Most of the discussion following the passage of The 21st Century Cures Act has focused on the law’s increased funding for biomedical research, and its more controversial changes to drug and device approval. Little attention has been paid to the legislation’s changes to reimbursement for home infusion drugs. However, this less publicized aspect may ultimately limit patient access to home inotropic infusions, which are associated with decreased hospital admissions, lengths of hospital stay, and total spending for advanced heart failure patients.1
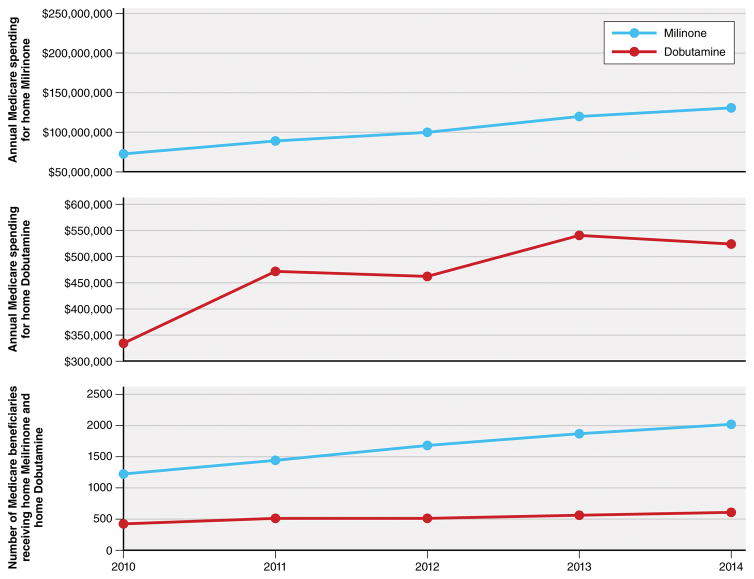
The epidemiology of heart failure is changing and with it, the epidemiology of home inotrope use. Patients are living longer with heart failure, now often not requiring advanced therapies, until they have become medically ineligible for ventricular assist devices or transplantation. At the same time, hospitals are facing increasing pressure to decrease lengths of stay and readmission rates. Home inotropic infusions can offer a practical solution to both challenges. As a result, the recent diffusion of home inotropic drugs, which include dobutamine or milrinone, has increased. Between 2010 and 2014, there was a 63% increase in the number of beneficiaries receiving home milrinone and a 44% increase in the number receiving home dobutamine. As a result, in 2014, Medicare spent $243 million ($64,152 per beneficiary on average) for home milrinone, an increase of 55% from 2010 and $3.8 million ($848 per beneficiary on average) for home dobutamine, an increase of 57% from 2010 (Figure).
The Cures Act changes the reimbursement methodology for all home infusion drugs, including home inotropes, covered under the Part B durable medical equipment (DME) benefit from an average wholesale price minus 5% (AWP-5%) method to an average sales price plus 6% (ASP+6%) method. Practically, this means that the reimbursement for some drugs will increase, but most will decrease. The net effect is an estimated $660 million savings to Medicare over 10 years, much of which will be achieved by a substantial decrease in home milrinone reimbursement.2
Why is milrinone reimbursement so significantly affected? In 2003, the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) made this same reimbursement change (going from an AWP-5% method to a ASP+6% method) to almost all Part B drugs. At the time however, the law exempted a few drugs, including home milrinone. As a result of the exemption, Medicare’s reimbursement for home milrinone was “locked in” at the 2003, AWP-5% price. Since milrinone became generically available however, home infusion companies have able to acquire it for 1/20th the amount Medicare is reimbursing– creating substantial profits.3 This “overpayment” is what the Cures Act aims to correct.
While that initially sounds like a wonderful idea, it is not quite so simple. During the last 15 years of overpayment, home infusion companies used much of those monies to cross-subsidize other necessary services that Medicare didn’t cover. These unreimbursed “professional services” include things like drug compounding, drug delivery, equipment maintenance, and patient education. For office-based infusions, they are separately reimbursed. No such provision has ever existed for home infusions. Drastically cutting milrinone funding without making allowance for these necessary, but otherwise unreimbursed professional services risks a significant, financial shock to the home infusion industry, the ramifications of which are unknown.
Fortunately, it turns out that the Cures Act does finally creates a separate provision for the reimbursement of home infusion professional services. Unfortunately, this section of the bill does not go into effect until 2021. This leaves a 4-year gap and does little to minimize the financial impact of the change in this short term. During the next four years, home infusion companies will have their most lucrative drug’s reimbursement cut by 92% (from $64,152 to $5,403 per beneficiary per year) and simultaneously be responsible for an estimated cost of $120 per day per beneficiary ($43,800 per beneficiary per year) in unreimbursed professional services.4 While large, diversified companies may be able to withstand the financial shock, other, smaller providers may not.
At present, the home infusion industry is highly fragmented with a few large, national companies covering about 25% of the market and the remainder supplied by some 800–1,000 smaller, independent businesses.5 One potential outcome of these economic changes is that some smaller home infusion companies, many of which are likely in rural and underserved areas, may not financially be able to continue supporting home inotropic infusions. This could result in some patients losing access temporarily or permanently, to home infusion services.
In the long run, these economic changes may not all be bad. Price corrections such as these are a necessary part of efficient market functioning. However, in the short term, a shrinking number of suppliers may impact patient access and have farther reaching effects the healthcare system. Even if larger companies ultimately do move into these areas and fill the market gap, there would almost certainly be significant disruption in home inotropic service. Without access to home therapy, many of these advanced heart failure patients may face longer and more frequent hospital stays, expensive rehabilitation hospitals or the loss of therapy. Despite Medicare’s projected savings, contraction of the home infusion industry may paradoxically increase spending as inpatient and long term acute rehabilitation hospitals see increased lengths of stay and higher admission and readmission rates.
We believe that the ultimate solution is to create a rational reimbursement system for home infusion drugs where all necessary costs, both drug and service costs, are appropriately identified and reimbursed fairly. Until then, attention must be paid to the next four years. It is important to remember that while this change most significantly impacts home inotropic therapy, the larger issue of access to home infusion services affects patients with rheumatologic, dermatologic, neurologic and many other diseases as well. Ways to expedite enactment of the Cures Act professional services provision or creation of a new stop gap measure should be explored. Until a solution can be reached, we all must remain alert to unintended consequences of this change, both for our patients’ health and well-being, and the costs to our system.
Figure 1.
Number of Medicare Fee-For-Service Beneficiaries and Annual Spending for Home Inotropic Therapy, 2010–2014
The total number of Medicare fee-for-service beneficiaries receiving home inotropic therapy and Medicare’s total annual spending on home inotropic therapy has increased from 2010 to 2014.
Acknowledgments
The authors would like to acknowledge Dr. Haiden Huskamp and Dr. Sharon-Lise Normand for their mentorship and guidance in the writing of this manuscript.
Footnotes
Conflict of Interest Disclosures
None
References
- 1.Harjai KJ, Mehra MR, Ventura HO, Lapeyre YM, Murgo JP, Stapleton DD, Smart FW. Home inotropic therapy in advanced heart failure: cost analysis and clinical outcomes. Chest. 1997;112:1298–303. doi: 10.1378/chest.112.5.1298. [DOI] [PubMed] [Google Scholar]
- 2.Direct spending and revenue effects for H.R. 34. [Accessed on: 4/26/2017];Congressional Budget Office. 2016 Available at: https://www.cbo.gov/sites/default/files/114th-congress-2015-2016/costestimate/hr34.pdf.
- 3.Murrin S. Recommendation Follow Up Memorandum Report: Implementing OIG Recommendation Would Have Reduced Payments for DME Infusion Drugs by Hundreds of Millions of Dollars. [Accessed on: 4/26/2017];Office of the Inspector General. 2015 Available at: https://oig.hhs.gov/oei/reports/oei-12-15-00110.pdf.
- 4.Drozd EM, Hoban N. Impact on Medicare Expenditures From Expanding Coverage of Infusion Therapy of Anti-Infective Drugs to the Home Setting. [Accessed on: 4/26/2017];Avalere. 2014 Available at: http://www.nhia.org/resource/legislative/documents/AvalereFinalHomeInfusionReport.pdf.
- 5.Home Infusion Industry Overview. Harris Williams and Company; 2014. [Accessed on: 4/26/2017]. Available at: http://www.harriswilliams.com/system/files/industry_update/2014.6.24_home_infusion_industry_overview.pdf. [Google Scholar]