aps.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–20. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antiox Redox Signal. 2006;8:152–62. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 98.Peng N, Meng N, Wang S, et al. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E−/− mice. Sci Rep. 2014;4:5519. doi: 10.1038/srep05519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinet W, Schrijvers DM, Timmermans JP, Bult H. Interactions between cell death induced by statins and 7-ketocholesterol in rabbit aorta smooth muscle cells. Br J Pharmacol. 2008;154:1236–46. doi: 10.1038/bjp.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schrijvers DM, De Meyer GR, Martinet W. Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol. 2011;31:2787–91. doi: 10.1161/ATVBAHA.111.224899. [DOI] [PubMed] [Google Scholar]
- 101.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dinkins C, Pilli M, Kehrl JH. Roles of autophagy in HIV infection. Immunol Cell Biol. 2015;93:11–7. doi: 10.1038/icb.2014.88. [DOI] [PubMed] [Google Scholar]
- 103.Blanchet FP, Moris A, Nikolic DS, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–69. doi: 10.1016/j.immuni.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kyei GB, Dinkins C, Davis AS, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186:255–68. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo W, Wong S, Pudney J, et al. Acipimox, an inhibitor of lipolysis, attenuates atherogenesis in LDLR-null mice treated with HIV protease inhibitor ritonavir. Arterioscler Thromb Vasc Biol. 2009;29:2028–32. doi: 10.1161/ATVBAHA.109.191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Caulk AW, Soler J, Platt MO, Gleason RL., Jr Efavirenz treatment causes arterial stiffening in apolipoprotein E-null mice. J Biomech. 2015;48:2176–80. doi: 10.1016/j.jbiomech.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 107.Kopp JB, Klotman ME, Adler SH, et al. Progressive glomerulosclerosis and enhanced renal accumulation of basement membrane components in mice transgenic for human immunodeficiency virus type 1 genes. Proc Natl Acad Sci U S A. 1992;89:1577–81. doi: 10.1073/pnas.89.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Raidel SM, Haase C, Jansen NR, et al. Targeted myocardial transgenic expression of HIV Tat causes cardiomyopathy and mitochondrial damage. Am J Physiol Heart Circ Physiolo. 2002;282:H1672–8. doi: 10.1152/ajpheart.00955.2001. [DOI] [PubMed] [Google Scholar]
- 109.Fang Q, Kan H, Lewis W, Chen F, Sharma P, Finkel MS. Dilated cardiomyopathy in transgenic mice expressing HIV Tat. Cardiovasc Toxicol. 2009;9:39–45. doi: 10.1007/s12012-009-9035-5. [DOI] [PubMed] [Google Scholar]
- 110.Pushkarsky T, Shilov E, Kruglova N, et al. Accumulation of neutral lipids in liver and aorta of Nef-transgenic mice. AIDS Res Hum Retroviruses. 2017;33:57–60. doi: 10.1089/aid.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Denton PW, Garcia JV. Novel humanized murine models for HIV research. Curr HIV/AIDS Rep. 2009;6:13–9. doi: 10.1007/s11904-009-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Curreli S, Krishnan S, Reitz M, et al. B cell lymphoma in HIV transgenic mice. Retrovirology. 2013;10:92. doi: 10.1186/1742-4690-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haque S, Lan X, Wen H, et al. HIV promotes NLRP3 inflammasome complex activation in murine HIV-associated nephropathy. Am J Pathol. 2016;186:347–58. doi: 10.1016/j.ajpath.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hansen L, Parker I, Sutliff RL, Platt MO, Gleason RL., Jr Endothelial dysfunction, arterial stiffening, and intima-media thickening in large arteries from HIV-1 transgenic mice. Ann Biomed Eng. 2013;41:682–93. doi: 10.1007/s10439-012-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mallipattu SK, Liu R, Zhong Y, et al. Expression of HIV transgene aggravates kidney injury in diabetic mice. Kidney Int. 2013;83:626–34. doi: 10.1038/ki.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kearns A, Dai S, Liu F, et al. P75: Transgenic expression of HIV-1 in Tg26 mice accelerates atherosclerosis; and implications for vascular dementia. J Neurovirol. 2016;22(Suppl 1):s36. (abstr) [Google Scholar]
- 117.Kim WK, Sun Y, Do H, et al. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J Leukoc Biol. 2010;87:557–67. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pandrea I, Xu C, Stock JL, et al. Antibiotic antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathog. 2016;12:e1005384. doi: 10.1371/journal.ppat.1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yearley JH, Pearson C, Shannon RP, Mansfield KG. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses. 2007;23:515–24. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 120.Walker JA, Sulciner ML, Nowicki KD, Miller AD, Burdo TH, Williams KC. Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res Hum Retroviruses. 2014;30:685–94. doi: 10.1089/aid.2013.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Panigrahi S, Freeman ML, Funderburg NT, et al. SIV/SHIV infection triggers vascular inflammation, diminished expression of Krüppel-like factor 2 and endothelial dysfunction. J Infect Dis. 2016;213:1419–27. doi: 10.1093/infdis/jiv749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mansfield KG, Carville A, Wachtman L, et al. A diet high in saturated fat and cholesterol accelerates simian immunodeficiency virus disease progression. J Infect Dis. 2007;196:1202–10. doi: 10.1086/521680. [DOI] [PubMed] [Google Scholar]
- 123.Banach M, Dinca M, Ursoniu S, et al. Lipid Blood Pressure Meta-analysis Collaboration Group. A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials investigating the effects of statin therapy on plasma lipid concentrations in HIV-infected patients. Pharmacol Res. 2016;111:343–56. doi: 10.1016/j.phrs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SC, Thompson MA. Pitavastatin vs. pravastatin in adults with HIV-1 infection and dyslipidemia (INTREPID): 12 week and 52 week results of a phase 4, multicenter, randomised, double-blind superiority trial. Lancet HIV. 2017 Apr 12; doi: 10.1016/S2352-3018(17)30075-9. [E-pub ahead of print], http://dx.doi.org/10.1016/S2352-3018(17)30075-9. [DOI] [PubMed]
- 125.Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23:146–9. [PMC free article] [PubMed] [Google Scholar]
- 126.Hsue P, Deeks SG, Amorina E. IL-1β inhibition significantly reduces atherosclerotic inflammation in treated HIV. Paper presented at: Conference on Retroviruses and Opportunisitc Infections (CROI); February 13–16, 2017; Seattle, Washington. [Google Scholar]
- 127.Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS. 2016;30:65–73. doi: 10.1097/QAD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS. 2016;30:2195–203. doi: 10.1097/QAD.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feng D, Dai S, Liu F, et al. Cre-inducible human CD59 mediates rapid cell ablation after intermedilysin administration. J Clin Invest. 2016;126:2321–33. doi: 10.1172/JCI84921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu W, Ferris SP, Tweten RK, et al. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat Med. 2008;14:98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- 131.Falcão Mda C, Zirpoli JC, Albuquerque VM, et al. Association of biomarkers with atherosclerosis and risk for coronary artery disease in patients with HIV. Arq Bras Cardiol. 2012;99:971–8. doi: 10.1590/s0066-782x2012005000093. [DOI] [PubMed] [Google Scholar]
- 132.Goulenok T, Boyd A, Larsen M, et al. CHIC Study group. Increased carotid intima-media thickness is not associated with T-cell activation nor with cytomegalovirus in HIV-infected never-smoker patients. AIDS. 2015;29:287–93. doi: 10.1097/QAD.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 133.Longenecker CT, Jiang Y, Yun CH, et al. Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int J Cardiol. 2013;168:4039–45. doi: 10.1016/j.ijcard.2013.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Papasavvas E, Hsue P, Reynolds G, et al. Increased CD34+/KDR+ cells are not associated with carotid artery intima-media thickness progression in chronic HIV-positive subjects. Antiviral Ther. 2012;17:557–63. doi: 10.3851/IMP2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rönsholt FF, Ostrowski SR, Katzenstein TL, Ullum H, Gerstoft J. T cell subsets in HIV infected patients after successful combination antiretroviral therapy: impact on survival after 12 years. PloS One. 2012;7:e39356. doi: 10.1371/journal.pone.0039356. [DOI] [PMC free article] [PubMed] [Google Scholar]